Solar Cells and Quantum Dots
VerifiedAdded on 2022/10/04
|24
|7144
|382
AI Summary
This report provides an overview of solar energy, quantum dot solar cells, core-shell quantum dots, and more. It discusses the three generations of solar cells and their efficiencies. The report also covers the photo electrochemistry at sensitized films and the quenching of fluorescence peak of cdte qds with selenide and electrolyte to the qds part.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: SOLAR CELLS AND QUANTUM DOTS
Solar Cells and Quantum Dots
Name of the Student
Name of the University
Author Note
Solar Cells and Quantum Dots
Name of the Student
Name of the University
Author Note
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1
SOLAR CELLS AND QUANTUM DOTS
Table of Contents
Introduction......................................................................................................................................2
Discussion........................................................................................................................................2
Energy transfer in the semiconductor..........................................................................................2
A general overview of solar energy.............................................................................................2
Quantum dot solar cells...............................................................................................................4
Core-shell quantum dots..............................................................................................................6
Solar cells.....................................................................................................................................8
Quantum confinement.................................................................................................................9
Self-assembled photonic structures for light localization..........................................................13
Ordered structure “Photonic crystals”.......................................................................................14
Photo electrochemistry at Q.D.s sensitized films......................................................................14
Quenching of fluorescence peak of cdte qds with selenide and electrolyte to the qds part.......15
Conclusion.....................................................................................................................................17
SOLAR CELLS AND QUANTUM DOTS
Table of Contents
Introduction......................................................................................................................................2
Discussion........................................................................................................................................2
Energy transfer in the semiconductor..........................................................................................2
A general overview of solar energy.............................................................................................2
Quantum dot solar cells...............................................................................................................4
Core-shell quantum dots..............................................................................................................6
Solar cells.....................................................................................................................................8
Quantum confinement.................................................................................................................9
Self-assembled photonic structures for light localization..........................................................13
Ordered structure “Photonic crystals”.......................................................................................14
Photo electrochemistry at Q.D.s sensitized films......................................................................14
Quenching of fluorescence peak of cdte qds with selenide and electrolyte to the qds part.......15
Conclusion.....................................................................................................................................17

2
SOLAR CELLS AND QUANTUM DOTS
Introduction
Semiconductors are the materials that have their levels of resistance between that of a conductor
as well as an insulator. By the introduction of the impurities into the lattice of the crystals, the
modification of the conductivity of the semiconductors can be done. These are common and are
found in all electronic devices. This report deals with an overview of the solar energy along with
the kinds of solar cells along with all the three generations of the solar cells, along with their
percentages of efficiencies. The concept of the quantum dot solar cells has also been discussed
along with the core-shell of quantum dots and the quantum confinement is also present. The
photo electrochemistry at sensitized films are also mentioned in this report.
Basically solar cell is any kind of device that helps in the conversion of light energy to
the electrical energy by the effect that is photovoltaic in nature as photovoltaics is the method
involved for converting the sunlight in the form of electricity directly with utilization of solar
cells which is rapidly growing nowadays and is becoming increasingly important day by day.
Discussion
Energy transfer in the semiconductor
Transfer of Energy refers to the energy transformation, involves the transfer of heat, collision
electric power transmission and many more. The removal of energy in the semiconductor is
significant.
A general overview of solar energy
The Solar Energy is very advantageous in many ways, one of the reasons is that all the
radioactive, as well as chemical by-products of the thermonuclear reactions which creates
SOLAR CELLS AND QUANTUM DOTS
Introduction
Semiconductors are the materials that have their levels of resistance between that of a conductor
as well as an insulator. By the introduction of the impurities into the lattice of the crystals, the
modification of the conductivity of the semiconductors can be done. These are common and are
found in all electronic devices. This report deals with an overview of the solar energy along with
the kinds of solar cells along with all the three generations of the solar cells, along with their
percentages of efficiencies. The concept of the quantum dot solar cells has also been discussed
along with the core-shell of quantum dots and the quantum confinement is also present. The
photo electrochemistry at sensitized films are also mentioned in this report.
Basically solar cell is any kind of device that helps in the conversion of light energy to
the electrical energy by the effect that is photovoltaic in nature as photovoltaics is the method
involved for converting the sunlight in the form of electricity directly with utilization of solar
cells which is rapidly growing nowadays and is becoming increasingly important day by day.
Discussion
Energy transfer in the semiconductor
Transfer of Energy refers to the energy transformation, involves the transfer of heat, collision
electric power transmission and many more. The removal of energy in the semiconductor is
significant.
A general overview of solar energy
The Solar Energy is very advantageous in many ways, one of the reasons is that all the
radioactive, as well as chemical by-products of the thermonuclear reactions which creates

3
SOLAR CELLS AND QUANTUM DOTS
pollution in the sun, are retained itself, and only the transmission of the radiant energy that is
pure takes place on the earth. Solar Energy can be considered as non-polluting in nature under
the above mentioned conditions. The most important issue is energy production, which includes
the growth of population, quality of life as well as fair energy distribution.
Nowadays, energy is obtained primarily mainly with the help of the fossil fuels that are
non-renewable like fuel, natural gases, oil. It has been indicated that if the similar trend of energy
consumption is continued, in 2035 the contribution of fossil fuels to the needs of the world will
highly increase. A dynamic process of transition of energy is initiated due to the depletion of the
natural resources and the necessity of the protection of the environment. Consumption of global
Energy is strongly dependent on fossil fuels is now moving towards a system which is firmly
based on renewable resources.
On a worldwide scale, huge methods are being implemented for renewable energy
development. The utilization of the semiconductor nanoparticles which are situated in these thin
films is fully expanded along with the utilization of the huge bandgap semiconductors due to the
usage of titanium mostly. When the U.V. light source irradiates the nanoparticles of titanium
dioxide, the excited electrons are transferred to the conduction band from the valence band.
SOLAR CELLS AND QUANTUM DOTS
pollution in the sun, are retained itself, and only the transmission of the radiant energy that is
pure takes place on the earth. Solar Energy can be considered as non-polluting in nature under
the above mentioned conditions. The most important issue is energy production, which includes
the growth of population, quality of life as well as fair energy distribution.
Nowadays, energy is obtained primarily mainly with the help of the fossil fuels that are
non-renewable like fuel, natural gases, oil. It has been indicated that if the similar trend of energy
consumption is continued, in 2035 the contribution of fossil fuels to the needs of the world will
highly increase. A dynamic process of transition of energy is initiated due to the depletion of the
natural resources and the necessity of the protection of the environment. Consumption of global
Energy is strongly dependent on fossil fuels is now moving towards a system which is firmly
based on renewable resources.
On a worldwide scale, huge methods are being implemented for renewable energy
development. The utilization of the semiconductor nanoparticles which are situated in these thin
films is fully expanded along with the utilization of the huge bandgap semiconductors due to the
usage of titanium mostly. When the U.V. light source irradiates the nanoparticles of titanium
dioxide, the excited electrons are transferred to the conduction band from the valence band.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
4
SOLAR CELLS AND QUANTUM DOTS
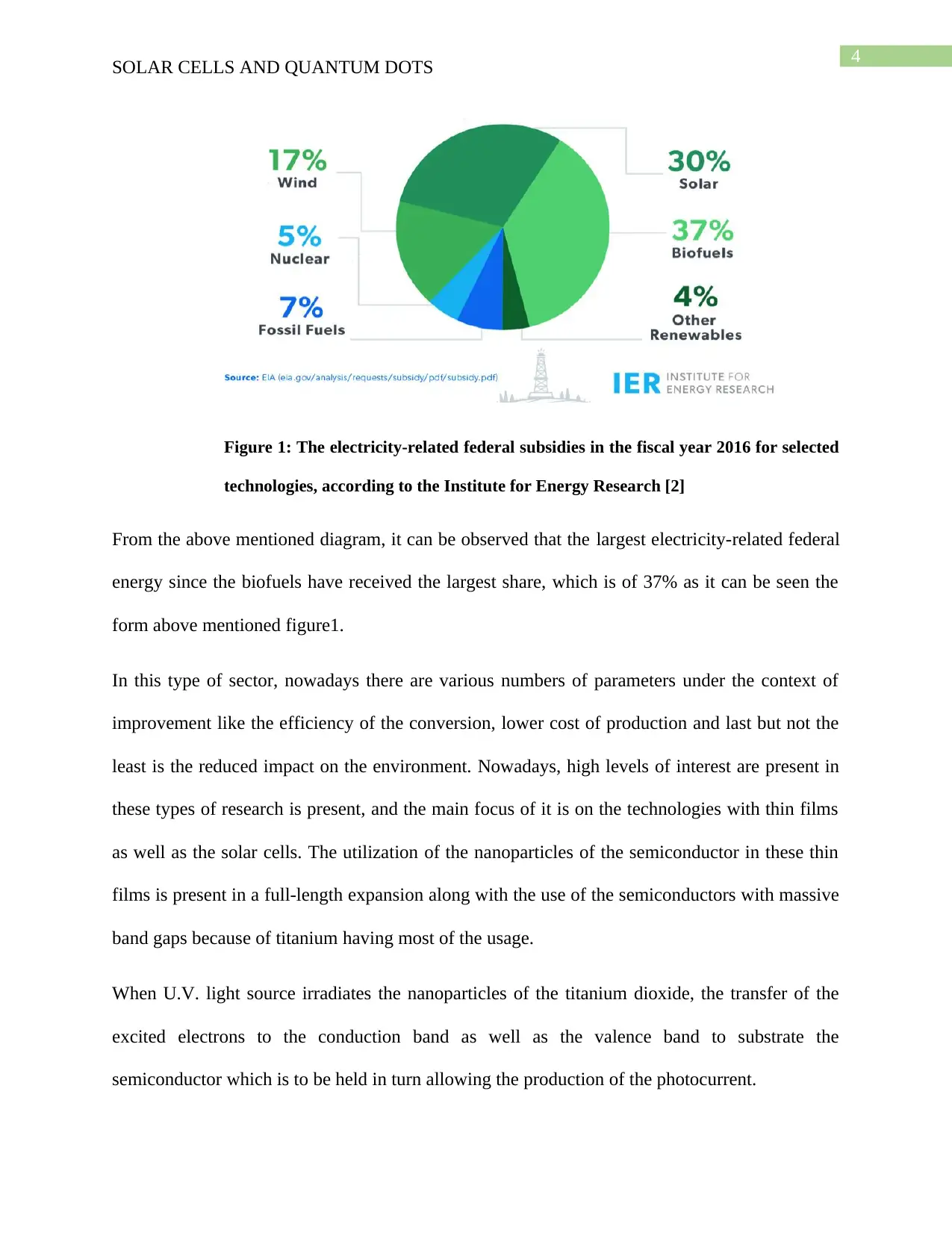
Figure 1: The electricity-related federal subsidies in the fiscal year 2016 for selected
technologies, according to the Institute for Energy Research [2]
From the above mentioned diagram, it can be observed that the largest electricity-related federal
energy since the biofuels have received the largest share, which is of 37% as it can be seen the
form above mentioned figure1.
In this type of sector, nowadays there are various numbers of parameters under the context of
improvement like the efficiency of the conversion, lower cost of production and last but not the
least is the reduced impact on the environment. Nowadays, high levels of interest are present in
these types of research is present, and the main focus of it is on the technologies with thin films
as well as the solar cells. The utilization of the nanoparticles of the semiconductor in these thin
films is present in a full-length expansion along with the use of the semiconductors with massive
band gaps because of titanium having most of the usage.
When U.V. light source irradiates the nanoparticles of the titanium dioxide, the transfer of the
excited electrons to the conduction band as well as the valence band to substrate the
semiconductor which is to be held in turn allowing the production of the photocurrent.
SOLAR CELLS AND QUANTUM DOTS
Figure 1: The electricity-related federal subsidies in the fiscal year 2016 for selected
technologies, according to the Institute for Energy Research [2]
From the above mentioned diagram, it can be observed that the largest electricity-related federal
energy since the biofuels have received the largest share, which is of 37% as it can be seen the
form above mentioned figure1.
In this type of sector, nowadays there are various numbers of parameters under the context of
improvement like the efficiency of the conversion, lower cost of production and last but not the
least is the reduced impact on the environment. Nowadays, high levels of interest are present in
these types of research is present, and the main focus of it is on the technologies with thin films
as well as the solar cells. The utilization of the nanoparticles of the semiconductor in these thin
films is present in a full-length expansion along with the use of the semiconductors with massive
band gaps because of titanium having most of the usage.
When U.V. light source irradiates the nanoparticles of the titanium dioxide, the transfer of the
excited electrons to the conduction band as well as the valence band to substrate the
semiconductor which is to be held in turn allowing the production of the photocurrent.

5
SOLAR CELLS AND QUANTUM DOTS
Quantum dot solar cells
A QDSC or quantum dot solar cell is a design of the solar cell which utilizes the quantum
dots in the form of photovoltaic material that can be absorbed. It takes an initiative for replacing
the materials that are bulk like cadmium telluride (CdTe), silicon and Copper indium gallium
selenide (CIGS). The quantum dots consists of band gap which are tunable across a massive
range of levels of energy by the method of altering their size. The band gap is constant by the
selection of the materials in context of the bulk materials. The quantum dots is made more
attractive by this property for the solar cells with multiple junctions where various kinds of
materials are utilized for the improvement of the efficiency by the process of harvesting of the
multiple parts of the solar spectrum. Basically the quantum dots are the particles which are
semiconducting in nature.
This dichotomy has motivated investigations of a novel protocols to localize and increase
light energy conversion by enhancing the absorbance of ultra-thin films; photonic crystals [7][8]
[9][10][11][12][13][14][15][16][17] and plasmonic particles [18][7][8][9][10][11][12][13][14]
[15][16][17] [19] are examples of systems that have been explored for this purpose. Investigation
of the novel protocols is motivated by the dichotomy for increasing as well as localizing the
conversion of the energy of light by the process of enhancement of the absorbance of those films
that are ultra-thin in nature.
The common examples of such kind of systems which have been explored for this
purpose are plasmonic particles as well as photonic crystals. Quantum dots (Q.D.) are one of the
emerging materials[20][21][22][23][24].
The quantum dots which can be also be abbreviated in the form of Q.D. are one of such
materials which are emerging and which have been studied for the solar cells of the third
generation because of the advantage of unique tunable electronic of it along with the theoretical
SOLAR CELLS AND QUANTUM DOTS
Quantum dot solar cells
A QDSC or quantum dot solar cell is a design of the solar cell which utilizes the quantum
dots in the form of photovoltaic material that can be absorbed. It takes an initiative for replacing
the materials that are bulk like cadmium telluride (CdTe), silicon and Copper indium gallium
selenide (CIGS). The quantum dots consists of band gap which are tunable across a massive
range of levels of energy by the method of altering their size. The band gap is constant by the
selection of the materials in context of the bulk materials. The quantum dots is made more
attractive by this property for the solar cells with multiple junctions where various kinds of
materials are utilized for the improvement of the efficiency by the process of harvesting of the
multiple parts of the solar spectrum. Basically the quantum dots are the particles which are
semiconducting in nature.
This dichotomy has motivated investigations of a novel protocols to localize and increase
light energy conversion by enhancing the absorbance of ultra-thin films; photonic crystals [7][8]
[9][10][11][12][13][14][15][16][17] and plasmonic particles [18][7][8][9][10][11][12][13][14]
[15][16][17] [19] are examples of systems that have been explored for this purpose. Investigation
of the novel protocols is motivated by the dichotomy for increasing as well as localizing the
conversion of the energy of light by the process of enhancement of the absorbance of those films
that are ultra-thin in nature.
The common examples of such kind of systems which have been explored for this
purpose are plasmonic particles as well as photonic crystals. Quantum dots (Q.D.) are one of the
emerging materials[20][21][22][23][24].
The quantum dots which can be also be abbreviated in the form of Q.D. are one of such
materials which are emerging and which have been studied for the solar cells of the third
generation because of the advantage of unique tunable electronic of it along with the theoretical

6
SOLAR CELLS AND QUANTUM DOTS
predictions as well as the optical properties with which the thermodynamic limit can be exceeded
for the sunlight conversion at a bulk semiconductor that is single resulting in the effects of the
quantum confinement. These kinds of nanoparticles are characterized by semiconductors with
small bandgap which can be able to absorb light over a spectral range which is wide from the
nearest U.V. to the visible as well as the infrared light.
Core-shell quantum dots
Now comes the turn of the core-shell quantum dots. The Nanocrystals of the core-shell are
considered or assumed as the nanoparticles of the semiconductor. These are unique, and the
reason behind this is the modulated properties of it which are related to the confinement of the
quantum. In the case of the core-shell quantum dot, the electronic structure of the semiconductor
has to be considered for the core of that nanoparticle as well as in case of the bulb.
There are four types of heterostructure core-shell which can always be encountered in
accordance with the difference in the energy levels in between the valence bands as well as the
conduction bands of both the core along with the bulb of the semiconductor with quantum dots.
These are defined in accordance with the position of the bandgap of the energy with respect to
one another. They are indicated as type I, type II as well as inverted type 1 core-shell Q.D.s as
shown below-mentioned figure.
SOLAR CELLS AND QUANTUM DOTS
predictions as well as the optical properties with which the thermodynamic limit can be exceeded
for the sunlight conversion at a bulk semiconductor that is single resulting in the effects of the
quantum confinement. These kinds of nanoparticles are characterized by semiconductors with
small bandgap which can be able to absorb light over a spectral range which is wide from the
nearest U.V. to the visible as well as the infrared light.
Core-shell quantum dots
Now comes the turn of the core-shell quantum dots. The Nanocrystals of the core-shell are
considered or assumed as the nanoparticles of the semiconductor. These are unique, and the
reason behind this is the modulated properties of it which are related to the confinement of the
quantum. In the case of the core-shell quantum dot, the electronic structure of the semiconductor
has to be considered for the core of that nanoparticle as well as in case of the bulb.
There are four types of heterostructure core-shell which can always be encountered in
accordance with the difference in the energy levels in between the valence bands as well as the
conduction bands of both the core along with the bulb of the semiconductor with quantum dots.
These are defined in accordance with the position of the bandgap of the energy with respect to
one another. They are indicated as type I, type II as well as inverted type 1 core-shell Q.D.s as
shown below-mentioned figure.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
7
SOLAR CELLS AND QUANTUM DOTS
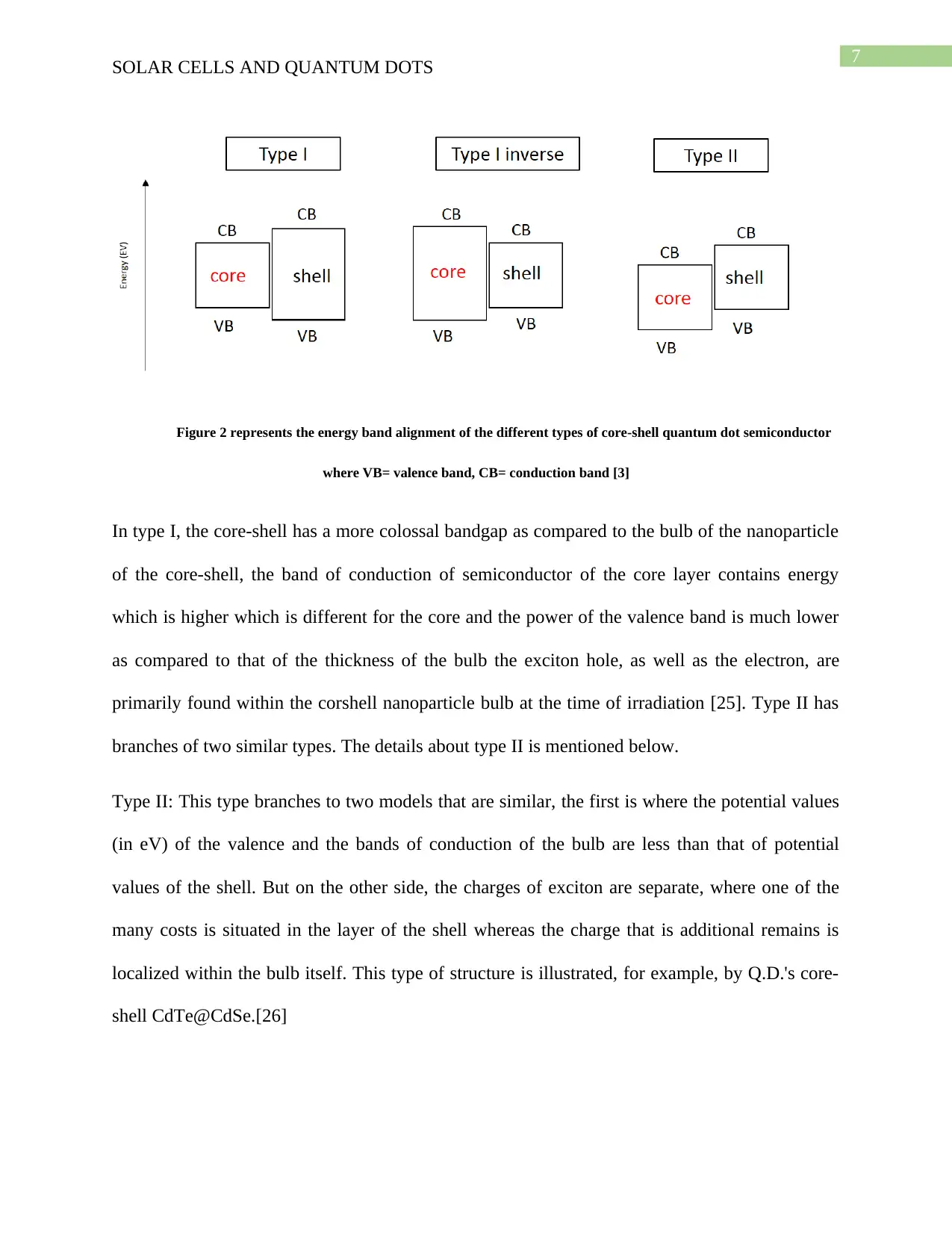
Figure 2 represents the energy band alignment of the different types of core-shell quantum dot semiconductor
where VB= valence band, CB= conduction band [3]
In type I, the core-shell has a more colossal bandgap as compared to the bulb of the nanoparticle
of the core-shell, the band of conduction of semiconductor of the core layer contains energy
which is higher which is different for the core and the power of the valence band is much lower
as compared to that of the thickness of the bulb the exciton hole, as well as the electron, are
primarily found within the corshell nanoparticle bulb at the time of irradiation [25]. Type II has
branches of two similar types. The details about type II is mentioned below.
Type II: This type branches to two models that are similar, the first is where the potential values
(in eV) of the valence and the bands of conduction of the bulb are less than that of potential
values of the shell. But on the other side, the charges of exciton are separate, where one of the
many costs is situated in the layer of the shell whereas the charge that is additional remains is
localized within the bulb itself. This type of structure is illustrated, for example, by Q.D.'s core-
shell CdTe@CdSe.[26]
SOLAR CELLS AND QUANTUM DOTS
Figure 2 represents the energy band alignment of the different types of core-shell quantum dot semiconductor
where VB= valence band, CB= conduction band [3]
In type I, the core-shell has a more colossal bandgap as compared to the bulb of the nanoparticle
of the core-shell, the band of conduction of semiconductor of the core layer contains energy
which is higher which is different for the core and the power of the valence band is much lower
as compared to that of the thickness of the bulb the exciton hole, as well as the electron, are
primarily found within the corshell nanoparticle bulb at the time of irradiation [25]. Type II has
branches of two similar types. The details about type II is mentioned below.
Type II: This type branches to two models that are similar, the first is where the potential values
(in eV) of the valence and the bands of conduction of the bulb are less than that of potential
values of the shell. But on the other side, the charges of exciton are separate, where one of the
many costs is situated in the layer of the shell whereas the charge that is additional remains is
localized within the bulb itself. This type of structure is illustrated, for example, by Q.D.'s core-
shell CdTe@CdSe.[26]

8
SOLAR CELLS AND QUANTUM DOTS
Inverted type I: This type has a gap smaller than the bulb of the Q.D.'s. The conduction band of
the shell has a potential value lower than the lamp of the Q.D.s. Conversely, the group of the
valence shell has a higher potential profit than that of the bulb. The electron and the exciton
holes are located only within the semiconductor shell during irradiation. CdS@CdSe core-shell
quantum dot, for example, belong to this category.[27]
The core-shell quantum dot used in this thesis is CdTe@CdSe type-II quantum dot. We will
present in more details the impacts of a shell on all electronic structures in this type of
configuration.
Core/shell Q.D.s with type-II energy levels alignment further offer enhanced separation of
charge across the interface of core-shell and absorbance of light with energy smaller as
compared to the the difference of core and shell materials.[28][29][30][31][32] Various Q.D.s
such as CdS, CdSe, CdTe, ZnSe, InP, CuIn2S, CdSe1-xTe and including Q-CdTe/CdSe and Q-
ZnTe/CdSe core/shell particles have been reported for sensitizing a wide band gap
semiconductor film which includes nanocrystalline (nc-) TiO2 with a liquid junction in a majorly
studied architecture of QDSC resembling a DSSC, but with the best efficiencies around 7%.[33]
[34][35][36][37][38][39][40][41]
Solar cells
The solar cells play a role for converting energy related to the solar photon into electrical
energy. This is executed by the method of absorbing the energy of the photon by the
semiconductors and the generation of the pairs of electron-hole. This particular idea was first
capitalized in the year 1905 with the photoelectric theory given by Albert Einstein. The first
demonstration of this phenomenon was done in the year 1839. Various researches in the solar
SOLAR CELLS AND QUANTUM DOTS
Inverted type I: This type has a gap smaller than the bulb of the Q.D.'s. The conduction band of
the shell has a potential value lower than the lamp of the Q.D.s. Conversely, the group of the
valence shell has a higher potential profit than that of the bulb. The electron and the exciton
holes are located only within the semiconductor shell during irradiation. CdS@CdSe core-shell
quantum dot, for example, belong to this category.[27]
The core-shell quantum dot used in this thesis is CdTe@CdSe type-II quantum dot. We will
present in more details the impacts of a shell on all electronic structures in this type of
configuration.
Core/shell Q.D.s with type-II energy levels alignment further offer enhanced separation of
charge across the interface of core-shell and absorbance of light with energy smaller as
compared to the the difference of core and shell materials.[28][29][30][31][32] Various Q.D.s
such as CdS, CdSe, CdTe, ZnSe, InP, CuIn2S, CdSe1-xTe and including Q-CdTe/CdSe and Q-
ZnTe/CdSe core/shell particles have been reported for sensitizing a wide band gap
semiconductor film which includes nanocrystalline (nc-) TiO2 with a liquid junction in a majorly
studied architecture of QDSC resembling a DSSC, but with the best efficiencies around 7%.[33]
[34][35][36][37][38][39][40][41]
Solar cells
The solar cells play a role for converting energy related to the solar photon into electrical
energy. This is executed by the method of absorbing the energy of the photon by the
semiconductors and the generation of the pairs of electron-hole. This particular idea was first
capitalized in the year 1905 with the photoelectric theory given by Albert Einstein. The first
demonstration of this phenomenon was done in the year 1839. Various researches in the solar

9
SOLAR CELLS AND QUANTUM DOTS
cells have been developing at a rapid rate along with the recent efforts for incrementing the
efficiency of power conversion in order to achieve the energy in order to fulfil the needs of the
world.
There are two kinds of solar cells, namely the photovoltaic solar cells and PEC. The
photovoltaic cell is an electrical device which is responsible for converting light energy into
electricity by the process of the photovoltaic effect,that is chemical as well as a physical
phenomenon. It is a kind of photoelectric cell which in turn can be described as a device and the
electrical characteristics of that particular device like the voltage, current or resistance vary
whenever it is exposed to light. The individual devices of the solar cells can be in turn combined
in order to form modules but otherwise are known as the solar panels.
The solar cells are discussed as photovoltaic regardless of the source like whether it is an
artificial light or sunlight. These are also used in the form of the photodetectors like the infrared
detectors which are used for light detection purposes or other radiations that are electromagnetic
in nature near the range which is visible or in measuring the intensity of light. The operation of
the photovoltaic cell involves three characteristics that are basic which are the separation of the
carriers of charge of opposite types, light absorption which either generates excitons or electron-
hole pairs along with the extraction of these carriers of charge to external circuits.
PEC or photoelectrochemical cell refers to one of the present two the distinct classes of a
device where the first one produces the electrical energy. The second one is the photoelectrolysis
cell which is a device that makes the usage of the light incident on semiconductor,
photosensitizer or an aqueous metal in an electronic device which is immersed in a solution of
electrolyte in order to cause a chemical reaction directly for the production of hydrogen via the
water electrolysis.
SOLAR CELLS AND QUANTUM DOTS
cells have been developing at a rapid rate along with the recent efforts for incrementing the
efficiency of power conversion in order to achieve the energy in order to fulfil the needs of the
world.
There are two kinds of solar cells, namely the photovoltaic solar cells and PEC. The
photovoltaic cell is an electrical device which is responsible for converting light energy into
electricity by the process of the photovoltaic effect,that is chemical as well as a physical
phenomenon. It is a kind of photoelectric cell which in turn can be described as a device and the
electrical characteristics of that particular device like the voltage, current or resistance vary
whenever it is exposed to light. The individual devices of the solar cells can be in turn combined
in order to form modules but otherwise are known as the solar panels.
The solar cells are discussed as photovoltaic regardless of the source like whether it is an
artificial light or sunlight. These are also used in the form of the photodetectors like the infrared
detectors which are used for light detection purposes or other radiations that are electromagnetic
in nature near the range which is visible or in measuring the intensity of light. The operation of
the photovoltaic cell involves three characteristics that are basic which are the separation of the
carriers of charge of opposite types, light absorption which either generates excitons or electron-
hole pairs along with the extraction of these carriers of charge to external circuits.
PEC or photoelectrochemical cell refers to one of the present two the distinct classes of a
device where the first one produces the electrical energy. The second one is the photoelectrolysis
cell which is a device that makes the usage of the light incident on semiconductor,
photosensitizer or an aqueous metal in an electronic device which is immersed in a solution of
electrolyte in order to cause a chemical reaction directly for the production of hydrogen via the
water electrolysis.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

10
SOLAR CELLS AND QUANTUM DOTS
The solar cells can be classified into three generations, which are first-generation, second
generation, third generation. The first generation is mainly based on the wafers of the silicon,
which consists of polycrystalline as well as single silicon [5]. The second generation which
consists of solar cells with thin films which is based on the thin semiconductor layers which are
few micrometers thick. The last but not the least is the third generation, which consists of various
technologies such as tandem solar cells, concentrator solar cells and the organic solar cells also
[6][7]. Among the solar cells of the second generation, Cadmium Telluride or CdTe solar cells
consist of an ideal bandgap of 1.44 eV, and hence a good match is shown by their absorption
along with the spectrum of absorption. The efficiency of first generation solar cell is 24.2%. The
efficiency of second generation solar cell is 22.6%. The efficiency of third generation solar cell
is 48%. The most efficient is the third generation solar cells.
Quantum confinement
The quantum dots which can be also be abbreviated in the form of Q.D. are one of such
materials which are emerging and which have been studied for the solar cells of the third
generation because of the advantage of unique tunable electronic of it along with the theoretical
predictions as well as the optical properties with which the thermodynamic limit can be exceeded
for the sunlight conversion at a bulk semiconductor that is single resulting in the effects of the
quantum confinement. This kind of nanoparticles are characterized by semiconductors with a
small bandgap which can be able to absorb light over a spectral range which is extensive from
the nearest U.V. to the visible as well as the infrared light.
The spectrum of absorbance as well as luminescence with the decreasing particle size is
shifted. An individual can change all the particle’s energetics by controlling the particle size of
the quantum dots. The band energies of the quantum dots which are increased can then be used
SOLAR CELLS AND QUANTUM DOTS
The solar cells can be classified into three generations, which are first-generation, second
generation, third generation. The first generation is mainly based on the wafers of the silicon,
which consists of polycrystalline as well as single silicon [5]. The second generation which
consists of solar cells with thin films which is based on the thin semiconductor layers which are
few micrometers thick. The last but not the least is the third generation, which consists of various
technologies such as tandem solar cells, concentrator solar cells and the organic solar cells also
[6][7]. Among the solar cells of the second generation, Cadmium Telluride or CdTe solar cells
consist of an ideal bandgap of 1.44 eV, and hence a good match is shown by their absorption
along with the spectrum of absorption. The efficiency of first generation solar cell is 24.2%. The
efficiency of second generation solar cell is 22.6%. The efficiency of third generation solar cell
is 48%. The most efficient is the third generation solar cells.
Quantum confinement
The quantum dots which can be also be abbreviated in the form of Q.D. are one of such
materials which are emerging and which have been studied for the solar cells of the third
generation because of the advantage of unique tunable electronic of it along with the theoretical
predictions as well as the optical properties with which the thermodynamic limit can be exceeded
for the sunlight conversion at a bulk semiconductor that is single resulting in the effects of the
quantum confinement. This kind of nanoparticles are characterized by semiconductors with a
small bandgap which can be able to absorb light over a spectral range which is extensive from
the nearest U.V. to the visible as well as the infrared light.
The spectrum of absorbance as well as luminescence with the decreasing particle size is
shifted. An individual can change all the particle’s energetics by controlling the particle size of
the quantum dots. The band energies of the quantum dots which are increased can then be used
11
SOLAR CELLS AND QUANTUM DOTS
to rectify, remote as well as suppress the transferring of the electrons between nanostructures of
the two semiconductors. This kind of composite structures help in correcting the flow of the
charge carriers as well as improve the performance of the photocatalytic or behaviour of
nanostructure related to the photoelectron-chemical of those systems that are based on the
semiconductors. For instance, nearly the ten times of enhancement in the efficiency of photo
catalyst has been achieved by the process of coupling of systems TiO2 and SnO2.
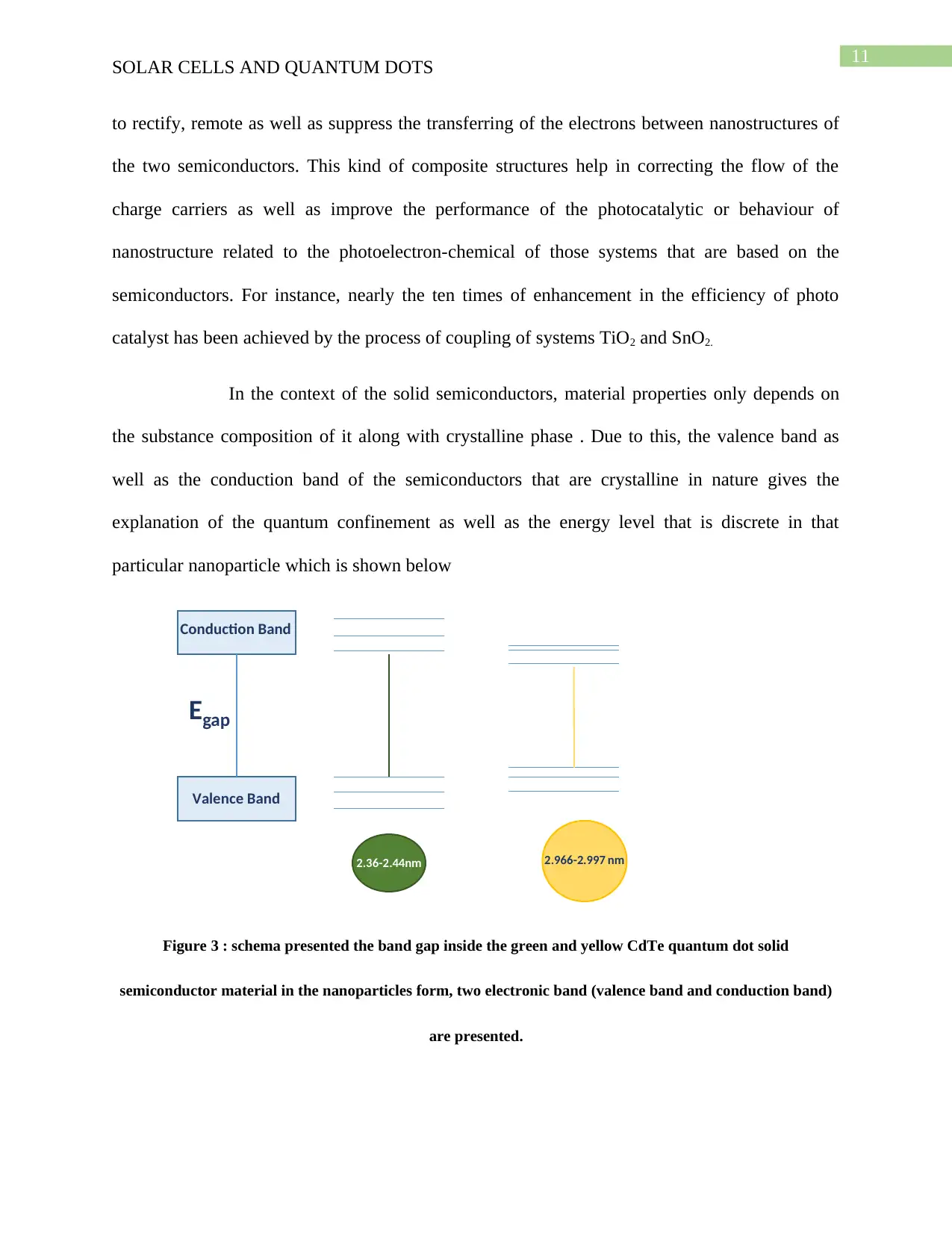
In the context of the solid semiconductors, material properties only depends on
the substance composition of it along with crystalline phase . Due to this, the valence band as
well as the conduction band of the semiconductors that are crystalline in nature gives the
explanation of the quantum confinement as well as the energy level that is discrete in that
particular nanoparticle which is shown below
Valence Band
Conduction Band
Egap
2.36-2.44nm 2.966-2.997 nm
Figure 3 : schema presented the band gap inside the green and yellow CdTe quantum dot solid
semiconductor material in the nanoparticles form, two electronic band (valence band and conduction band)
are presented.
SOLAR CELLS AND QUANTUM DOTS
to rectify, remote as well as suppress the transferring of the electrons between nanostructures of
the two semiconductors. This kind of composite structures help in correcting the flow of the
charge carriers as well as improve the performance of the photocatalytic or behaviour of
nanostructure related to the photoelectron-chemical of those systems that are based on the
semiconductors. For instance, nearly the ten times of enhancement in the efficiency of photo
catalyst has been achieved by the process of coupling of systems TiO2 and SnO2.
In the context of the solid semiconductors, material properties only depends on
the substance composition of it along with crystalline phase . Due to this, the valence band as
well as the conduction band of the semiconductors that are crystalline in nature gives the
explanation of the quantum confinement as well as the energy level that is discrete in that
particular nanoparticle which is shown below
Valence Band
Conduction Band
Egap
2.36-2.44nm 2.966-2.997 nm
Figure 3 : schema presented the band gap inside the green and yellow CdTe quantum dot solid
semiconductor material in the nanoparticles form, two electronic band (valence band and conduction band)
are presented.

12
SOLAR CELLS AND QUANTUM DOTS
When a semiconductor nanoparticle is excited by a photon, an electron-hole pair
(exciton) is constructed for a solid semiconductor material. The wavefunction associated with the
electron and exciton hole will therefore be confined to the inorganic bulb of the quantum dot.
This effect is called quantum confinement[44]. Its first consequence is the variation of the size of
the nanoparticle with the variation in the bandgap of the semiconductor (Energy separating the
valence band and the conduction band of the semiconductor).They are more notable in
semiconductor because it has energy gap in their electronic band structure, where it is tunable
due to their different size. Although with having intermediate bands, their absorbance of energy
is lower than the bandgap, which is, not allowed in bulk semiconductor, since there are no
discrete energy levels through the band gap. The energy of the discrete energy levels is also
varied when the size of the quantum dot nanoparticle varies.
Changing the geometry and size of the Q.D.s can change the electronic structure and the
optical properties like the band gap energy, the emission wavelength, the width of the gain
spectrum. The smaller the size of the nanoparticles the larger the band gap. Since they exhibit 3-
dimensional quantum confinements, which mean a spatial confinement between electron-hole
pairs "excitons" in one or more dimensions within the material, the resulting electron energy
levels are quantized and they are no longer treated as continuous, but as discrete (figure 2).
The excess kinetic energy, due to the absorbance elhe>eg, transforms to heat through
phonon emission leading to thermalization ref. The rates of excited state exciton cooling can be
slowed down in quantized energy levels. Then high-energy electrons and holes in higher excited
quantized states in the Q.D.s cool by relaxing from a given quantum level to the next lower level,
several phonons must be emitted simultaneously via electron-phonon scattering to satisfy energy
SOLAR CELLS AND QUANTUM DOTS
When a semiconductor nanoparticle is excited by a photon, an electron-hole pair
(exciton) is constructed for a solid semiconductor material. The wavefunction associated with the
electron and exciton hole will therefore be confined to the inorganic bulb of the quantum dot.
This effect is called quantum confinement[44]. Its first consequence is the variation of the size of
the nanoparticle with the variation in the bandgap of the semiconductor (Energy separating the
valence band and the conduction band of the semiconductor).They are more notable in
semiconductor because it has energy gap in their electronic band structure, where it is tunable
due to their different size. Although with having intermediate bands, their absorbance of energy
is lower than the bandgap, which is, not allowed in bulk semiconductor, since there are no
discrete energy levels through the band gap. The energy of the discrete energy levels is also
varied when the size of the quantum dot nanoparticle varies.
Changing the geometry and size of the Q.D.s can change the electronic structure and the
optical properties like the band gap energy, the emission wavelength, the width of the gain
spectrum. The smaller the size of the nanoparticles the larger the band gap. Since they exhibit 3-
dimensional quantum confinements, which mean a spatial confinement between electron-hole
pairs "excitons" in one or more dimensions within the material, the resulting electron energy
levels are quantized and they are no longer treated as continuous, but as discrete (figure 2).
The excess kinetic energy, due to the absorbance elhe>eg, transforms to heat through
phonon emission leading to thermalization ref. The rates of excited state exciton cooling can be
slowed down in quantized energy levels. Then high-energy electrons and holes in higher excited
quantized states in the Q.D.s cool by relaxing from a given quantum level to the next lower level,
several phonons must be emitted simultaneously via electron-phonon scattering to satisfy energy
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
13
SOLAR CELLS AND QUANTUM DOTS
conservation ref, which lead to multiple exciton generation, through impact ionization which
decreases electron recombination rate.
Whenever in bulk semiconductor, the photogenerated carrier thermalized to the band
edge and the rates of hot carrier cooling are very fast. The diagram as well as the discussion
regarding this are as follows:-
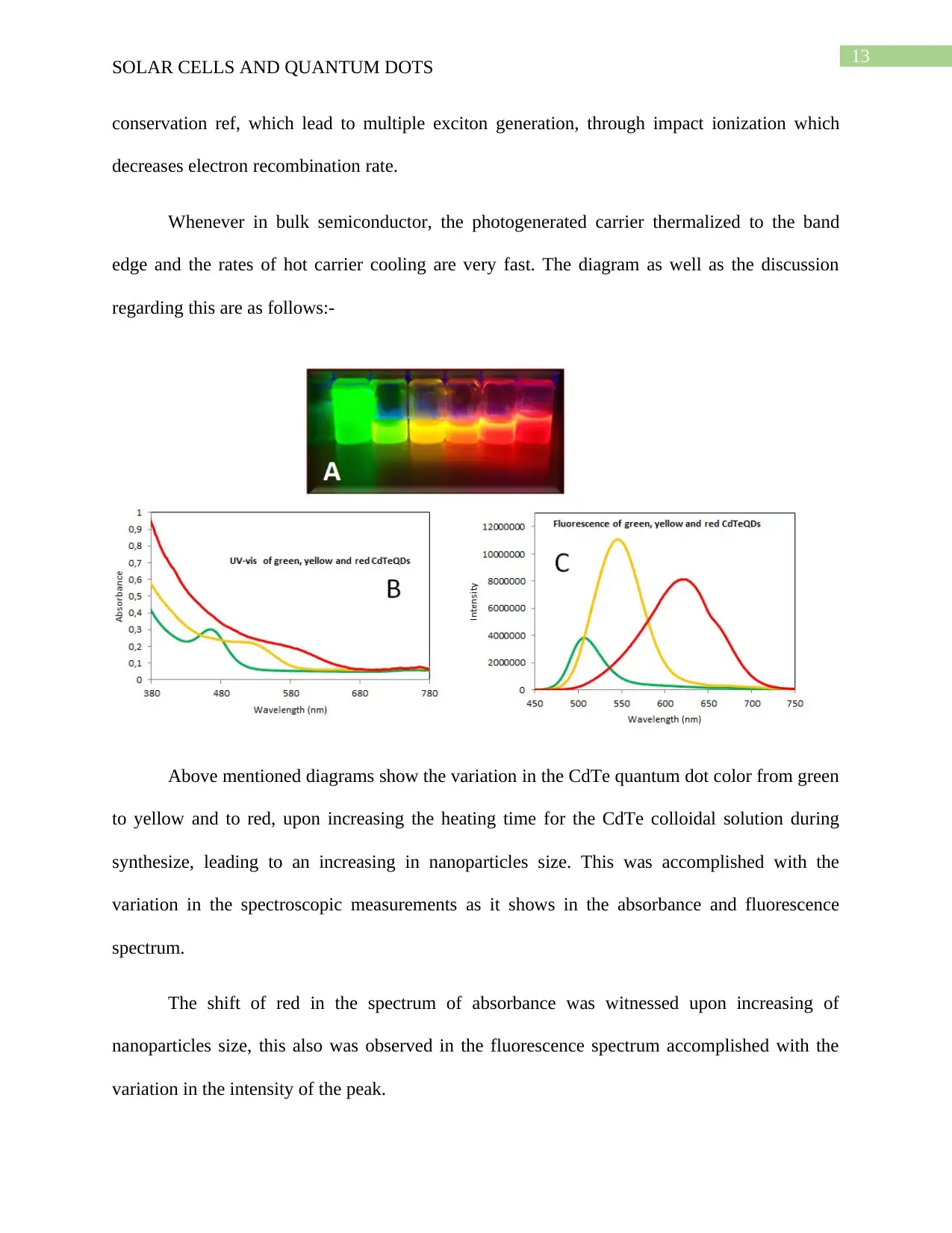
Above mentioned diagrams show the variation in the CdTe quantum dot color from green
to yellow and to red, upon increasing the heating time for the CdTe colloidal solution during
synthesize, leading to an increasing in nanoparticles size. This was accomplished with the
variation in the spectroscopic measurements as it shows in the absorbance and fluorescence
spectrum.
The shift of red in the spectrum of absorbance was witnessed upon increasing of
nanoparticles size, this also was observed in the fluorescence spectrum accomplished with the
variation in the intensity of the peak.
SOLAR CELLS AND QUANTUM DOTS
conservation ref, which lead to multiple exciton generation, through impact ionization which
decreases electron recombination rate.
Whenever in bulk semiconductor, the photogenerated carrier thermalized to the band
edge and the rates of hot carrier cooling are very fast. The diagram as well as the discussion
regarding this are as follows:-
Above mentioned diagrams show the variation in the CdTe quantum dot color from green
to yellow and to red, upon increasing the heating time for the CdTe colloidal solution during
synthesize, leading to an increasing in nanoparticles size. This was accomplished with the
variation in the spectroscopic measurements as it shows in the absorbance and fluorescence
spectrum.
The shift of red in the spectrum of absorbance was witnessed upon increasing of
nanoparticles size, this also was observed in the fluorescence spectrum accomplished with the
variation in the intensity of the peak.

14
SOLAR CELLS AND QUANTUM DOTS
Self-assembled photonic structures for light localization
The self-assembled structures in most of the areas like technology and science are
pervasive and same in case of the photonics. A photonic crystal is an optical nanostructure which
is reason behind affecting the motions of the photons mostly in the same way as compared to the
way in which the ionic lattices affects all the electrons in solids. Photonic crystals occur in the
order of a structural coloration or the reflector of the animals in nature and also occurs in
different forms and also promises to be utilized in a various range of applications.
Ordered structure “Photonic crystals”
The titanium dioxide is a semiconductor that has a capability of being used widely. Its
nonmetric scale, tio2 can always occur in various crystalline forms like in the form of anatase,
brookite as well as rutile forms. It can be applied in various sectors like construction, textiles,
cosmetics. They can also be applied in the field of air treatment as well as the food automobile.
On photo exciting the semiconductor of titanium dioxide, the electron creates an exciton which
was excited to the conduction band from the valence band which have the tendency of
transferring as well as recombining to the valence band of another semiconductor (Even et al.
2014). A technique allows an individual in changing the amount of energy which is needed in
order to construct a semiconductor make the efficiency level of it more or less which in turn can
be applied by the doping or sensitizing a particular semiconductor with any other sort of metal.
In these particular fields, it can be observed that the capability of the manipulation of the
directions of light as well as the constituent material structure. The photonic crystals which are
periodic nanostructures are designed in order to affect the motion of the photons that are situated
in the lattice crystalline structures. Various numbers of experiments were carried in order to
SOLAR CELLS AND QUANTUM DOTS
Self-assembled photonic structures for light localization
The self-assembled structures in most of the areas like technology and science are
pervasive and same in case of the photonics. A photonic crystal is an optical nanostructure which
is reason behind affecting the motions of the photons mostly in the same way as compared to the
way in which the ionic lattices affects all the electrons in solids. Photonic crystals occur in the
order of a structural coloration or the reflector of the animals in nature and also occurs in
different forms and also promises to be utilized in a various range of applications.
Ordered structure “Photonic crystals”
The titanium dioxide is a semiconductor that has a capability of being used widely. Its
nonmetric scale, tio2 can always occur in various crystalline forms like in the form of anatase,
brookite as well as rutile forms. It can be applied in various sectors like construction, textiles,
cosmetics. They can also be applied in the field of air treatment as well as the food automobile.
On photo exciting the semiconductor of titanium dioxide, the electron creates an exciton which
was excited to the conduction band from the valence band which have the tendency of
transferring as well as recombining to the valence band of another semiconductor (Even et al.
2014). A technique allows an individual in changing the amount of energy which is needed in
order to construct a semiconductor make the efficiency level of it more or less which in turn can
be applied by the doping or sensitizing a particular semiconductor with any other sort of metal.
In these particular fields, it can be observed that the capability of the manipulation of the
directions of light as well as the constituent material structure. The photonic crystals which are
periodic nanostructures are designed in order to affect the motion of the photons that are situated
in the lattice crystalline structures. Various numbers of experiments were carried in order to

15
SOLAR CELLS AND QUANTUM DOTS
study the group velocity which is the reduced one and approaches to zero at the photonic gap
edges.
Photo electrochemistry at Q.D.s sensitized films
The films of the quantum dots or Q.D.s possess short length of charge diffusion and
hence would get benefitted from the protocols of the light localization for enhancing their
absorbance in the assemblies which are ultra-thin in nature or else at the loading of the lower
surface. One method for achieving this is by structuring of the band gap semiconductor which is
wide and at the same time sensitized with the quantum dots or the Q.D.s as a personal computer.
The effects of light trapping in the photonic crystals on the conversion of the light to current
efficiency were studied by the process of replacing a nc-TiO2 film with TiO2 inverse opals.
Along with the light absorbance extent, there are others factors that affects the efficiency of light
to current conversion with a junction of liquid and in this context the charge separation
efficiency is included which is dependent on the hole scavenging efficiency related to the species
that are redox in nature.
Quenching of fluorescence peak of cdte qds with selenide and electrolyte to the qds part
The redox electrolyte Se2– is capable of separating the photoholes and stabilizing the
Q-CdTe quantum dots of the solar cells with the help of the junction which is liquid in nature.
The photophysical as well as photoelectrochemical behavior of the Q-CdTe quantum dot solar
cell is examined in two different sizes which consists of the green emitting dots and the red
emitting dots. The diameter range in the green emitting dots is between the 2.3–2.7 nm whereas
the diameter range in the red emitting dots is 4nm when the Se2– electrolyte is present and is
alkaline in nature which is prepared under the inert conditions of the atmosphere. The presence
SOLAR CELLS AND QUANTUM DOTS
study the group velocity which is the reduced one and approaches to zero at the photonic gap
edges.
Photo electrochemistry at Q.D.s sensitized films
The films of the quantum dots or Q.D.s possess short length of charge diffusion and
hence would get benefitted from the protocols of the light localization for enhancing their
absorbance in the assemblies which are ultra-thin in nature or else at the loading of the lower
surface. One method for achieving this is by structuring of the band gap semiconductor which is
wide and at the same time sensitized with the quantum dots or the Q.D.s as a personal computer.
The effects of light trapping in the photonic crystals on the conversion of the light to current
efficiency were studied by the process of replacing a nc-TiO2 film with TiO2 inverse opals.
Along with the light absorbance extent, there are others factors that affects the efficiency of light
to current conversion with a junction of liquid and in this context the charge separation
efficiency is included which is dependent on the hole scavenging efficiency related to the species
that are redox in nature.
Quenching of fluorescence peak of cdte qds with selenide and electrolyte to the qds part
The redox electrolyte Se2– is capable of separating the photoholes and stabilizing the
Q-CdTe quantum dots of the solar cells with the help of the junction which is liquid in nature.
The photophysical as well as photoelectrochemical behavior of the Q-CdTe quantum dot solar
cell is examined in two different sizes which consists of the green emitting dots and the red
emitting dots. The diameter range in the green emitting dots is between the 2.3–2.7 nm whereas
the diameter range in the red emitting dots is 4nm when the Se2– electrolyte is present and is
alkaline in nature which is prepared under the inert conditions of the atmosphere. The presence
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

16
SOLAR CELLS AND QUANTUM DOTS
of condition of dependence of size on the surface of the electrolyte of Se2– is revealed by the
absorbance, photoelectron chemical and measurements of the emission quenching for binding of
the Q-CdTe, along with the growth of type II Q-CdTe/CdSe and the establishment of the stability
in the PEC cells. The measurements of the quenching of emmisions showcases the photohole of
Q-CdTe is scavenged by the electrolyte of Se2– with the method which is in turn dependent on
the size as well as the concentration of the quencher. Binding the Se2– to green-emitting Q-CdTe
took place along with a larger binding constant as compared to that of the red-emitting dots
which in turn results in the formation of the type II Q-CdTe/CdSe at the core which is smaller
and indicated in the absorption which is red-shifted and spectra of emission with the incremented
addition of the Se2– electrolyte at the room temperature. The measurements of the
photoelectrochemical which are acquired at the level of Q-CdTe sensitized nc-TiO2 and TiO2
inverses the opal with a stop band of 600nm, 600-i-TiO2-o, in Se2– electrolyte which confirmed
the capabilities of the redox species for scavenging the photohole and protecting the Q-CdTe
from the photoanodic dissolution which is fast. It consists of larger stability which is witnessed
for all the huge dots. The gains in the conversion efficiency of photon-to-current is assigned to
the light trapping purposes were counted at Q-CdTe sensitized 600-i-TiO2-o relative to nc-TiO2.
Conclusion
Semiconductors are quite common in all the electronic devices. The semiconductors or
the semiconductor devices in fact possess some of the characteristics that are unique in nature
like the high resistance to temperature, low consumption of power, breakdown of high voltage,
improved thermal stability. With mobility of electrons getting higher this leads to the mentioned
activities more applicable which is very useful in the aerospace industry. The solar power acts as
SOLAR CELLS AND QUANTUM DOTS
of condition of dependence of size on the surface of the electrolyte of Se2– is revealed by the
absorbance, photoelectron chemical and measurements of the emission quenching for binding of
the Q-CdTe, along with the growth of type II Q-CdTe/CdSe and the establishment of the stability
in the PEC cells. The measurements of the quenching of emmisions showcases the photohole of
Q-CdTe is scavenged by the electrolyte of Se2– with the method which is in turn dependent on
the size as well as the concentration of the quencher. Binding the Se2– to green-emitting Q-CdTe
took place along with a larger binding constant as compared to that of the red-emitting dots
which in turn results in the formation of the type II Q-CdTe/CdSe at the core which is smaller
and indicated in the absorption which is red-shifted and spectra of emission with the incremented
addition of the Se2– electrolyte at the room temperature. The measurements of the
photoelectrochemical which are acquired at the level of Q-CdTe sensitized nc-TiO2 and TiO2
inverses the opal with a stop band of 600nm, 600-i-TiO2-o, in Se2– electrolyte which confirmed
the capabilities of the redox species for scavenging the photohole and protecting the Q-CdTe
from the photoanodic dissolution which is fast. It consists of larger stability which is witnessed
for all the huge dots. The gains in the conversion efficiency of photon-to-current is assigned to
the light trapping purposes were counted at Q-CdTe sensitized 600-i-TiO2-o relative to nc-TiO2.
Conclusion
Semiconductors are quite common in all the electronic devices. The semiconductors or
the semiconductor devices in fact possess some of the characteristics that are unique in nature
like the high resistance to temperature, low consumption of power, breakdown of high voltage,
improved thermal stability. With mobility of electrons getting higher this leads to the mentioned
activities more applicable which is very useful in the aerospace industry. The solar power acts as

17
SOLAR CELLS AND QUANTUM DOTS
an immense source of energy which is directly useable. Both the photovoltaic and photoelectron
chemical cell plays a massive role behind the conversion of the energy. Sufficient amount of
solar energy is received by most part of the surface of earth for permitting heating of water at
lower grade. Simple mirror devices tends to concentrate the solar energy for driving the steam
turbines. The most part of the surface of the earth
Direct utilization of the solar energy is only the renewable means which has the
capability of replacing the supply of the current global energy from the resources that are non-
renewable in nature. The solar power ultimately leads to the creation of various other energy
resources which includes wind, biomass, wave energy, hydropower to name a few. In the other
hand the current efficiency of the photovoltaic solar cells is very low. Due to this, areas are
demanded by them which are large in size for supplying the demands related to electricity. The
quantum confinement is very important as the effect of it is witnessed when the particle size
becomes much smaller which is to be comparable with each and every electron wavelength.
SOLAR CELLS AND QUANTUM DOTS
an immense source of energy which is directly useable. Both the photovoltaic and photoelectron
chemical cell plays a massive role behind the conversion of the energy. Sufficient amount of
solar energy is received by most part of the surface of earth for permitting heating of water at
lower grade. Simple mirror devices tends to concentrate the solar energy for driving the steam
turbines. The most part of the surface of the earth
Direct utilization of the solar energy is only the renewable means which has the
capability of replacing the supply of the current global energy from the resources that are non-
renewable in nature. The solar power ultimately leads to the creation of various other energy
resources which includes wind, biomass, wave energy, hydropower to name a few. In the other
hand the current efficiency of the photovoltaic solar cells is very low. Due to this, areas are
demanded by them which are large in size for supplying the demands related to electricity. The
quantum confinement is very important as the effect of it is witnessed when the particle size
becomes much smaller which is to be comparable with each and every electron wavelength.

18
SOLAR CELLS AND QUANTUM DOTS
References
[1] M. D. Archer and R. Hill, Clean Electricity from Photovoltaics, vol. 1, no. Clean
Electricity from Photovoltaics. PUBLISHED BY IMPERIAL COLLEGE PRESS AND
DISTRIBUTED BY WORLD SCIENTIFIC PUBLISHING CO., 2001.
[2] IER, “No Title,” Foss. Fuels Domin. U.S. Energy Prod. But Receiv. a Small Percent. Fed.
Fuel Subsid., no. share of energy fuel subsides.
[3] A. B. Arons and M. B. Peppard, “Einstein’s Proposal of the Photon Concept—a
Translation of the Annalen der Physik Paper of 1905,” Am. J. Phys., 1965.
[4] W. W. Anderson and Y. G. Chai, “Becquerel effect solar cell,” Energy Convers., vol. 15,
no. 3–4, pp. 85–94, Jan. 1976.
[5] E. efficiency and renewable energy, "No Title," Hist. Sol., 2002.
[6] P. Reiss, M. Protière, and L. Li, “Core/shell semiconductor nanocrystals,” Small. 2009.
[7] L. I. Halaoui, N. M. Abrams, and T. E. Mallouk, “Increasing the Conversion Efficiency of
Dye-Sensitized TiO 2 Photoelectrochemical Cells by Coupling to Photonic Crystals,” J.
Phys. Chem. B, vol. 109, no. 13, pp. 6334–6342, Apr. 2005.
[8] S.-H. A. Lee, N. M. Abrams, P. G. Hoertz, G. D. Barber, L. I. Halaoui, and T. E. Mallouk,
“Coupling of Titania Inverse Opals to Nanocrystalline Titania Layers in Dye-Sensitized
Solar Cells †,” J. Phys. Chem. B, vol. 112, no. 46, pp. 14415–14421, Nov. 2008.
[9] H. A. Atwater and A. Polman, “Plasmonics for improved photovoltaic devices.,” Nat.
SOLAR CELLS AND QUANTUM DOTS
References
[1] M. D. Archer and R. Hill, Clean Electricity from Photovoltaics, vol. 1, no. Clean
Electricity from Photovoltaics. PUBLISHED BY IMPERIAL COLLEGE PRESS AND
DISTRIBUTED BY WORLD SCIENTIFIC PUBLISHING CO., 2001.
[2] IER, “No Title,” Foss. Fuels Domin. U.S. Energy Prod. But Receiv. a Small Percent. Fed.
Fuel Subsid., no. share of energy fuel subsides.
[3] A. B. Arons and M. B. Peppard, “Einstein’s Proposal of the Photon Concept—a
Translation of the Annalen der Physik Paper of 1905,” Am. J. Phys., 1965.
[4] W. W. Anderson and Y. G. Chai, “Becquerel effect solar cell,” Energy Convers., vol. 15,
no. 3–4, pp. 85–94, Jan. 1976.
[5] E. efficiency and renewable energy, "No Title," Hist. Sol., 2002.
[6] P. Reiss, M. Protière, and L. Li, “Core/shell semiconductor nanocrystals,” Small. 2009.
[7] L. I. Halaoui, N. M. Abrams, and T. E. Mallouk, “Increasing the Conversion Efficiency of
Dye-Sensitized TiO 2 Photoelectrochemical Cells by Coupling to Photonic Crystals,” J.
Phys. Chem. B, vol. 109, no. 13, pp. 6334–6342, Apr. 2005.
[8] S.-H. A. Lee, N. M. Abrams, P. G. Hoertz, G. D. Barber, L. I. Halaoui, and T. E. Mallouk,
“Coupling of Titania Inverse Opals to Nanocrystalline Titania Layers in Dye-Sensitized
Solar Cells †,” J. Phys. Chem. B, vol. 112, no. 46, pp. 14415–14421, Nov. 2008.
[9] H. A. Atwater and A. Polman, “Plasmonics for improved photovoltaic devices.,” Nat.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

19
SOLAR CELLS AND QUANTUM DOTS
Mater., 2010.
[10] L. I. Halaoui, N. M. Abrams, and T. E. Mallouk, “Increasing the conversion efficiency of
dye-sensitized TiO 2 photoelectrochemical cells by coupling to photonic crystals,” J.
Phys. Chem. B, 2005.
[11] R. Fayad and L. Halaoui, “The Role of Order in the Amplification of Light-Energy
Conversion in a Dye-Sensitized Solar Cell Coupled to a Photonic Crystal,”
ChemPhysChem, 2016.
[12] A. Mihi and H. Míguez, “Origin of light-harvesting enhancement in colloidal-photonic-
crystal-based dye-sensitized solar cells,” J. Phys. Chem. B, 2005.
[13] A. Mihi, F. J. López-Alcaraz, and H. Míguez, “Full spectrum enhancement of the light
harvesting efficiency of dye sensitized solar cells by including colloidal photonic crystal
multilayers,” Appl. Phys. Lett., 2006.
[14] T. Suezaki, H. Yano, T. Hatayama, G. A. Ozin, and T. Fuyuki, “Photoconductivity in
inverse silicon opals enhanced by slow photon effect: Yet another step towards optically
amplified silicon photonic crystal solar cells,” Appl. Phys. Lett., 2011.
[15] M. El Harakeh and L. Halaoui, “Enhanced conversion of light at TiO2 photonic crystals to
the blue of a stop band and at TiO2 random films sensitized with Q-CdS: Order and
disorder,” J. Phys. Chem. C, 2010.
[16] A. S. Nehme, F. Haydous, and L. Halaoui, “Amplification in Light Energy Conversion at
Q-CdTe Sensitized TiO2 Photonic Crystal, Photoelectrochemical Stability in Se2-
Electrolyte, and Size-Dependent Type II Q-CdTe/CdSe Formation,” J. Phys. Chem. C,
2016.
[17] S. Bayram and L. Halaoui, “Amplification of solar energy conversion in quantum-
confined CdSe-sensitized TiO2 photonic crystals by trapping light,” Part. Part. Syst.
Charact., 2013.
[18] S. Nishimura et al., “Standing Wave Enhancement of Red Absorbance and Photocurrent
in Dye-Sensitized Titanium Dioxide Photoelectrodes Coupled to Photonic Crystals,” J.
Am. Chem. Soc., vol. 125, no. 20, pp. 6306–6310, May 2003.
SOLAR CELLS AND QUANTUM DOTS
Mater., 2010.
[10] L. I. Halaoui, N. M. Abrams, and T. E. Mallouk, “Increasing the conversion efficiency of
dye-sensitized TiO 2 photoelectrochemical cells by coupling to photonic crystals,” J.
Phys. Chem. B, 2005.
[11] R. Fayad and L. Halaoui, “The Role of Order in the Amplification of Light-Energy
Conversion in a Dye-Sensitized Solar Cell Coupled to a Photonic Crystal,”
ChemPhysChem, 2016.
[12] A. Mihi and H. Míguez, “Origin of light-harvesting enhancement in colloidal-photonic-
crystal-based dye-sensitized solar cells,” J. Phys. Chem. B, 2005.
[13] A. Mihi, F. J. López-Alcaraz, and H. Míguez, “Full spectrum enhancement of the light
harvesting efficiency of dye sensitized solar cells by including colloidal photonic crystal
multilayers,” Appl. Phys. Lett., 2006.
[14] T. Suezaki, H. Yano, T. Hatayama, G. A. Ozin, and T. Fuyuki, “Photoconductivity in
inverse silicon opals enhanced by slow photon effect: Yet another step towards optically
amplified silicon photonic crystal solar cells,” Appl. Phys. Lett., 2011.
[15] M. El Harakeh and L. Halaoui, “Enhanced conversion of light at TiO2 photonic crystals to
the blue of a stop band and at TiO2 random films sensitized with Q-CdS: Order and
disorder,” J. Phys. Chem. C, 2010.
[16] A. S. Nehme, F. Haydous, and L. Halaoui, “Amplification in Light Energy Conversion at
Q-CdTe Sensitized TiO2 Photonic Crystal, Photoelectrochemical Stability in Se2-
Electrolyte, and Size-Dependent Type II Q-CdTe/CdSe Formation,” J. Phys. Chem. C,
2016.
[17] S. Bayram and L. Halaoui, “Amplification of solar energy conversion in quantum-
confined CdSe-sensitized TiO2 photonic crystals by trapping light,” Part. Part. Syst.
Charact., 2013.
[18] S. Nishimura et al., “Standing Wave Enhancement of Red Absorbance and Photocurrent
in Dye-Sensitized Titanium Dioxide Photoelectrodes Coupled to Photonic Crystals,” J.
Am. Chem. Soc., vol. 125, no. 20, pp. 6306–6310, May 2003.

20
SOLAR CELLS AND QUANTUM DOTS
[19] J. I. L. Chen, G. Von Freymann, S. Y. Choi, V. Kitaev, and G. A. Ozin, “Amplified
photochemistry with slow photons,” Adv. Mater., 2006.
[20] R. D. Meade, A. M. Rappe, K. D. Brommer, J. D. Joannopoulos, and O. L. Alerhand,
"Accurate theoretical analysis of photonic bandgap materials," Phys. Rev. B, 1993.
[21] R. Rengarajan, D. Mittleman, C. Rich, and V. Colvin, “Effect of disorder on the optical
properties of colloidal crystals,” Phys. Rev. E - Stat. Nonlinear, Soft Matter Phys., 2005.
[22] A. Diamantopoulou et al., “Titania photonic crystal photocatalysts functionalized by
graphene oxide nanocolloids,” Appl. Catal. B Environ., 2019.
[23] L. J. Diguna, Q. Shen, J. Kobayashi, and T. Toyoda, “High efficiency of CdSe quantum-
dot-sensitized Ti O2 inverse opal solar cells,” Appl. Phys. Lett., 2007.
[24] W. Wang and S. A. Asher, “Photochemical incorporation of silver quantum dots in
monodisperse silica colloids for photonic crystal applications,” J. Am. Chem. Soc., 2001.
[25] B. O. Dabbousi et al., “(CdSe)ZnS Core−Shell Quantum Dots: Synthesis and
Characterization of a Size Series of Highly Luminescent Nanocrystallites,” J. Phys. Chem.
B, 1997.
[26] J. Wang and H. Han, “Hydrothermal synthesis of high-quality type-II CdTe/CdSe
quantum dots with near-infrared fluorescence,” J. Colloid Interface Sci., 2010.
[27] Z. Pan, H. Zhang, K. Cheng, Y. Hou, J. Hua, and X. Zhong, “Highly efficient inverted
type-I CdS/CdSe core/shell structure QD-sensitized solar cells,” ACS Nano, 2012.
[28] K. Yoshino, S. B. Lee, S. Tatsuhara, Y. Kawagishi, M. Ozaki, and A. A. Zakhidov,
“Observation of inhibited spontaneous emission and stimulated emission of rhodamine 6G
in polymer replica of synthetic opal,” Appl. Phys. Lett., 1998.
[29] Y. A. Vlasov, K. Luterova, I. Pelant, B. Hönerlage, and V. N. Astratov, “Enhancement of
optical gain of semiconductors embedded in three-dimensional photonic crystals,” Appl.
Phys. Lett., 1997.
[30] M. A. Green, “Photovoltaic principles,” in Physica E: Low-Dimensional Systems and
Nanostructures, 2002.
SOLAR CELLS AND QUANTUM DOTS
[19] J. I. L. Chen, G. Von Freymann, S. Y. Choi, V. Kitaev, and G. A. Ozin, “Amplified
photochemistry with slow photons,” Adv. Mater., 2006.
[20] R. D. Meade, A. M. Rappe, K. D. Brommer, J. D. Joannopoulos, and O. L. Alerhand,
"Accurate theoretical analysis of photonic bandgap materials," Phys. Rev. B, 1993.
[21] R. Rengarajan, D. Mittleman, C. Rich, and V. Colvin, “Effect of disorder on the optical
properties of colloidal crystals,” Phys. Rev. E - Stat. Nonlinear, Soft Matter Phys., 2005.
[22] A. Diamantopoulou et al., “Titania photonic crystal photocatalysts functionalized by
graphene oxide nanocolloids,” Appl. Catal. B Environ., 2019.
[23] L. J. Diguna, Q. Shen, J. Kobayashi, and T. Toyoda, “High efficiency of CdSe quantum-
dot-sensitized Ti O2 inverse opal solar cells,” Appl. Phys. Lett., 2007.
[24] W. Wang and S. A. Asher, “Photochemical incorporation of silver quantum dots in
monodisperse silica colloids for photonic crystal applications,” J. Am. Chem. Soc., 2001.
[25] B. O. Dabbousi et al., “(CdSe)ZnS Core−Shell Quantum Dots: Synthesis and
Characterization of a Size Series of Highly Luminescent Nanocrystallites,” J. Phys. Chem.
B, 1997.
[26] J. Wang and H. Han, “Hydrothermal synthesis of high-quality type-II CdTe/CdSe
quantum dots with near-infrared fluorescence,” J. Colloid Interface Sci., 2010.
[27] Z. Pan, H. Zhang, K. Cheng, Y. Hou, J. Hua, and X. Zhong, “Highly efficient inverted
type-I CdS/CdSe core/shell structure QD-sensitized solar cells,” ACS Nano, 2012.
[28] K. Yoshino, S. B. Lee, S. Tatsuhara, Y. Kawagishi, M. Ozaki, and A. A. Zakhidov,
“Observation of inhibited spontaneous emission and stimulated emission of rhodamine 6G
in polymer replica of synthetic opal,” Appl. Phys. Lett., 1998.
[29] Y. A. Vlasov, K. Luterova, I. Pelant, B. Hönerlage, and V. N. Astratov, “Enhancement of
optical gain of semiconductors embedded in three-dimensional photonic crystals,” Appl.
Phys. Lett., 1997.
[30] M. A. Green, “Photovoltaic principles,” in Physica E: Low-Dimensional Systems and
Nanostructures, 2002.

21
SOLAR CELLS AND QUANTUM DOTS
[31] A. J. Nozik, “Nanoscience and nanostructures for photovoltaics and solar fuels,” Nano
Lett., 2010.
[32] A. J. Nozik, “Exciton multiplication and relaxation dynamics in quantum dots:
Applications to ultra-high efficiency solar photon conversion,” in Conference Record of
the 2006 IEEE 4th World Conference on Photovoltaic Energy Conversion, WCPEC-4,
2007.
[33] J. T. Stewart et al., “Comparison of carrier multiplication yields in PbS and PbSe
nanocrystals: The role of competing energy-loss processes,” Nano Lett., 2012.
[34] M. C. Beard, J. M. Luther, O. E. Semonin, and A. J. Nozik, “Third generation
photovoltaics based on multiple exciton generation in quantum confined semiconductors,”
Acc. Chem. Res., 2013.
[35] S. Kim, B. Fisher, H. J. Eisler, and M. Bawendi, “Type-II quantum dots:
CdTe/CdSe(core/shell) and CdSe/ZnTe(core/shell) heterostructures,” J. Am. Chem. Soc.,
2003.
[36] K. Yu, B. Zaman, S. Romanova, D. S. Wang, and J. A. Ripmeester, “Sequential synthesis
of type II colloidal CdTe/CdSe core-shell nanocrystals,” Small, 2005.
[37] A. Samanta, Z. Deng, and Y. Liu, “Aqueous synthesis of glutathione-capped
CdTe/CdS/ZnS and CdTe/CdSe/ZnS core/shell/shell nanocrystal heterostructures,”
Langmuir, 2012.
[38] C. H. Chuang, S. S. Lo, G. D. Scholes, and C. Burda, “Charge separation and
recombination in CdTe/CdSe core/shell nanocrystals as a function of shell coverage:
Probing the onset of the quasi type-II regime,” J. Phys. Chem. Lett., 2010.
[39] S. S. Lo, T. Mirkovic, C. H. Chuang, C. Burda, and G. D. Scholes, “Emergent properties
resulting from type-II band alignment in semiconductor nanoheterostructures,” Adv.
Mater., 2011.
[40] S. Jiao et al., “Band engineering in core/shell ZnTe/cdse for photovoltage and efficiency
enhancement in exciplex quantum dot sensitized solar cells,” ACS Nano, 2015.
SOLAR CELLS AND QUANTUM DOTS
[31] A. J. Nozik, “Nanoscience and nanostructures for photovoltaics and solar fuels,” Nano
Lett., 2010.
[32] A. J. Nozik, “Exciton multiplication and relaxation dynamics in quantum dots:
Applications to ultra-high efficiency solar photon conversion,” in Conference Record of
the 2006 IEEE 4th World Conference on Photovoltaic Energy Conversion, WCPEC-4,
2007.
[33] J. T. Stewart et al., “Comparison of carrier multiplication yields in PbS and PbSe
nanocrystals: The role of competing energy-loss processes,” Nano Lett., 2012.
[34] M. C. Beard, J. M. Luther, O. E. Semonin, and A. J. Nozik, “Third generation
photovoltaics based on multiple exciton generation in quantum confined semiconductors,”
Acc. Chem. Res., 2013.
[35] S. Kim, B. Fisher, H. J. Eisler, and M. Bawendi, “Type-II quantum dots:
CdTe/CdSe(core/shell) and CdSe/ZnTe(core/shell) heterostructures,” J. Am. Chem. Soc.,
2003.
[36] K. Yu, B. Zaman, S. Romanova, D. S. Wang, and J. A. Ripmeester, “Sequential synthesis
of type II colloidal CdTe/CdSe core-shell nanocrystals,” Small, 2005.
[37] A. Samanta, Z. Deng, and Y. Liu, “Aqueous synthesis of glutathione-capped
CdTe/CdS/ZnS and CdTe/CdSe/ZnS core/shell/shell nanocrystal heterostructures,”
Langmuir, 2012.
[38] C. H. Chuang, S. S. Lo, G. D. Scholes, and C. Burda, “Charge separation and
recombination in CdTe/CdSe core/shell nanocrystals as a function of shell coverage:
Probing the onset of the quasi type-II regime,” J. Phys. Chem. Lett., 2010.
[39] S. S. Lo, T. Mirkovic, C. H. Chuang, C. Burda, and G. D. Scholes, “Emergent properties
resulting from type-II band alignment in semiconductor nanoheterostructures,” Adv.
Mater., 2011.
[40] S. Jiao et al., “Band engineering in core/shell ZnTe/cdse for photovoltage and efficiency
enhancement in exciplex quantum dot sensitized solar cells,” ACS Nano, 2015.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

22
SOLAR CELLS AND QUANTUM DOTS
[41] A. Kongkanand, K. Tvrdy, K. Takechi, M. Kuno, and P. V. Kamat, “Quantum dot solar
cells. Tuning photoresponse through size and shape control of CdSe-TiO2 architecture,”
J. Am. Chem. Soc., 2008.
[42] A. J. Nozik, “Quantum dot solar cells,” Phys. E Low-Dimens. Syst Nanostruct., 2002.
[43] N. Kanai, T. Nuida, K. Ueta, K. Hashimoto, T. Watanabe, and H. Ohsaki, "Photocatalytic
efficiency of TiO2/SnO2 thin film stacks prepared by D.C. magnetron sputtering,"
Vacuum, 2004.
[44] J. Jasieniak, L. Smith, J. Van Embden, P. Mulvaney, and M. Califano, “Re-examination of
the size-dependent absorption properties of CdSe quantum dots,” J. Phys. Chem. C, 2009.
[45] G. Von Freymann, V. Kitaev, B. V. Lotsch, and G. A. Ozin, “Bottom-up assembly of
photonic crystals,” Chemical Society Reviews. 2013.
[46] E. Yablonovitch, “Inhibited spontaneous emission in solid-state physics and electronics,”
Phys. Rev. Lett., 1987.
[47] S. John, “Strong localization of photons in certain disordered dielectric superlattices,”
Phys. Rev. Lett., 1987.
[48] J. I. L. Chen and G. A. Ozin, “Tracing the Effect of Slow Photons in Photoisomerization
of Azobenzene,” Adv. Mater., 2008.
[49] M. Scalora, J. P. Dowling, C. M. Bowden, and M. J. Bloemer, “The photonic band edge
optical diode,” J. Appl. Phys., 1994.
[50] Y. A. Vlasov, S. Petit, G. Klein, B. Hönerlage, and C. Hirlimann, “Femtosecond
measurements of the time of flight of photons in a three-dimensional photonic crystal,”
Phys. Rev. E - Stat. Physics, Plasmas, Fluids, Relat. Interdiscip. Top., 1999.
[51] A. Imhof, W. L. Vos, R. Sprik, and A. Lagendijk, “Large dispersive effects near the band
edges of photonic crystals,” Phys. Rev. Lett., 1999.
[52] G. Von Freymann, S. John, S. Wong, V. Kitaev, and G. A. Ozin, “Measurement of group
velocity dispersion for finite size three-dimensional photonic crystals in the near-infrared
spectral region,” Appl. Phys. Lett., 2005.
SOLAR CELLS AND QUANTUM DOTS
[41] A. Kongkanand, K. Tvrdy, K. Takechi, M. Kuno, and P. V. Kamat, “Quantum dot solar
cells. Tuning photoresponse through size and shape control of CdSe-TiO2 architecture,”
J. Am. Chem. Soc., 2008.
[42] A. J. Nozik, “Quantum dot solar cells,” Phys. E Low-Dimens. Syst Nanostruct., 2002.
[43] N. Kanai, T. Nuida, K. Ueta, K. Hashimoto, T. Watanabe, and H. Ohsaki, "Photocatalytic
efficiency of TiO2/SnO2 thin film stacks prepared by D.C. magnetron sputtering,"
Vacuum, 2004.
[44] J. Jasieniak, L. Smith, J. Van Embden, P. Mulvaney, and M. Califano, “Re-examination of
the size-dependent absorption properties of CdSe quantum dots,” J. Phys. Chem. C, 2009.
[45] G. Von Freymann, V. Kitaev, B. V. Lotsch, and G. A. Ozin, “Bottom-up assembly of
photonic crystals,” Chemical Society Reviews. 2013.
[46] E. Yablonovitch, “Inhibited spontaneous emission in solid-state physics and electronics,”
Phys. Rev. Lett., 1987.
[47] S. John, “Strong localization of photons in certain disordered dielectric superlattices,”
Phys. Rev. Lett., 1987.
[48] J. I. L. Chen and G. A. Ozin, “Tracing the Effect of Slow Photons in Photoisomerization
of Azobenzene,” Adv. Mater., 2008.
[49] M. Scalora, J. P. Dowling, C. M. Bowden, and M. J. Bloemer, “The photonic band edge
optical diode,” J. Appl. Phys., 1994.
[50] Y. A. Vlasov, S. Petit, G. Klein, B. Hönerlage, and C. Hirlimann, “Femtosecond
measurements of the time of flight of photons in a three-dimensional photonic crystal,”
Phys. Rev. E - Stat. Physics, Plasmas, Fluids, Relat. Interdiscip. Top., 1999.
[51] A. Imhof, W. L. Vos, R. Sprik, and A. Lagendijk, “Large dispersive effects near the band
edges of photonic crystals,” Phys. Rev. Lett., 1999.
[52] G. Von Freymann, S. John, S. Wong, V. Kitaev, and G. A. Ozin, “Measurement of group
velocity dispersion for finite size three-dimensional photonic crystals in the near-infrared
spectral region,” Appl. Phys. Lett., 2005.

23
SOLAR CELLS AND QUANTUM DOTS
[53] F. di Stasio et al., “Tuning optical properties of opal photonic crystals by structural defects
engineering,” J. Eur. Opt. Soc., 2009.
[54] E. Ozbay, “Plasmonics: Merging photonics and electronics at nanoscale dimensions,”
Science. 2006.
[55] J. Homola, “Present and future of surface plasmon resonance biosensors,” Analytical and
Bioanalytical Chemistry. 2003.
[56] M. Kolle et al., “Mimicking the colourful wing scale structure of the Papilio blumei
butterfly,” Nat. Nanotechnol., 2010.
[57] T. Suezaki, P. C. O’Brien, J. I. L. Chen, E. Loso, N. P. Kherani, and G. A. Ozin,
“Tailoring the electrical properties of inverse silicon opals - A step towards optically
amplified silicon solar cells,” Adv. Mater., 2009.
[58] X. Li, Y. Wu, Y. Shen, Y. Sun, Y. Yang, and A. Xie, “A novel bifunctional Ni-doped TiO
2 inverse opal with enhanced SERS performance and excellent photocatalytic activity,”
Appl. Surf. Sci., 2018.
[59] M. Müller, R. Zentel, T. Maka, S. G. Romanov, and C. M. S. Torres, “Photonic crystal
films with high refractive index contrast,” Adv. Mater., 2000.
[60] X.-F. Gao, H.-B. Li, W.-T. Sun, Q. Chen, F.-Q. Tang, and L.-M. Peng, “CdTe Quantum
Dots-Sensitized TiO 2 Nanotube Array Photoelectrodes,” J. Phys. Chem. C, 2009.
[61] Z. Pan et al., "High-efficiency 'green' quantum dot solar cells," J. Am. Chem. Soc., 2014.
[62] H. Wei et al., “Fumed SiO2 modified electrolytes for quantum dot sensitized solar cells
with efficiency exceeding 11% and better stability,” J. Mater. Chem. A, 2016.
SOLAR CELLS AND QUANTUM DOTS
[53] F. di Stasio et al., “Tuning optical properties of opal photonic crystals by structural defects
engineering,” J. Eur. Opt. Soc., 2009.
[54] E. Ozbay, “Plasmonics: Merging photonics and electronics at nanoscale dimensions,”
Science. 2006.
[55] J. Homola, “Present and future of surface plasmon resonance biosensors,” Analytical and
Bioanalytical Chemistry. 2003.
[56] M. Kolle et al., “Mimicking the colourful wing scale structure of the Papilio blumei
butterfly,” Nat. Nanotechnol., 2010.
[57] T. Suezaki, P. C. O’Brien, J. I. L. Chen, E. Loso, N. P. Kherani, and G. A. Ozin,
“Tailoring the electrical properties of inverse silicon opals - A step towards optically
amplified silicon solar cells,” Adv. Mater., 2009.
[58] X. Li, Y. Wu, Y. Shen, Y. Sun, Y. Yang, and A. Xie, “A novel bifunctional Ni-doped TiO
2 inverse opal with enhanced SERS performance and excellent photocatalytic activity,”
Appl. Surf. Sci., 2018.
[59] M. Müller, R. Zentel, T. Maka, S. G. Romanov, and C. M. S. Torres, “Photonic crystal
films with high refractive index contrast,” Adv. Mater., 2000.
[60] X.-F. Gao, H.-B. Li, W.-T. Sun, Q. Chen, F.-Q. Tang, and L.-M. Peng, “CdTe Quantum
Dots-Sensitized TiO 2 Nanotube Array Photoelectrodes,” J. Phys. Chem. C, 2009.
[61] Z. Pan et al., "High-efficiency 'green' quantum dot solar cells," J. Am. Chem. Soc., 2014.
[62] H. Wei et al., “Fumed SiO2 modified electrolytes for quantum dot sensitized solar cells
with efficiency exceeding 11% and better stability,” J. Mater. Chem. A, 2016.
1 out of 24
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.