University Chemistry Assignment: Acid-Base Equilibria and Kinetics
VerifiedAdded on 2023/01/09
|5
|1232
|75
Homework Assignment
AI Summary
This chemistry assignment solution covers a range of topics including pH and pOH calculations, acid-base reactions, and equilibrium. It explores concepts such as the determination of pH for various solutions, including strong bases like NaOH and weak acids like HNO3, and calculations involving the dissociation constant (Ka) for acids. The solution also delves into buffer action and the effects of temperature, concentration, and catalysts on reaction rates. Furthermore, it examines the rate laws for different reaction orders and the mechanisms of SN1 and SN2 reactions. Overall, the assignment provides a comprehensive overview of fundamental chemical principles, offering detailed explanations and calculations for each problem.

1.
pH=-log10(activity of H+)
pOH=-log10(activity of OH-)
and pH+pOH=14
a. NaOH, 0245 mol dm-3
OH- concentration=0.245
pOH=-log10(0.245) =0.611
pH=14-0.611=13.389
b. HNO3
Concentration of H+=0.500M
pH=-log10(0.50) =0.301
c. Sr(OH)2
RFM=(87.6)+((16+1)*2)=121.6
Number of moles= Mass
RFM = 0.450
121.6 =3.701× 10−3 mols
molarity= moles
volume(dm3 )=3.701 ×10−3
0.1 =0.037 M
Sr ( OH 2 ) → Sr+2 ( aq ) +2 OH−¿ ¿
From the equation, the concentration of OH−¿¿=0.037 ×2=0.074 M
pOH=-log10(0.074) =1.131
pH=14-1.131=12.869
2.
a. HCOOH(aq) (Acid)+H2O(base)> HCOO-(aq) (conjugate acid) +H3O+(aq) (conjugate acid)
b. H2SO4(aq)(acid) +HNO3(aq)(base)>H2SO4-(aq) (conjugate base) +H2NO3+(aq) (conjugate
acid)
3. pKa=-log(Ka)
a.
CH3CH2COOH
4.88=-log10(Ka)
Ka=antilog(-4.88)=1.3183 ×10−5
C6H5COOH
Ka=antilog(-4.2)= 6.3096×10−5
b. C6H5COOH is a stronger acid than CH3CH2COOH because it has a larger value of
Ka and larger ka implies that more of the acid dissociates to form hydrogen ions.
c. pH = ½ × pK – ½ × log [AH]
pH=-log10(activity of H+)
pOH=-log10(activity of OH-)
and pH+pOH=14
a. NaOH, 0245 mol dm-3
OH- concentration=0.245
pOH=-log10(0.245) =0.611
pH=14-0.611=13.389
b. HNO3
Concentration of H+=0.500M
pH=-log10(0.50) =0.301
c. Sr(OH)2
RFM=(87.6)+((16+1)*2)=121.6
Number of moles= Mass
RFM = 0.450
121.6 =3.701× 10−3 mols
molarity= moles
volume(dm3 )=3.701 ×10−3
0.1 =0.037 M
Sr ( OH 2 ) → Sr+2 ( aq ) +2 OH−¿ ¿
From the equation, the concentration of OH−¿¿=0.037 ×2=0.074 M
pOH=-log10(0.074) =1.131
pH=14-1.131=12.869
2.
a. HCOOH(aq) (Acid)+H2O(base)> HCOO-(aq) (conjugate acid) +H3O+(aq) (conjugate acid)
b. H2SO4(aq)(acid) +HNO3(aq)(base)>H2SO4-(aq) (conjugate base) +H2NO3+(aq) (conjugate
acid)
3. pKa=-log(Ka)
a.
CH3CH2COOH
4.88=-log10(Ka)
Ka=antilog(-4.88)=1.3183 ×10−5
C6H5COOH
Ka=antilog(-4.2)= 6.3096×10−5
b. C6H5COOH is a stronger acid than CH3CH2COOH because it has a larger value of
Ka and larger ka implies that more of the acid dissociates to form hydrogen ions.
c. pH = ½ × pK – ½ × log [AH]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

CH3CH2COOH
pK=-log(1.3183 ×10−5)=4.88
pH=0.5(4.88)-0.5(log (0.25))=2.741
C6H5COOH
pK=-log(6.3096×10−5)=4.2
pH=0.5(4.2) -0.5(log(0.25))=2.401
4.
Ethanoic acid is a weak acid and its equilibrium is to the left. Addition of CH3COONa
introduces more ethanoate ions in the system. From Le Chatelier’s principle, addition of
more ethanoate ions makes the system to shift equilibrium so as to oppose addition of
more ethanoate ions. The equilibrium thus shifts more to the left. The resulting solution is
made up of un-ionized ethanoic acid, more ethanoate ions from sodium ethanoate and
hydrogen ions.
Buffer action
Adding an acid.
The resulting solution eliminates majority of the new hydrogen ions thus preventing
drastic drop of pH. H+ from acid reacts with ethanoate ions to form ethanoic acid an
although the reaction is reversible, ethanoic acid is a weak acid.
Because majority of H+ are removed in this manner the pH will not vary much
Addition of a base
Two reactions will remove hydroxide ion
1. Reaction with ethanoic acid to form water
2. Reaction with hydrogen ions. There are some H+ ions present in the when ethanoic
acid is ionized. OH- react with them to form water and the process continues until
most of the hydroxide are removed
pK=-log(1.3183 ×10−5)=4.88
pH=0.5(4.88)-0.5(log (0.25))=2.741
C6H5COOH
pK=-log(6.3096×10−5)=4.2
pH=0.5(4.2) -0.5(log(0.25))=2.401
4.
Ethanoic acid is a weak acid and its equilibrium is to the left. Addition of CH3COONa
introduces more ethanoate ions in the system. From Le Chatelier’s principle, addition of
more ethanoate ions makes the system to shift equilibrium so as to oppose addition of
more ethanoate ions. The equilibrium thus shifts more to the left. The resulting solution is
made up of un-ionized ethanoic acid, more ethanoate ions from sodium ethanoate and
hydrogen ions.
Buffer action
Adding an acid.
The resulting solution eliminates majority of the new hydrogen ions thus preventing
drastic drop of pH. H+ from acid reacts with ethanoate ions to form ethanoic acid an
although the reaction is reversible, ethanoic acid is a weak acid.
Because majority of H+ are removed in this manner the pH will not vary much
Addition of a base
Two reactions will remove hydroxide ion
1. Reaction with ethanoic acid to form water
2. Reaction with hydrogen ions. There are some H+ ions present in the when ethanoic
acid is ionized. OH- react with them to form water and the process continues until
most of the hydroxide are removed

5. Increase in temperature and rate of reaction. Increasing temperature increases kinetic
energy and velocity of the particles. The particles will thus collide more frequently since
they move faster hence encountering more reactant particles. Increased also ensures that
the particles that collide have enough amount of energy required for effective collision.
More particles will thus have the required activation energy thus increased chemical
reaction.
Concentration and rates of reaction. In a chemical reaction, the rate of reaction is
proportional to the number of collissions per unit time. Increasing the concentration of
either of the reactant increases the number of collisions. This increases the number of
successful collisions and reaction rate. This explains why increasing concentration
increases reaction rate.
Catalyst. Addition of a catalyst lowers the activation energy because they provide a new
pathway with relatively lower activation energy. This has an effect of increasing
successful collisions thus increasing the rate of reaction.
6. A third order reaction is one in which the initial rate of reaction increases eightfold when
the reactant is increased. The 4 different rate law equations that might apply to this type
of reaction include
rate=k[A]3----------------------------1
rate=k[B]3-------------------------------2
rate=k[A][B]2------------------3
rate=k[A]2[B]----------------4
7. Generally, for a two reactant reaction, the rate of reaction,
Rate= k[A]a[B]b
In order of reaction with respect to O2 (g) and NO(g), a and b have to be evaluated. From
the given values,
3.2 ×10−3 =k (0.011)a 0.013b−−−−−−1
6 .4 × 10−3=k (0.022)a 0.013b−−−−−−2
6 .4 × 10−3=k (0.01)a 0.026b−−−−−−3
Dividing enq 1 by 2
0.011a
0.022a =0.5
a=1
Substituting a in eqn 2 and 3 and equating the two equations,
k (0.022)1 0.013b=k (0.01)1 0.026b
b=1.138
Substituting the values of a and be in any of the equation, say
energy and velocity of the particles. The particles will thus collide more frequently since
they move faster hence encountering more reactant particles. Increased also ensures that
the particles that collide have enough amount of energy required for effective collision.
More particles will thus have the required activation energy thus increased chemical
reaction.
Concentration and rates of reaction. In a chemical reaction, the rate of reaction is
proportional to the number of collissions per unit time. Increasing the concentration of
either of the reactant increases the number of collisions. This increases the number of
successful collisions and reaction rate. This explains why increasing concentration
increases reaction rate.
Catalyst. Addition of a catalyst lowers the activation energy because they provide a new
pathway with relatively lower activation energy. This has an effect of increasing
successful collisions thus increasing the rate of reaction.
6. A third order reaction is one in which the initial rate of reaction increases eightfold when
the reactant is increased. The 4 different rate law equations that might apply to this type
of reaction include
rate=k[A]3----------------------------1
rate=k[B]3-------------------------------2
rate=k[A][B]2------------------3
rate=k[A]2[B]----------------4
7. Generally, for a two reactant reaction, the rate of reaction,
Rate= k[A]a[B]b
In order of reaction with respect to O2 (g) and NO(g), a and b have to be evaluated. From
the given values,
3.2 ×10−3 =k (0.011)a 0.013b−−−−−−1
6 .4 × 10−3=k (0.022)a 0.013b−−−−−−2
6 .4 × 10−3=k (0.01)a 0.026b−−−−−−3
Dividing enq 1 by 2
0.011a
0.022a =0.5
a=1
Substituting a in eqn 2 and 3 and equating the two equations,
k (0.022)1 0.013b=k (0.01)1 0.026b
b=1.138
Substituting the values of a and be in any of the equation, say
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
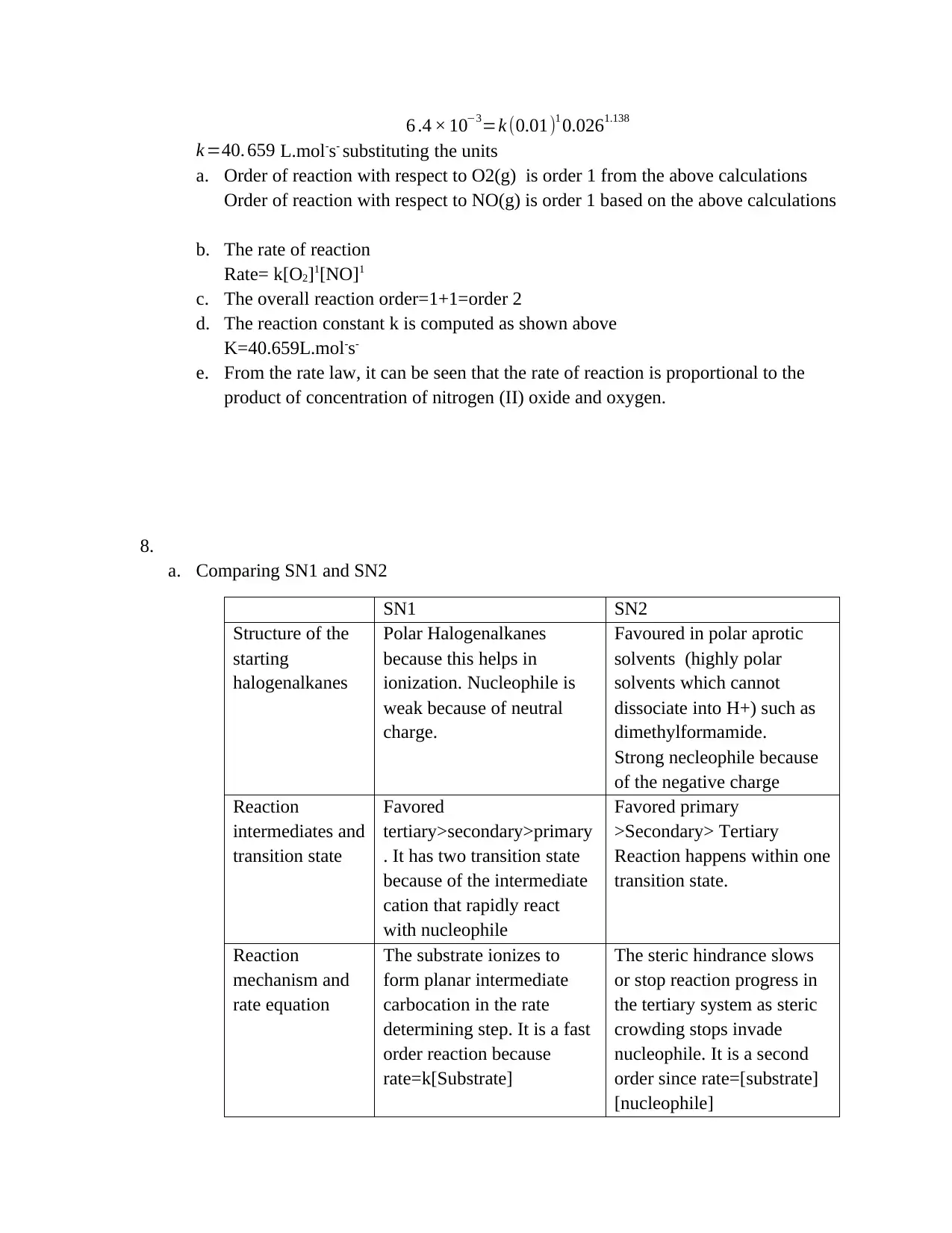
6 .4 × 10−3=k (0.01)1 0.0261.138
k =40. 659 L.mol-s- substituting the units
a. Order of reaction with respect to O2(g) is order 1 from the above calculations
Order of reaction with respect to NO(g) is order 1 based on the above calculations
b. The rate of reaction
Rate= k[O2]1[NO]1
c. The overall reaction order=1+1=order 2
d. The reaction constant k is computed as shown above
K=40.659L.mol-s-
e. From the rate law, it can be seen that the rate of reaction is proportional to the
product of concentration of nitrogen (II) oxide and oxygen.
8.
a. Comparing SN1 and SN2
SN1 SN2
Structure of the
starting
halogenalkanes
Polar Halogenalkanes
because this helps in
ionization. Nucleophile is
weak because of neutral
charge.
Favoured in polar aprotic
solvents (highly polar
solvents which cannot
dissociate into H+) such as
dimethylformamide.
Strong necleophile because
of the negative charge
Reaction
intermediates and
transition state
Favored
tertiary>secondary>primary
. It has two transition state
because of the intermediate
cation that rapidly react
with nucleophile
Favored primary
>Secondary> Tertiary
Reaction happens within one
transition state.
Reaction
mechanism and
rate equation
The substrate ionizes to
form planar intermediate
carbocation in the rate
determining step. It is a fast
order reaction because
rate=k[Substrate]
The steric hindrance slows
or stop reaction progress in
the tertiary system as steric
crowding stops invade
nucleophile. It is a second
order since rate=[substrate]
[nucleophile]
k =40. 659 L.mol-s- substituting the units
a. Order of reaction with respect to O2(g) is order 1 from the above calculations
Order of reaction with respect to NO(g) is order 1 based on the above calculations
b. The rate of reaction
Rate= k[O2]1[NO]1
c. The overall reaction order=1+1=order 2
d. The reaction constant k is computed as shown above
K=40.659L.mol-s-
e. From the rate law, it can be seen that the rate of reaction is proportional to the
product of concentration of nitrogen (II) oxide and oxygen.
8.
a. Comparing SN1 and SN2
SN1 SN2
Structure of the
starting
halogenalkanes
Polar Halogenalkanes
because this helps in
ionization. Nucleophile is
weak because of neutral
charge.
Favoured in polar aprotic
solvents (highly polar
solvents which cannot
dissociate into H+) such as
dimethylformamide.
Strong necleophile because
of the negative charge
Reaction
intermediates and
transition state
Favored
tertiary>secondary>primary
. It has two transition state
because of the intermediate
cation that rapidly react
with nucleophile
Favored primary
>Secondary> Tertiary
Reaction happens within one
transition state.
Reaction
mechanism and
rate equation
The substrate ionizes to
form planar intermediate
carbocation in the rate
determining step. It is a fast
order reaction because
rate=k[Substrate]
The steric hindrance slows
or stop reaction progress in
the tertiary system as steric
crowding stops invade
nucleophile. It is a second
order since rate=[substrate]
[nucleophile]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
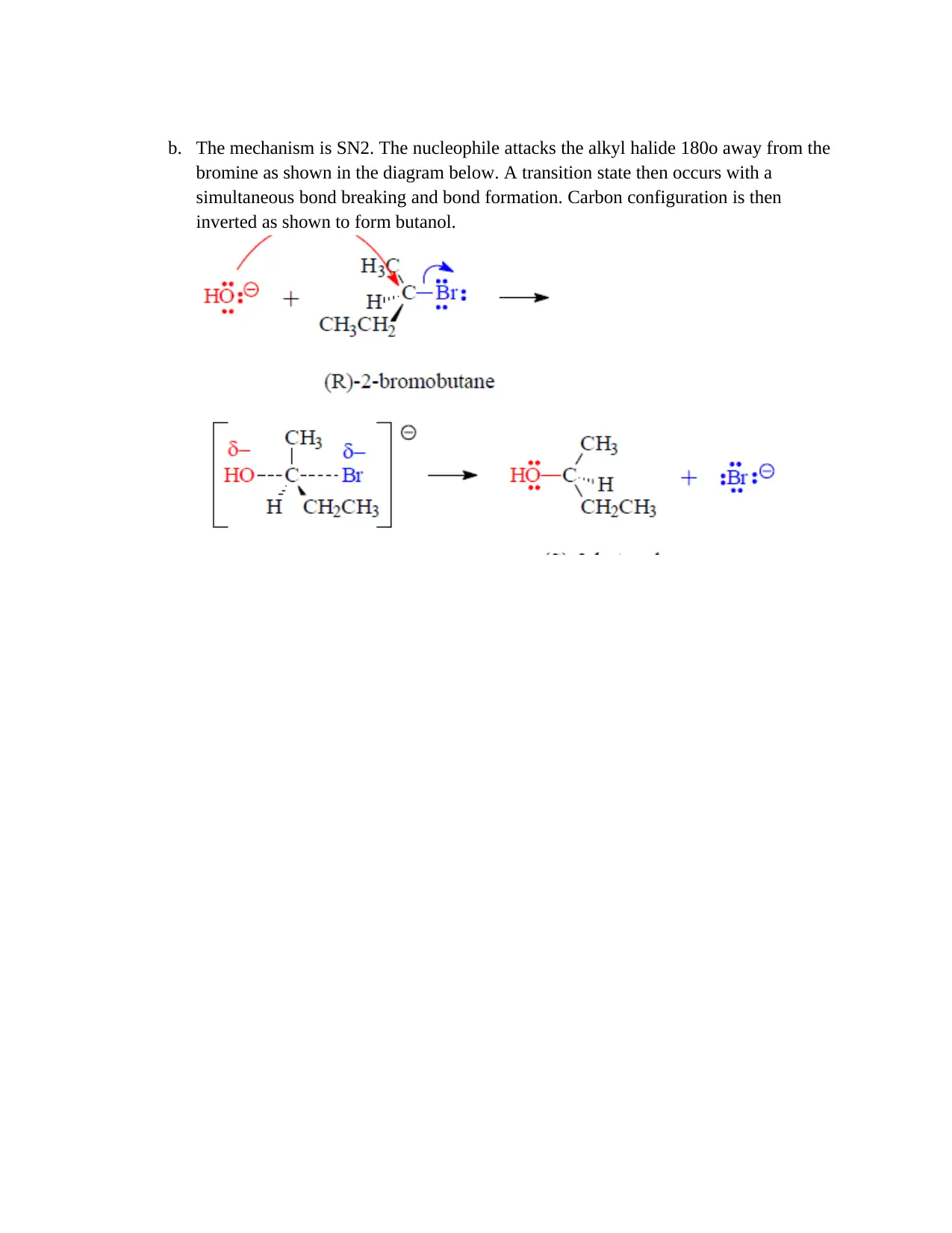
b. The mechanism is SN2. The nucleophile attacks the alkyl halide 180o away from the
bromine as shown in the diagram below. A transition state then occurs with a
simultaneous bond breaking and bond formation. Carbon configuration is then
inverted as shown to form butanol.
bromine as shown in the diagram below. A transition state then occurs with a
simultaneous bond breaking and bond formation. Carbon configuration is then
inverted as shown to form butanol.
1 out of 5
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
