Increased Risk of Intraoperative Awareness
VerifiedAdded on 2022/08/31
|9
|7597
|18
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Anesthesiology, V 119 • No 6 1275 December 2013
ABSTRACT
Background:Patientswith a historyof intraoperative
awareness with explicit recall (AWR) are hypothesize
be at higher risk for AWR than the general surgical popu-
lation. In this study, the authors assessed whether patien
with a history of AWR (1) are actually at higher risk
AWR; (2) receive different anesthetic management; and
are relatively resistant to the hypnotic actions of vo
anesthetics.
Methods: Patients with a history of AWR and match
controls from three randomized clinical trials investigatin
prevention of AWR were compared for relative risk of AW
Anesthetic management was compared with the use of t
Hotelling’s T2 statistic. A linear mixed model, including pre-
viously identified covariates, assessed the effects of a his
What We Already Know about This Topic
• It is not clear whether patients with
awareness with explicit recall are at high
during general anesthesia
What This Article Tells Us That Is New
• In a matched cohort analysis of
trials including more than 25,000 patients,
tory of intraoperative awareness had a
incidence of awareness compared with
controls who did not have a history of
• Anesthetic management did not differ
view of the likely increased risk of
consider modifying anesthetic management in
history of awareness
◇ This article is featured in “This
Please see this issue of AnesThesIology , page 1A.
◆ This article is accompanied by an editori
Pryor Ko, hemmings hC: Increased risk
der anesthesia: An issue of consciousnes
AnesThesIology 2013; 119:1236–8.Copyright © 2013, the American Society of Anesthesiologists, Inc. Lippincott
Williams & Wilkins. Anesthesiology 2013; 119:1275-83
* Medical Student and Predoctoral Research Trainee, Division of
Cardiothoracic Anesthesiology and Department of Anesthesiology,
║ Assistant Professor of Anesthesiology, Department of Anesthesiol-
ogy, §§ Professor, Division Chief, and Director of INQUIRI, Division of
Cardiothoracic Anesthesiology, Washington University School of Med-
icine, Saint Louis, Missouri. † Research Assistant Professor, ‡‡ Henry
E. Mallinckrodt Professor and Department Head, University School
of Medicine, Saint Louis, Missouri. ‡ Assistant Professor, Division of
Biostatistics, Department of Mathematics, Washington University in
Saint Louis, Saint Louis, Missouri. § Statistician Lead, ║║ Associate
Chair for Faculty Affairs; Associate Professor of Anesthesiology and
Neurosurgery; Faculty, Neuroscience Graduate Program, Depart-
ment of Anesthesiology, University of Michigan Medical School, Ann
Arbor, Michigan. # Associate Professor of Anesthesia and Critical
Care, Department of Anesthesiology, University of Chicago, Chicago,
Illinois. ** Professor and Department Head, †† Research Technician,
Department of Anesthesiology and Perioperative Medicine, Faculty
of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
Received from the Department of Anesthesiology, University of
Michigan Medical School, Ann Arbor, Michigan, and the Department of
Anesthesiology, Washington University, Saint Louis, Missouri. Submitted
for publication December 9, 2012. Accepted for publication April 18,
2013. The Michigan Awareness Control Study was generously funded by
the Cerebral Function Monitoring grant (Dr. Mashour, Principal Inves-
tigator) from the Foundation for Anesthesia Education and Research,
Rochester, Minnesota; the American Society of Anesthesiologists, Park
Ridge, Illinois; the National Institutes of Health (NIH), Bethesda, Mary-
land (KL2 RR024987-01) (to Dr. Mashour); and the Department of Anes-
thesiology, University of Michigan Medical School, Ann Arbor, Michigan.
The B-Unaware Trial was supported by grant 604302 from the Barnes-
Jewish Hospital Foundation (to Dr. Avidan), St. Louis, Missouri, as well
as institutional and departmental sources. The BAG-RECALL trial was
supported by a grant (CFM-08/15/2007-Avidan) awarded by the Foun-
dation for Anesthesia Education and Research, Rochester, Minnesota;
and the American Society of Anesthesiologists, Park Ridge, Illinois (Dr.
Avidan, Principal Investigator); as well as institutional and departmental
sources. Amrita Aranake is a predoctoral research trainee who received
a grant (grant number UL1 RR024992 and sub-award number TL1
RR024995) from the National Center for Research Resources (NCRR),
Bethesda, Maryland, a component of the NIH and the NIH Roadmap for
Medical Research. The contents of this article are solely the responsibil-
ity of the authors and do not necessarily represent the official view of
NCRR or NIH. The authors declare no competing interests. Drs. Avidan
and Mashour contributed equally to this article.
Address correspondence to Dr. Mashour: Division of Neuroan-
esthesiology, Department of Anesthesiology, University of Michigan
Medical School, 1H247 UH/SPC-5048, 1500 East Medical Center
Drive, Ann Arbor, Michigan 48109-5048. gmashour@umich.edu. This
article may be accessed for personal use at no charge through the
Journal Web site, www.anesthesiology.org.
Increased Risk of Intraoperative Awareness in Patien
with a History of Awareness
Amrita Aranake, B.A.,* stephen gradwohl, B.s.,* Arbi Ben-Abdallah, Ph.D.
Amy shanks, M.s.,§ Daniell. helsten, M.D.,║David B. glick, M.D., M.B.A.,#
eric Jacobsohn, M.B., Ch.B.,** Alex J. Villafranca, M.sc.,†† Alex s. evers
Michael s. Avidan, M.B., B.Ch,§§ george A. Mashour, M.D., Ph.D.
PERIOPERATIVE MEDICINE
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
ABSTRACT
Background:Patientswith a historyof intraoperative
awareness with explicit recall (AWR) are hypothesize
be at higher risk for AWR than the general surgical popu-
lation. In this study, the authors assessed whether patien
with a history of AWR (1) are actually at higher risk
AWR; (2) receive different anesthetic management; and
are relatively resistant to the hypnotic actions of vo
anesthetics.
Methods: Patients with a history of AWR and match
controls from three randomized clinical trials investigatin
prevention of AWR were compared for relative risk of AW
Anesthetic management was compared with the use of t
Hotelling’s T2 statistic. A linear mixed model, including pre-
viously identified covariates, assessed the effects of a his
What We Already Know about This Topic
• It is not clear whether patients with
awareness with explicit recall are at high
during general anesthesia
What This Article Tells Us That Is New
• In a matched cohort analysis of
trials including more than 25,000 patients,
tory of intraoperative awareness had a
incidence of awareness compared with
controls who did not have a history of
• Anesthetic management did not differ
view of the likely increased risk of
consider modifying anesthetic management in
history of awareness
◇ This article is featured in “This
Please see this issue of AnesThesIology , page 1A.
◆ This article is accompanied by an editori
Pryor Ko, hemmings hC: Increased risk
der anesthesia: An issue of consciousnes
AnesThesIology 2013; 119:1236–8.Copyright © 2013, the American Society of Anesthesiologists, Inc. Lippincott
Williams & Wilkins. Anesthesiology 2013; 119:1275-83
* Medical Student and Predoctoral Research Trainee, Division of
Cardiothoracic Anesthesiology and Department of Anesthesiology,
║ Assistant Professor of Anesthesiology, Department of Anesthesiol-
ogy, §§ Professor, Division Chief, and Director of INQUIRI, Division of
Cardiothoracic Anesthesiology, Washington University School of Med-
icine, Saint Louis, Missouri. † Research Assistant Professor, ‡‡ Henry
E. Mallinckrodt Professor and Department Head, University School
of Medicine, Saint Louis, Missouri. ‡ Assistant Professor, Division of
Biostatistics, Department of Mathematics, Washington University in
Saint Louis, Saint Louis, Missouri. § Statistician Lead, ║║ Associate
Chair for Faculty Affairs; Associate Professor of Anesthesiology and
Neurosurgery; Faculty, Neuroscience Graduate Program, Depart-
ment of Anesthesiology, University of Michigan Medical School, Ann
Arbor, Michigan. # Associate Professor of Anesthesia and Critical
Care, Department of Anesthesiology, University of Chicago, Chicago,
Illinois. ** Professor and Department Head, †† Research Technician,
Department of Anesthesiology and Perioperative Medicine, Faculty
of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
Received from the Department of Anesthesiology, University of
Michigan Medical School, Ann Arbor, Michigan, and the Department of
Anesthesiology, Washington University, Saint Louis, Missouri. Submitted
for publication December 9, 2012. Accepted for publication April 18,
2013. The Michigan Awareness Control Study was generously funded by
the Cerebral Function Monitoring grant (Dr. Mashour, Principal Inves-
tigator) from the Foundation for Anesthesia Education and Research,
Rochester, Minnesota; the American Society of Anesthesiologists, Park
Ridge, Illinois; the National Institutes of Health (NIH), Bethesda, Mary-
land (KL2 RR024987-01) (to Dr. Mashour); and the Department of Anes-
thesiology, University of Michigan Medical School, Ann Arbor, Michigan.
The B-Unaware Trial was supported by grant 604302 from the Barnes-
Jewish Hospital Foundation (to Dr. Avidan), St. Louis, Missouri, as well
as institutional and departmental sources. The BAG-RECALL trial was
supported by a grant (CFM-08/15/2007-Avidan) awarded by the Foun-
dation for Anesthesia Education and Research, Rochester, Minnesota;
and the American Society of Anesthesiologists, Park Ridge, Illinois (Dr.
Avidan, Principal Investigator); as well as institutional and departmental
sources. Amrita Aranake is a predoctoral research trainee who received
a grant (grant number UL1 RR024992 and sub-award number TL1
RR024995) from the National Center for Research Resources (NCRR),
Bethesda, Maryland, a component of the NIH and the NIH Roadmap for
Medical Research. The contents of this article are solely the responsibil-
ity of the authors and do not necessarily represent the official view of
NCRR or NIH. The authors declare no competing interests. Drs. Avidan
and Mashour contributed equally to this article.
Address correspondence to Dr. Mashour: Division of Neuroan-
esthesiology, Department of Anesthesiology, University of Michigan
Medical School, 1H247 UH/SPC-5048, 1500 East Medical Center
Drive, Ann Arbor, Michigan 48109-5048. gmashour@umich.edu. This
article may be accessed for personal use at no charge through the
Journal Web site, www.anesthesiology.org.
Increased Risk of Intraoperative Awareness in Patien
with a History of Awareness
Amrita Aranake, B.A.,* stephen gradwohl, B.s.,* Arbi Ben-Abdallah, Ph.D.
Amy shanks, M.s.,§ Daniell. helsten, M.D.,║David B. glick, M.D., M.B.A.,#
eric Jacobsohn, M.B., Ch.B.,** Alex J. Villafranca, M.sc.,†† Alex s. evers
Michael s. Avidan, M.B., B.Ch,§§ george A. Mashour, M.D., Ph.D.
PERIOPERATIVE MEDICINE
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Anesthesiology 2013; 119:1275-83 1276 Aranake et al.
Increased Risk of Intraoperative Awareness
of AWR on the relationship between end-tidal anesthetic
concentration and bispectral index.
Results: The incidence of AWR was 1.7% (4 of 241) in
patients with a history of AWR and 0.3% (4 of 1,205) in
control patients (relative risk = 5.0; 95% CI, 1.3–19.9).
Anesthetic management did not differ between cohorts, but
there was a significant effect of a history of AWR on the
end-tidal anesthetic concentration versus bispectral index
relationship.
Conclusions: Surgical patients with a history of AWR
are five times more likely to experience AWR than similar
patients without a history of AWR. Further consideration
should be given to modifying perioperative care and postop-
erative evaluation of patients with a history of AWR.
I NTRAOPERATIVE awarenesswith explicitrecall
(AWR) occurs in 0.1–0.2% of patients undergoing gen-
eral anesthesia1 and may result in devastating psychologi-
cal symptoms. Patients often experience significant anxiety
and stress after an AWR event, and up to 70% of patients
may develop posttraumatic stress disorder.2–4 It has been
suggested that patients who have experienced AWR dur-
ing a previous surgery are at increased risk for AWR.3,5 A
review of 271 case reports of AWR indicated that 1.6% of
these patients reported a prior history of AWR.3 However,
this review lacked a comparison group and was not able
to estimate the increased risk attributable to a history of
AWR. In the bispectral index (BIS) or Anesthetic Gas to
Reduce Explicit Recall (BAG-RECALL) study, the percent-
age of patients reporting a prior history of AWR was sig-
nificantly higher in those who experienced AWR compared
with those who did not. However, this difference may be
explained by unequal distributions of other risk factors for
AWR.6 To date, there are no compelling data that establish
a history of AWR as an independent risk factor for AWR. A
better understanding of the risk for AWR in patients with
a history of AWR could positively impact clinical care by
guiding changes in intraoperative management as well as
systematic postoperative screening for AWR and its psycho-
logical sequelae.
This substudy of three randomized controlled trials of
AWR prevention—B-Unaware,7 BAG-RECALL,6 and
Michigan Awareness Control Study (MACS)8—investigates
whether patients with a history of AWR (1) have a higher
risk for AWR; (2) are cared for differently by anesthesia prac-
titioners; and (3) require a higher concentration of volatile
anesthetic to achieve BIS values suggested to be consistent
with surgical anesthesia compared with a matched surgical
cohort without a history of AWR.
Materials and Methods
Patient Cohort
The B-Unaware, BAG-RECALL, and MACS trials compared
protocols based either on the BIS monitor® (Covidien,
Boulder, CO) (a processed electroencephalographic index)
or on end-tidal anesthetic concentration (ETAC) alarms to
prevent AWR.6,7,9In the current retrospective cohort study,
we performed secondary data analyses of the patients en
in these three randomized clinical trials to compare the in
dence of AWR, the anesthetic management, and the rela
ship between BIS and ETAC in patients with a histor
AWR to a matched control group without a history of AW
The B-Unaware trial, a single-center study, enrolled an
assessed outcomes for 1,941 surgical patients underg
general anesthesia between September 2005 and Oc
2006. The BAG-RECALL and MACS trials enrolled and
assessed outcomes for 5,713 and 18,836 patients, re
tively, between May 2008 and May 2010. The B-Unaware
and BAG-RECALL trials studied patients considered to
be at high risk for AWR, whereas the MACS trial studied
an unselected surgical population. Further details of
studies have been previously described.6–8 Each trial received
approval from the appropriate institutional review bo
Among the 26,490 patients enrolled in the three trials, w
identified 241 patients who self-reported a history of AWR
in prior surgeries. To control for potential imbalances
baseline characteristics between patients with and w
a history of AWR, each patient with a history of AWR was
matched to five controls based on demographic characte
tics, comorbid conditions, and other risk factors for AWR.
A ratio of 5:1 was selected for matching due to the low in
dence of AWR. Selection of 1,205 control patients yielded
total sample size of 1,446.
Outcomes Measured
There were three main outcomes of interest in the
rent study: incidence of AWR, anesthetic managemen
and BIS–ETAC relationships in patients with a history of
AWR compared with controls. Data regarding potenti
risk factors for AWR, including a prior history of AW
daily alcohol consumption, and regular use of opiate
benzodiazepines, or anticonvulsants were obtained durin
the parent trials. For participants in the B-Unaware a
BAG-RECALL trials, which comprise 52.6% of the sample
in this study, this information was obtained by interview
upon recruitment. For participants in the MACS trial,
which comprise the remaining 47.4% of the study sample
this information was obtained by querying the medic
record retrospectively. Postoperatively, interviewers ev
ated patients for AWR with the modified Brice interview
(appendix 1 for questions).10 All patients reporting AWR in
this screening had a follow-up interview by trained inter-
viewers and anesthesiologists with experience in assessi
AWR; data from the first two interviews were review
independently by members of a committee of senior ane
thesiologists that determined whether patient reports we
definite, possible, or no AWR. Reported memories judged
to have a very high likelihood of occurring during th
anesthetic and surgical periods were classified as de
AWR, whereas credible reports without compelling detail
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
Increased Risk of Intraoperative Awareness
of AWR on the relationship between end-tidal anesthetic
concentration and bispectral index.
Results: The incidence of AWR was 1.7% (4 of 241) in
patients with a history of AWR and 0.3% (4 of 1,205) in
control patients (relative risk = 5.0; 95% CI, 1.3–19.9).
Anesthetic management did not differ between cohorts, but
there was a significant effect of a history of AWR on the
end-tidal anesthetic concentration versus bispectral index
relationship.
Conclusions: Surgical patients with a history of AWR
are five times more likely to experience AWR than similar
patients without a history of AWR. Further consideration
should be given to modifying perioperative care and postop-
erative evaluation of patients with a history of AWR.
I NTRAOPERATIVE awarenesswith explicitrecall
(AWR) occurs in 0.1–0.2% of patients undergoing gen-
eral anesthesia1 and may result in devastating psychologi-
cal symptoms. Patients often experience significant anxiety
and stress after an AWR event, and up to 70% of patients
may develop posttraumatic stress disorder.2–4 It has been
suggested that patients who have experienced AWR dur-
ing a previous surgery are at increased risk for AWR.3,5 A
review of 271 case reports of AWR indicated that 1.6% of
these patients reported a prior history of AWR.3 However,
this review lacked a comparison group and was not able
to estimate the increased risk attributable to a history of
AWR. In the bispectral index (BIS) or Anesthetic Gas to
Reduce Explicit Recall (BAG-RECALL) study, the percent-
age of patients reporting a prior history of AWR was sig-
nificantly higher in those who experienced AWR compared
with those who did not. However, this difference may be
explained by unequal distributions of other risk factors for
AWR.6 To date, there are no compelling data that establish
a history of AWR as an independent risk factor for AWR. A
better understanding of the risk for AWR in patients with
a history of AWR could positively impact clinical care by
guiding changes in intraoperative management as well as
systematic postoperative screening for AWR and its psycho-
logical sequelae.
This substudy of three randomized controlled trials of
AWR prevention—B-Unaware,7 BAG-RECALL,6 and
Michigan Awareness Control Study (MACS)8—investigates
whether patients with a history of AWR (1) have a higher
risk for AWR; (2) are cared for differently by anesthesia prac-
titioners; and (3) require a higher concentration of volatile
anesthetic to achieve BIS values suggested to be consistent
with surgical anesthesia compared with a matched surgical
cohort without a history of AWR.
Materials and Methods
Patient Cohort
The B-Unaware, BAG-RECALL, and MACS trials compared
protocols based either on the BIS monitor® (Covidien,
Boulder, CO) (a processed electroencephalographic index)
or on end-tidal anesthetic concentration (ETAC) alarms to
prevent AWR.6,7,9In the current retrospective cohort study,
we performed secondary data analyses of the patients en
in these three randomized clinical trials to compare the in
dence of AWR, the anesthetic management, and the rela
ship between BIS and ETAC in patients with a histor
AWR to a matched control group without a history of AW
The B-Unaware trial, a single-center study, enrolled an
assessed outcomes for 1,941 surgical patients underg
general anesthesia between September 2005 and Oc
2006. The BAG-RECALL and MACS trials enrolled and
assessed outcomes for 5,713 and 18,836 patients, re
tively, between May 2008 and May 2010. The B-Unaware
and BAG-RECALL trials studied patients considered to
be at high risk for AWR, whereas the MACS trial studied
an unselected surgical population. Further details of
studies have been previously described.6–8 Each trial received
approval from the appropriate institutional review bo
Among the 26,490 patients enrolled in the three trials, w
identified 241 patients who self-reported a history of AWR
in prior surgeries. To control for potential imbalances
baseline characteristics between patients with and w
a history of AWR, each patient with a history of AWR was
matched to five controls based on demographic characte
tics, comorbid conditions, and other risk factors for AWR.
A ratio of 5:1 was selected for matching due to the low in
dence of AWR. Selection of 1,205 control patients yielded
total sample size of 1,446.
Outcomes Measured
There were three main outcomes of interest in the
rent study: incidence of AWR, anesthetic managemen
and BIS–ETAC relationships in patients with a history of
AWR compared with controls. Data regarding potenti
risk factors for AWR, including a prior history of AW
daily alcohol consumption, and regular use of opiate
benzodiazepines, or anticonvulsants were obtained durin
the parent trials. For participants in the B-Unaware a
BAG-RECALL trials, which comprise 52.6% of the sample
in this study, this information was obtained by interview
upon recruitment. For participants in the MACS trial,
which comprise the remaining 47.4% of the study sample
this information was obtained by querying the medic
record retrospectively. Postoperatively, interviewers ev
ated patients for AWR with the modified Brice interview
(appendix 1 for questions).10 All patients reporting AWR in
this screening had a follow-up interview by trained inter-
viewers and anesthesiologists with experience in assessi
AWR; data from the first two interviews were review
independently by members of a committee of senior ane
thesiologists that determined whether patient reports we
definite, possible, or no AWR. Reported memories judged
to have a very high likelihood of occurring during th
anesthetic and surgical periods were classified as de
AWR, whereas credible reports without compelling detail
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020

Anesthesiology 2013; 119:1275-83 1277 Aranake et al.
PERIOPERATIVE MEDICINE
were classified as possible AWR. Finally, reported memories
considered to have occurred in the preoperative or post-
operative period were classified as no AWR. The outcome
of anesthetic management was based on doses of sedative,
analgesic, hypnotic, and paralytic medications adminis-
tered and recorded by practitioners.
The measurement of ETAC and algorithm-based analysis
of the electroencephalogram are commonly used surrogates
for depth of anesthesia.6,7,11The BIS® monitor processes a
frontal electroencephalographic signal to produce a num-
ber that is intended to reflect the depth of anesthesia or the
hypnotic component of anesthesia. The BIS value ranges
from 0, reflecting electroencephalographic suppression, to
values approaching 100, which are consistent with wake-
fulness. We assessed BIS-ETAC relationships in a subset
of patients at high risk of AWR from the B-Unaware and
BAG-RECALL trials. ETAC of volatile agents and BIS val-
ues were recorded electronically at 1-s, 1-min, or 5-min
intervals using TrendFace (ixellence GmbH, Wildau, Ger-
many) or MetaVision (iMDsoft, Needham, MA) software.
Variables Analyzed
We converted doses of drugs in the same class to equivalents
of one agent: opioid analgesics were converted to morphine
equivalents, hypnotic agents to propofol equivalents, and
neuromuscular-blocking agents to vecuronium equivalents.
Opioid-conversion factors were obtained from the Alberta
Hospice Palliative Care Resource Manual.12 Doses of etomi-
date and thiopental were converted to propofol equivalents
by using mean values of the dose range for induction pro-
vided in Cusick’s Anesthesia & Critical Care Reference Sheet.13
Doses of midazolam were considered separately. The 95%
effective dose was used to convert neuromuscular-blocking
agents to vecuronium equivalents.13 ETAC values for volatile
agents were converted into age-adjusted minimum alveolar
concentration (aaMAC) values.14 If patients received more
than one drug from each class, the sum of equivalent doses
was calculated. Any dose values outside of a pharmacologi-
cally plausible range were excluded (appendix 2). Outliers
skewing the distribution were truncated.
Pharmacokinetically stable epochs of ETAC were identi-
fied to compare the relationship between BIS and ETAC,
because steady-state ETAC levels take time to establish after
changes in inspired anesthetic concentration. Stable epochs
were defined as periods in which aaMAC values had not
fluctuated more than 0.05 in the preceding 10 min and were
identified using a MATLAB program (MathWorks, Natick,
MA) previously described.15 Data collected during pharma-
cokinetically stable epochs were then resampled to reduce
BIS and ETAC measurements to 1-min intervals.
Statistical Analysis
We comparedbaselinepatientcharacteristics,comor-
bidities, and other risk factors for AWR with independent
samples t tests for continuous variables and chi-square tests
for categorical variables. All continuous variables were no
mally distributed. Logistic regression was used to calcula
propensity scores based on patient characteristics (age,
body mass index, American Society of Anesthesiologi
physical status [ASA-PS], and smoking status), individual
comorbidities(valvularheartdisease,diabetesmellitus,
coronary heart disease, dysrhythmias, chronic obstruc
pulmonary disease, prior stroke, congestive heart fail
peripheral vascular disease, and hypertension), and indiv
ual risk factors for AWR (planned heart surgery, pulmona
hypertension, regular opiate use, regular benzodiazep
use, regular anticonvulsant use, and daily alcohol use). W
the exception of the continuous variables age and body m
index, all variables included in the propensity score
dichotomous. By using the greedy matching algorithm16
each patient with a history of AWR was matched to
controls on sex, age, ASA, body mass index, a composite
comorbidities, a composite of risk factors for AWR, and th
propensity score. In the matching algorithm, the followin
calipers were used: 0.005 for propensity score, 5 for
1 for composite of comorbidities, and 1 for composite of
risk factors for AWR. All other factors were matched exac
Comparisons of baseline patient characteristics, comorbi
ties, and risk factors for AWR were repeated to ensure su
cessful matching. In addition, a comprehensive balan
test, the standardized difference in means of the propens
scores, was used to evaluate whether the matching algor
produced cohorts with the same covariate distributio17
The primary outcome of this study was a comparison usin
relative risk of the incidence of AWR in patients with and
without a history of AWR.
Routine perioperative management by anesthesia p
viders typically involves different types of drugs, suc
benzodiazepines,intravenousinductionagents,opioid
analgesics, neuromuscular blockers, and volatile anesthe
agents. To compare multiple related dependent varia
between the two groups, we calculated the Hotelling
T2 statistic. This multivariate test computes a canoni
derived mean using a linear combination of the depende
variables, representing overall anesthetic management,
compares this canonical variate between cohorts. To ach
multivariate normality, the dependent variables were tra
formed using the Box-Cox transformation procedure
then standardized. The Box’s M assessed the homogenei
of the variance-covariance matrix at a significance level
0.005 per previously published guidelines.18 For all other
statistical analyses, P value less than 0.05 was cons
significant.
To determine whether patients with a history of AWR h
an increased requirement for volatile anesthetic to achie
values suggested to be consistent with surgical anesthes
assessed the relationship between ETAC and BIS. After re
ing pharmacokinetically unstable data, 594 patients rem
in the analysis. A linear mixed-effects model was chosen
this analysis due to within-subject repeated measuremen
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
PERIOPERATIVE MEDICINE
were classified as possible AWR. Finally, reported memories
considered to have occurred in the preoperative or post-
operative period were classified as no AWR. The outcome
of anesthetic management was based on doses of sedative,
analgesic, hypnotic, and paralytic medications adminis-
tered and recorded by practitioners.
The measurement of ETAC and algorithm-based analysis
of the electroencephalogram are commonly used surrogates
for depth of anesthesia.6,7,11The BIS® monitor processes a
frontal electroencephalographic signal to produce a num-
ber that is intended to reflect the depth of anesthesia or the
hypnotic component of anesthesia. The BIS value ranges
from 0, reflecting electroencephalographic suppression, to
values approaching 100, which are consistent with wake-
fulness. We assessed BIS-ETAC relationships in a subset
of patients at high risk of AWR from the B-Unaware and
BAG-RECALL trials. ETAC of volatile agents and BIS val-
ues were recorded electronically at 1-s, 1-min, or 5-min
intervals using TrendFace (ixellence GmbH, Wildau, Ger-
many) or MetaVision (iMDsoft, Needham, MA) software.
Variables Analyzed
We converted doses of drugs in the same class to equivalents
of one agent: opioid analgesics were converted to morphine
equivalents, hypnotic agents to propofol equivalents, and
neuromuscular-blocking agents to vecuronium equivalents.
Opioid-conversion factors were obtained from the Alberta
Hospice Palliative Care Resource Manual.12 Doses of etomi-
date and thiopental were converted to propofol equivalents
by using mean values of the dose range for induction pro-
vided in Cusick’s Anesthesia & Critical Care Reference Sheet.13
Doses of midazolam were considered separately. The 95%
effective dose was used to convert neuromuscular-blocking
agents to vecuronium equivalents.13 ETAC values for volatile
agents were converted into age-adjusted minimum alveolar
concentration (aaMAC) values.14 If patients received more
than one drug from each class, the sum of equivalent doses
was calculated. Any dose values outside of a pharmacologi-
cally plausible range were excluded (appendix 2). Outliers
skewing the distribution were truncated.
Pharmacokinetically stable epochs of ETAC were identi-
fied to compare the relationship between BIS and ETAC,
because steady-state ETAC levels take time to establish after
changes in inspired anesthetic concentration. Stable epochs
were defined as periods in which aaMAC values had not
fluctuated more than 0.05 in the preceding 10 min and were
identified using a MATLAB program (MathWorks, Natick,
MA) previously described.15 Data collected during pharma-
cokinetically stable epochs were then resampled to reduce
BIS and ETAC measurements to 1-min intervals.
Statistical Analysis
We comparedbaselinepatientcharacteristics,comor-
bidities, and other risk factors for AWR with independent
samples t tests for continuous variables and chi-square tests
for categorical variables. All continuous variables were no
mally distributed. Logistic regression was used to calcula
propensity scores based on patient characteristics (age,
body mass index, American Society of Anesthesiologi
physical status [ASA-PS], and smoking status), individual
comorbidities(valvularheartdisease,diabetesmellitus,
coronary heart disease, dysrhythmias, chronic obstruc
pulmonary disease, prior stroke, congestive heart fail
peripheral vascular disease, and hypertension), and indiv
ual risk factors for AWR (planned heart surgery, pulmona
hypertension, regular opiate use, regular benzodiazep
use, regular anticonvulsant use, and daily alcohol use). W
the exception of the continuous variables age and body m
index, all variables included in the propensity score
dichotomous. By using the greedy matching algorithm16
each patient with a history of AWR was matched to
controls on sex, age, ASA, body mass index, a composite
comorbidities, a composite of risk factors for AWR, and th
propensity score. In the matching algorithm, the followin
calipers were used: 0.005 for propensity score, 5 for
1 for composite of comorbidities, and 1 for composite of
risk factors for AWR. All other factors were matched exac
Comparisons of baseline patient characteristics, comorbi
ties, and risk factors for AWR were repeated to ensure su
cessful matching. In addition, a comprehensive balan
test, the standardized difference in means of the propens
scores, was used to evaluate whether the matching algor
produced cohorts with the same covariate distributio17
The primary outcome of this study was a comparison usin
relative risk of the incidence of AWR in patients with and
without a history of AWR.
Routine perioperative management by anesthesia p
viders typically involves different types of drugs, suc
benzodiazepines,intravenousinductionagents,opioid
analgesics, neuromuscular blockers, and volatile anesthe
agents. To compare multiple related dependent varia
between the two groups, we calculated the Hotelling
T2 statistic. This multivariate test computes a canoni
derived mean using a linear combination of the depende
variables, representing overall anesthetic management,
compares this canonical variate between cohorts. To ach
multivariate normality, the dependent variables were tra
formed using the Box-Cox transformation procedure
then standardized. The Box’s M assessed the homogenei
of the variance-covariance matrix at a significance level
0.005 per previously published guidelines.18 For all other
statistical analyses, P value less than 0.05 was cons
significant.
To determine whether patients with a history of AWR h
an increased requirement for volatile anesthetic to achie
values suggested to be consistent with surgical anesthes
assessed the relationship between ETAC and BIS. After re
ing pharmacokinetically unstable data, 594 patients rem
in the analysis. A linear mixed-effects model was chosen
this analysis due to within-subject repeated measuremen
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
Anesthesiology 2013; 119:1275-83 1278 Aranake et al.
Increased Risk of Intraoperative Awareness
both ETAC and BIS. Both history of AWR and the inter-
action between ETAC and a history of AWR were included
as predictors in the model. Age, sex, ASA-PS ≥4 (categorical
variable: yes or no), nitrous oxide use (categorical variable: yes
or no), midazolam greater than 2 mg (categorical variable: yes
or no), and morphine equivalents greater than 50 mg (categor-
ical variable: yes or no) were previously shown to be significant
predictors of BIS values and were included as covariates in
the model.15 Residual plots were tested for homoscedascity.
Results with a P value less than 0.05 were considered signifi-
cant. All above statistical analyses were performed using SAS
9.2 (SAS Institute Inc., Cary, NC) and SPSS Statistics version
19 (IBM Corporation, Somers, NY).
Results
Of the 26,490 patients enrolled in the parent trials,
patients (0.9%)had a history of AWR.Characteristics
for the overall sample (separated by history of AWR
reported in table 1. Patients with a history of AWR
younger and had a higher body mass index than those w
out a history of AWR. In addition, a higher proportion of
patients with a history of AWR were female, current smok
ers, opiate users, and anticonvulsant users. A lower prop
tion of patients with a history of AWR reported to be daily
alcohol users compared with control patients. Furthermo
significant differences in ASA-PS between cohorts sugges
Table 1. Patient Demographic Characteristics, Comorbid Conditions, and Risk Factors for AWR for Total Sample
Overall Sample
(n = 26,257)
History of Awareness
(n = 241)
No History of
Awareness
(n = 26,016) P Value
Male 48.5% 36.9% 48.6% <0.01
Age 54.2 ± 15.8 52.2 ± 14.6 54.3 ± 15.8 0.04
BMI (kg/m2) 29.6 ± 7.7 30.8 ± 8.1 29.6 ± 7.7 0.02
ASA-PS
=1 9.4% 2.5% 9.4%
2–3 79.0% 82.6% 78.9%
>3 11.7% 14.9% 11.6% <0.01
Comorbid conditions (n = 24,349)
Valvular heart disease 7.5% 6.2% 7.5% 0.47
Diabetes mellitus 14.0% 17.4% 14.0% 0.13
Coronary disease 16.3% 19.5% 16.3% 0.17
Dysrhythmias 7.8% 8.7% 7.8% 0.58
COPD 4.7% 9.1% 4.7% <0.01
CVA/stroke 3.4% 5.8% 3.4% 0.04
Congestive heart failure 5.6% 7.5% 5.6% 0.20
Peripheral vascular disease 4.3% 6.2% 4.3% 0.14
Hypertension 45.1% 47.3% 45.1% 0.50
Number of comorbidities
None 62.6% 53.1% 62.7%
One 21.2% 27.8% 21.1%
Two or more 16.2% 19.1% 16.2% 0.01
Current smoker 14.3% 21.2% 14.3% <0.01
Risk factors for awareness
Planned heart surgery 12.0% 10.0% 12.0% 0.33
Pulmonary hypertension 1.6% 2.1% 1.6% 0.52
Regular opiate use 24.5% 31.1% 24.5% 0.02
Regular benzodiazepine use 30.6% 32.0% 30.6% 0.64
Regular anticonvulsant use 3.7% 6.6% 3.7% 0.02
Daily alcohol use 10.6% 5.4% 10.6% 0.01
Number of risk factors
None 39.7% 37.8% 39.8%
One 40.2% 39.4% 40.2%
Two or more 20.1% 22.8% 20.1% 0.56
Value presented are % or mean ± SD. P values were calculated using chi-square tests for categorical variables and independent sam
ples t tests for continuous variables.
ASA-PS = American Society of Anesthesiologists physical status; AWR = awareness with explicit recall; BMI = body mass
COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident.
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
Increased Risk of Intraoperative Awareness
both ETAC and BIS. Both history of AWR and the inter-
action between ETAC and a history of AWR were included
as predictors in the model. Age, sex, ASA-PS ≥4 (categorical
variable: yes or no), nitrous oxide use (categorical variable: yes
or no), midazolam greater than 2 mg (categorical variable: yes
or no), and morphine equivalents greater than 50 mg (categor-
ical variable: yes or no) were previously shown to be significant
predictors of BIS values and were included as covariates in
the model.15 Residual plots were tested for homoscedascity.
Results with a P value less than 0.05 were considered signifi-
cant. All above statistical analyses were performed using SAS
9.2 (SAS Institute Inc., Cary, NC) and SPSS Statistics version
19 (IBM Corporation, Somers, NY).
Results
Of the 26,490 patients enrolled in the parent trials,
patients (0.9%)had a history of AWR.Characteristics
for the overall sample (separated by history of AWR
reported in table 1. Patients with a history of AWR
younger and had a higher body mass index than those w
out a history of AWR. In addition, a higher proportion of
patients with a history of AWR were female, current smok
ers, opiate users, and anticonvulsant users. A lower prop
tion of patients with a history of AWR reported to be daily
alcohol users compared with control patients. Furthermo
significant differences in ASA-PS between cohorts sugges
Table 1. Patient Demographic Characteristics, Comorbid Conditions, and Risk Factors for AWR for Total Sample
Overall Sample
(n = 26,257)
History of Awareness
(n = 241)
No History of
Awareness
(n = 26,016) P Value
Male 48.5% 36.9% 48.6% <0.01
Age 54.2 ± 15.8 52.2 ± 14.6 54.3 ± 15.8 0.04
BMI (kg/m2) 29.6 ± 7.7 30.8 ± 8.1 29.6 ± 7.7 0.02
ASA-PS
=1 9.4% 2.5% 9.4%
2–3 79.0% 82.6% 78.9%
>3 11.7% 14.9% 11.6% <0.01
Comorbid conditions (n = 24,349)
Valvular heart disease 7.5% 6.2% 7.5% 0.47
Diabetes mellitus 14.0% 17.4% 14.0% 0.13
Coronary disease 16.3% 19.5% 16.3% 0.17
Dysrhythmias 7.8% 8.7% 7.8% 0.58
COPD 4.7% 9.1% 4.7% <0.01
CVA/stroke 3.4% 5.8% 3.4% 0.04
Congestive heart failure 5.6% 7.5% 5.6% 0.20
Peripheral vascular disease 4.3% 6.2% 4.3% 0.14
Hypertension 45.1% 47.3% 45.1% 0.50
Number of comorbidities
None 62.6% 53.1% 62.7%
One 21.2% 27.8% 21.1%
Two or more 16.2% 19.1% 16.2% 0.01
Current smoker 14.3% 21.2% 14.3% <0.01
Risk factors for awareness
Planned heart surgery 12.0% 10.0% 12.0% 0.33
Pulmonary hypertension 1.6% 2.1% 1.6% 0.52
Regular opiate use 24.5% 31.1% 24.5% 0.02
Regular benzodiazepine use 30.6% 32.0% 30.6% 0.64
Regular anticonvulsant use 3.7% 6.6% 3.7% 0.02
Daily alcohol use 10.6% 5.4% 10.6% 0.01
Number of risk factors
None 39.7% 37.8% 39.8%
One 40.2% 39.4% 40.2%
Two or more 20.1% 22.8% 20.1% 0.56
Value presented are % or mean ± SD. P values were calculated using chi-square tests for categorical variables and independent sam
ples t tests for continuous variables.
ASA-PS = American Society of Anesthesiologists physical status; AWR = awareness with explicit recall; BMI = body mass
COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident.
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Anesthesiology 2013; 119:1275-83 1279 Aranake et al.
PERIOPERATIVE MEDICINE
that control patients were healthier, with a higher percentage
of patients with ASA-PS1 and lower percentages of patients
with ASA-PS 2–3 or ≥4. An attempt was made to decrease
confounds attributable to other covariates potentially associ-
ated with AWR risk by matching as described in the Mate-
rials and Methods.No significantdifferencesremained
between cohorts after matching (table 2). Comparison of
the standardized means of the propensity scores demon-
strated no significant difference in the overall distributional
balance of covariates between cohorts (t(324) = −1.93; P >
0.05), indicating that the greedy matching algorithm pro-
duced an adequate matched sample. The absolute number
and incidence of AWR events in each cohort are shown in
table 3. The incidence of AWR differed significantly betwe
cohorts (P = 0.03); patients with a history of AWR were fi
times more likely to experience AWR than control patient
(relative risk = 5.0; 95% CI, 1.3–19.9).
Approximately 4% of patients (N = 64) had incomplete
information describing drug administration. For the 1,382
remaining patients, the means and SEM for each drug typ
are presented in table 4. The proportion of patients
received each drug type did not differ significantly betwe
cohorts. All patients in this study received volatile anesth
agents. Because drug doses were not normally distri
these variables were transformed. Evaluation of the
variate distribution of the transformed variables displ
Table 2. Patient Demographic Characteristics, Comorbid Conditions, and Risk Factors for AWR for Matched Sample
Overall Sample
(n = 1,446)
History of Awareness
(n = 241)
No History of
Awareness
(n = 1,205) P Value
Male 36.9% 36.9% 36.9% 1.00
Age (yr), mean ± SD 52.3 ± 14.5 52.2 ± 14.6 52.3 ± 14.5 0.94
BMI (kg/m2), mean ± SD 30.8 ± 8.2 30.8 ± 8.1 30.8 ± 8.2 0.98
ASA-PS
=1 2.4% 2.5% 2.4%
2–3 83.3% 82.6% 83.4%
>3 14.3% 14.9% 14.2% 0.95
Comorbid conditions
Valvular heart disease 7.1% 6.2% 7.3% 0.55
Diabetes mellitus 18.1% 17.4% 18.3% 0.76
Coronary disease 18.9% 19.5% 18.8% 0.79
Dysrhythmias 9.1% 8.7% 9.2% 0.81
COPD 7.1% 9.1% 6.6% 0.17
CVA/stroke 4.0% 5.8% 3.7% 0.12
Congestive heart failure 6.5% 7.5% 6.3% 0.50
Peripheral vascular disease 6.6% 6.2% 6.6% 0.81
Hypertension 48.3% 47.3% 48.5% 0.73
Number of comorbidities
None 53.8% 53.1% 53.9%
One 26.7% 27.8% 26.5%
Two or more 19.5% 19.1% 19.6% 0.91
Current smoker 21.2% 21.2% 21.2% 1.00
Risk factors for awareness
Planned heart surgery 11.6% 10.0% 12.0% 0.38
Pulmonary hypertension 1.8% 2.1% 1.7% 0.72
Regular opiate use 32.0% 31.1% 32.2% 0.74
Regular benzodiazepine use 29.7% 32.0% 29.3% 0.41
Regular anticonvulsant use 5.2% 6.6% 4.9% 0.27
Daily alcohol use 5.2% 5.4% 5.2% 0.87
Number of risk factors
None 36.5% 37.8% 36.2%
One 42.9% 39.4% 43.6%
Two or more 20.7% 22.8% 20.3% 0.45
Value presented are % or mean ± SD. P values were calculated using chi-square tests for categorical variables and independent sam
ples t tests for continuous variables.
ASA-PS = American Society of Anesthesiologists physical status; AWR = awareness with explicit recall; BMI = body mass
COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident.
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
PERIOPERATIVE MEDICINE
that control patients were healthier, with a higher percentage
of patients with ASA-PS1 and lower percentages of patients
with ASA-PS 2–3 or ≥4. An attempt was made to decrease
confounds attributable to other covariates potentially associ-
ated with AWR risk by matching as described in the Mate-
rials and Methods.No significantdifferencesremained
between cohorts after matching (table 2). Comparison of
the standardized means of the propensity scores demon-
strated no significant difference in the overall distributional
balance of covariates between cohorts (t(324) = −1.93; P >
0.05), indicating that the greedy matching algorithm pro-
duced an adequate matched sample. The absolute number
and incidence of AWR events in each cohort are shown in
table 3. The incidence of AWR differed significantly betwe
cohorts (P = 0.03); patients with a history of AWR were fi
times more likely to experience AWR than control patient
(relative risk = 5.0; 95% CI, 1.3–19.9).
Approximately 4% of patients (N = 64) had incomplete
information describing drug administration. For the 1,382
remaining patients, the means and SEM for each drug typ
are presented in table 4. The proportion of patients
received each drug type did not differ significantly betwe
cohorts. All patients in this study received volatile anesth
agents. Because drug doses were not normally distri
these variables were transformed. Evaluation of the
variate distribution of the transformed variables displ
Table 2. Patient Demographic Characteristics, Comorbid Conditions, and Risk Factors for AWR for Matched Sample
Overall Sample
(n = 1,446)
History of Awareness
(n = 241)
No History of
Awareness
(n = 1,205) P Value
Male 36.9% 36.9% 36.9% 1.00
Age (yr), mean ± SD 52.3 ± 14.5 52.2 ± 14.6 52.3 ± 14.5 0.94
BMI (kg/m2), mean ± SD 30.8 ± 8.2 30.8 ± 8.1 30.8 ± 8.2 0.98
ASA-PS
=1 2.4% 2.5% 2.4%
2–3 83.3% 82.6% 83.4%
>3 14.3% 14.9% 14.2% 0.95
Comorbid conditions
Valvular heart disease 7.1% 6.2% 7.3% 0.55
Diabetes mellitus 18.1% 17.4% 18.3% 0.76
Coronary disease 18.9% 19.5% 18.8% 0.79
Dysrhythmias 9.1% 8.7% 9.2% 0.81
COPD 7.1% 9.1% 6.6% 0.17
CVA/stroke 4.0% 5.8% 3.7% 0.12
Congestive heart failure 6.5% 7.5% 6.3% 0.50
Peripheral vascular disease 6.6% 6.2% 6.6% 0.81
Hypertension 48.3% 47.3% 48.5% 0.73
Number of comorbidities
None 53.8% 53.1% 53.9%
One 26.7% 27.8% 26.5%
Two or more 19.5% 19.1% 19.6% 0.91
Current smoker 21.2% 21.2% 21.2% 1.00
Risk factors for awareness
Planned heart surgery 11.6% 10.0% 12.0% 0.38
Pulmonary hypertension 1.8% 2.1% 1.7% 0.72
Regular opiate use 32.0% 31.1% 32.2% 0.74
Regular benzodiazepine use 29.7% 32.0% 29.3% 0.41
Regular anticonvulsant use 5.2% 6.6% 4.9% 0.27
Daily alcohol use 5.2% 5.4% 5.2% 0.87
Number of risk factors
None 36.5% 37.8% 36.2%
One 42.9% 39.4% 43.6%
Two or more 20.7% 22.8% 20.3% 0.45
Value presented are % or mean ± SD. P values were calculated using chi-square tests for categorical variables and independent sam
ples t tests for continuous variables.
ASA-PS = American Society of Anesthesiologists physical status; AWR = awareness with explicit recall; BMI = body mass
COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident.
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
Anesthesiology 2013; 119:1275-83 1280 Aranake et al.
Increased Risk of Intraoperative Awareness
no substantial outliers. A Hotelling’s T2, using mean doses
for each of the five drug types, was performed to determine
whether the overall management differed between cohorts.
The Box’s M value was 23.1 with a P value of 0.085 indicating
that the data satisfied the equality of covariance assumption.
There was no difference in overall anesthetic management in
patients with a history of AWR compared with matched con-
trols (F(5, 1,376) = 1.4; P = 0.239; Hotelling’s T2 = 6.9).
After pharmacokinetic censoring of repeated measurements
of ETAC and BIS, 35,801 BIS-aaMAC datapoints derived
from 594 patients were analyzed in the mixed-effects model. A
history of AWR was a significant predictor of BIS in the model
(β = −5.4; P = 0.0001), indicating that after controlling for
the other factors in the model, the presence of this risk factor
decreases the projected intercept of the BIS–ETAC relation-
ship by 5 units. The interaction between aaMAC and history
of AWR was also significant (β = 3.7; P = 0.0018), indicating
that in patients with a history of AWR, the magnitude of the
slope of the BIS–ETAC relationship is less than that of the
control patients. According to this model, increasing aaMAC
by 0.1 in patients without a history of AWR would result in
an average decrease in BIS of 1.8; whereas the same change
in patients with a history of AWR would result in an aver-
age decrease in BIS of 1.5. The difference in the correlation
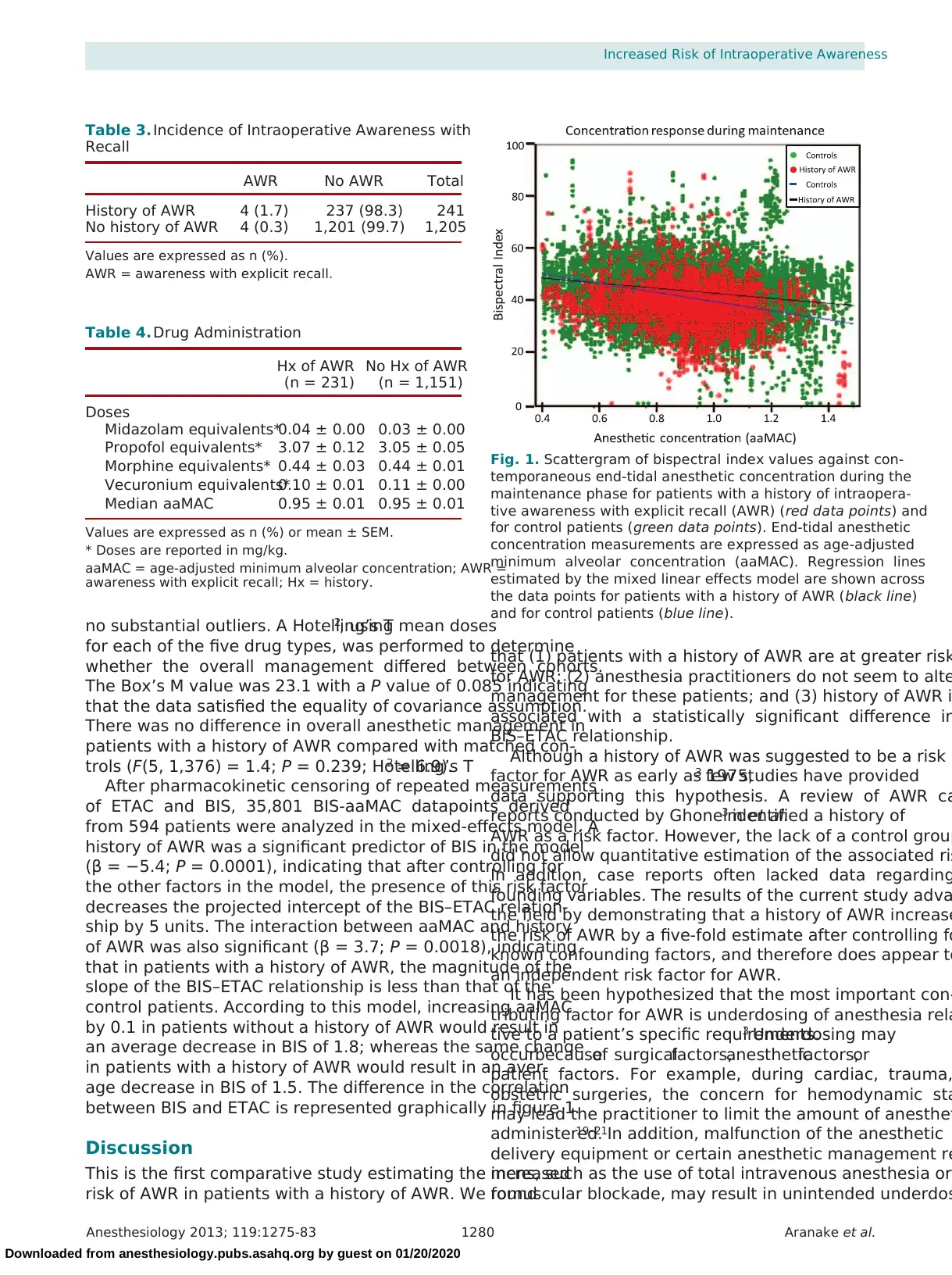
between BIS and ETAC is represented graphically in figure 1.
Discussion
This is the first comparative study estimating the increased
risk of AWR in patients with a history of AWR. We found
that (1) patients with a history of AWR are at greater risk
for AWR; (2) anesthesia practitioners do not seem to alte
management for these patients; and (3) history of AWR i
associated with a statistically significant difference in
BIS–ETAC relationship.
Although a history of AWR was suggested to be a risk
factor for AWR as early as 1975,3 few studies have provided
data supporting this hypothesis. A review of AWR ca
reports conducted by Ghoneim et al.3 identified a history of
AWR as a risk factor. However, the lack of a control group
did not allow quantitative estimation of the associated ris
In addition, case reports often lacked data regarding
founding variables. The results of the current study adva
the field by demonstrating that a history of AWR increase
the risk of AWR by a five-fold estimate after controlling fo
known confounding factors, and therefore does appear to
an independent risk factor for AWR.
It has been hypothesized that the most important con-
tributing factor for AWR is underdosing of anesthesia rela
tive to a patient’s specific requirements.3 Underdosing may
occurbecauseof surgicalfactors,anestheticfactors,or
patient factors. For example, during cardiac, trauma,
obstetric surgeries, the concern for hemodynamic sta
may lead the practitioner to limit the amount of anesthet
administered.19–21In addition, malfunction of the anesthetic
delivery equipment or certain anesthetic management re
mens, such as the use of total intravenous anesthesia or
romuscular blockade, may result in unintended underdos
Fig. 1. Scattergram of bispectral index values against con-
temporaneous end-tidal anesthetic concentration during the
maintenance phase for patients with a history of intraopera-
tive awareness with explicit recall (AWR) (red data points) and
for control patients (green data points). End-tidal anesthetic
concentration measurements are expressed as age-adjusted
minimum alveolar concentration (aaMAC). Regression lines
estimated by the mixed linear effects model are shown across
the data points for patients with a history of AWR (black line)
and for control patients (blue line).
Table 4. Drug Administration
Hx of AWR
(n = 231)
No Hx of AWR
(n = 1,151)
Doses
Midazolam equivalents*0.04 ± 0.00 0.03 ± 0.00
Propofol equivalents* 3.07 ± 0.12 3.05 ± 0.05
Morphine equivalents* 0.44 ± 0.03 0.44 ± 0.01
Vecuronium equivalents*0.10 ± 0.01 0.11 ± 0.00
Median aaMAC 0.95 ± 0.01 0.95 ± 0.01
Values are expressed as n (%) or mean ± SEM.
* Doses are reported in mg/kg.
aaMAC = age-adjusted minimum alveolar concentration; AWR =
awareness with explicit recall; Hx = history.
Table 3. Incidence of Intraoperative Awareness with
Recall
AWR No AWR Total
History of AWR 4 (1.7) 237 (98.3) 241
No history of AWR 4 (0.3) 1,201 (99.7) 1,205
Values are expressed as n (%).
AWR = awareness with explicit recall.
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
Increased Risk of Intraoperative Awareness
no substantial outliers. A Hotelling’s T2, using mean doses
for each of the five drug types, was performed to determine
whether the overall management differed between cohorts.
The Box’s M value was 23.1 with a P value of 0.085 indicating
that the data satisfied the equality of covariance assumption.
There was no difference in overall anesthetic management in
patients with a history of AWR compared with matched con-
trols (F(5, 1,376) = 1.4; P = 0.239; Hotelling’s T2 = 6.9).
After pharmacokinetic censoring of repeated measurements
of ETAC and BIS, 35,801 BIS-aaMAC datapoints derived
from 594 patients were analyzed in the mixed-effects model. A
history of AWR was a significant predictor of BIS in the model
(β = −5.4; P = 0.0001), indicating that after controlling for
the other factors in the model, the presence of this risk factor
decreases the projected intercept of the BIS–ETAC relation-
ship by 5 units. The interaction between aaMAC and history
of AWR was also significant (β = 3.7; P = 0.0018), indicating
that in patients with a history of AWR, the magnitude of the
slope of the BIS–ETAC relationship is less than that of the
control patients. According to this model, increasing aaMAC
by 0.1 in patients without a history of AWR would result in
an average decrease in BIS of 1.8; whereas the same change
in patients with a history of AWR would result in an aver-
age decrease in BIS of 1.5. The difference in the correlation
between BIS and ETAC is represented graphically in figure 1.
Discussion
This is the first comparative study estimating the increased
risk of AWR in patients with a history of AWR. We found
that (1) patients with a history of AWR are at greater risk
for AWR; (2) anesthesia practitioners do not seem to alte
management for these patients; and (3) history of AWR i
associated with a statistically significant difference in
BIS–ETAC relationship.
Although a history of AWR was suggested to be a risk
factor for AWR as early as 1975,3 few studies have provided
data supporting this hypothesis. A review of AWR ca
reports conducted by Ghoneim et al.3 identified a history of
AWR as a risk factor. However, the lack of a control group
did not allow quantitative estimation of the associated ris
In addition, case reports often lacked data regarding
founding variables. The results of the current study adva
the field by demonstrating that a history of AWR increase
the risk of AWR by a five-fold estimate after controlling fo
known confounding factors, and therefore does appear to
an independent risk factor for AWR.
It has been hypothesized that the most important con-
tributing factor for AWR is underdosing of anesthesia rela
tive to a patient’s specific requirements.3 Underdosing may
occurbecauseof surgicalfactors,anestheticfactors,or
patient factors. For example, during cardiac, trauma,
obstetric surgeries, the concern for hemodynamic sta
may lead the practitioner to limit the amount of anesthet
administered.19–21In addition, malfunction of the anesthetic
delivery equipment or certain anesthetic management re
mens, such as the use of total intravenous anesthesia or
romuscular blockade, may result in unintended underdos
Fig. 1. Scattergram of bispectral index values against con-
temporaneous end-tidal anesthetic concentration during the
maintenance phase for patients with a history of intraopera-
tive awareness with explicit recall (AWR) (red data points) and
for control patients (green data points). End-tidal anesthetic
concentration measurements are expressed as age-adjusted
minimum alveolar concentration (aaMAC). Regression lines
estimated by the mixed linear effects model are shown across
the data points for patients with a history of AWR (black line)
and for control patients (blue line).
Table 4. Drug Administration
Hx of AWR
(n = 231)
No Hx of AWR
(n = 1,151)
Doses
Midazolam equivalents*0.04 ± 0.00 0.03 ± 0.00
Propofol equivalents* 3.07 ± 0.12 3.05 ± 0.05
Morphine equivalents* 0.44 ± 0.03 0.44 ± 0.01
Vecuronium equivalents*0.10 ± 0.01 0.11 ± 0.00
Median aaMAC 0.95 ± 0.01 0.95 ± 0.01
Values are expressed as n (%) or mean ± SEM.
* Doses are reported in mg/kg.
aaMAC = age-adjusted minimum alveolar concentration; AWR =
awareness with explicit recall; Hx = history.
Table 3. Incidence of Intraoperative Awareness with
Recall
AWR No AWR Total
History of AWR 4 (1.7) 237 (98.3) 241
No history of AWR 4 (0.3) 1,201 (99.7) 1,205
Values are expressed as n (%).
AWR = awareness with explicit recall.
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020

Anesthesiology 2013; 119:1275-83 1281 Aranake et al.
PERIOPERATIVE MEDICINE
of anesthesia.3 Finally, an acquired or genetic resistance to
the hypnotic or amnesic actions of certain anesthetic agents
may increaseanestheticrequirementsor rendercertain
anesthetics ineffective in some individuals.3,22–24
Modifying
anesthetic management in patients with an increased risk for
developing AWR might help prevent an AWR event; how-
ever, there are no guidelines for treating these patients due to
the paucity of evidence regarding whether or how anesthesia
providers change their anesthetic regimen.25
Clinical Implications
The 2006 ASA Practice Advisory for intraoperative aware-
ness included a history of AWR as a potential risk factor for
AWR. Although there is a lack of evidence supporting the
efficacy of specific pharmacologic interventions to prevent
AWR, several modifications of anesthetic administration
have been proposed for high-risk patients. These include
increased inhaled anesthetic concentration to ensure uncon-
sciousness,increaseduse of benzodiazepinesto prevent
memory, and the avoidance of neuromuscular blockers to
preserve motility.26 Suggestions to increase the dosage of
certain anesthetic agents are based on the hypothesis that
patients with a relative resistance to these agents may require
higher concentrations for adequate anesthesia.
The current study, from several tertiary academic medi-
cal centers, suggests that there is no substantial modifica-
tion of anesthetic care for patients with a history of AWR.
The adjusted five-fold increase in AWR risk with a history of
the complication provides the first compelling evidence that
changes in anesthesia practice are necessary to reduce what is
one of the highest incidences of AWR (1.7%) reported in the
modern literature. The methodology of this study may even
have resulted in an underestimation of the problem in that vir-
tually all patients recruited to the parent trials were entered
into a treatment arm aimed at preventing AWR. As such, a
history of AWR should prompt consideration of measures to
prevent AWR and minimize patient distress including: (1) a
preoperative discussion with the patient regarding further risk,
(2) increased doses and multimodal approaches to anesthesia
and analgesia, (3) use of a brain monitor that can assist in sug-
gesting adequate depth of anesthesia, and (4) postoperative
screening for AWR at multiple time points for psychiatric
referral if experienced. However, precise recommendations
regarding intraoperative management should be tempered at
this time, as the mechanism responsible for the increased risk
of AWR in patients with a history of AWR is unclear. We spec-
ulate that the interventions of the parent trials (BIS or MAC
alarms) prevented the majority of preventable reasons for AWR
(e.g., empty vaporizer) and therefore unmasked some individu-
als that might be intrinsically resistant to hypnotic or amne-
sic effects of general anesthesia. The current study identified a
reduced sensitivity of BIS values to changes in ETAC, but it is
unlikely that this statistical difference has major clinical impact
and is sufficient to account for the increased incidence of AWR
experienced by the patients with a history of AWR.
Scientific Implications
Whitlock et al.15 demonstrated that although BIS corre-
lates unpredictably with aaMAC for individual patient
there is an average negative correlation between aa
and BIS values for the population during the maintenanc
phase of anesthesia. Resistance to the hypnotic actions o
inhaled anesthetics could be manifest as either redu
potency or efficacy and would result in predictable shifts
the relationship between BIS and aaMAC (fig. 2). If resis-
tance to anesthetic effect is due to a decrease in anesthe
potency, this might produce a right shift in the relationsh
between BIS and ETAC (fig. 2). Alternatively, if resistance
is due to decreased anesthetic efficacy, this might result
higher BIS values at all ETAC concentrations with a highe
(nonzero) BIS value at the highest ETAC concentrations.
Although there was a small significant difference in the re
tionship between BIS and aaMAC, this difference was not
strongly suggestive of a resistance to the hypnotic action
of anesthetics in patients with a history of AWR. Genetic
variations might result in resistance to the hypnotic
amnesic actions of certain anesthetic agents although su
variations have not yet been identified in humans. In an
experimental model, mutations of the α5 subunit of the
γ-aminobutyric acidA receptor render mice resistant to the
amnesic, but not hypnotic, actions of etomidate.24 It is
conceivable that a genetic resistance to some or all
thetic agents may account for the predisposition for AWR
in patients with a history of AWR. Although challenging
given the rarity of the complication, pharmacogenom
analysis and other mechanistic details could help gu
clinical practice. Previous suggestions to increase doses o
inhaled anesthetics and benzodiazepines would only
effective if a reduction in drug potency was the underlyin
Fig. 2. Hypothetical concentration–response curves. The solid
line represents the relationship between processed electroen-
cephalography (EEG) value and the anesthetic concentration
for controls. The dotted line represents the expected rela-
tionship for patients who are resistant to anesthesia due to
decreased potency of the anesthetic. The hashed line repre-
sents the expected relationship for patients who are resistant
to anesthesia due to decreased efficacy of the anesthetic.
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
PERIOPERATIVE MEDICINE
of anesthesia.3 Finally, an acquired or genetic resistance to
the hypnotic or amnesic actions of certain anesthetic agents
may increaseanestheticrequirementsor rendercertain
anesthetics ineffective in some individuals.3,22–24
Modifying
anesthetic management in patients with an increased risk for
developing AWR might help prevent an AWR event; how-
ever, there are no guidelines for treating these patients due to
the paucity of evidence regarding whether or how anesthesia
providers change their anesthetic regimen.25
Clinical Implications
The 2006 ASA Practice Advisory for intraoperative aware-
ness included a history of AWR as a potential risk factor for
AWR. Although there is a lack of evidence supporting the
efficacy of specific pharmacologic interventions to prevent
AWR, several modifications of anesthetic administration
have been proposed for high-risk patients. These include
increased inhaled anesthetic concentration to ensure uncon-
sciousness,increaseduse of benzodiazepinesto prevent
memory, and the avoidance of neuromuscular blockers to
preserve motility.26 Suggestions to increase the dosage of
certain anesthetic agents are based on the hypothesis that
patients with a relative resistance to these agents may require
higher concentrations for adequate anesthesia.
The current study, from several tertiary academic medi-
cal centers, suggests that there is no substantial modifica-
tion of anesthetic care for patients with a history of AWR.
The adjusted five-fold increase in AWR risk with a history of
the complication provides the first compelling evidence that
changes in anesthesia practice are necessary to reduce what is
one of the highest incidences of AWR (1.7%) reported in the
modern literature. The methodology of this study may even
have resulted in an underestimation of the problem in that vir-
tually all patients recruited to the parent trials were entered
into a treatment arm aimed at preventing AWR. As such, a
history of AWR should prompt consideration of measures to
prevent AWR and minimize patient distress including: (1) a
preoperative discussion with the patient regarding further risk,
(2) increased doses and multimodal approaches to anesthesia
and analgesia, (3) use of a brain monitor that can assist in sug-
gesting adequate depth of anesthesia, and (4) postoperative
screening for AWR at multiple time points for psychiatric
referral if experienced. However, precise recommendations
regarding intraoperative management should be tempered at
this time, as the mechanism responsible for the increased risk
of AWR in patients with a history of AWR is unclear. We spec-
ulate that the interventions of the parent trials (BIS or MAC
alarms) prevented the majority of preventable reasons for AWR
(e.g., empty vaporizer) and therefore unmasked some individu-
als that might be intrinsically resistant to hypnotic or amne-
sic effects of general anesthesia. The current study identified a
reduced sensitivity of BIS values to changes in ETAC, but it is
unlikely that this statistical difference has major clinical impact
and is sufficient to account for the increased incidence of AWR
experienced by the patients with a history of AWR.
Scientific Implications
Whitlock et al.15 demonstrated that although BIS corre-
lates unpredictably with aaMAC for individual patient
there is an average negative correlation between aa
and BIS values for the population during the maintenanc
phase of anesthesia. Resistance to the hypnotic actions o
inhaled anesthetics could be manifest as either redu
potency or efficacy and would result in predictable shifts
the relationship between BIS and aaMAC (fig. 2). If resis-
tance to anesthetic effect is due to a decrease in anesthe
potency, this might produce a right shift in the relationsh
between BIS and ETAC (fig. 2). Alternatively, if resistance
is due to decreased anesthetic efficacy, this might result
higher BIS values at all ETAC concentrations with a highe
(nonzero) BIS value at the highest ETAC concentrations.
Although there was a small significant difference in the re
tionship between BIS and aaMAC, this difference was not
strongly suggestive of a resistance to the hypnotic action
of anesthetics in patients with a history of AWR. Genetic
variations might result in resistance to the hypnotic
amnesic actions of certain anesthetic agents although su
variations have not yet been identified in humans. In an
experimental model, mutations of the α5 subunit of the
γ-aminobutyric acidA receptor render mice resistant to the
amnesic, but not hypnotic, actions of etomidate.24 It is
conceivable that a genetic resistance to some or all
thetic agents may account for the predisposition for AWR
in patients with a history of AWR. Although challenging
given the rarity of the complication, pharmacogenom
analysis and other mechanistic details could help gu
clinical practice. Previous suggestions to increase doses o
inhaled anesthetics and benzodiazepines would only
effective if a reduction in drug potency was the underlyin
Fig. 2. Hypothetical concentration–response curves. The solid
line represents the relationship between processed electroen-
cephalography (EEG) value and the anesthetic concentration
for controls. The dotted line represents the expected rela-
tionship for patients who are resistant to anesthesia due to
decreased potency of the anesthetic. The hashed line repre-
sents the expected relationship for patients who are resistant
to anesthesia due to decreased efficacy of the anesthetic.
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Anesthesiology 2013; 119:1275-83 1282 Aranake et al.
Increased Risk of Intraoperative Awareness
problem. However, such an increase would make little or
no difference in resistance due to decreased drug efficacy.
Mechanistic studies may clarify whether it is possible to
reduce the incidence of AWR in patients with a history of
AWR with specific alterations in anesthetic management.
Limitations
AWR is a rare postoperative complication and thus this study
is limited by the small number of AWR events in each cohort.
Previous AWR events were self-reported by patients during
enrollment for the parent trials. As such, review of records was
not adequate to determine whether these patients truly expe-
rienced AWR during a prior surgery. Furthermore, to explore
resistance to anesthesia, we assume that BIS is a reliable sur-
rogate for depth of anesthesia. Given the limitations of our
understanding of the neural correlates of consciousness, we
do not yet have a validated (surrogate) metric for the hyp-
notic effect of volatile anesthetics. Although the relationship
between BIS and ETAC displays marked inter- and intrain-
dividual variability, there is a significant negative correlation
for a population in the maintenance range. Extrapolation of
this linear relationship beyond this range is invalid; thus, the
estimated intercept is meaningless because the relationship
is not linear for the entire range of anesthetic concentration.
In addition, this analysis did not provide any data regard-
ing amnesic actions of anesthetics in these patients. Finally,
although the parent trials were randomized, the current study
was retrospective and used a matched cohort. There is the
potential for hidden confounders that explain the observed
differences between the two groups.
Conclusion
History of AWR confers an adjusted five-fold increase in
risk of AWR, even in the setting of preventive interventions.
These data should prompt a careful preoperative discussion
of AWR risk in patients reporting a history of the compli-
cation, intraoperative vigilance for potentially insufficient
anesthesia or analgesia, and systematic postoperative assess-
ment to screen for AWR and its psychological consequences.
Further translational research is required to clarify whether
genetic variations contribute to the increased risk of AWR in
this vulnerable surgical population.
References
1. Sebel PS, Bowdle TA, Ghoneim MM, Rampil IJ, Padilla RE,
Gan TJ, Domino KB: The incidence of awareness during
anesthesia: A multicenter United States study. Anesth Analg
2004; 99:833–9
2. Samuelsson P, Brudin L, Sandin RH: Late psychological
symptoms after awareness among consecutively included
surgical patients. ANesthesiology 2007; 106:26–32
3. Ghoneim MM, Block RI, Haffarnan M, Mathews MJ:
Awareness during anesthesia:Risk factors, causes and
sequelae: A review of reported cases in the literature. Anesth
Analg 2009; 108:527–35
4. Leslie K, Chan MT, Myles PS, Forbes A, McCulloch TJ:
Posttraumatic stress disorder in aware patients from the
B-aware trial. Anesth Analg 2010; 110:823–8
5. Utting JE: Phillip Gett memorial lecture awareness in anaes-
thesia. Anaesth Intensive Care 1975; 3:334–40
6. Avidan Ms, Jacobsohn e, glick D, Burnside BA, Zhang l,
Villafranca A, Karl l, Kamal s, torres B, o’Connor M, evers
As, gradwohl s, lin N, Palanca BJ, Mashour gA; BAg-
RECALL Research Group: Prevention of intraoperative
awareness in a high-risk surgical population. N engl J Med
2011; 365:591–600
7. Avidan Ms, Zhang l, Burnside BA, Finkel KJ, searleman AC,
Selvidge JA, Saager L, Turner MS, Rao S, Bottros M, Hantler C,
Jacobsohn E, Evers AS: Anesthesia awareness and the bispec-
tral index. N engl J Med 2008; 358:1097–108
8. Mashour GA, Shanks A, Tremper KK, Kheterpal S, Turner
CR, Ramachandran SK, Picton P, Schueller C, Morris M,
Vandervest JC, lin N, Avidan Ms: Prevention of intraopera-
tive awareness with explicit recall in an unselected surgical
population: A randomized comparative effectiveness trial.
ANesthesiology 2012; 117:717–25
9. Mashour GA, Tremper KK, Avidan MS: Protocol for the
“Michigan Awareness Control Study”: A prospective, random-
ized, controlled trial comparing electronic alerts based on
bispectral index monitoring or minimum alveolar concen-
tration for the prevention of intraoperative awareness. BMC
Anesthesiol 2009; 9:7
10. Brice DD, Hetherington RR, Utting JE: A simple study of
awareness and dreaming during anaesthesia. Br J Anaesth
1970; 42:535–42
11. Myles Ps, leslie K, McNeil J, Forbes A, Chan Mt: Bispectral
index monitoring to prevent awareness during anaesthe-
sia: The B-Aware randomised controlled trial. Lancet 2004;
363:1757–63
12. Alberta Hospice Palliative Care Resource Manual, 2nd edi-
tion. edited by Pereira J, Berry otfinowski P, hagen N,
Bruera e, Fainsinger R, summers N. Calgary, Alberta Cancer
Board, 2001
13. Cusick JM: Anesthesia & Critical Care Reference Sheet.
Goodyear, Jeffery Cusick, 2009
14. Nickalls RW, Mapleson WW: Age-related iso-MAC charts for
isoflurane, sevoflurane and desflurane in man. Br J Anaesth
2003; 91:170–4
15. Whitlock el, Villafranca AJ, lin N, Palanca BJ, Jacobsohn e,
Finkel KJ, Zhang l, Burnside BA, Kaiser hA, evers As, Avidan
MS: Relationship between bispectral index values and vola-
tile anesthetic concentrations during the maintenance phase
of anesthesia in the B-Unaware trial. ANesthesiology 2011;
115:1209–18
16. Parsons l: Reducing bias in a propensity score matched-pair
sample using greedy matching techniques, Proceedings of
the Twenty-Sixth Annual SAS® Users Group International
Conference. Long Beach, SAS Institute Inc., 2001, pp
214–26
17. Stuart EA: Matching methods for causal inference: A review
and a look forward. Stat Sci 2010; 25:1–21
18. Huberty CJ, Petoskey MD: Multivariate analysis of variance
and covariance, Handbook of Applied Multivariate Statistics
and Mathematical Modeling. Edited by Tinsley HEA, Brown
sD. New york, Academic Press, 2000, pp 183–208
19. Phillips AA, McLean RF, Devitt JH, Harrington EM: Recall of
intraoperative events after general anaesthesia and cardio-
pulmonary bypass. Can J Anaesth 1993; 40:922–6
20. Bogetz MS, Katz JA: Recall of surgery for major trauma.
ANesthesiology 1984; 61:6–9
21. Lyons G, Macdonald R: Awareness during caesarean section.
Anaesthesia 1991; 46:62–4
22. Liem EB, Lin CM, Suleman MI, Doufas AG, Gregg RG,
Veauthier JM, Loyd G, Sessler DI: Anesthetic requirement is
increased in redheads. ANesthesiology 2004; 101:279–83
23. Brundidge PK, Leavell ME, Tempelhoff R: EEG-controlled
“overdosage” of anesthetics in a patient with a history of
intra-anesthetic awareness. J Clin Anesth 1994; 6:496–9
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
Increased Risk of Intraoperative Awareness
problem. However, such an increase would make little or
no difference in resistance due to decreased drug efficacy.
Mechanistic studies may clarify whether it is possible to
reduce the incidence of AWR in patients with a history of
AWR with specific alterations in anesthetic management.
Limitations
AWR is a rare postoperative complication and thus this study
is limited by the small number of AWR events in each cohort.
Previous AWR events were self-reported by patients during
enrollment for the parent trials. As such, review of records was
not adequate to determine whether these patients truly expe-
rienced AWR during a prior surgery. Furthermore, to explore
resistance to anesthesia, we assume that BIS is a reliable sur-
rogate for depth of anesthesia. Given the limitations of our
understanding of the neural correlates of consciousness, we
do not yet have a validated (surrogate) metric for the hyp-
notic effect of volatile anesthetics. Although the relationship
between BIS and ETAC displays marked inter- and intrain-
dividual variability, there is a significant negative correlation
for a population in the maintenance range. Extrapolation of
this linear relationship beyond this range is invalid; thus, the
estimated intercept is meaningless because the relationship
is not linear for the entire range of anesthetic concentration.
In addition, this analysis did not provide any data regard-
ing amnesic actions of anesthetics in these patients. Finally,
although the parent trials were randomized, the current study
was retrospective and used a matched cohort. There is the
potential for hidden confounders that explain the observed
differences between the two groups.
Conclusion
History of AWR confers an adjusted five-fold increase in
risk of AWR, even in the setting of preventive interventions.
These data should prompt a careful preoperative discussion
of AWR risk in patients reporting a history of the compli-
cation, intraoperative vigilance for potentially insufficient
anesthesia or analgesia, and systematic postoperative assess-
ment to screen for AWR and its psychological consequences.
Further translational research is required to clarify whether
genetic variations contribute to the increased risk of AWR in
this vulnerable surgical population.
References
1. Sebel PS, Bowdle TA, Ghoneim MM, Rampil IJ, Padilla RE,
Gan TJ, Domino KB: The incidence of awareness during
anesthesia: A multicenter United States study. Anesth Analg
2004; 99:833–9
2. Samuelsson P, Brudin L, Sandin RH: Late psychological
symptoms after awareness among consecutively included
surgical patients. ANesthesiology 2007; 106:26–32
3. Ghoneim MM, Block RI, Haffarnan M, Mathews MJ:
Awareness during anesthesia:Risk factors, causes and
sequelae: A review of reported cases in the literature. Anesth
Analg 2009; 108:527–35
4. Leslie K, Chan MT, Myles PS, Forbes A, McCulloch TJ:
Posttraumatic stress disorder in aware patients from the
B-aware trial. Anesth Analg 2010; 110:823–8
5. Utting JE: Phillip Gett memorial lecture awareness in anaes-
thesia. Anaesth Intensive Care 1975; 3:334–40
6. Avidan Ms, Jacobsohn e, glick D, Burnside BA, Zhang l,
Villafranca A, Karl l, Kamal s, torres B, o’Connor M, evers
As, gradwohl s, lin N, Palanca BJ, Mashour gA; BAg-
RECALL Research Group: Prevention of intraoperative
awareness in a high-risk surgical population. N engl J Med
2011; 365:591–600
7. Avidan Ms, Zhang l, Burnside BA, Finkel KJ, searleman AC,
Selvidge JA, Saager L, Turner MS, Rao S, Bottros M, Hantler C,
Jacobsohn E, Evers AS: Anesthesia awareness and the bispec-
tral index. N engl J Med 2008; 358:1097–108
8. Mashour GA, Shanks A, Tremper KK, Kheterpal S, Turner
CR, Ramachandran SK, Picton P, Schueller C, Morris M,
Vandervest JC, lin N, Avidan Ms: Prevention of intraopera-
tive awareness with explicit recall in an unselected surgical
population: A randomized comparative effectiveness trial.
ANesthesiology 2012; 117:717–25
9. Mashour GA, Tremper KK, Avidan MS: Protocol for the
“Michigan Awareness Control Study”: A prospective, random-
ized, controlled trial comparing electronic alerts based on
bispectral index monitoring or minimum alveolar concen-
tration for the prevention of intraoperative awareness. BMC
Anesthesiol 2009; 9:7
10. Brice DD, Hetherington RR, Utting JE: A simple study of
awareness and dreaming during anaesthesia. Br J Anaesth
1970; 42:535–42
11. Myles Ps, leslie K, McNeil J, Forbes A, Chan Mt: Bispectral
index monitoring to prevent awareness during anaesthe-
sia: The B-Aware randomised controlled trial. Lancet 2004;
363:1757–63
12. Alberta Hospice Palliative Care Resource Manual, 2nd edi-
tion. edited by Pereira J, Berry otfinowski P, hagen N,
Bruera e, Fainsinger R, summers N. Calgary, Alberta Cancer
Board, 2001
13. Cusick JM: Anesthesia & Critical Care Reference Sheet.
Goodyear, Jeffery Cusick, 2009
14. Nickalls RW, Mapleson WW: Age-related iso-MAC charts for
isoflurane, sevoflurane and desflurane in man. Br J Anaesth
2003; 91:170–4
15. Whitlock el, Villafranca AJ, lin N, Palanca BJ, Jacobsohn e,
Finkel KJ, Zhang l, Burnside BA, Kaiser hA, evers As, Avidan
MS: Relationship between bispectral index values and vola-
tile anesthetic concentrations during the maintenance phase
of anesthesia in the B-Unaware trial. ANesthesiology 2011;
115:1209–18
16. Parsons l: Reducing bias in a propensity score matched-pair
sample using greedy matching techniques, Proceedings of
the Twenty-Sixth Annual SAS® Users Group International
Conference. Long Beach, SAS Institute Inc., 2001, pp
214–26
17. Stuart EA: Matching methods for causal inference: A review
and a look forward. Stat Sci 2010; 25:1–21
18. Huberty CJ, Petoskey MD: Multivariate analysis of variance
and covariance, Handbook of Applied Multivariate Statistics
and Mathematical Modeling. Edited by Tinsley HEA, Brown
sD. New york, Academic Press, 2000, pp 183–208
19. Phillips AA, McLean RF, Devitt JH, Harrington EM: Recall of
intraoperative events after general anaesthesia and cardio-
pulmonary bypass. Can J Anaesth 1993; 40:922–6
20. Bogetz MS, Katz JA: Recall of surgery for major trauma.
ANesthesiology 1984; 61:6–9
21. Lyons G, Macdonald R: Awareness during caesarean section.
Anaesthesia 1991; 46:62–4
22. Liem EB, Lin CM, Suleman MI, Doufas AG, Gregg RG,
Veauthier JM, Loyd G, Sessler DI: Anesthetic requirement is
increased in redheads. ANesthesiology 2004; 101:279–83
23. Brundidge PK, Leavell ME, Tempelhoff R: EEG-controlled
“overdosage” of anesthetics in a patient with a history of
intra-anesthetic awareness. J Clin Anesth 1994; 6:496–9
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020

Anesthesiology 2013; 119:1275-83 1283 Aranake et al.
PERIOPERATIVE MEDICINE
24. Cheng Vy, Martin lJ, elliott eM, Kim Jh, Mount ht, taverna
FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, Wafford
KA, orser BA: Alpha5gABAA receptors mediate the amnes-
tic but not sedative-hypnotic effects of the general anesthetic
etomidate. J Neurosci 2006; 26:3713–20
25. AmericanSociety of AnesthesiologistsTask Force on
Intraoperative A: Practice advisory for intraoperative aware-
ness and brain function monitoring: A report by the American
Society of Anesthesiologists Task Force on Intraoperative
Awareness. ANesthesiology 2006; 104:847–64
26. Mashour gA, Avidan Ms: Pharmacologic approaches to
the prevention of intraoperative awareness. Expert Rev
Neurother 2011; 11:611–3
Appendix 1. Modified Brice Questionnaire
1. What was the last thing you remember before going to
sleep?
2. What is the first thing you remember after waking up?
3. Do you remember anything between going to sleep and
waking up?
4. Did you dream during your procedure?
5. What was the worst thing about your operation?
Appendix 2.Upper Dose Limit for Drug Conversion
(Beyond Which Values Were Excluded)
Drug Dose (mg)
Atracurium 500
Cisatracurium 200
Etomidate 50
Fentanyl 20
Hydromorphone 20
Meperidine 300
Methadone 100
Midazolam 100
Morphine 200
Pancuronium 50
Propofol 6,200
Rocuronium 250
Sufentanil 10
Thiopental 2,000
Vecuronium 50
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
PERIOPERATIVE MEDICINE
24. Cheng Vy, Martin lJ, elliott eM, Kim Jh, Mount ht, taverna
FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, Wafford
KA, orser BA: Alpha5gABAA receptors mediate the amnes-
tic but not sedative-hypnotic effects of the general anesthetic
etomidate. J Neurosci 2006; 26:3713–20
25. AmericanSociety of AnesthesiologistsTask Force on
Intraoperative A: Practice advisory for intraoperative aware-
ness and brain function monitoring: A report by the American
Society of Anesthesiologists Task Force on Intraoperative
Awareness. ANesthesiology 2006; 104:847–64
26. Mashour gA, Avidan Ms: Pharmacologic approaches to
the prevention of intraoperative awareness. Expert Rev
Neurother 2011; 11:611–3
Appendix 1. Modified Brice Questionnaire
1. What was the last thing you remember before going to
sleep?
2. What is the first thing you remember after waking up?
3. Do you remember anything between going to sleep and
waking up?
4. Did you dream during your procedure?
5. What was the worst thing about your operation?
Appendix 2.Upper Dose Limit for Drug Conversion
(Beyond Which Values Were Excluded)
Drug Dose (mg)
Atracurium 500
Cisatracurium 200
Etomidate 50
Fentanyl 20
Hydromorphone 20
Meperidine 300
Methadone 100
Midazolam 100
Morphine 200
Pancuronium 50
Propofol 6,200
Rocuronium 250
Sufentanil 10
Thiopental 2,000
Vecuronium 50
Downloaded from anesthesiology.pubs.asahq.org by guest on 01/20/2020
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.