Breast Cancer Detection Using Deep Learning
VerifiedAdded on 2020/11/23
|16
|14251
|98
Practical Assignment
AI Summary
This assignment examines the application of deep learning, particularly convolutional neural networks (CNNs), in breast cancer detection. It covers various aspects, including CNN architectures suited for image analysis, methods for extracting relevant features from mammograms, and evaluation metrics commonly used to assess the performance of these models in classifying cancerous and non-cancerous tissues. The aim is to demonstrate how deep learning can contribute to improved accuracy and efficiency in breast cancer diagnosis.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

A Robust Deep Neural Network Based Breast Cancer
Detection And Classification
Abstract— The exponential rise in breast cancer cases across
the globe has alarmed academia-industries to achieve certain more
efficient and robust Breast Cancer Computer Aided Diagnosis (BC-
CAD) system for breast cancer detection. A number of techniques
have been developed with focus on case (i.e., data type) centric
segmentation, feature extraction and classification of breast cancer
Histopathological images. However, rising complexity and accuracy
often demands more robust solution. Recently, Convolutional
Neural Network (CNN) has emerged as one of the most efficient
techniques for medical data analysis and various image
classification problems. In this paper a highly robust and efficient
BC-CAD solution has been proposed. Our proposed system
incorporates pre-processing, enhanced adaptive learning based
Gaussian Mixture Model (GMM), connected component analysis
based region of interest localization, AlexNet-DNN based feature
extraction. The Principle Component Analysis (PCA) and Linear
Discriminant Analysis (LDA) based on feature selection which is
used as dimensional reduction. One of the advantages of the
proposed method is that none of the current dimensional reduction
algorithms employed with SVM to perform breast cancer detection
and classification. The overall results obtained signify that the
AlexNet-DNN based features at fully connected layer; FC6 in
conjunction with LDA dimensional reduction and SVM based
classification outperforms other state-of-art techniques for breast
cancer detection. The proposed BC-CAD system has been
performed over real world data BreakHis having significant
diversity and complexity, and therefore we suggest it to be used for
other real-world applications. The proposed method achieved 96.15
for AlexNet-FC6 and 96.20 for AlexNet-FC7 in term of evaluation
measures.
Keywords—Breast Cancer Detection; Computer Aided
Diagnosis; Convolutional Neural Network; AlexNet-DNN; Linear
Discriminant Analysis.
INTRODUCTION
Cancer is a type of disease involving the development and
growth of abnormal cell that invades or spreads to other parts of a
human body. In last few decades, cancer has emerged as one of
the deadliest heath diseases claiming huge death rate. There are
different types of cancers such as breast cancer, blood cancer,
melanoma or skin cancer…etc. However, in last few years, breast
cancer has emerged as the second most common cancer type after
skin cancer in women. Literatures reveal that almost 50% of the
breast cancer cases are found in the developing countries, while
approximate 58% of deaths take place in less developed
countries. It is estimated that around the world over 5,08,000
women died in 2011 due to breast cancer [1]. Various case
studies [1] have revealed that the earlier cancer identification and
its diagnosis can play vital role in providing success diagnosis or
treatment. A similar survey [2] also indicated high pace rise in
deaths caused due to breast cancer in 2012, which is even
increasing with vast pace [2]. The study revealed that the death
rate can reach up to 27 million till 2030 [3]. The exponential rise
in breast cancer has alarmed academia-industries to develop
certain vision based approach for earlier breast cancer detection
and diagnosis. The rise in advanced computing techniques, vision
based computations and decision process…etc. has given rise to a
new dimension having immense potential to meet major
demands. Non-deniably such development often gives a hope for
better human life, health and decision process. In fact, the
development of science and technology often intends to enable
human life secure, healthy and productive. On the other hand,
gigantically rising population across world requires optimal
healthcare solutions. In fact, the traditional manual diagnosis
processes are either confined or less productive to meet the
demands and enable optimal healthcare solution. This as a result
has motivated academia-industries to achieve more efficient
computer aided diagnosis (CAD) solutions for earlier health-
diagnosis. The development of Histopathological analysis and
molecular biology has made vision based breast cancer CAD
system more efficient [4]. Unlike traditional manual microscopic
analysis based approaches vision based CAD system can be of
paramount significance for breast cancer detection and
classification [5].
In last few years, efforts have been made to exploit X-ray
mammography and ultrasound technique to assist breast cancer
detection and diagnosis; however, mammography usually
exhibits poor sensitivity to the minute size cancers. In addition, it
is insignificant especially for augmented breast or dense breast
conditions [60]. Similarly, poor specificity and the complexity in
whole breast-imaging process make ultrasound method confined
and ineffective. As an enhanced solution, authors proposed
Magnetic Resonance Imagining (MRI) systems that possess
better soft tissue resolution capacity and enhanced sensitivity for
breast cancer detection [6]. The MRI was method used to avoid
any ionization radiation and hence it can be suitable for patients
with implants. The key usefulness of MRI method is its ability of
quantification of tumor volume, multi-focal and multi-centric
breast cancer detection [7]. Vision based CAD systems have been
1
Detection And Classification
Abstract— The exponential rise in breast cancer cases across
the globe has alarmed academia-industries to achieve certain more
efficient and robust Breast Cancer Computer Aided Diagnosis (BC-
CAD) system for breast cancer detection. A number of techniques
have been developed with focus on case (i.e., data type) centric
segmentation, feature extraction and classification of breast cancer
Histopathological images. However, rising complexity and accuracy
often demands more robust solution. Recently, Convolutional
Neural Network (CNN) has emerged as one of the most efficient
techniques for medical data analysis and various image
classification problems. In this paper a highly robust and efficient
BC-CAD solution has been proposed. Our proposed system
incorporates pre-processing, enhanced adaptive learning based
Gaussian Mixture Model (GMM), connected component analysis
based region of interest localization, AlexNet-DNN based feature
extraction. The Principle Component Analysis (PCA) and Linear
Discriminant Analysis (LDA) based on feature selection which is
used as dimensional reduction. One of the advantages of the
proposed method is that none of the current dimensional reduction
algorithms employed with SVM to perform breast cancer detection
and classification. The overall results obtained signify that the
AlexNet-DNN based features at fully connected layer; FC6 in
conjunction with LDA dimensional reduction and SVM based
classification outperforms other state-of-art techniques for breast
cancer detection. The proposed BC-CAD system has been
performed over real world data BreakHis having significant
diversity and complexity, and therefore we suggest it to be used for
other real-world applications. The proposed method achieved 96.15
for AlexNet-FC6 and 96.20 for AlexNet-FC7 in term of evaluation
measures.
Keywords—Breast Cancer Detection; Computer Aided
Diagnosis; Convolutional Neural Network; AlexNet-DNN; Linear
Discriminant Analysis.
INTRODUCTION
Cancer is a type of disease involving the development and
growth of abnormal cell that invades or spreads to other parts of a
human body. In last few decades, cancer has emerged as one of
the deadliest heath diseases claiming huge death rate. There are
different types of cancers such as breast cancer, blood cancer,
melanoma or skin cancer…etc. However, in last few years, breast
cancer has emerged as the second most common cancer type after
skin cancer in women. Literatures reveal that almost 50% of the
breast cancer cases are found in the developing countries, while
approximate 58% of deaths take place in less developed
countries. It is estimated that around the world over 5,08,000
women died in 2011 due to breast cancer [1]. Various case
studies [1] have revealed that the earlier cancer identification and
its diagnosis can play vital role in providing success diagnosis or
treatment. A similar survey [2] also indicated high pace rise in
deaths caused due to breast cancer in 2012, which is even
increasing with vast pace [2]. The study revealed that the death
rate can reach up to 27 million till 2030 [3]. The exponential rise
in breast cancer has alarmed academia-industries to develop
certain vision based approach for earlier breast cancer detection
and diagnosis. The rise in advanced computing techniques, vision
based computations and decision process…etc. has given rise to a
new dimension having immense potential to meet major
demands. Non-deniably such development often gives a hope for
better human life, health and decision process. In fact, the
development of science and technology often intends to enable
human life secure, healthy and productive. On the other hand,
gigantically rising population across world requires optimal
healthcare solutions. In fact, the traditional manual diagnosis
processes are either confined or less productive to meet the
demands and enable optimal healthcare solution. This as a result
has motivated academia-industries to achieve more efficient
computer aided diagnosis (CAD) solutions for earlier health-
diagnosis. The development of Histopathological analysis and
molecular biology has made vision based breast cancer CAD
system more efficient [4]. Unlike traditional manual microscopic
analysis based approaches vision based CAD system can be of
paramount significance for breast cancer detection and
classification [5].
In last few years, efforts have been made to exploit X-ray
mammography and ultrasound technique to assist breast cancer
detection and diagnosis; however, mammography usually
exhibits poor sensitivity to the minute size cancers. In addition, it
is insignificant especially for augmented breast or dense breast
conditions [60]. Similarly, poor specificity and the complexity in
whole breast-imaging process make ultrasound method confined
and ineffective. As an enhanced solution, authors proposed
Magnetic Resonance Imagining (MRI) systems that possess
better soft tissue resolution capacity and enhanced sensitivity for
breast cancer detection [6]. The MRI was method used to avoid
any ionization radiation and hence it can be suitable for patients
with implants. The key usefulness of MRI method is its ability of
quantification of tumor volume, multi-focal and multi-centric
breast cancer detection [7]. Vision based CAD systems have been
1
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

found efficient to perform more time efficient and accurate breast
cancer detection to assist early diagnosis. Exploring in depth of
the CAD systems, it can be found that its efficacy primarily
depends on the efficiency of the Region Of Interest (ROI)
segmentation, feature extraction, feature mapping and
classification techniques. There are a number of techniques, such
as wavelet transform [8][9][10], Gabor transform [11], applied
for feature extraction while classification is done using standard
support vector machine (SVM) or other artificial intelligence
approaches [9][12][13]. However, most of the traditional
approaches are confined due to limited feature extraction
efficiency and classification for lesion of different shape, size,
and orientation density. The low information availability too
confines the efficacy of the traditional feature extraction and
cancer classification models. In addition, there is an inevitable
need to develop efficient approaches to deal with high
dimensional features, feature selection and classification or
prediction. Considering large size unannotated data (i.e., breast
cancer image data), the use of CNN as feature extraction tool can
ensure better breast cancer CAD (BC-CAD) solution.
In this paper, a highly robust and efficient CNN based Deep
Neural Network (DNN) model has been developed for breast
cancer detection and classification. To enable an optimal
solution, our proposed model encompasses various enhancements
including pre-processing, ROI identification, Caffe-enet-AlexNet
DNN based feature extraction, PCA and LDA based feature
selection and SVM based classification. To examine the
effectiveness of the proposed DNN model, a parallel BC-CAD
model using Spatial Invariant Fourier Transform (SIFT) feature
extraction has been developed. In addition, to perform breast
cancer prediction, a two class classification model is developed;
where the overall result affirms that the AlexNet-DNN with LDA
feature extraction outperforms other state-of-art techniques for
BC-CAD purpose.
The other sections of the presented manuscript are divided as
follows: Section two presents the related work, which is followed
by the discussion of the proposed AlexNet DNN based breast
cancer detection and classification in Section three. Section four
presents the results and discussion, while the overall research
conclusion is presented in Section five.
RELATED WORK
This section briefs some of the key literatures pertaining to
the breast cancer detection and related technologies.
Rehman et al. [12] derived a diverse feature based breast
cancer detection model, where they applied phylogenetic trees,
various statistical features and local binary patterns for
generating certain distinctive and discriminative features to
perform classification. Authors applied radial basis function
based SVM for two class classification: cancerous and non-
cancerous. Pramanik et al. [8] assessed the breast thermogram to
perform cancer detection, where they at first applied Initial
Feature point Image (IFI) to perform feature extraction for each
segmented breast thermogram. They applied DWT to perform
feature extraction, which was followed by feature classification
using feed-forward Artificial Neural Network (ANN). A similar
effort was made in [9], where authors applied wavelet and
counterlet transformation for feature extraction and SVM
classifier based classification of the mammograms. Yousefi et al.
[11] used multi-channel Gabor wavelet filter for feature
extraction over mammograms. Authors applied Gabor filter
design to perform feature extraction, which was then followed by
two class classifications using Bayesian classifier. Caorsi et al.
[14] proposed an ANN based radar data processing model for
breast cancer detection where they emphasized not only on the
detection of the cancerous tumour but also its geometric features
such as depth and width. A similar effort was made in [15],
where authors proposed a data-driven matched field model to
assist microwave breast cancer detection. George et al. [16]
developed robust and intelligent breast cancer detection and
classification model using cytological images. Authors applied
different classifiers including back-propagation based multilayer
perceptron, Probabilistic Neural Network (PNN), SVM and
learning vector quantization to perform breast cancer
classification. Considering mitosis as a cancer signifier, Tashk et
al. [17] developed an automatic BC-CAD model, where at first
they applied 2-D anisotropic diffusion for noise removal and
image morphological process. To achieve pixel-wise features
from the ROI (i.e., mitosis region), authors derived a statistical
feature extraction model. In addition, to alleviate the issue of
misclassification of mitosis and non-mitosis objects, authors
developed an object-wise Completed Local Binary Pattern
(CLBP) that enabled efficient textural features extraction. The
predominant novelty of the CLBP model was that it is robust
against positional variation, color changes …etc. Authors applied
SVM to perform feature classification. A space processing model
was developed in [18] that enabled time reversal imaging
approach to perform breast cancer detection. To perform
malignant lesion detection, authors applied FDTD breast model
that comprises dense breast tissues with differing fibro-glandular
tissue composition. To achieve better accuracy, Naeemabadi et
al. [13] applied LS and SMO techniques rather applying
traditional SVM for classification. Li et al. [19] developed an
MFSVM-FKNN ensemble classifier for breast cancer detection,
where authors applied the concept of mixture membership
function. Fuzzy classifier based breast cancer detection and
classification model was proposed in [20]. A bio-inspired
immunological scheme was proposed for mammographic mass
classification that classifies malignant tumors from the benign
ones. Gaike et al. [21] at first focused on retrieving the higher
order (particularly third order) features that was later processed
for clustering to perform malignant breast cancer detection. Ali et
al. [22] developed an automatic segmentation model using the
concept of the data acquisition protocol parameter over the image
statistics of DMR-IR database. A similar effort was made in [23],
where authors developed a two phase BC-CAD system. In their
approach at first they applied Neutrosophic Sets (NS) and
optimized Fast Fuzzy C-Mean (F-FCM) algorithm for ROI
segmentation, which was followed by classification to perform
two class classifications, i.e. normal and abnormal tissue.
2
cancer detection to assist early diagnosis. Exploring in depth of
the CAD systems, it can be found that its efficacy primarily
depends on the efficiency of the Region Of Interest (ROI)
segmentation, feature extraction, feature mapping and
classification techniques. There are a number of techniques, such
as wavelet transform [8][9][10], Gabor transform [11], applied
for feature extraction while classification is done using standard
support vector machine (SVM) or other artificial intelligence
approaches [9][12][13]. However, most of the traditional
approaches are confined due to limited feature extraction
efficiency and classification for lesion of different shape, size,
and orientation density. The low information availability too
confines the efficacy of the traditional feature extraction and
cancer classification models. In addition, there is an inevitable
need to develop efficient approaches to deal with high
dimensional features, feature selection and classification or
prediction. Considering large size unannotated data (i.e., breast
cancer image data), the use of CNN as feature extraction tool can
ensure better breast cancer CAD (BC-CAD) solution.
In this paper, a highly robust and efficient CNN based Deep
Neural Network (DNN) model has been developed for breast
cancer detection and classification. To enable an optimal
solution, our proposed model encompasses various enhancements
including pre-processing, ROI identification, Caffe-enet-AlexNet
DNN based feature extraction, PCA and LDA based feature
selection and SVM based classification. To examine the
effectiveness of the proposed DNN model, a parallel BC-CAD
model using Spatial Invariant Fourier Transform (SIFT) feature
extraction has been developed. In addition, to perform breast
cancer prediction, a two class classification model is developed;
where the overall result affirms that the AlexNet-DNN with LDA
feature extraction outperforms other state-of-art techniques for
BC-CAD purpose.
The other sections of the presented manuscript are divided as
follows: Section two presents the related work, which is followed
by the discussion of the proposed AlexNet DNN based breast
cancer detection and classification in Section three. Section four
presents the results and discussion, while the overall research
conclusion is presented in Section five.
RELATED WORK
This section briefs some of the key literatures pertaining to
the breast cancer detection and related technologies.
Rehman et al. [12] derived a diverse feature based breast
cancer detection model, where they applied phylogenetic trees,
various statistical features and local binary patterns for
generating certain distinctive and discriminative features to
perform classification. Authors applied radial basis function
based SVM for two class classification: cancerous and non-
cancerous. Pramanik et al. [8] assessed the breast thermogram to
perform cancer detection, where they at first applied Initial
Feature point Image (IFI) to perform feature extraction for each
segmented breast thermogram. They applied DWT to perform
feature extraction, which was followed by feature classification
using feed-forward Artificial Neural Network (ANN). A similar
effort was made in [9], where authors applied wavelet and
counterlet transformation for feature extraction and SVM
classifier based classification of the mammograms. Yousefi et al.
[11] used multi-channel Gabor wavelet filter for feature
extraction over mammograms. Authors applied Gabor filter
design to perform feature extraction, which was then followed by
two class classifications using Bayesian classifier. Caorsi et al.
[14] proposed an ANN based radar data processing model for
breast cancer detection where they emphasized not only on the
detection of the cancerous tumour but also its geometric features
such as depth and width. A similar effort was made in [15],
where authors proposed a data-driven matched field model to
assist microwave breast cancer detection. George et al. [16]
developed robust and intelligent breast cancer detection and
classification model using cytological images. Authors applied
different classifiers including back-propagation based multilayer
perceptron, Probabilistic Neural Network (PNN), SVM and
learning vector quantization to perform breast cancer
classification. Considering mitosis as a cancer signifier, Tashk et
al. [17] developed an automatic BC-CAD model, where at first
they applied 2-D anisotropic diffusion for noise removal and
image morphological process. To achieve pixel-wise features
from the ROI (i.e., mitosis region), authors derived a statistical
feature extraction model. In addition, to alleviate the issue of
misclassification of mitosis and non-mitosis objects, authors
developed an object-wise Completed Local Binary Pattern
(CLBP) that enabled efficient textural features extraction. The
predominant novelty of the CLBP model was that it is robust
against positional variation, color changes …etc. Authors applied
SVM to perform feature classification. A space processing model
was developed in [18] that enabled time reversal imaging
approach to perform breast cancer detection. To perform
malignant lesion detection, authors applied FDTD breast model
that comprises dense breast tissues with differing fibro-glandular
tissue composition. To achieve better accuracy, Naeemabadi et
al. [13] applied LS and SMO techniques rather applying
traditional SVM for classification. Li et al. [19] developed an
MFSVM-FKNN ensemble classifier for breast cancer detection,
where authors applied the concept of mixture membership
function. Fuzzy classifier based breast cancer detection and
classification model was proposed in [20]. A bio-inspired
immunological scheme was proposed for mammographic mass
classification that classifies malignant tumors from the benign
ones. Gaike et al. [21] at first focused on retrieving the higher
order (particularly third order) features that was later processed
for clustering to perform malignant breast cancer detection. Ali et
al. [22] developed an automatic segmentation model using the
concept of the data acquisition protocol parameter over the image
statistics of DMR-IR database. A similar effort was made in [23],
where authors developed a two phase BC-CAD system. In their
approach at first they applied Neutrosophic Sets (NS) and
optimized Fast Fuzzy C-Mean (F-FCM) algorithm for ROI
segmentation, which was followed by classification to perform
two class classifications, i.e. normal and abnormal tissue.
2

Ismahan et al. [24] used a mathematical morphology concept to
perform masses detection over digitized mammograms. Texture
analysis based ROI detection for thermal imaging based BC-
CAD was performed in [25]. Considering the significance of
feature extraction for mammograms classification, Sanae et al.
[10] exploited comprehensive statistical Block-Based features,
which were extracted from sub-bands of the discrete wavelet
transformation. Once mapping the extraction features, SVM was
applied to perform classification. Unlike traditional approaches,
Maken et al. [26] examined the efficacy of Multiple Instance
Learning (MIL) algorithm for mammograms classification,
where they developed MIL using tile-based spatio-temporal
features. Rosa et al. [27] and Hatipoglu et al. [28] intended to
exploit MIL in conjunction with SVM for mammograms
classification. Non-deniably, MIL approaches have performed
better, particularly for unannotated data; however the likelihood
of better performance with deep features cannot be ignored.
To enable more efficient feature extraction and accurate
performance, DNN has gained significant attention. CNN has
gained widespread recognition to extract features from the
complex image data, such as mammograms, MRI medical data or
even histopathological datasets. CNN techniques which are
inspired biologically by the organization of human visual cortex
possess robust efficiency due to its ability to learn features
invariant to translation, rotation and shifting is their great
advantage. Spanhol et al. [29] applied CNN approach to classify
breast cancer Histopathological images. Their proposed model
performed learning CNN by means of training patches generated
through varied approaches. Authors obtained the best
classification accuracy of 89.6% for images with 40x
enlargement. To achieve this result, authors considered the
patches of the size 64x64 pixels. Hatipoglu et al. [28] used CNN
to classify cellular and non-cellular structures in breast cancer
histopathological images. Authors applied neural network to
perform classification, where they achieved the best classification
accuracy of 86.88%. Recently, Wang et al. [30] used Googlenet
CNN model to perform Histopathological image classification,
where they achieved the accuracy of 98.4% patch classification.
To detect the invasive ductal carcinoma tissue in histological
images for BC-CAD, Cruz-Roa et al. [31] applied CNN feature
extraction approach. Ertosun et al. [32] developed BC-CAD
model using Deep Learning with three distinct CNN models so as
to localize masses in mammography images. To enhance
accuracy of segmentation or ROI identification, authors
incorporated additional novelties such as cropping, translation,
rotation, flipping and scaling techniques. Arevalo et al. [33]
applied CNN based feature extraction followed by SVM based
classification for BC-CAD solution. Authors achieved Receiver
Operating Characteristics (ROC) of 86%. A similar work was
performed in [34], where authors achieved classification
accuracy of 96.7%. Russakovsky et al. [35] and Zuiderveld et al.
[40] trained CNN over ImageNet to perform breast cancer
classification. Authors Abdel-Zaher et al. [36] and Wang et al.
[41] performed breast cancer classification, developing a
classifier by means of the weights of a previously trained Deep
Belief Network (DBN). They applied Levenberg Marquardt
learning based ANN to perform classification. Amongst the
various researches it has been realized that in addition to the ROI
identification and feature extraction efficiency, selecting optimal
features is equally significant to ensure efficient performance. To
achieve this, dimensional reduction and feature selection
measures are of utmost significance. With these objectives, Olfati
et al. [37] applied Genetic Algorithm (GA) to enhanced PCA by
selecting optimal principal components analysis (GA-SPCA).
They applied PCA for dimension reduction, GA for feature
selection [38] and SVM for classification which facilitates to
achieve better performance in order to gain desired results.
The developed system is much effective as it is prepared by
utilizing advanced technology but it has a risk which may create
major problems due to minor technical issues. Moreover, it is
suggested that they can utilize Computerized tomography (CT)
scan and Positron emission tomography (PET) scan to detrmine
actual situation of breats cancer appropriately.
I. PROPOSED METHODOLOGY
This section primarily discusses the proposed research
work and implementation model to achieve intended BC-CAD
solution. This solution provide an accurate breast cancer
detection to assist early diagnosis in more efficient way. My
contribution is all about to make highly robust and efficient CNN
based on a model named as Deep Neural Network (DNN) model.
Moreover, it has been developed for breast cancer detection and
classification. In the current research work, a novel deep learning
based Breast Cancer detection and classification algorithm is
developed. In our work, the predominant emphasis is made on
developing a novel and robust automated CAD system for Breast
Cancer (BC) detection and classification. Our research method
applied on multi-dimensional shape to increase the performance
of the proposed BC-CAD system, where enhancement has been
made for major functional processes including pre-processing,
ROI detection and segmentation, feature extraction, feature
selection and classification. In addition, a standard breast cancer
dataset named BreaKHis [39], which is the Histopathological
image data for breast cancer, has been considered for our study.
To ensure better efficiency enriching input data or medical image
quality is often a better solution. With this objective, the input
Histopathological images are processed for noise removal and
resizing. Once retrieving the suitable input images, it has been
further processed for ROI segmentation, where an enhanced
GMM approach has been taken into consideration. Unlike
generic GMM algorithm, we have derived an adaptive learning
based Gaussian approach that enables swift and accurate ROI
identification. This as a result can play vital role in accurate and
significant feature retrieval for optimal BC-CAD solution.
Noticeably, our applied datasets BreaKHis [39] possesses key
features as marked cellular atypia, mitosis, disruption of
basement membranes, metastasize …etc. These features have
been applied to perform two class classifications. Realizing the
non-deniable fact that inaccurate ROI localization and
insignificant pixel conjuncture might lead inaccurate
3
perform masses detection over digitized mammograms. Texture
analysis based ROI detection for thermal imaging based BC-
CAD was performed in [25]. Considering the significance of
feature extraction for mammograms classification, Sanae et al.
[10] exploited comprehensive statistical Block-Based features,
which were extracted from sub-bands of the discrete wavelet
transformation. Once mapping the extraction features, SVM was
applied to perform classification. Unlike traditional approaches,
Maken et al. [26] examined the efficacy of Multiple Instance
Learning (MIL) algorithm for mammograms classification,
where they developed MIL using tile-based spatio-temporal
features. Rosa et al. [27] and Hatipoglu et al. [28] intended to
exploit MIL in conjunction with SVM for mammograms
classification. Non-deniably, MIL approaches have performed
better, particularly for unannotated data; however the likelihood
of better performance with deep features cannot be ignored.
To enable more efficient feature extraction and accurate
performance, DNN has gained significant attention. CNN has
gained widespread recognition to extract features from the
complex image data, such as mammograms, MRI medical data or
even histopathological datasets. CNN techniques which are
inspired biologically by the organization of human visual cortex
possess robust efficiency due to its ability to learn features
invariant to translation, rotation and shifting is their great
advantage. Spanhol et al. [29] applied CNN approach to classify
breast cancer Histopathological images. Their proposed model
performed learning CNN by means of training patches generated
through varied approaches. Authors obtained the best
classification accuracy of 89.6% for images with 40x
enlargement. To achieve this result, authors considered the
patches of the size 64x64 pixels. Hatipoglu et al. [28] used CNN
to classify cellular and non-cellular structures in breast cancer
histopathological images. Authors applied neural network to
perform classification, where they achieved the best classification
accuracy of 86.88%. Recently, Wang et al. [30] used Googlenet
CNN model to perform Histopathological image classification,
where they achieved the accuracy of 98.4% patch classification.
To detect the invasive ductal carcinoma tissue in histological
images for BC-CAD, Cruz-Roa et al. [31] applied CNN feature
extraction approach. Ertosun et al. [32] developed BC-CAD
model using Deep Learning with three distinct CNN models so as
to localize masses in mammography images. To enhance
accuracy of segmentation or ROI identification, authors
incorporated additional novelties such as cropping, translation,
rotation, flipping and scaling techniques. Arevalo et al. [33]
applied CNN based feature extraction followed by SVM based
classification for BC-CAD solution. Authors achieved Receiver
Operating Characteristics (ROC) of 86%. A similar work was
performed in [34], where authors achieved classification
accuracy of 96.7%. Russakovsky et al. [35] and Zuiderveld et al.
[40] trained CNN over ImageNet to perform breast cancer
classification. Authors Abdel-Zaher et al. [36] and Wang et al.
[41] performed breast cancer classification, developing a
classifier by means of the weights of a previously trained Deep
Belief Network (DBN). They applied Levenberg Marquardt
learning based ANN to perform classification. Amongst the
various researches it has been realized that in addition to the ROI
identification and feature extraction efficiency, selecting optimal
features is equally significant to ensure efficient performance. To
achieve this, dimensional reduction and feature selection
measures are of utmost significance. With these objectives, Olfati
et al. [37] applied Genetic Algorithm (GA) to enhanced PCA by
selecting optimal principal components analysis (GA-SPCA).
They applied PCA for dimension reduction, GA for feature
selection [38] and SVM for classification which facilitates to
achieve better performance in order to gain desired results.
The developed system is much effective as it is prepared by
utilizing advanced technology but it has a risk which may create
major problems due to minor technical issues. Moreover, it is
suggested that they can utilize Computerized tomography (CT)
scan and Positron emission tomography (PET) scan to detrmine
actual situation of breats cancer appropriately.
I. PROPOSED METHODOLOGY
This section primarily discusses the proposed research
work and implementation model to achieve intended BC-CAD
solution. This solution provide an accurate breast cancer
detection to assist early diagnosis in more efficient way. My
contribution is all about to make highly robust and efficient CNN
based on a model named as Deep Neural Network (DNN) model.
Moreover, it has been developed for breast cancer detection and
classification. In the current research work, a novel deep learning
based Breast Cancer detection and classification algorithm is
developed. In our work, the predominant emphasis is made on
developing a novel and robust automated CAD system for Breast
Cancer (BC) detection and classification. Our research method
applied on multi-dimensional shape to increase the performance
of the proposed BC-CAD system, where enhancement has been
made for major functional processes including pre-processing,
ROI detection and segmentation, feature extraction, feature
selection and classification. In addition, a standard breast cancer
dataset named BreaKHis [39], which is the Histopathological
image data for breast cancer, has been considered for our study.
To ensure better efficiency enriching input data or medical image
quality is often a better solution. With this objective, the input
Histopathological images are processed for noise removal and
resizing. Once retrieving the suitable input images, it has been
further processed for ROI segmentation, where an enhanced
GMM approach has been taken into consideration. Unlike
generic GMM algorithm, we have derived an adaptive learning
based Gaussian approach that enables swift and accurate ROI
identification. This as a result can play vital role in accurate and
significant feature retrieval for optimal BC-CAD solution.
Noticeably, our applied datasets BreaKHis [39] possesses key
features as marked cellular atypia, mitosis, disruption of
basement membranes, metastasize …etc. These features have
been applied to perform two class classifications. Realizing the
non-deniable fact that inaccurate ROI localization and
insignificant pixel conjuncture might lead inaccurate
3

classification accuracy, we have applied Connected Component
Analysis (CCA) model over segmented region. It eliminates
those image components that percept to be connected with the
target ROI; however, it is insignificant toward BC-CAD
classification. Once obtaining the CCA processed segmented
ROI, we have processed it for feature extraction followed by
feature selection and classification to achieve anticipated BC-
CAD solution. Unlike existing feature extraction models, such as
Gabor filter [11], Wavelet Transform [8][9][10], MIL [26-28] or
even classical CNNs [28-33][35], in our research a CNN model
is derived. In the proposed research work, an enhanced DNN
model named AlexNet DNN has been applied to extract
significant features from the Histopathological image datasets.
Realizing the fact that high dimensional features often provide
more significant information to make precise classification or
analysis; in our research work, we have developed AlexNet-DNN
to extract high dimensional features with 4096 Kernels or
dimensions. The proposed AlexNet-DNN model comprises five
convolutional layers in succession with three Fully Connected
(FC) layers (FC6, FC7 and FC8). The feature extracted at the
higher layer provides more significant information that helps in
achieving accurate BC-CAD performance. Considering major
classical approaches, where the probability of over-fitting and
accuracy is often ignored, we have applied a supplementary
model called Caffe-enet that assists AlexNet-DNN to overcome
existing limitations and enables its (AlexNet-DNN) execution
over general purpose computers without any need of
sophisticated Graphical Processing Units (GPUs). The use of
Caffe-enet in conjunction with AlexNet-DNN plays a vital role in
assuring efficient feature extraction even under large scale
unannotated data. It makes a suitable environment for major
breast cancer detection applications. Specifically, owing to high
unannotated breast cancer data, performing DNN learning and
further classification is a mammoth task. Hence to alleviate such
issues, in our proposed model AlexNet applied multilayered
DNN architecture, where at each layer we retrieve the features
for further mapping and classification. However, to achieve more
efficient results, we have considered 4096-dimensional features
extracted at the Fully Connected (FC) layers, FC-6 and FC-7 of
the AlexNet-DNN. The detailed discussion of the developed
DNN model and its implementation for BC-CAD is given in the
next section. In addition, to the proposed AlexNet-DNN based
BC-CAD solution, in this research we have developed a parallel
feature extraction model, called Scale Invariant Fourier
Transform (SIFT). We have applied Fully-Connected (FC)
layers: FC6 and FC7 features, which are 4096-dimensional
features and hence amounts a huge data to process. To enhance
computational efficiency, in this research work, we have applied
PCA and LDA techniques to perform dimension reduction or
feature selection. Once processing for the dimensional reduction,
the selected features are projected to polynomial kernel based
classifier that performs two class classifications: Malignant and
Benign. To further strengthen the efficiency of our proposed
work, 10-fold cross validation approach is applied that ensures
optimal classification accuracy for BC-CAD solution.
The overall proposed research methodology and
implementation schematic is given in the next section.
PROPOSED SYSTEM DESIGN
In this section, the detailed discussion of the proposed
research work and implementation schematic is presented. Before
discussing the proposed BC-CAD solution, introducing key
terminologies is must. Table I presents the nomenclature of
different abbreviated terms.
BC Data
Pre/Post
Processing
Breast Cancer
region
Segmentation
SIFT
Caffee-AlexNet
DNN
FC6
FC7
AlexNet-FC6-LDA
AlexNet-FC6-PCA
AlexNet-FC7-LDA
AlexNet-FC7-PCA
SIFT-FV--PCA
SIFT-FV-LDA
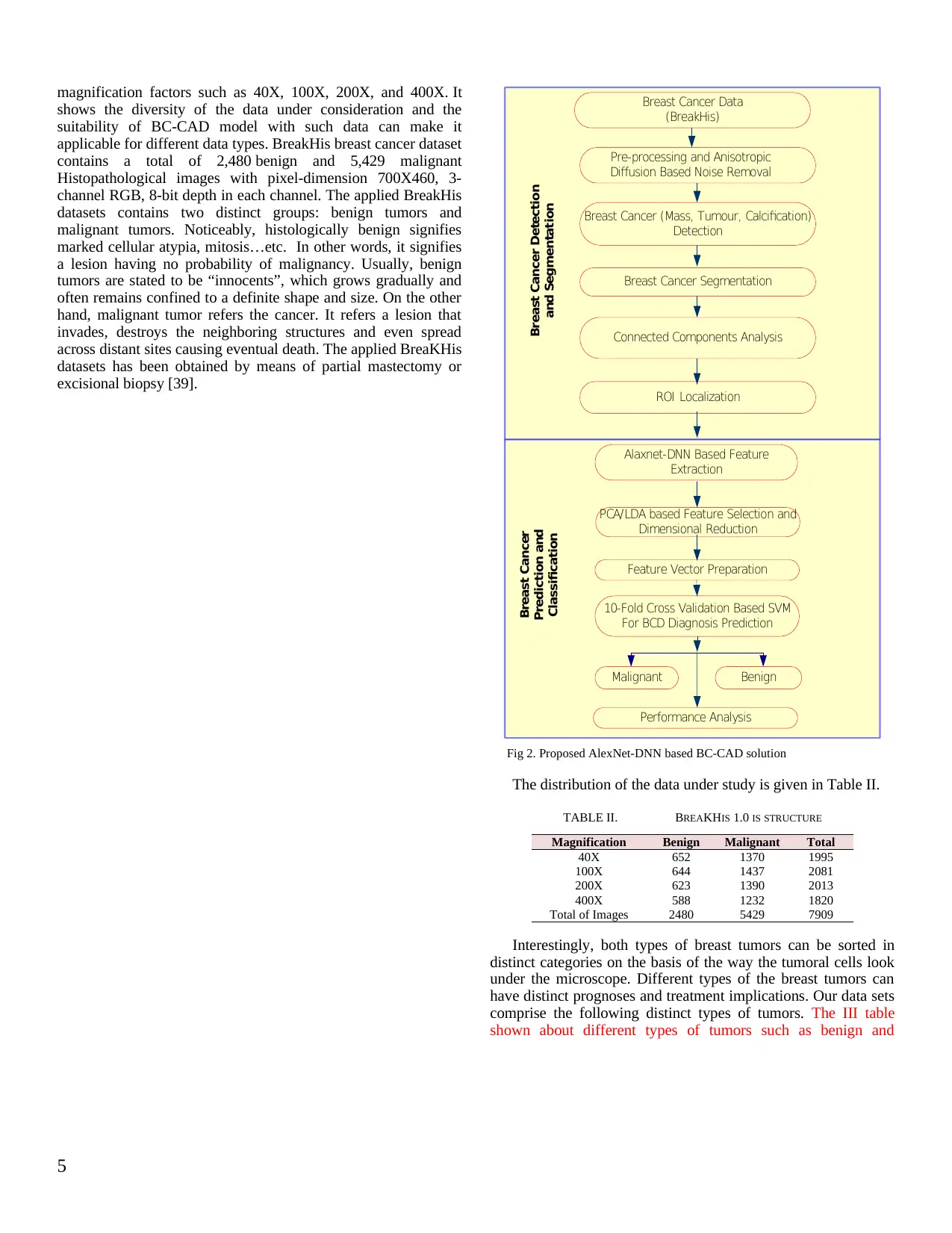
Fig. 1. Propsoed BC-CAD System
A detailed discussion of the proposed research model is
given, as presented in Fig. 2, as follows:
.a Breast Cancer Histopathological Image Data
Collection
.b Pre-processing
.c ROI Detection and Localization
.d Feature Extraction
.e Dimensional reduction of Feature Selection,
and
.f BC-CAD Two-Class Classification.
Which will be discussed in details:
1. Breast Cancer Histopathological Image Data
collection
In this research or study, we have used standard datasets
named Breast Cancer Histopathological Image Classification
(BreakHis) [39] that contains a total of 9,109 Histopathological
(say, microscopic) images of breast cancer tissue. The historical
perspective of the BreaKHis states that the microscopic images
are retrieved from 82 patients. BreakHis dataset has been built in
association with the P&D Laboratory - Pathological Anatomy
and Cytopathology, Parana, Brazil (http://www.
prevencaoediagnose.com.br). Unlike generic singe size or single
feature datasets, BreaKHis data are obtained with varied
4
Analysis (CCA) model over segmented region. It eliminates
those image components that percept to be connected with the
target ROI; however, it is insignificant toward BC-CAD
classification. Once obtaining the CCA processed segmented
ROI, we have processed it for feature extraction followed by
feature selection and classification to achieve anticipated BC-
CAD solution. Unlike existing feature extraction models, such as
Gabor filter [11], Wavelet Transform [8][9][10], MIL [26-28] or
even classical CNNs [28-33][35], in our research a CNN model
is derived. In the proposed research work, an enhanced DNN
model named AlexNet DNN has been applied to extract
significant features from the Histopathological image datasets.
Realizing the fact that high dimensional features often provide
more significant information to make precise classification or
analysis; in our research work, we have developed AlexNet-DNN
to extract high dimensional features with 4096 Kernels or
dimensions. The proposed AlexNet-DNN model comprises five
convolutional layers in succession with three Fully Connected
(FC) layers (FC6, FC7 and FC8). The feature extracted at the
higher layer provides more significant information that helps in
achieving accurate BC-CAD performance. Considering major
classical approaches, where the probability of over-fitting and
accuracy is often ignored, we have applied a supplementary
model called Caffe-enet that assists AlexNet-DNN to overcome
existing limitations and enables its (AlexNet-DNN) execution
over general purpose computers without any need of
sophisticated Graphical Processing Units (GPUs). The use of
Caffe-enet in conjunction with AlexNet-DNN plays a vital role in
assuring efficient feature extraction even under large scale
unannotated data. It makes a suitable environment for major
breast cancer detection applications. Specifically, owing to high
unannotated breast cancer data, performing DNN learning and
further classification is a mammoth task. Hence to alleviate such
issues, in our proposed model AlexNet applied multilayered
DNN architecture, where at each layer we retrieve the features
for further mapping and classification. However, to achieve more
efficient results, we have considered 4096-dimensional features
extracted at the Fully Connected (FC) layers, FC-6 and FC-7 of
the AlexNet-DNN. The detailed discussion of the developed
DNN model and its implementation for BC-CAD is given in the
next section. In addition, to the proposed AlexNet-DNN based
BC-CAD solution, in this research we have developed a parallel
feature extraction model, called Scale Invariant Fourier
Transform (SIFT). We have applied Fully-Connected (FC)
layers: FC6 and FC7 features, which are 4096-dimensional
features and hence amounts a huge data to process. To enhance
computational efficiency, in this research work, we have applied
PCA and LDA techniques to perform dimension reduction or
feature selection. Once processing for the dimensional reduction,
the selected features are projected to polynomial kernel based
classifier that performs two class classifications: Malignant and
Benign. To further strengthen the efficiency of our proposed
work, 10-fold cross validation approach is applied that ensures
optimal classification accuracy for BC-CAD solution.
The overall proposed research methodology and
implementation schematic is given in the next section.
PROPOSED SYSTEM DESIGN
In this section, the detailed discussion of the proposed
research work and implementation schematic is presented. Before
discussing the proposed BC-CAD solution, introducing key
terminologies is must. Table I presents the nomenclature of
different abbreviated terms.
BC Data
Pre/Post
Processing
Breast Cancer
region
Segmentation
SIFT
Caffee-AlexNet
DNN
FC6
FC7
AlexNet-FC6-LDA
AlexNet-FC6-PCA
AlexNet-FC7-LDA
AlexNet-FC7-PCA
SIFT-FV--PCA
SIFT-FV-LDA
Fig. 1. Propsoed BC-CAD System
A detailed discussion of the proposed research model is
given, as presented in Fig. 2, as follows:
.a Breast Cancer Histopathological Image Data
Collection
.b Pre-processing
.c ROI Detection and Localization
.d Feature Extraction
.e Dimensional reduction of Feature Selection,
and
.f BC-CAD Two-Class Classification.
Which will be discussed in details:
1. Breast Cancer Histopathological Image Data
collection
In this research or study, we have used standard datasets
named Breast Cancer Histopathological Image Classification
(BreakHis) [39] that contains a total of 9,109 Histopathological
(say, microscopic) images of breast cancer tissue. The historical
perspective of the BreaKHis states that the microscopic images
are retrieved from 82 patients. BreakHis dataset has been built in
association with the P&D Laboratory - Pathological Anatomy
and Cytopathology, Parana, Brazil (http://www.
prevencaoediagnose.com.br). Unlike generic singe size or single
feature datasets, BreaKHis data are obtained with varied
4
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
magnification factors such as 40X, 100X, 200X, and 400X. It
shows the diversity of the data under consideration and the
suitability of BC-CAD model with such data can make it
applicable for different data types. BreakHis breast cancer dataset
contains a total of 2,480 benign and 5,429 malignant
Histopathological images with pixel-dimension 700X460, 3-
channel RGB, 8-bit depth in each channel. The applied BreakHis
datasets contains two distinct groups: benign tumors and
malignant tumors. Noticeably, histologically benign signifies
marked cellular atypia, mitosis…etc. In other words, it signifies
a lesion having no probability of malignancy. Usually, benign
tumors are stated to be “innocents”, which grows gradually and
often remains confined to a definite shape and size. On the other
hand, malignant tumor refers the cancer. It refers a lesion that
invades, destroys the neighboring structures and even spread
across distant sites causing eventual death. The applied BreaKHis
datasets has been obtained by means of partial mastectomy or
excisional biopsy [39].
Breast Cancer Segmentation
Connected Components Analysis
ROI Localization
Alaxnet-DNN Based Feature
Extraction
Pre-processing and Anisotropic
Diffusion Based Noise Removal
Breast Cancer (Mass, Tumour, Calcification)
Detection
PCA/LDA based Feature Selection and
Dimensional Reduction
Feature Vector Preparation
10-Fold Cross Validation Based SVM
For BCD Diagnosis Prediction
Malignant
Breast Cancer Detection
and Segmentation
Breast Cancer
Prediction and
Classification
Breast Cancer Data
(BreakHis)
Benign
Performance Analysis
Fig 2. Proposed AlexNet-DNN based BC-CAD solution
The distribution of the data under study is given in Table II.
TABLE II. BREAKHIS 1.0 IS STRUCTURE
Magnification Benign Malignant Total
40X 652 1370 1995
100X 644 1437 2081
200X 623 1390 2013
400X 588 1232 1820
Total of Images 2480 5429 7909
Interestingly, both types of breast tumors can be sorted in
distinct categories on the basis of the way the tumoral cells look
under the microscope. Different types of the breast tumors can
have distinct prognoses and treatment implications. Our data sets
comprise the following distinct types of tumors. The III table
shown about different types of tumors such as benign and
5
shows the diversity of the data under consideration and the
suitability of BC-CAD model with such data can make it
applicable for different data types. BreakHis breast cancer dataset
contains a total of 2,480 benign and 5,429 malignant
Histopathological images with pixel-dimension 700X460, 3-
channel RGB, 8-bit depth in each channel. The applied BreakHis
datasets contains two distinct groups: benign tumors and
malignant tumors. Noticeably, histologically benign signifies
marked cellular atypia, mitosis…etc. In other words, it signifies
a lesion having no probability of malignancy. Usually, benign
tumors are stated to be “innocents”, which grows gradually and
often remains confined to a definite shape and size. On the other
hand, malignant tumor refers the cancer. It refers a lesion that
invades, destroys the neighboring structures and even spread
across distant sites causing eventual death. The applied BreaKHis
datasets has been obtained by means of partial mastectomy or
excisional biopsy [39].
Breast Cancer Segmentation
Connected Components Analysis
ROI Localization
Alaxnet-DNN Based Feature
Extraction
Pre-processing and Anisotropic
Diffusion Based Noise Removal
Breast Cancer (Mass, Tumour, Calcification)
Detection
PCA/LDA based Feature Selection and
Dimensional Reduction
Feature Vector Preparation
10-Fold Cross Validation Based SVM
For BCD Diagnosis Prediction
Malignant
Breast Cancer Detection
and Segmentation
Breast Cancer
Prediction and
Classification
Breast Cancer Data
(BreakHis)
Benign
Performance Analysis
Fig 2. Proposed AlexNet-DNN based BC-CAD solution
The distribution of the data under study is given in Table II.
TABLE II. BREAKHIS 1.0 IS STRUCTURE
Magnification Benign Malignant Total
40X 652 1370 1995
100X 644 1437 2081
200X 623 1390 2013
400X 588 1232 1820
Total of Images 2480 5429 7909
Interestingly, both types of breast tumors can be sorted in
distinct categories on the basis of the way the tumoral cells look
under the microscope. Different types of the breast tumors can
have distinct prognoses and treatment implications. Our data sets
comprise the following distinct types of tumors. The III table
shown about different types of tumors such as benign and
5
malignant along with their examples including overall breast
tumors which may or may not be cancer.
TABLE III. TUMOR CONTAINTS (DISTRIBUTION) IN BREAKHIS 1.0 IS
Benign Tumors: adenosis Malignant Tumors
Fibroadenoma carcinoma
phyllodes tumor lobular carcinoma
tubular adenona mucinous carcinoma
adenosis papillary carcinoma
In the dataset, individual Histopathological image filename
stores significant image information containing biopsy method,
type of tumor, patient information (Patient ID), and
magnification factor. For illustration, for an image SOB_B_TA-
14-4659-40-001.png, as illustrated in Fig. 3, it signifies a benign
tumor of tubular adenoma type, obtained at the magnification
factor 40. The biopsy method used was SOB.
(a) (b)
(c) (d)
Fig 3. Snippet of the breast malignent cancer at different magnification
factors: (a) 40X, (b) 100X, (c) 200X, and (d) 400X [39].
.g Pre-processing
Non-deniably, the quality of data influences the final
classification accuracy and efficiency. To enable suitable data for
further processing, we have performed pre-processing. In our
research model, the pre-processing model is exceptionally
significant to ensure precise nuclei and allied vicinity
identification and characterization. In breast cancer detection, the
features, such as the shape and size of nuclei, glandular shape …
etc. is expected to be precisely identifiable. Performing pre-
processing assures that suppression of the original image
qualities required to estimate or identify texture representation
and other significant features, precisely [40]. Realizing
interchangeable nature of the pre-processing approaches, we have
performed pre-processing before performing candidate region
detection and feature extraction [40-41]. In our paper, we have
followed the pre-processing instructions provided in [35][41]
Once performing pre-processing, the concept region
identification or segmentation has been processed. In addition to
the above mentioned, in pre-processing, we have resized input
breast cancer Histopathological images to 224X224. A brief of
the proposed segmentation model or ROI identification approach
will be explained as follows.
.h Concept Region Identification
In this section, the process of breast cancer region detection
and segmentation is discussed. Unlike traditional approaches of
segmentation such as Otsu’s method [42], our model applies an
enhanced GMM technique. Here, we segment cancer tumor
region from the background so as to distinguish it from other
regions over Histopathological images. GMM has exhibited
better performance over other state-of-the-art techniques for
background subtraction and ROI identification because of b a
pixel-wise segmentation approach. In addition, GMM robustness
causes to be used for segmenting ROI from the background
region that can be further processed for feature extraction over
the detected ROI. A brief of the proposed GMM model for ROI
detection including several steps is given as follows:
Adaptive-Learning-Based GMM for Background Subtraction
The typical Histopathological images exhibits highly complex
color and textural features that makes classical segmentation
approaches, such as Otsu method, static thresholding based
segmentation and even generic GMM confined to enable optimal
ROI detection. One of the predominant reasons is the static
learning rate [43]. To deal with such limitations, in this research
model, we have developed an enhanced adaptive learning based
GMM model for ROI detection and segmentation. Consider is
the pixel-value at certain instant; GMM can be used to estimate
Probability Density Function (PDF) of. Noticeably, PDF can
have the all connected Gaussians. Here, the PDF of the Gaussian
mixture with components is estimated as: Where signifies
weight factor,. Here, y represents the output with respect to the
values of at nth instant. Here y refers the normalized density of
mean . In equation (1), the variable represents the covariance
matrix. In fact, this condition hypothesizes that the pixel values
of the color components (i.e., red, green, and blue) in the breast
cancer image are independent and have equal variances. On the
contrary, it is infeasible and therefore the distribution of recently
occurred pixel values in the Histopathological image can be
defined in the Gaussian mixture form. In our model, the value of
a new pixel is characterized by means of one of the predominant
components of the mixture model, which is further applied to
update the model. Stauffer et al. [43] applied this concept to
estimate background where initially they initialized these
variables with zero. Once detecting any similarity, such as, with
and as a threshold, the operating variables of the GMM model
are obtained as:
Initially, Neural Networks which are internediatde (1)
6
tumors which may or may not be cancer.
TABLE III. TUMOR CONTAINTS (DISTRIBUTION) IN BREAKHIS 1.0 IS
Benign Tumors: adenosis Malignant Tumors
Fibroadenoma carcinoma
phyllodes tumor lobular carcinoma
tubular adenona mucinous carcinoma
adenosis papillary carcinoma
In the dataset, individual Histopathological image filename
stores significant image information containing biopsy method,
type of tumor, patient information (Patient ID), and
magnification factor. For illustration, for an image SOB_B_TA-
14-4659-40-001.png, as illustrated in Fig. 3, it signifies a benign
tumor of tubular adenoma type, obtained at the magnification
factor 40. The biopsy method used was SOB.
(a) (b)
(c) (d)
Fig 3. Snippet of the breast malignent cancer at different magnification
factors: (a) 40X, (b) 100X, (c) 200X, and (d) 400X [39].
.g Pre-processing
Non-deniably, the quality of data influences the final
classification accuracy and efficiency. To enable suitable data for
further processing, we have performed pre-processing. In our
research model, the pre-processing model is exceptionally
significant to ensure precise nuclei and allied vicinity
identification and characterization. In breast cancer detection, the
features, such as the shape and size of nuclei, glandular shape …
etc. is expected to be precisely identifiable. Performing pre-
processing assures that suppression of the original image
qualities required to estimate or identify texture representation
and other significant features, precisely [40]. Realizing
interchangeable nature of the pre-processing approaches, we have
performed pre-processing before performing candidate region
detection and feature extraction [40-41]. In our paper, we have
followed the pre-processing instructions provided in [35][41]
Once performing pre-processing, the concept region
identification or segmentation has been processed. In addition to
the above mentioned, in pre-processing, we have resized input
breast cancer Histopathological images to 224X224. A brief of
the proposed segmentation model or ROI identification approach
will be explained as follows.
.h Concept Region Identification
In this section, the process of breast cancer region detection
and segmentation is discussed. Unlike traditional approaches of
segmentation such as Otsu’s method [42], our model applies an
enhanced GMM technique. Here, we segment cancer tumor
region from the background so as to distinguish it from other
regions over Histopathological images. GMM has exhibited
better performance over other state-of-the-art techniques for
background subtraction and ROI identification because of b a
pixel-wise segmentation approach. In addition, GMM robustness
causes to be used for segmenting ROI from the background
region that can be further processed for feature extraction over
the detected ROI. A brief of the proposed GMM model for ROI
detection including several steps is given as follows:
Adaptive-Learning-Based GMM for Background Subtraction
The typical Histopathological images exhibits highly complex
color and textural features that makes classical segmentation
approaches, such as Otsu method, static thresholding based
segmentation and even generic GMM confined to enable optimal
ROI detection. One of the predominant reasons is the static
learning rate [43]. To deal with such limitations, in this research
model, we have developed an enhanced adaptive learning based
GMM model for ROI detection and segmentation. Consider is
the pixel-value at certain instant; GMM can be used to estimate
Probability Density Function (PDF) of. Noticeably, PDF can
have the all connected Gaussians. Here, the PDF of the Gaussian
mixture with components is estimated as: Where signifies
weight factor,. Here, y represents the output with respect to the
values of at nth instant. Here y refers the normalized density of
mean . In equation (1), the variable represents the covariance
matrix. In fact, this condition hypothesizes that the pixel values
of the color components (i.e., red, green, and blue) in the breast
cancer image are independent and have equal variances. On the
contrary, it is infeasible and therefore the distribution of recently
occurred pixel values in the Histopathological image can be
defined in the Gaussian mixture form. In our model, the value of
a new pixel is characterized by means of one of the predominant
components of the mixture model, which is further applied to
update the model. Stauffer et al. [43] applied this concept to
estimate background where initially they initialized these
variables with zero. Once detecting any similarity, such as, with
and as a threshold, the operating variables of the GMM model
are obtained as:
Initially, Neural Networks which are internediatde (1)
6

varicables that are not observable known as hidden
units.
Support Vector Machine can be formulated in
context of binary classification to find decision
boudary for maximising margin between two
data classes.
(2)
Partial Least Square include principal
component analysis which has same concept as
PLS.
(3)
LDA technique which are combined in one
objective function.
(4)
where
If no element matches together then the element with the
minimal is re-initialized, and the variables (2-4) are updated as:
SVM and PLS (5)
In above expression (5), refers to the learning rate. Here, is
obtained as:
Support ventor machines is formulatde in order
to for dula optimization problem whihc ensures
unique global solution.
(6)
In our proposed GMM model, we normalize in a way that it
converges toward unit value (i.e., 1). To obtain background
region, authors [43] sorted Gaussians in decreasing order by
using threshold in conjunction with the sums of the weights. As
a result, this achieves a set, where is obtained as:
It summarise turning parameter of all models
which are inlcuded in proposed theory.
(7)
With decrease in variances, usually the Gaussian distribution
gains increases. This as a result makes it more evident to perform
background subtraction. Once estimating GMM parameters,
sorting is performed from the matched mixture distribution
toward most probable background. This is feasible only because
only the relative value of the matched model changes. In our
applied model, ordering is performed in a way that the most
feasible background distributions remains on the top, while the
low probability distributions move towards bottom. In such
manner, the initial value of (7) is considered as the background.
In (7), parameter signifies the result of the least section of the
data responsible for the background. With the minimal possible
value of , the background model is stated to be unimodal, where
using only the most probable distribution enhances
computational efficiency. On the contrary, the high signifies
multimodal distribution, is typically caused due to the iterative
background motion. In this paper, the breast cancer
Histopathological images with static features are taken as input
and hence the use of unimodal approach with low ensures
computationally efficient process. In our approach, the Gaussian
with the maximum and minimal refer to the background region.
Thus, applying aforementioned approach the background region
can be identified that consequently helps in segmenting the ROI.
In major cases, classical GMM updates with certain fixed
learning rate [43] are used. While, considering breast cancer
detection requirement, the static learning rate approach seems
questionable, particularly when there can be excessive texture
variation over the breast cancer Histopathological images. To
deal with such limitation, authors [44] developed an adaptive
learning rate based GMM for ROI segmentation over varying
surface or texture conditions. Non-deniably, there can be certain
texture or the surface conditions, where the pixel might neither be
the breast can tumor (i.e., ROI) nor the background; however, it
could be classified as either type Fibroadenoma and phyllodes
tumor. This as a result may influence accuracy of the proposed
BC-CAD solution. Increasing learning rate might cause high-
rate pixel feature changes [44] that consequently may make
breast cancer tumor or ROI detection susceptible. Though, the
adaptive learning based GMM model [43] can perform ROI
identification under varying background conditions. However,
the very minute and fine-connected texture or surface features
still make existing approach limited. Precisely for breast cancer
Histopathological images, textural or structural features, such as
cellular atypia, mitosis, disruption of basement membranes,
metastasize …etc. are highly intricate and minute feature that
requires more efficient ROI identification model. An enhanced
Gaussian mixture learning based GMM model was proposed in
[44]. However, authors could not address the saturation scenario
particularly during convergence. As an enhanced solution, in this
paper an adaptive learning based GMM model is developed that
detects precise ROI. In our proposed approach, initially, we have
decoupled learning rate from other components such as and. We
have applied a new learning rate variable called adaptive learning
rate that updates mean component using a probability
parameter. Here, states whether a pixel is a part of the nth
Gaussian distribution or not. In our model, the adaptive learning
rate parameter is estimated as follows:
Thresold (8)
Where, states the number of distributions.
Can provide fast and accurate Gaussian update that may
enhance the system to adapt with swift textural variations. As a
result, this can enable optimal ROI detection for the targeted BC-
CAD solution.
Now, substituting as in (3), it is observed that variance
updating might avoid Gaussian saturation. On the other hand, the
fast learning rate might cause degeneracy [44]. To deal with this
issue, we have developed a semi-parametric model to estimate
7
units.
Support Vector Machine can be formulated in
context of binary classification to find decision
boudary for maximising margin between two
data classes.
(2)
Partial Least Square include principal
component analysis which has same concept as
PLS.
(3)
LDA technique which are combined in one
objective function.
(4)
where
If no element matches together then the element with the
minimal is re-initialized, and the variables (2-4) are updated as:
SVM and PLS (5)
In above expression (5), refers to the learning rate. Here, is
obtained as:
Support ventor machines is formulatde in order
to for dula optimization problem whihc ensures
unique global solution.
(6)
In our proposed GMM model, we normalize in a way that it
converges toward unit value (i.e., 1). To obtain background
region, authors [43] sorted Gaussians in decreasing order by
using threshold in conjunction with the sums of the weights. As
a result, this achieves a set, where is obtained as:
It summarise turning parameter of all models
which are inlcuded in proposed theory.
(7)
With decrease in variances, usually the Gaussian distribution
gains increases. This as a result makes it more evident to perform
background subtraction. Once estimating GMM parameters,
sorting is performed from the matched mixture distribution
toward most probable background. This is feasible only because
only the relative value of the matched model changes. In our
applied model, ordering is performed in a way that the most
feasible background distributions remains on the top, while the
low probability distributions move towards bottom. In such
manner, the initial value of (7) is considered as the background.
In (7), parameter signifies the result of the least section of the
data responsible for the background. With the minimal possible
value of , the background model is stated to be unimodal, where
using only the most probable distribution enhances
computational efficiency. On the contrary, the high signifies
multimodal distribution, is typically caused due to the iterative
background motion. In this paper, the breast cancer
Histopathological images with static features are taken as input
and hence the use of unimodal approach with low ensures
computationally efficient process. In our approach, the Gaussian
with the maximum and minimal refer to the background region.
Thus, applying aforementioned approach the background region
can be identified that consequently helps in segmenting the ROI.
In major cases, classical GMM updates with certain fixed
learning rate [43] are used. While, considering breast cancer
detection requirement, the static learning rate approach seems
questionable, particularly when there can be excessive texture
variation over the breast cancer Histopathological images. To
deal with such limitation, authors [44] developed an adaptive
learning rate based GMM for ROI segmentation over varying
surface or texture conditions. Non-deniably, there can be certain
texture or the surface conditions, where the pixel might neither be
the breast can tumor (i.e., ROI) nor the background; however, it
could be classified as either type Fibroadenoma and phyllodes
tumor. This as a result may influence accuracy of the proposed
BC-CAD solution. Increasing learning rate might cause high-
rate pixel feature changes [44] that consequently may make
breast cancer tumor or ROI detection susceptible. Though, the
adaptive learning based GMM model [43] can perform ROI
identification under varying background conditions. However,
the very minute and fine-connected texture or surface features
still make existing approach limited. Precisely for breast cancer
Histopathological images, textural or structural features, such as
cellular atypia, mitosis, disruption of basement membranes,
metastasize …etc. are highly intricate and minute feature that
requires more efficient ROI identification model. An enhanced
Gaussian mixture learning based GMM model was proposed in
[44]. However, authors could not address the saturation scenario
particularly during convergence. As an enhanced solution, in this
paper an adaptive learning based GMM model is developed that
detects precise ROI. In our proposed approach, initially, we have
decoupled learning rate from other components such as and. We
have applied a new learning rate variable called adaptive learning
rate that updates mean component using a probability
parameter. Here, states whether a pixel is a part of the nth
Gaussian distribution or not. In our model, the adaptive learning
rate parameter is estimated as follows:
Thresold (8)
Where, states the number of distributions.
Can provide fast and accurate Gaussian update that may
enhance the system to adapt with swift textural variations. As a
result, this can enable optimal ROI detection for the targeted BC-
CAD solution.
Now, substituting as in (3), it is observed that variance
updating might avoid Gaussian saturation. On the other hand, the
fast learning rate might cause degeneracy [44]. To deal with this
issue, we have developed a semi-parametric model to estimate
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
the variance that can effectively enable quasi-linear adaptation. It
is significant for the condition where even a minute change from
the average and certain reduced response might cause decisive
variation. To achieve it, in our model we have applied a sigmoid
function (9):
Sigmoid Curve whihc inlcudes logistic and
hyperbolic tangent functions.
(9)
In (9), the variable refers to a controller called sigmoid slope
controller (SSC).
Now, substituting (9) in (2), we obtain the variance update as
(10).
The sigmoid functions are used for as activation
function or artificila neurons.
(10)
In our study, performing try and assess approach, we find
suitable to perform better. To estimate, we have applied a
mathematical approach, where the two real positive variables
and are selected in such manner that expand over one th of the
pixel range. Here, caps to a range. In (9), we have used a new
variable rather using as applied in (2)-(4). Thus, applying above
discussed proposed background subtraction model the breast
cancer ROI is detected and segmented to further process
connected component analysis and feature extraction. Fig. 5
represents the breast cancer region (i.e. breast cancer ROI)
segmentation process. In Fig. 5, the first column refers the
original breast cancer Histopathological image from the
BreakHis dataset. The other two consecutive columns signify the
gray images with noise filtering as processed through [41].
Column 4 presents the subtracted BC-ROI regions and allied
internal geometries.
Fig 4. Examples of real textures present in histopathological images (HE
staining).
(a)
(b)
Fig 5. (a) Example of breast malignant tumor acquired at 40× magnification and
(b) 32 × 32 patch images.
.i Breast Cancer Region Localization
Once performing ROI segmentation, we have applied CCA
over the segmented breast cancer region that considers the
region, size, and location of the key BC features such like
mitosis, marked cellular atypia, disruption of basement
membranes, metastasize, and their shape, size, and proximity to
the normal breast regions. In our model, we consider that the
disconnected region referring to the Gaussian components
belongs to the breast cancer ROI. The significant feature
information such as the dimension and proximity to the BC ROI
has been normalized. In our model, the normalized dimensional
feature is estimated as the width of the connected component
region divided by the width of the cancer regions at the centroid
of the connected region. In this manner, using the normalized
width it becomes easier to compare the sizes of marked cellular
atypia, mitosis, disruption of basement membranes, metastasize
…etc., at distinct locations on the breast cancer Histopathological
image. Thus, applying above mentioned enhanced GMM and the
8
is significant for the condition where even a minute change from
the average and certain reduced response might cause decisive
variation. To achieve it, in our model we have applied a sigmoid
function (9):
Sigmoid Curve whihc inlcudes logistic and
hyperbolic tangent functions.
(9)
In (9), the variable refers to a controller called sigmoid slope
controller (SSC).
Now, substituting (9) in (2), we obtain the variance update as
(10).
The sigmoid functions are used for as activation
function or artificila neurons.
(10)
In our study, performing try and assess approach, we find
suitable to perform better. To estimate, we have applied a
mathematical approach, where the two real positive variables
and are selected in such manner that expand over one th of the
pixel range. Here, caps to a range. In (9), we have used a new
variable rather using as applied in (2)-(4). Thus, applying above
discussed proposed background subtraction model the breast
cancer ROI is detected and segmented to further process
connected component analysis and feature extraction. Fig. 5
represents the breast cancer region (i.e. breast cancer ROI)
segmentation process. In Fig. 5, the first column refers the
original breast cancer Histopathological image from the
BreakHis dataset. The other two consecutive columns signify the
gray images with noise filtering as processed through [41].
Column 4 presents the subtracted BC-ROI regions and allied
internal geometries.
Fig 4. Examples of real textures present in histopathological images (HE
staining).
(a)
(b)
Fig 5. (a) Example of breast malignant tumor acquired at 40× magnification and
(b) 32 × 32 patch images.
.i Breast Cancer Region Localization
Once performing ROI segmentation, we have applied CCA
over the segmented breast cancer region that considers the
region, size, and location of the key BC features such like
mitosis, marked cellular atypia, disruption of basement
membranes, metastasize, and their shape, size, and proximity to
the normal breast regions. In our model, we consider that the
disconnected region referring to the Gaussian components
belongs to the breast cancer ROI. The significant feature
information such as the dimension and proximity to the BC ROI
has been normalized. In our model, the normalized dimensional
feature is estimated as the width of the connected component
region divided by the width of the cancer regions at the centroid
of the connected region. In this manner, using the normalized
width it becomes easier to compare the sizes of marked cellular
atypia, mitosis, disruption of basement membranes, metastasize
…etc., at distinct locations on the breast cancer Histopathological
image. Thus, applying above mentioned enhanced GMM and the
8

CCA approaches of accurate BC-ROI has been identified for
further feature extraction. The following section briefs the
proposed CNN based feature extraction model for BC-CAD
solution.
.j Feature Extraction
Once identifying or localizing the BC-ROI the detected
region has been projected further for feature extraction. Unlike
traditional approaches, in this research work we have applied
AlexNet-DNN model for feature extraction and learning. In
addition, our proposed AlexNet-DNN model functions in
conjunction with the Caffe-enet DNN that makes feature
extraction even for large size unannotated data over general
purpose computers. A brief of the proposed Caffe-enet-AlexNet-
DNN model for BC-ROI feature extraction is given as follows:
AlexNet DNN
Unlike generic feature extraction techniques, such as wavelet
transform or Gabor filter …etc., we have applied a recently
developed and robust DNN model called AlexNet. In fact,
AlexNet was at first developed by Alex Krizhevsky for color
image classification, where it was trained over millions of images
[61]. However, its efficacy over other approaches enabled it to be
used in major image classification problems. In fact, AlexNet-
DNN represents a multilayered DNN architecture that applies
CNN concept to learn features over ImageNet [45]. The
transferable nature of AlexNet enables it to be used for major
image classification applications, including medical data analysis
and classification [46]. Considering these robustness, in our
model we have applied AlexNet-DNN to perform feature
extraction. In our proposed model, the transferability of AlexNet
has enabled the extracted features to be projected over ImageNet
[45], which has been further processed for training and learning.
The proposed AlexNet DNN model enables learning of the rich
midlevel breast cancer image features and corresponding
semantic representations that signify breast cancer perception. As
already stated, to enable ease of implementation of the AlexNet
DNN model over general purpose computers, we have applied
Caffe-enet-DNN [47]. It does not only enable easy
implementation but also ensures fast feature extraction over large
size unannotated data. A typical presentation of AlexNet-DNN
architecture is given as follows:
Max
pooling
224
3
5
27
128 192
11
Stride
of 4
5
13
55
48
dense
4096
1000
11
11
11 13
13
13
13
13
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
27
55
48
Max
pooling
224
128
192
192192 128
128
dense
4096
4096
4096
5
5
Max
pooling
Fig. 6. AlexNet CNN architecture. Extracted from [48].
Input Layer:
In AlexNet-DNN, this layer takes data as input and generates
output which is further fed as input to the convolutional layers.
To assure better performance, a few transformations like feature
scaling and mean-subtraction can also be performed at this layer.
In our proposed model, the inputs are the segmented images
while the parameters states its dimension (i.e., 32 ×32 or 64 ×64
pixels) and the total channel numbers (i.e., for RGB, 3).
Convolutional Layers:
This layer primarily convolves the input image with certain
filters, each generating single feature map in the output image. In
our proposed work, AlexNet-DNN with five convolutional layers
has been developed. Here, the receptive fields, also known as
kernels of respective size 5 × 5 are taken into consideration and
the zero-padding is fixed at 2. In addition, at convolutional layer
the stride is fixed at 3 which is known as long term decisive steps
that are required to be put in a correct direction. In our proposed
AlexNet-DNN model, the Convolution layers (CONV) have the
kernels specification as: CONV1-96 kernels, CONV2-256
kernels, CONV3-384 kernels, CONV4-384 kernels and CONV5-
256 kernels. Moreover, these kernels are specified as all of them
has single accurate feature which is mandatory for creation of an
output image.
Pooling Layers:
These layers in AlexNet usually functions for down-sampling
the spatial dimension of the input provided. In our proposed
model, we have incorporated one pooling-layer after each CONV
layer. Here, each of these is defined to apply a 3×3 receptive field
having a stride of 3. Here, receptive filed states spatial extent.
Usually, the first pooling layer applies the most generic max-
operation over the defined spatial extent, while the other
remaining exhibit average pooling.
ReLU Layers:
In addition to the above mentioned layers, there is a layer
called ReLU that typically functions as an activation function. In
fact, it is a non-linear element-wise operator that functions as a
layer. In AlexNet, there are three distinct ReLU layers. Provided
an input y, the ReLU layer estimates the output of the neuron as
if and if. Here, states whether to escape the negative component
by performing multiplication with it with slope (such as 0.01…)
or setting it to 0. In our case, we have set as the default case. In
case, is not defined it behaves similar to a classical ReLU
function. In another way, in this case the activation is performed
at the zero threshold value.
Fully Connected (FC) Layers:
In AlexNet, FC layer usually treats input data as a simple
vector and generates output as a single vector. In our applied
AlexNet-DNN model, three FC layers are applied: FC6, FC7 and
FC8; they have kernels of 4096, 4096 and 1000, respectively. In
other words, FC6-4096 kernels, FC7-4096 kernels and FC8-1000
kernels are used in proposed AlexNet-DNN because three layers
are applied including FC6, FC7, FC8 are combined with selected
9
further feature extraction. The following section briefs the
proposed CNN based feature extraction model for BC-CAD
solution.
.j Feature Extraction
Once identifying or localizing the BC-ROI the detected
region has been projected further for feature extraction. Unlike
traditional approaches, in this research work we have applied
AlexNet-DNN model for feature extraction and learning. In
addition, our proposed AlexNet-DNN model functions in
conjunction with the Caffe-enet DNN that makes feature
extraction even for large size unannotated data over general
purpose computers. A brief of the proposed Caffe-enet-AlexNet-
DNN model for BC-ROI feature extraction is given as follows:
AlexNet DNN
Unlike generic feature extraction techniques, such as wavelet
transform or Gabor filter …etc., we have applied a recently
developed and robust DNN model called AlexNet. In fact,
AlexNet was at first developed by Alex Krizhevsky for color
image classification, where it was trained over millions of images
[61]. However, its efficacy over other approaches enabled it to be
used in major image classification problems. In fact, AlexNet-
DNN represents a multilayered DNN architecture that applies
CNN concept to learn features over ImageNet [45]. The
transferable nature of AlexNet enables it to be used for major
image classification applications, including medical data analysis
and classification [46]. Considering these robustness, in our
model we have applied AlexNet-DNN to perform feature
extraction. In our proposed model, the transferability of AlexNet
has enabled the extracted features to be projected over ImageNet
[45], which has been further processed for training and learning.
The proposed AlexNet DNN model enables learning of the rich
midlevel breast cancer image features and corresponding
semantic representations that signify breast cancer perception. As
already stated, to enable ease of implementation of the AlexNet
DNN model over general purpose computers, we have applied
Caffe-enet-DNN [47]. It does not only enable easy
implementation but also ensures fast feature extraction over large
size unannotated data. A typical presentation of AlexNet-DNN
architecture is given as follows:
Max
pooling
224
3
5
27
128 192
11
Stride
of 4
5
13
55
48
dense
4096
1000
11
11
11 13
13
13
13
13
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
27
55
48
Max
pooling
224
128
192
192192 128
128
dense
4096
4096
4096
5
5
Max
pooling
Fig. 6. AlexNet CNN architecture. Extracted from [48].
Input Layer:
In AlexNet-DNN, this layer takes data as input and generates
output which is further fed as input to the convolutional layers.
To assure better performance, a few transformations like feature
scaling and mean-subtraction can also be performed at this layer.
In our proposed model, the inputs are the segmented images
while the parameters states its dimension (i.e., 32 ×32 or 64 ×64
pixels) and the total channel numbers (i.e., for RGB, 3).
Convolutional Layers:
This layer primarily convolves the input image with certain
filters, each generating single feature map in the output image. In
our proposed work, AlexNet-DNN with five convolutional layers
has been developed. Here, the receptive fields, also known as
kernels of respective size 5 × 5 are taken into consideration and
the zero-padding is fixed at 2. In addition, at convolutional layer
the stride is fixed at 3 which is known as long term decisive steps
that are required to be put in a correct direction. In our proposed
AlexNet-DNN model, the Convolution layers (CONV) have the
kernels specification as: CONV1-96 kernels, CONV2-256
kernels, CONV3-384 kernels, CONV4-384 kernels and CONV5-
256 kernels. Moreover, these kernels are specified as all of them
has single accurate feature which is mandatory for creation of an
output image.
Pooling Layers:
These layers in AlexNet usually functions for down-sampling
the spatial dimension of the input provided. In our proposed
model, we have incorporated one pooling-layer after each CONV
layer. Here, each of these is defined to apply a 3×3 receptive field
having a stride of 3. Here, receptive filed states spatial extent.
Usually, the first pooling layer applies the most generic max-
operation over the defined spatial extent, while the other
remaining exhibit average pooling.
ReLU Layers:
In addition to the above mentioned layers, there is a layer
called ReLU that typically functions as an activation function. In
fact, it is a non-linear element-wise operator that functions as a
layer. In AlexNet, there are three distinct ReLU layers. Provided
an input y, the ReLU layer estimates the output of the neuron as
if and if. Here, states whether to escape the negative component
by performing multiplication with it with slope (such as 0.01…)
or setting it to 0. In our case, we have set as the default case. In
case, is not defined it behaves similar to a classical ReLU
function. In another way, in this case the activation is performed
at the zero threshold value.
Fully Connected (FC) Layers:
In AlexNet, FC layer usually treats input data as a simple
vector and generates output as a single vector. In our applied
AlexNet-DNN model, three FC layers are applied: FC6, FC7 and
FC8; they have kernels of 4096, 4096 and 1000, respectively. In
other words, FC6-4096 kernels, FC7-4096 kernels and FC8-1000
kernels are used in proposed AlexNet-DNN because three layers
are applied including FC6, FC7, FC8 are combined with selected
9

kernels to form the same. The last one layer is a fully connected
output layer with softmax activation, which primarily depends on
the total number of classes required for classification. Here, we
have considered two class classifications which is generally
known as binary classification. Hence, there are two output filters
for the binary classification of the results.
Now, applying the derived DNN model, the feature extraction
has been performed. A brief of the AlexNet-DNN based feature
extraction is given as follows:
AlexNet DNN-Based BC-ROI Feature Extraction
In our BC-CAD model, AlexNet DNN model is applied for
extracting 4096 dimensional features from breast cancer
Histopathological images (particularly, BC-ROI localized
images). For ease of implementation on general purpose
computer, Caffe-enet-DNN has also been applied in conjunction
with AlexNet. Here, Caffe-enet is trained over the localized BC-
ROI features of the used BC dataset. A simplified model of the
applied multi-layered AlexNet-DNN is given as follows:
Conv1
Conv 2
Conv 3
Conv 4
FC6
FC7
Feature Selection and
Dimensional Reduction
Breast Cancer
Segmented Image
10-fold Cross validation
based SVM for DR
Prediction
Conv 5
Convolution Layers Fully Connected
Layers
96 K 256 K 384 K384 K 4096 N256 K 4096 N
K=Kernals
N=Neurons
Fig. 7. Simplified AlexNet-DNN Convolutional layers
In typical AlexNet model, the features extracted at the low
layer are similar to that of extracted through the classical
approaches, such as Gabor information, blob features …etc. On
the contrary, the features extracted at the higher layer usually
comprise significant information that play vital role in targeted
classification purposes (here, BC detection and classification). In
our work, we have considered features at the FC6 and FC7 layers
of the AlexNet DNN model. As already stated in above
discussion, the features can be extracted at five CONV layers
(i.e., CONV1–CONV5) and the FC layers (i.e., FC6 and FC7) of
AlexNet provide extracted BC-ROI features. As depicted above,
the individual CONV layer comprises multiple kernels that
define a 3D filter connected to the outputs of the preceding layer.
As depicted in Fig. 7, FC layers comprise multiple neurons
having the real positive value and each neuron is connected to the
all neurons of the previous layer. In our model, FC6 and FC7
features are considered that possess a total of 4096-dimensional
features to be further processed for classification. Once retrieving
the features, it is projected to the feature vector. This is because
the extracted features have the huge quantity and hence there can
be the probability that many instances could have the same
significances or do not give different meaning. Hence,
suppressing those insignificant features can be vital to enhance
accuracy as well as computational efficiency. In our work, once
obtaining the features, it has been projected further for feature
selection or dimensional reduction.
In addition to the proposed AlexNet DNN based feature
extraction model, we have developed a parallel approach called
SIFT to extract features from the segmented BC-ROI Before
discussing the feature selection or the dimensional reduction
approaches, a brief of SIFT based BC-ROI features extraction is
presented as follows:
SIFT-Based BC-ROI Feature Extraction
Scale Invariant Feature Transform (SIFT) is an algorithm in
computer vision, which detects and identifies local features in an
image for further decision. Typically, SIFT landmark points or
the keypoints of ROI are retrieved from a reference image, which
are then stored in a database. Thus, the ROI is detected in a new
image by performing comparison of the individual feature from
the new image to this database. It then finds the candidate
matching features on the basis of Euclidean distance of their
feature vectors. Thus, from the available complete set of matches,
keypoint-(in subsets) agreeing the ROI or object and its position,
or other features, such as scale, orientation…etc., in the new
image are obtained to retrieve good matches. The estimation of
constant clusters is performed rapidly by using an efficient hash
table implementation of the generalized Hough transform. Each
cluster of 3 or more features that agree on an object and its pose
is then subject to further detailed model verification and
subsequently outliers are discarded. Finally, the probability that a
particular set of features indicates the presence of an object is
computed, given the accuracy of fit and number of probable false
matches. Object matches that pass all these tests can be identified
as correct with high confidence.
To perform SIFT based feature extraction, at first SIFT
descriptors have been obtained for each BC-ROI or the breast
cancer Histopathological image. Here, the individual SIFT
feature descriptor signifies a 128-dimensional feature vector. In
our proposed work, only half of the 128-dimensional feature
vectors are considered. In other words, only 64 dimensions are
considered for features analysis. In our model, we have processed
Fisher encoding with the 32 Gaussian distributions that as a result
generates a 4096-dimensional Fisher vector equivalent to the
AlexNet DNN features at FC6 and FC7.
Once, the features have been extracted from the datasets, it
has been processed for the feature selection or the dimensional
reduction. A brief of the proposed feature selection or the
dimensional reduction approaches is given as follows.
.a Feature Selection and Dimensional Reduction
In this paper, we have applied two feature selection or the
dimensional reduction approaches: named Principle Component
10
output layer with softmax activation, which primarily depends on
the total number of classes required for classification. Here, we
have considered two class classifications which is generally
known as binary classification. Hence, there are two output filters
for the binary classification of the results.
Now, applying the derived DNN model, the feature extraction
has been performed. A brief of the AlexNet-DNN based feature
extraction is given as follows:
AlexNet DNN-Based BC-ROI Feature Extraction
In our BC-CAD model, AlexNet DNN model is applied for
extracting 4096 dimensional features from breast cancer
Histopathological images (particularly, BC-ROI localized
images). For ease of implementation on general purpose
computer, Caffe-enet-DNN has also been applied in conjunction
with AlexNet. Here, Caffe-enet is trained over the localized BC-
ROI features of the used BC dataset. A simplified model of the
applied multi-layered AlexNet-DNN is given as follows:
Conv1
Conv 2
Conv 3
Conv 4
FC6
FC7
Feature Selection and
Dimensional Reduction
Breast Cancer
Segmented Image
10-fold Cross validation
based SVM for DR
Prediction
Conv 5
Convolution Layers Fully Connected
Layers
96 K 256 K 384 K384 K 4096 N256 K 4096 N
K=Kernals
N=Neurons
Fig. 7. Simplified AlexNet-DNN Convolutional layers
In typical AlexNet model, the features extracted at the low
layer are similar to that of extracted through the classical
approaches, such as Gabor information, blob features …etc. On
the contrary, the features extracted at the higher layer usually
comprise significant information that play vital role in targeted
classification purposes (here, BC detection and classification). In
our work, we have considered features at the FC6 and FC7 layers
of the AlexNet DNN model. As already stated in above
discussion, the features can be extracted at five CONV layers
(i.e., CONV1–CONV5) and the FC layers (i.e., FC6 and FC7) of
AlexNet provide extracted BC-ROI features. As depicted above,
the individual CONV layer comprises multiple kernels that
define a 3D filter connected to the outputs of the preceding layer.
As depicted in Fig. 7, FC layers comprise multiple neurons
having the real positive value and each neuron is connected to the
all neurons of the previous layer. In our model, FC6 and FC7
features are considered that possess a total of 4096-dimensional
features to be further processed for classification. Once retrieving
the features, it is projected to the feature vector. This is because
the extracted features have the huge quantity and hence there can
be the probability that many instances could have the same
significances or do not give different meaning. Hence,
suppressing those insignificant features can be vital to enhance
accuracy as well as computational efficiency. In our work, once
obtaining the features, it has been projected further for feature
selection or dimensional reduction.
In addition to the proposed AlexNet DNN based feature
extraction model, we have developed a parallel approach called
SIFT to extract features from the segmented BC-ROI Before
discussing the feature selection or the dimensional reduction
approaches, a brief of SIFT based BC-ROI features extraction is
presented as follows:
SIFT-Based BC-ROI Feature Extraction
Scale Invariant Feature Transform (SIFT) is an algorithm in
computer vision, which detects and identifies local features in an
image for further decision. Typically, SIFT landmark points or
the keypoints of ROI are retrieved from a reference image, which
are then stored in a database. Thus, the ROI is detected in a new
image by performing comparison of the individual feature from
the new image to this database. It then finds the candidate
matching features on the basis of Euclidean distance of their
feature vectors. Thus, from the available complete set of matches,
keypoint-(in subsets) agreeing the ROI or object and its position,
or other features, such as scale, orientation…etc., in the new
image are obtained to retrieve good matches. The estimation of
constant clusters is performed rapidly by using an efficient hash
table implementation of the generalized Hough transform. Each
cluster of 3 or more features that agree on an object and its pose
is then subject to further detailed model verification and
subsequently outliers are discarded. Finally, the probability that a
particular set of features indicates the presence of an object is
computed, given the accuracy of fit and number of probable false
matches. Object matches that pass all these tests can be identified
as correct with high confidence.
To perform SIFT based feature extraction, at first SIFT
descriptors have been obtained for each BC-ROI or the breast
cancer Histopathological image. Here, the individual SIFT
feature descriptor signifies a 128-dimensional feature vector. In
our proposed work, only half of the 128-dimensional feature
vectors are considered. In other words, only 64 dimensions are
considered for features analysis. In our model, we have processed
Fisher encoding with the 32 Gaussian distributions that as a result
generates a 4096-dimensional Fisher vector equivalent to the
AlexNet DNN features at FC6 and FC7.
Once, the features have been extracted from the datasets, it
has been processed for the feature selection or the dimensional
reduction. A brief of the proposed feature selection or the
dimensional reduction approaches is given as follows.
.a Feature Selection and Dimensional Reduction
In this paper, we have applied two feature selection or the
dimensional reduction approaches: named Principle Component
10
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Analysis (PCA) and Linear Discriminate Analysis (LDA). In
major existing approaches, PCA based feature selection approach
has been applied; however, the efficacy of LDA towards most
discriminating features makes process more efficient.
Considering this fact, we have applied both algorithms distinctly
and respective performance has been assessed. A brief of these
techniques is given, as follows:
Principle Component Analysis (PCA)
It mainly projects feature space of a high dimension to the
lower dimension while ensuring that the obtained feature space
contains most significant features. In function, PCA rotates the p-
dimensional feature space’s axes to a new position called
principle axes in such way that the principle axis 1 has the
highest variance followed by axis 2, and so on. In practice among
extracted breast cancer features there can be the highly correlated
feature attributes of the elements. These highly correlated
features (sometimes called homogeneous feature) are known as
the most expressive features (MEF). In this case, PCA intends to
transfer the feature attributes or the elements to a new feature set
or feature vector having no correlation among the elements.
Thus, applying this approach PCA reduces a significant amount
of feature elements that makes computation more efficient. The
finally retrieved feature vector is then projected for classification.
Linear Discriminant Analysis (LDA)
Unlike PCA that uses MEF to perform feature selection or the
dimensional reduction, LDA employs the most discriminating
features (MDF) to perform dimensional reduction. LDA performs
dimensional reduction automatically. Executing PCA, the LDA
dimensional reduction approach projects extracted features to a
single principle component regardless of its class label. To
perform feature selection and the dimensional reduction, LDA
applied two distinct matrix called intra-class scatter matrix and
inter-class scatter matrix. These matrix values are estimated, as
follows:
(11)
(12)
In (12), refers the total number of classes, signifies average
vector of class , and presents the overall samples in class . We
estimate the as
(13)
In (13), refers the total number of classes.
LDA intends to increase the inter-class scatter and
decrease the intra-class scatter. In our model, it was
achieved by increasing a factor , given in equation
(14).
(14)
For a non-singular, is increased provided the column vectors
of the projection matrix are the eigenvectors of. Here, the
projection matrix with dimension projects the training data to
form Fisher vector (FV). In this way, the final Fischer Vector
obtained is given as input to the classifier. Noticeably, the overall
Fischer Vectors projected for classification be .
Once obtaining the final feature vectors, it is processed for
breast cancer classification. In our proposed work, a two class
classification has been done: BENIGN and MALIGNANT. To
further enhance performance, 10-fold cross validation has been
performed. A brief of the classification method used is presented
as follows:
.a Classification
Considering ease of implementation and better non-linear
pattern learning efficiency, in our model a Radial Basis Function
(RBF) kernel based SVM classifier is applied to perform two-
class BC-CAD classification. In our model, SVM has been
trained over extracted feature vectors that intend to achieve the
maximum margin by obtaining low as given in equation (15).
(15)
Where, ≥ 0 and signify the extent of error resiliency.
In our model, to perform two-class classification, FV is
grouped in the labeled pairs, say where presents the training
vector. The class label of the training vector is given by. In
classification, the hyper plane classifies the maximum possible
points of the same class on the same side of hyperplane. The use
of 10-fold cross-validation ensures higher accuracy and reliability
of the results. The overall performance of the proposed BC-CAD
model is examined in terms of classification accuracy and the
obtained results are compared with other state-of-art techniques.
The results obtained in this research work are discussed in the
next section.
V. RESULTS AND DISCUSSION
To assess the performance of our proposed BC-CAD
solution, we consider BreakHis dataset [39] that contains 9,109
Histopathological (say, microscopic) images of breast cancer
tissue with different tumor types or associated traits, such as
fibroadenoma, phyllodes tumor, tubular adenona, adenosis,
carcinoma, lobular carcinoma, mucinous carcinoma, and
papillary carcinoma. For ease of implementation, we consider
random 2000 Histopathological images for our study. The
original Histopathological images are in the size of 700X460,
which is further reduced using resampling approach (i.e., pixel
area relation) and finally the images with 350X230 dimensions
are obtained. It is then followed by the extraction of patches from
the considered Histopathological images. In our work, we apply a
sliding window approach with 50% overlapping. In addition, the
patches can be obtained randomly too without incorporating any
overlapping control between patches. In our simulation test, the
patches with 32X32 dimensions are obtained. As pre-processing,
11
major existing approaches, PCA based feature selection approach
has been applied; however, the efficacy of LDA towards most
discriminating features makes process more efficient.
Considering this fact, we have applied both algorithms distinctly
and respective performance has been assessed. A brief of these
techniques is given, as follows:
Principle Component Analysis (PCA)
It mainly projects feature space of a high dimension to the
lower dimension while ensuring that the obtained feature space
contains most significant features. In function, PCA rotates the p-
dimensional feature space’s axes to a new position called
principle axes in such way that the principle axis 1 has the
highest variance followed by axis 2, and so on. In practice among
extracted breast cancer features there can be the highly correlated
feature attributes of the elements. These highly correlated
features (sometimes called homogeneous feature) are known as
the most expressive features (MEF). In this case, PCA intends to
transfer the feature attributes or the elements to a new feature set
or feature vector having no correlation among the elements.
Thus, applying this approach PCA reduces a significant amount
of feature elements that makes computation more efficient. The
finally retrieved feature vector is then projected for classification.
Linear Discriminant Analysis (LDA)
Unlike PCA that uses MEF to perform feature selection or the
dimensional reduction, LDA employs the most discriminating
features (MDF) to perform dimensional reduction. LDA performs
dimensional reduction automatically. Executing PCA, the LDA
dimensional reduction approach projects extracted features to a
single principle component regardless of its class label. To
perform feature selection and the dimensional reduction, LDA
applied two distinct matrix called intra-class scatter matrix and
inter-class scatter matrix. These matrix values are estimated, as
follows:
(11)
(12)
In (12), refers the total number of classes, signifies average
vector of class , and presents the overall samples in class . We
estimate the as
(13)
In (13), refers the total number of classes.
LDA intends to increase the inter-class scatter and
decrease the intra-class scatter. In our model, it was
achieved by increasing a factor , given in equation
(14).
(14)
For a non-singular, is increased provided the column vectors
of the projection matrix are the eigenvectors of. Here, the
projection matrix with dimension projects the training data to
form Fisher vector (FV). In this way, the final Fischer Vector
obtained is given as input to the classifier. Noticeably, the overall
Fischer Vectors projected for classification be .
Once obtaining the final feature vectors, it is processed for
breast cancer classification. In our proposed work, a two class
classification has been done: BENIGN and MALIGNANT. To
further enhance performance, 10-fold cross validation has been
performed. A brief of the classification method used is presented
as follows:
.a Classification
Considering ease of implementation and better non-linear
pattern learning efficiency, in our model a Radial Basis Function
(RBF) kernel based SVM classifier is applied to perform two-
class BC-CAD classification. In our model, SVM has been
trained over extracted feature vectors that intend to achieve the
maximum margin by obtaining low as given in equation (15).
(15)
Where, ≥ 0 and signify the extent of error resiliency.
In our model, to perform two-class classification, FV is
grouped in the labeled pairs, say where presents the training
vector. The class label of the training vector is given by. In
classification, the hyper plane classifies the maximum possible
points of the same class on the same side of hyperplane. The use
of 10-fold cross-validation ensures higher accuracy and reliability
of the results. The overall performance of the proposed BC-CAD
model is examined in terms of classification accuracy and the
obtained results are compared with other state-of-art techniques.
The results obtained in this research work are discussed in the
next section.
V. RESULTS AND DISCUSSION
To assess the performance of our proposed BC-CAD
solution, we consider BreakHis dataset [39] that contains 9,109
Histopathological (say, microscopic) images of breast cancer
tissue with different tumor types or associated traits, such as
fibroadenoma, phyllodes tumor, tubular adenona, adenosis,
carcinoma, lobular carcinoma, mucinous carcinoma, and
papillary carcinoma. For ease of implementation, we consider
random 2000 Histopathological images for our study. The
original Histopathological images are in the size of 700X460,
which is further reduced using resampling approach (i.e., pixel
area relation) and finally the images with 350X230 dimensions
are obtained. It is then followed by the extraction of patches from
the considered Histopathological images. In our work, we apply a
sliding window approach with 50% overlapping. In addition, the
patches can be obtained randomly too without incorporating any
overlapping control between patches. In our simulation test, the
patches with 32X32 dimensions are obtained. As pre-processing,
11
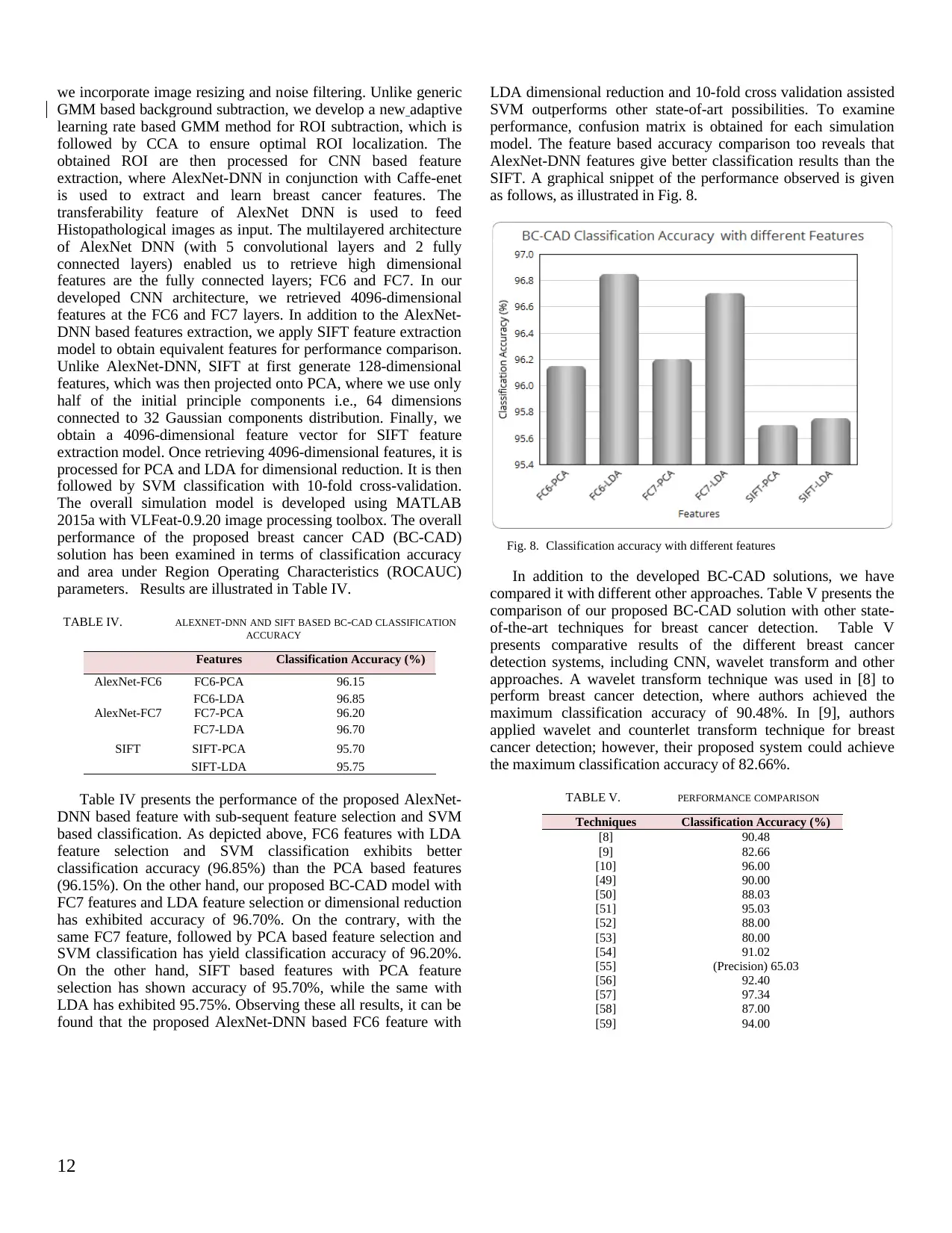
we incorporate image resizing and noise filtering. Unlike generic
GMM based background subtraction, we develop a new adaptive
learning rate based GMM method for ROI subtraction, which is
followed by CCA to ensure optimal ROI localization. The
obtained ROI are then processed for CNN based feature
extraction, where AlexNet-DNN in conjunction with Caffe-enet
is used to extract and learn breast cancer features. The
transferability feature of AlexNet DNN is used to feed
Histopathological images as input. The multilayered architecture
of AlexNet DNN (with 5 convolutional layers and 2 fully
connected layers) enabled us to retrieve high dimensional
features are the fully connected layers; FC6 and FC7. In our
developed CNN architecture, we retrieved 4096-dimensional
features at the FC6 and FC7 layers. In addition to the AlexNet-
DNN based features extraction, we apply SIFT feature extraction
model to obtain equivalent features for performance comparison.
Unlike AlexNet-DNN, SIFT at first generate 128-dimensional
features, which was then projected onto PCA, where we use only
half of the initial principle components i.e., 64 dimensions
connected to 32 Gaussian components distribution. Finally, we
obtain a 4096-dimensional feature vector for SIFT feature
extraction model. Once retrieving 4096-dimensional features, it is
processed for PCA and LDA for dimensional reduction. It is then
followed by SVM classification with 10-fold cross-validation.
The overall simulation model is developed using MATLAB
2015a with VLFeat-0.9.20 image processing toolbox. The overall
performance of the proposed breast cancer CAD (BC-CAD)
solution has been examined in terms of classification accuracy
and area under Region Operating Characteristics (ROCAUC)
parameters. Results are illustrated in Table IV.
TABLE IV. ALEXNET-DNN AND SIFT BASED BC-CAD CLASSIFICATION
ACCURACY
Features Classification Accuracy (%)
AlexNet-FC6 FC6-PCA 96.15
FC6-LDA 96.85
AlexNet-FC7 FC7-PCA 96.20
FC7-LDA 96.70
SIFT SIFT-PCA 95.70
SIFT-LDA 95.75
Table IV presents the performance of the proposed AlexNet-
DNN based feature with sub-sequent feature selection and SVM
based classification. As depicted above, FC6 features with LDA
feature selection and SVM classification exhibits better
classification accuracy (96.85%) than the PCA based features
(96.15%). On the other hand, our proposed BC-CAD model with
FC7 features and LDA feature selection or dimensional reduction
has exhibited accuracy of 96.70%. On the contrary, with the
same FC7 feature, followed by PCA based feature selection and
SVM classification has yield classification accuracy of 96.20%.
On the other hand, SIFT based features with PCA feature
selection has shown accuracy of 95.70%, while the same with
LDA has exhibited 95.75%. Observing these all results, it can be
found that the proposed AlexNet-DNN based FC6 feature with
LDA dimensional reduction and 10-fold cross validation assisted
SVM outperforms other state-of-art possibilities. To examine
performance, confusion matrix is obtained for each simulation
model. The feature based accuracy comparison too reveals that
AlexNet-DNN features give better classification results than the
SIFT. A graphical snippet of the performance observed is given
as follows, as illustrated in Fig. 8.
Fig. 8. Classification accuracy with different features
In addition to the developed BC-CAD solutions, we have
compared it with different other approaches. Table V presents the
comparison of our proposed BC-CAD solution with other state-
of-the-art techniques for breast cancer detection. Table V
presents comparative results of the different breast cancer
detection systems, including CNN, wavelet transform and other
approaches. A wavelet transform technique was used in [8] to
perform breast cancer detection, where authors achieved the
maximum classification accuracy of 90.48%. In [9], authors
applied wavelet and counterlet transform technique for breast
cancer detection; however, their proposed system could achieve
the maximum classification accuracy of 82.66%.
TABLE V. PERFORMANCE COMPARISON
Techniques Classification Accuracy (%)
[8] 90.48
[9] 82.66
[10] 96.00
[49] 90.00
[50] 88.03
[51] 95.03
[52] 88.00
[53] 80.00
[54] 91.02
[55] (Precision) 65.03
[56] 92.40
[57] 97.34
[58] 87.00
[59] 94.00
12
GMM based background subtraction, we develop a new adaptive
learning rate based GMM method for ROI subtraction, which is
followed by CCA to ensure optimal ROI localization. The
obtained ROI are then processed for CNN based feature
extraction, where AlexNet-DNN in conjunction with Caffe-enet
is used to extract and learn breast cancer features. The
transferability feature of AlexNet DNN is used to feed
Histopathological images as input. The multilayered architecture
of AlexNet DNN (with 5 convolutional layers and 2 fully
connected layers) enabled us to retrieve high dimensional
features are the fully connected layers; FC6 and FC7. In our
developed CNN architecture, we retrieved 4096-dimensional
features at the FC6 and FC7 layers. In addition to the AlexNet-
DNN based features extraction, we apply SIFT feature extraction
model to obtain equivalent features for performance comparison.
Unlike AlexNet-DNN, SIFT at first generate 128-dimensional
features, which was then projected onto PCA, where we use only
half of the initial principle components i.e., 64 dimensions
connected to 32 Gaussian components distribution. Finally, we
obtain a 4096-dimensional feature vector for SIFT feature
extraction model. Once retrieving 4096-dimensional features, it is
processed for PCA and LDA for dimensional reduction. It is then
followed by SVM classification with 10-fold cross-validation.
The overall simulation model is developed using MATLAB
2015a with VLFeat-0.9.20 image processing toolbox. The overall
performance of the proposed breast cancer CAD (BC-CAD)
solution has been examined in terms of classification accuracy
and area under Region Operating Characteristics (ROCAUC)
parameters. Results are illustrated in Table IV.
TABLE IV. ALEXNET-DNN AND SIFT BASED BC-CAD CLASSIFICATION
ACCURACY
Features Classification Accuracy (%)
AlexNet-FC6 FC6-PCA 96.15
FC6-LDA 96.85
AlexNet-FC7 FC7-PCA 96.20
FC7-LDA 96.70
SIFT SIFT-PCA 95.70
SIFT-LDA 95.75
Table IV presents the performance of the proposed AlexNet-
DNN based feature with sub-sequent feature selection and SVM
based classification. As depicted above, FC6 features with LDA
feature selection and SVM classification exhibits better
classification accuracy (96.85%) than the PCA based features
(96.15%). On the other hand, our proposed BC-CAD model with
FC7 features and LDA feature selection or dimensional reduction
has exhibited accuracy of 96.70%. On the contrary, with the
same FC7 feature, followed by PCA based feature selection and
SVM classification has yield classification accuracy of 96.20%.
On the other hand, SIFT based features with PCA feature
selection has shown accuracy of 95.70%, while the same with
LDA has exhibited 95.75%. Observing these all results, it can be
found that the proposed AlexNet-DNN based FC6 feature with
LDA dimensional reduction and 10-fold cross validation assisted
SVM outperforms other state-of-art possibilities. To examine
performance, confusion matrix is obtained for each simulation
model. The feature based accuracy comparison too reveals that
AlexNet-DNN features give better classification results than the
SIFT. A graphical snippet of the performance observed is given
as follows, as illustrated in Fig. 8.
Fig. 8. Classification accuracy with different features
In addition to the developed BC-CAD solutions, we have
compared it with different other approaches. Table V presents the
comparison of our proposed BC-CAD solution with other state-
of-the-art techniques for breast cancer detection. Table V
presents comparative results of the different breast cancer
detection systems, including CNN, wavelet transform and other
approaches. A wavelet transform technique was used in [8] to
perform breast cancer detection, where authors achieved the
maximum classification accuracy of 90.48%. In [9], authors
applied wavelet and counterlet transform technique for breast
cancer detection; however, their proposed system could achieve
the maximum classification accuracy of 82.66%.
TABLE V. PERFORMANCE COMPARISON
Techniques Classification Accuracy (%)
[8] 90.48
[9] 82.66
[10] 96.00
[49] 90.00
[50] 88.03
[51] 95.03
[52] 88.00
[53] 80.00
[54] 91.02
[55] (Precision) 65.03
[56] 92.40
[57] 97.34
[58] 87.00
[59] 94.00
12
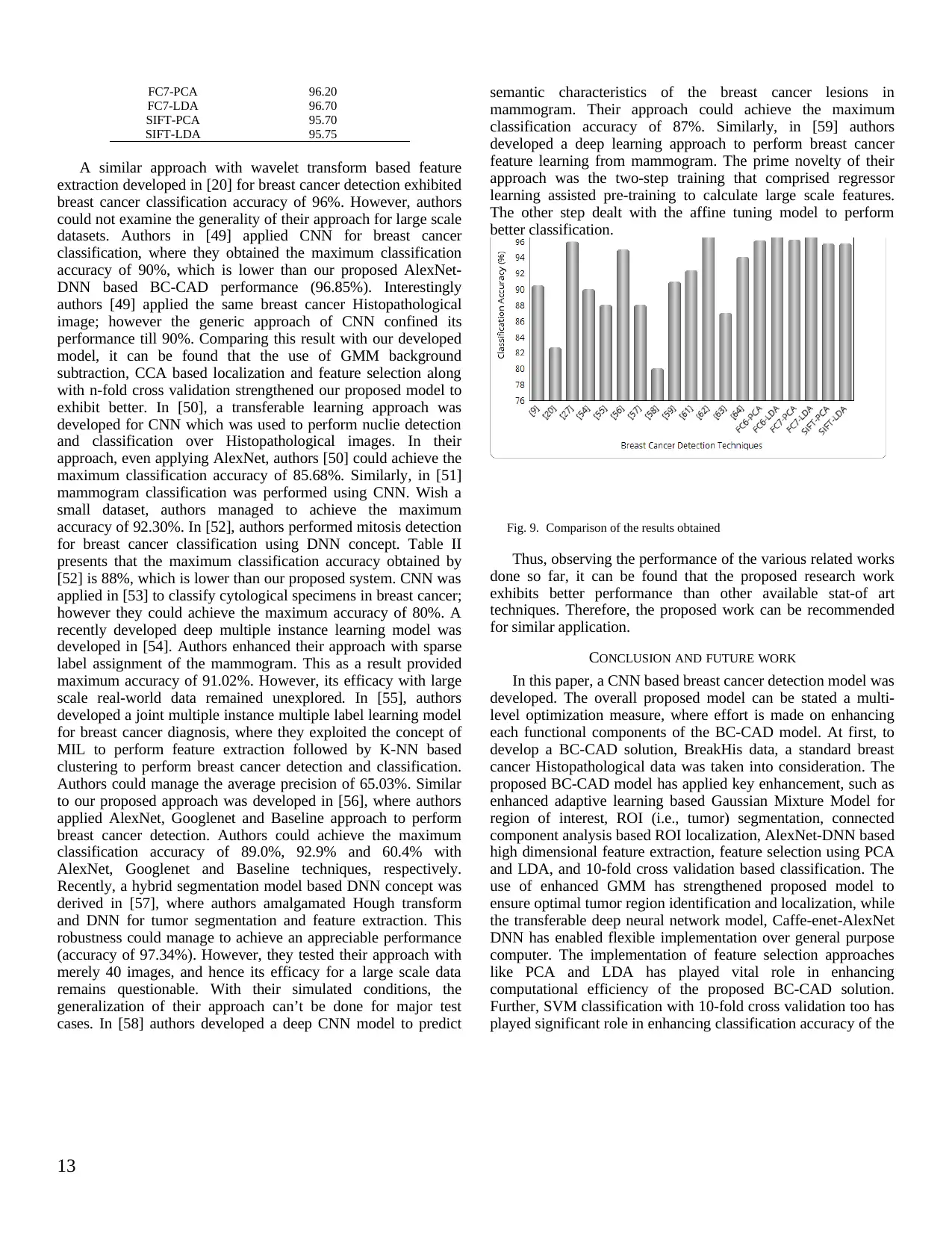
FC7-PCA 96.20
FC7-LDA 96.70
SIFT-PCA 95.70
SIFT-LDA 95.75
A similar approach with wavelet transform based feature
extraction developed in [20] for breast cancer detection exhibited
breast cancer classification accuracy of 96%. However, authors
could not examine the generality of their approach for large scale
datasets. Authors in [49] applied CNN for breast cancer
classification, where they obtained the maximum classification
accuracy of 90%, which is lower than our proposed AlexNet-
DNN based BC-CAD performance (96.85%). Interestingly
authors [49] applied the same breast cancer Histopathological
image; however the generic approach of CNN confined its
performance till 90%. Comparing this result with our developed
model, it can be found that the use of GMM background
subtraction, CCA based localization and feature selection along
with n-fold cross validation strengthened our proposed model to
exhibit better. In [50], a transferable learning approach was
developed for CNN which was used to perform nuclie detection
and classification over Histopathological images. In their
approach, even applying AlexNet, authors [50] could achieve the
maximum classification accuracy of 85.68%. Similarly, in [51]
mammogram classification was performed using CNN. Wish a
small dataset, authors managed to achieve the maximum
accuracy of 92.30%. In [52], authors performed mitosis detection
for breast cancer classification using DNN concept. Table II
presents that the maximum classification accuracy obtained by
[52] is 88%, which is lower than our proposed system. CNN was
applied in [53] to classify cytological specimens in breast cancer;
however they could achieve the maximum accuracy of 80%. A
recently developed deep multiple instance learning model was
developed in [54]. Authors enhanced their approach with sparse
label assignment of the mammogram. This as a result provided
maximum accuracy of 91.02%. However, its efficacy with large
scale real-world data remained unexplored. In [55], authors
developed a joint multiple instance multiple label learning model
for breast cancer diagnosis, where they exploited the concept of
MIL to perform feature extraction followed by K-NN based
clustering to perform breast cancer detection and classification.
Authors could manage the average precision of 65.03%. Similar
to our proposed approach was developed in [56], where authors
applied AlexNet, Googlenet and Baseline approach to perform
breast cancer detection. Authors could achieve the maximum
classification accuracy of 89.0%, 92.9% and 60.4% with
AlexNet, Googlenet and Baseline techniques, respectively.
Recently, a hybrid segmentation model based DNN concept was
derived in [57], where authors amalgamated Hough transform
and DNN for tumor segmentation and feature extraction. This
robustness could manage to achieve an appreciable performance
(accuracy of 97.34%). However, they tested their approach with
merely 40 images, and hence its efficacy for a large scale data
remains questionable. With their simulated conditions, the
generalization of their approach can’t be done for major test
cases. In [58] authors developed a deep CNN model to predict
semantic characteristics of the breast cancer lesions in
mammogram. Their approach could achieve the maximum
classification accuracy of 87%. Similarly, in [59] authors
developed a deep learning approach to perform breast cancer
feature learning from mammogram. The prime novelty of their
approach was the two-step training that comprised regressor
learning assisted pre-training to calculate large scale features.
The other step dealt with the affine tuning model to perform
better classification.
Fig. 9. Comparison of the results obtained
Thus, observing the performance of the various related works
done so far, it can be found that the proposed research work
exhibits better performance than other available stat-of art
techniques. Therefore, the proposed work can be recommended
for similar application.
CONCLUSION AND FUTURE WORK
In this paper, a CNN based breast cancer detection model was
developed. The overall proposed model can be stated a multi-
level optimization measure, where effort is made on enhancing
each functional components of the BC-CAD model. At first, to
develop a BC-CAD solution, BreakHis data, a standard breast
cancer Histopathological data was taken into consideration. The
proposed BC-CAD model has applied key enhancement, such as
enhanced adaptive learning based Gaussian Mixture Model for
region of interest, ROI (i.e., tumor) segmentation, connected
component analysis based ROI localization, AlexNet-DNN based
high dimensional feature extraction, feature selection using PCA
and LDA, and 10-fold cross validation based classification. The
use of enhanced GMM has strengthened proposed model to
ensure optimal tumor region identification and localization, while
the transferable deep neural network model, Caffe-enet-AlexNet
DNN has enabled flexible implementation over general purpose
computer. The implementation of feature selection approaches
like PCA and LDA has played vital role in enhancing
computational efficiency of the proposed BC-CAD solution.
Further, SVM classification with 10-fold cross validation too has
played significant role in enhancing classification accuracy of the
13
FC7-LDA 96.70
SIFT-PCA 95.70
SIFT-LDA 95.75
A similar approach with wavelet transform based feature
extraction developed in [20] for breast cancer detection exhibited
breast cancer classification accuracy of 96%. However, authors
could not examine the generality of their approach for large scale
datasets. Authors in [49] applied CNN for breast cancer
classification, where they obtained the maximum classification
accuracy of 90%, which is lower than our proposed AlexNet-
DNN based BC-CAD performance (96.85%). Interestingly
authors [49] applied the same breast cancer Histopathological
image; however the generic approach of CNN confined its
performance till 90%. Comparing this result with our developed
model, it can be found that the use of GMM background
subtraction, CCA based localization and feature selection along
with n-fold cross validation strengthened our proposed model to
exhibit better. In [50], a transferable learning approach was
developed for CNN which was used to perform nuclie detection
and classification over Histopathological images. In their
approach, even applying AlexNet, authors [50] could achieve the
maximum classification accuracy of 85.68%. Similarly, in [51]
mammogram classification was performed using CNN. Wish a
small dataset, authors managed to achieve the maximum
accuracy of 92.30%. In [52], authors performed mitosis detection
for breast cancer classification using DNN concept. Table II
presents that the maximum classification accuracy obtained by
[52] is 88%, which is lower than our proposed system. CNN was
applied in [53] to classify cytological specimens in breast cancer;
however they could achieve the maximum accuracy of 80%. A
recently developed deep multiple instance learning model was
developed in [54]. Authors enhanced their approach with sparse
label assignment of the mammogram. This as a result provided
maximum accuracy of 91.02%. However, its efficacy with large
scale real-world data remained unexplored. In [55], authors
developed a joint multiple instance multiple label learning model
for breast cancer diagnosis, where they exploited the concept of
MIL to perform feature extraction followed by K-NN based
clustering to perform breast cancer detection and classification.
Authors could manage the average precision of 65.03%. Similar
to our proposed approach was developed in [56], where authors
applied AlexNet, Googlenet and Baseline approach to perform
breast cancer detection. Authors could achieve the maximum
classification accuracy of 89.0%, 92.9% and 60.4% with
AlexNet, Googlenet and Baseline techniques, respectively.
Recently, a hybrid segmentation model based DNN concept was
derived in [57], where authors amalgamated Hough transform
and DNN for tumor segmentation and feature extraction. This
robustness could manage to achieve an appreciable performance
(accuracy of 97.34%). However, they tested their approach with
merely 40 images, and hence its efficacy for a large scale data
remains questionable. With their simulated conditions, the
generalization of their approach can’t be done for major test
cases. In [58] authors developed a deep CNN model to predict
semantic characteristics of the breast cancer lesions in
mammogram. Their approach could achieve the maximum
classification accuracy of 87%. Similarly, in [59] authors
developed a deep learning approach to perform breast cancer
feature learning from mammogram. The prime novelty of their
approach was the two-step training that comprised regressor
learning assisted pre-training to calculate large scale features.
The other step dealt with the affine tuning model to perform
better classification.
Fig. 9. Comparison of the results obtained
Thus, observing the performance of the various related works
done so far, it can be found that the proposed research work
exhibits better performance than other available stat-of art
techniques. Therefore, the proposed work can be recommended
for similar application.
CONCLUSION AND FUTURE WORK
In this paper, a CNN based breast cancer detection model was
developed. The overall proposed model can be stated a multi-
level optimization measure, where effort is made on enhancing
each functional components of the BC-CAD model. At first, to
develop a BC-CAD solution, BreakHis data, a standard breast
cancer Histopathological data was taken into consideration. The
proposed BC-CAD model has applied key enhancement, such as
enhanced adaptive learning based Gaussian Mixture Model for
region of interest, ROI (i.e., tumor) segmentation, connected
component analysis based ROI localization, AlexNet-DNN based
high dimensional feature extraction, feature selection using PCA
and LDA, and 10-fold cross validation based classification. The
use of enhanced GMM has strengthened proposed model to
ensure optimal tumor region identification and localization, while
the transferable deep neural network model, Caffe-enet-AlexNet
DNN has enabled flexible implementation over general purpose
computer. The implementation of feature selection approaches
like PCA and LDA has played vital role in enhancing
computational efficiency of the proposed BC-CAD solution.
Further, SVM classification with 10-fold cross validation too has
played significant role in enhancing classification accuracy of the
13
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

proposed system with 96.15 for AlexNet-FC6 and 96.20 for
AlexNet-FC7. The overall enhanced functional components as a
cumulative BC-CAD solution has exhibited better than other
state-of-art techniques, including spatial invariant feature
transformation, wavelet transform, generic CNN …etc. In future
work, the proposed approach can be assessed with more
sophisticated GPUs such as NVidia …etc. to get more time
efficient process to yield an optimal BC-CAD solution.
REFERENCES
[1] World Health Organization, “Breast cancer: prevention and control,” Jan
2016.
[2] Australian Institute of Health and Welfare & Cancer, Australia, “Breast
cancer in Australia: an overview,” Cancer series, no. 71, Cat. No. CAN 67,
Canberra: AIHW, 2012.
[3] P. Boyle and B. Levin, Eds., World Cancer Report 2008. Lyon: IARC, 2008.
[Online]. Available:
http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/wcr_2008.pdf
[4] S. R. Lakhani, E. I.O., S. Schnitt, P. Tan, and M. van de Vijver, WHO
classification of tumours of the breast, 4th ed. Lyon: WHO Press, 2012.
[5] Tabar L., Dean P.B., “Mammography and breast cancer: the new era,” Int J
Gynaecol Obstet (PubMed: 14499978), 82, no. 3, pp. 319–326, 2003.
[6] Rankin, S.C., “MRI of the breast,” Br J Radiol, 73, pp. 806–818, 2000.
[7] P.L. Davis, M.J. Staiger, K.B. Harris , M.A. Gannot , J. Klementaviciene ,
K.S. MacCarty , Jr., H. Tobon. “Breast cancer measurements with magnetic
resonance imaging, ultra sonography, and mammography,” Breast Cancer
Res. Treatment, vol 37, pp. 1–9, 1996.
[8] S. Pramanik, D. Bhattacharjee and M. Nasipuri, "Wavelet based thermogram
analysis for breast cancer detection," 2015 International Symposium on
Advanced Computing and Communication (ISACC), Silchar, pp. 205-212,
2015.
[9] Y. Nasiri, M. Hariri and M. Afzali, "Breast cancer detection in mammograms
using Wavelet and contourlet transformations," 2015 2nd International
Conference on Knowledge-Based Engineering and Innovation (KBEI),
Tehran, pp. 923-926, 2015.
[10] B. Sanae, E. M. Samira, A. K. Mounir and F. Youssef, "Statistical block-
based DWT features for digital mammograms classification," 2014 9th
International Conference on Intelligent Systems: Theories and Applications
(SITA-14), Rabat, pp. 1-7, 2014.
[11] B. Yousefi, Hua-Nong Ting, S. M. Mirhassani and M. Hosseini,
"Development of computer-aided detection of breast lesion using gabor-
wavelet BASED features in mammographic images," 2013 IEEE
International Conference on Control System, Computing and Engineering,
Mindeb, pp. 127-131, 2013.
[12] A. U. Rehman, N. Chouhan and A. Khan, "Diverse and Discrimintative
Features Based Breast Cancer Detection Using Digital
Mammography," 2015 13th International Conference on Frontiers of
Information Technology (FIT), Islamabad, pp. 234-239, 2015.
[13] M. Naeemabadi, M. A. Saleh, M. Zabihi, G. Mohseni and N. A.
Chomachar, "Malignant tumor detection using linear support vector machine
in breast cancer based on new optimization algorithms," 2012 International
Symposium on Instrumentation & Measurement, Sensor Network and
Automation (IMSNA), Sanya, pp. 80-84, 2012.
[14] S. Caorsi and C. Lenzi, "Can an ANN based radar data processing approach
be an aid in breast cancer detection?," 2015 International Conference on
Electromagnetics in Advanced Applications (ICEAA), Turin, pp. 1464-
1467, 2015.
[15] J. Moll, J. B. Harley and V. Krozer, "Data-driven matched field processing
for radar-based microwave breast cancer detection," 2015 9th European
Conference on Antennas and Propagation (EuCAP), Lisbon, pp. 1-4, 2015.
[16] Y. M. George, H. H. Zayed, M. I. Roushdy and B. M. Elbagoury, "Remote
Computer-Aided Breast Cancer Detection and Diagnosis System Based on
Cytological Images," in IEEE Systems Journal, vol. 8, no. 3, pp. 949-964,
Sept. 2014.
[17] A. Tashk, M. S. Helfroush, H. Danyali and M. Akbarzadeh, "An automatic
mitosis detection method for breast cancer histopathology slide images
based on objective and pixel-wise textural features classification," The 5th
Conference on Information and Knowledge Technology, Shiraz, pp. 406-
410, 2013.
[18] A. Sanagavarapu Mohan, M. D. Hossain and M. J. Abedin, "Beamspace
based time reversal processing for breast cancer detection," Proceedings of
the 2012 IEEE International Symposium on Antennas and Propagation,
Chicago, IL, pp. 1-2, 2012.
[19] Y. l. Li, J. Feng, Y. Ren, Q. p. Wang and B. y. Chen, "Breast cancer
detection based on mixture membership function with MFSVM-FKNN
ensemble classifier," 2012 9th International Conference on Fuzzy Systems
and Knowledge Discovery, Sichuan, pp. 297-301, 2012.
[20] K. Ohri, H. Singh and A. Sharma, "Fuzzy expert system for diagnosis of
Breast Cancer," 2016 International Conference on Wireless
Communications, Signal Processing and Networking (WiSPNET), Chennai,
pp. 2487-2492, 2016.
[21] V. Gaike, R. Mhaske, S. Sonawane, N. Akhter and P. D. Deshmukh,
"Clustering of breast cancer tumor using third order GLCM feature," 2015
International Conference on Green Computing and Internet of Things
(ICGCIoT), Noida, pp. 318-322, 2015.
[22] M. A. S. Ali, G. I. Sayed, T. Gaber, A. E. Hassanien, V. Snasel and L. F.
Silva, "Detection of breast abnormalities of thermograms based on a new
segmentation method," 2015 Federated Conference on Computer Science
and Information Systems (FedCSIS), Lodz, pp. 255-261, 2015.
[23] T. Gaber et al., "Thermogram breast cancer prediction approach based on
Neutrosophic sets and fuzzy c-means algorithm," 2015 37th Annual
International Conference of the IEEE Engineering in Medicine and Biology
Society (EMBC), Milan, pp. 4254-4257, 2015.
[24] H. Ismahan, F. Amel and B. Abdelhafid, "Mass segmentation in
mammograms for computer-aided diagnosis of breast cancer," 2015 3rd
International Conference on Control, Engineering & Information
Technology (CEIT), Tlemcen, pp. 1-5, 2015.
[25] T. M. Mejía, M. G. Pérez, V. H. Andaluz and A. Conci, "Automatic
Segmentation and Analysis of Thermograms Using Texture Descriptors for
Breast Cancer Detection," 2015 Asia-Pacific Conference on Computer
Aided System Engineering, Quito, pp. 24-29, 2015.
[26] F. A. Maken and A. P. Bradley, "Multiple instance learning for breast MRI
based on generic spatio-temporal features," 2015 IEEE International
Conference on Acoustics, Speech and Signal Processing (ICASSP), South
Brisbane, QLD, pp. 902-906, 2015.
[27] R. Sánchez de la Rosa, M. Lamard, G. Cazuguel, G. Coatrieux, M. Cozic
and G. Quellec, "Multiple-instance learning for breast cancer detection in
mammograms," 2015 37th Annual International Conference of the IEEE
Engineering in Medicine and Biology Society (EMBC), Milan, pp. 7055-
7058, 2015.
[28] Hatipoglu N., and Bilgin G, "Classification of histopathological images
using convolutional neural network," 2014 Proc. 4th Int. Conf. on Image
Processing Theory, Tools and Applications (IPTA) (Paris, France) pp 1-6,
2014.
[29] Spanhol F A, Oliveira L, Petitjean C and Heutte L 2016 Proc. Int. Joint
Conf. on Neural Networks (IJCNN 2016) (Vancouver, Canada) pp 123-128
[30] Wang, D., Khosla, A., Gargeya, R., Irshad, H. & Beck, A. H. “Deep
Learning for Identifying Metastatic Breast
Cancer,”. https://arxiv.org/abs/1606.05718 ,pp. 1-6, 2016.
[31] Cruz-Roa A, Basavanhally A, Gonzalez F, Gilmore H, Feldman M,
Ganesan S, Shih N, Tomaszewski J and Madabhushi A, “Automatic
detection of invasive ductal carcinoma in whole slide images with
convolutional neural networks” Proc. SPIE 9041, Medical Imaging 2014:
Digital Pathology, 904103, pp. 1-15, 20 March 2014.
14
AlexNet-FC7. The overall enhanced functional components as a
cumulative BC-CAD solution has exhibited better than other
state-of-art techniques, including spatial invariant feature
transformation, wavelet transform, generic CNN …etc. In future
work, the proposed approach can be assessed with more
sophisticated GPUs such as NVidia …etc. to get more time
efficient process to yield an optimal BC-CAD solution.
REFERENCES
[1] World Health Organization, “Breast cancer: prevention and control,” Jan
2016.
[2] Australian Institute of Health and Welfare & Cancer, Australia, “Breast
cancer in Australia: an overview,” Cancer series, no. 71, Cat. No. CAN 67,
Canberra: AIHW, 2012.
[3] P. Boyle and B. Levin, Eds., World Cancer Report 2008. Lyon: IARC, 2008.
[Online]. Available:
http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/wcr_2008.pdf
[4] S. R. Lakhani, E. I.O., S. Schnitt, P. Tan, and M. van de Vijver, WHO
classification of tumours of the breast, 4th ed. Lyon: WHO Press, 2012.
[5] Tabar L., Dean P.B., “Mammography and breast cancer: the new era,” Int J
Gynaecol Obstet (PubMed: 14499978), 82, no. 3, pp. 319–326, 2003.
[6] Rankin, S.C., “MRI of the breast,” Br J Radiol, 73, pp. 806–818, 2000.
[7] P.L. Davis, M.J. Staiger, K.B. Harris , M.A. Gannot , J. Klementaviciene ,
K.S. MacCarty , Jr., H. Tobon. “Breast cancer measurements with magnetic
resonance imaging, ultra sonography, and mammography,” Breast Cancer
Res. Treatment, vol 37, pp. 1–9, 1996.
[8] S. Pramanik, D. Bhattacharjee and M. Nasipuri, "Wavelet based thermogram
analysis for breast cancer detection," 2015 International Symposium on
Advanced Computing and Communication (ISACC), Silchar, pp. 205-212,
2015.
[9] Y. Nasiri, M. Hariri and M. Afzali, "Breast cancer detection in mammograms
using Wavelet and contourlet transformations," 2015 2nd International
Conference on Knowledge-Based Engineering and Innovation (KBEI),
Tehran, pp. 923-926, 2015.
[10] B. Sanae, E. M. Samira, A. K. Mounir and F. Youssef, "Statistical block-
based DWT features for digital mammograms classification," 2014 9th
International Conference on Intelligent Systems: Theories and Applications
(SITA-14), Rabat, pp. 1-7, 2014.
[11] B. Yousefi, Hua-Nong Ting, S. M. Mirhassani and M. Hosseini,
"Development of computer-aided detection of breast lesion using gabor-
wavelet BASED features in mammographic images," 2013 IEEE
International Conference on Control System, Computing and Engineering,
Mindeb, pp. 127-131, 2013.
[12] A. U. Rehman, N. Chouhan and A. Khan, "Diverse and Discrimintative
Features Based Breast Cancer Detection Using Digital
Mammography," 2015 13th International Conference on Frontiers of
Information Technology (FIT), Islamabad, pp. 234-239, 2015.
[13] M. Naeemabadi, M. A. Saleh, M. Zabihi, G. Mohseni and N. A.
Chomachar, "Malignant tumor detection using linear support vector machine
in breast cancer based on new optimization algorithms," 2012 International
Symposium on Instrumentation & Measurement, Sensor Network and
Automation (IMSNA), Sanya, pp. 80-84, 2012.
[14] S. Caorsi and C. Lenzi, "Can an ANN based radar data processing approach
be an aid in breast cancer detection?," 2015 International Conference on
Electromagnetics in Advanced Applications (ICEAA), Turin, pp. 1464-
1467, 2015.
[15] J. Moll, J. B. Harley and V. Krozer, "Data-driven matched field processing
for radar-based microwave breast cancer detection," 2015 9th European
Conference on Antennas and Propagation (EuCAP), Lisbon, pp. 1-4, 2015.
[16] Y. M. George, H. H. Zayed, M. I. Roushdy and B. M. Elbagoury, "Remote
Computer-Aided Breast Cancer Detection and Diagnosis System Based on
Cytological Images," in IEEE Systems Journal, vol. 8, no. 3, pp. 949-964,
Sept. 2014.
[17] A. Tashk, M. S. Helfroush, H. Danyali and M. Akbarzadeh, "An automatic
mitosis detection method for breast cancer histopathology slide images
based on objective and pixel-wise textural features classification," The 5th
Conference on Information and Knowledge Technology, Shiraz, pp. 406-
410, 2013.
[18] A. Sanagavarapu Mohan, M. D. Hossain and M. J. Abedin, "Beamspace
based time reversal processing for breast cancer detection," Proceedings of
the 2012 IEEE International Symposium on Antennas and Propagation,
Chicago, IL, pp. 1-2, 2012.
[19] Y. l. Li, J. Feng, Y. Ren, Q. p. Wang and B. y. Chen, "Breast cancer
detection based on mixture membership function with MFSVM-FKNN
ensemble classifier," 2012 9th International Conference on Fuzzy Systems
and Knowledge Discovery, Sichuan, pp. 297-301, 2012.
[20] K. Ohri, H. Singh and A. Sharma, "Fuzzy expert system for diagnosis of
Breast Cancer," 2016 International Conference on Wireless
Communications, Signal Processing and Networking (WiSPNET), Chennai,
pp. 2487-2492, 2016.
[21] V. Gaike, R. Mhaske, S. Sonawane, N. Akhter and P. D. Deshmukh,
"Clustering of breast cancer tumor using third order GLCM feature," 2015
International Conference on Green Computing and Internet of Things
(ICGCIoT), Noida, pp. 318-322, 2015.
[22] M. A. S. Ali, G. I. Sayed, T. Gaber, A. E. Hassanien, V. Snasel and L. F.
Silva, "Detection of breast abnormalities of thermograms based on a new
segmentation method," 2015 Federated Conference on Computer Science
and Information Systems (FedCSIS), Lodz, pp. 255-261, 2015.
[23] T. Gaber et al., "Thermogram breast cancer prediction approach based on
Neutrosophic sets and fuzzy c-means algorithm," 2015 37th Annual
International Conference of the IEEE Engineering in Medicine and Biology
Society (EMBC), Milan, pp. 4254-4257, 2015.
[24] H. Ismahan, F. Amel and B. Abdelhafid, "Mass segmentation in
mammograms for computer-aided diagnosis of breast cancer," 2015 3rd
International Conference on Control, Engineering & Information
Technology (CEIT), Tlemcen, pp. 1-5, 2015.
[25] T. M. Mejía, M. G. Pérez, V. H. Andaluz and A. Conci, "Automatic
Segmentation and Analysis of Thermograms Using Texture Descriptors for
Breast Cancer Detection," 2015 Asia-Pacific Conference on Computer
Aided System Engineering, Quito, pp. 24-29, 2015.
[26] F. A. Maken and A. P. Bradley, "Multiple instance learning for breast MRI
based on generic spatio-temporal features," 2015 IEEE International
Conference on Acoustics, Speech and Signal Processing (ICASSP), South
Brisbane, QLD, pp. 902-906, 2015.
[27] R. Sánchez de la Rosa, M. Lamard, G. Cazuguel, G. Coatrieux, M. Cozic
and G. Quellec, "Multiple-instance learning for breast cancer detection in
mammograms," 2015 37th Annual International Conference of the IEEE
Engineering in Medicine and Biology Society (EMBC), Milan, pp. 7055-
7058, 2015.
[28] Hatipoglu N., and Bilgin G, "Classification of histopathological images
using convolutional neural network," 2014 Proc. 4th Int. Conf. on Image
Processing Theory, Tools and Applications (IPTA) (Paris, France) pp 1-6,
2014.
[29] Spanhol F A, Oliveira L, Petitjean C and Heutte L 2016 Proc. Int. Joint
Conf. on Neural Networks (IJCNN 2016) (Vancouver, Canada) pp 123-128
[30] Wang, D., Khosla, A., Gargeya, R., Irshad, H. & Beck, A. H. “Deep
Learning for Identifying Metastatic Breast
Cancer,”. https://arxiv.org/abs/1606.05718 ,pp. 1-6, 2016.
[31] Cruz-Roa A, Basavanhally A, Gonzalez F, Gilmore H, Feldman M,
Ganesan S, Shih N, Tomaszewski J and Madabhushi A, “Automatic
detection of invasive ductal carcinoma in whole slide images with
convolutional neural networks” Proc. SPIE 9041, Medical Imaging 2014:
Digital Pathology, 904103, pp. 1-15, 20 March 2014.
14

[32] M. G. Ertosun and D. L. Rubin, “Probabilistic visual search for masses
within mammography images using deep learning,” in Bioinformatics and
Biomedicine (BIBM), 2015 IEEE International Conference on, pp. 1310–
1315, Nov 2015.
[33] J. Arevalo, F. A. Gonz´alez, R. Ramos-Poll´an, J. L. Oliveira, and M. A. G.
Lopez, “Convolutional neural networks for mammography mass lesion
classification,” in 2015 37th Annual International Conference of the IEEE
Engineering in Medicine and Biology Society (EMBC), pp. 797–800, Aug
2015.
[34] Z. Jiao, X. Gao, Y. Wang, and J. Li, “A deep feature based framework for
breast masses classification,” Neurocomputing, Vol. 197, pp. 221 – 231,
2016.
[35] O. Russakovsky, J. Deng, H. Su, J. Krause, S. Satheesh, S. Ma, Z. Huang,
A. Karpathy, A. Khosla, M. Bernstein, A. C. Berg, and L. Fei-Fei,
“ImageNet Large Scale Visual Recognition Challenge,” International
Journal of Computer Vision (IJCV), vol. 115, no. 3, pp. 211–252, 2015.
[36] A. M. Abdel-Zaher and A. M. Eldeib, “Breast cancer classification using
deep belief networks,” Expert Systems with Applications, vol. 46, pp. 139 –
144, 2016.
[37] E. Olfati, H. Zarabadipour and M. A. Shoorehdeli, "Feature subset selection
and parameters optimization for support vector machine in breast cancer
diagnosis," 2014 Iranian Conference on Intelligent Systems (ICIS), Bam, pp.
1-6, 2014.
[38] R. Nateghi, H. Danyali, M. SadeghHelfroush and F. P. Pour, "Automatic
detection of mitosis cell in breast cancer histopathology images using
genetic algorithm," 2014 21th Iranian Conference on Biomedical
Engineering (ICBME), Tehran, pp. 1-6, 2014.
[39] http://web.inf.ufpr.br/vri/breast-cancer-database
[40] K. Zuiderveld, “Contrast Limited Adaptive Histogram Equalization”,
Graphics gems, Vol IV, pp. 474-485, 1994.
[41] Z. Wang, G. Yu, Y. Kang, Y. Zhao, and Q. Qu, “Breast tumor detection in
digital mammography based on extreme learning machine,”
Neurocomputing, vol. 128, pp. 175 – 184, 2014.
[42] Wentao Zhu, Qi Lou, Yeeleng Scott Vang, and Xiaohui Xie “Deep Multi-
instance Networks with Sparse Label Assignment for Whole Mammogram
Classification” Deep MIL with Sparse Label Assignment for Whole Mamm.
Class.,pp 1-12, 18 Dec 2016.
[43] C. Stauffer and W. E. L. Grimson, “Adaptive background mixture models
for real-time tracking,” in Proc. of IEEE Conference on Computer Vision
and Pattern Recognition, vol. 2, pp. 246–252, 1999.
[44] D. S. Lee, “Effective gaussian mixture learning for video background
subtraction,” IEEE Trans. on Pattern Analysis and Machine Intelligence,
vol. 27, no. 5, pp. 827–832, 2005.
[45] A. Krizhevsky, I. Sutskever, and G. E. Hinton, “Imagenet classification
with deep convolutional neural networks,” in Advances in neural
information processing systems, pp. 1097–1105, 2012.
[46] J. Yosinski, J. Clune, Y. Bengio, and H. Lipson, “How transferable are
features in deep neural networks?” in Advances in Neural Information
Processing Systems, pp. 3320–3328, 2014.
[47] Y. Jia, et al., “Caffe: Convolutional architecture for fast feature
embedding,” in Proceedings of the ACM International Conference on
Multimedia. ACM, pp. 675–678, 2014.
[48] A. Krizhevsky, I. Sutskever, and G. E. Hinton, “Imagenet classification
with deep convolutional neural networks,” in Proceedings of 26th Annual
Conference on Neural Information Processing Systems 2012 (NIPS), P. L.
Bartlett, F. C. N. Pereira, C. J. C. Burges, L. Bottou, and K. Q. Weinberger,
Eds., pp. 1106–1114, Dec. 2012.
[49] Fabio A. Spanhol, Luiz S. Oliveira, Caroline Petitjean, and Laurent Heutte
“Breast Cancer Histopathological Image Classification using Convolutional
Neural Networks” 2016 International Joint Conference on Neural Networks
(IJCNN), Vancouver, BC, pp. 2560-2567, 2016.
[50] Neslihan Bayramoglu and Janne Heikkil “Transfer Learning for Cell Nuclei
Classi_cation in Histopathology Images” The 14th European Conference on
Computer Vision, pp.1-8, 2016.
[51] Henry Zhou ,Yuki Zaninovich, “Mammogram Classification Using
Convolutional Neural Networks”, pp. 1-8.
http://chrisgregory.me/projects/papers/cnn-mammogram.pdf
[52] Dan C. Cires¸an, Alessandro Giusti, Luca M. Gambardella, J¨urgen
Schmidhuber “Mitosis Detection in Breast Cancer Histology Images with
Deep Neural Networks” Medical Image Computing and Computer-Assisted
Intervention – MICCAI 2013, pp 411-418, 2013.
[53] Micha l Z_ ejmo, Marek Kowal, Jo_zef Korbicz and Roman Monczak
“Classiffication of breast cancer cytological specimen using convolutional
neural network” IOP Conf. Series: Journal of Physics: Conf. Series 783, pp.
1-11, 2017.
[54] Wentao Zhu, Qi Lou, Yeeleng Scott Vang, and Xiaohui Xie “Deep Multi-
instance Networks with Sparse Label Assignment for Whole Mammogram
Classification” Deep MIL with Sparse Label Assignment for Whole Mamm
Class, pp. 1-12, 2016.
[55] Baris Gecer, Ozge Yalcinkaya, Onur Tasar and Selim Aksoy “Evaluation of
Joint Multi-Instance Multi-Label Learning For Breast Cancer Diagnosis”,
Computer Vision and Pattern Recognition, pp. 1-4, 2015.
[56] Daniel Lévy, Arzav Jain “Breast Mass Classification from Mammograms
using Deep Convolutional Neural Networks” 30th Conference on Neural
Information Processing Systems (NIPS 2016), Barcelona, Spain, pp. 1-6,
2016.
[57] Rekha Chakravarthi, NM Nandhitha, S Emalda Roslin, N Selvarasu,
“Tumour extraction from breast mammographs through hough transform
and DNN hybrid segmentation technique” Biomedical Research 2016; 27
(4): pp. 1188-1193, 2016.
[58] Vibhu Agarwal, Clayton Carson, “Using Deep Convolutional Neural
Networks to Predict Semantic Feature of Lesions in Mammograms”,
semanticscholar, pp. 1-6, 2015.
[59] Neeraj Dhungel, Gustavo Carneiro, Andrew P. Bradley, “The Automated
Learning of Deep Features for Breast Mass Classification from
Mammograms” Medical Image Computing and Computer-Assisted
Intervention – MICCAI 2016, pp.106-114, 2016.
[60] S. J. Nass, I. C. Henderson, J. C. Lashof, “Mammography and Beyond:
Developing Technologies for the Early Detection of Breast Cancer,”
Institute of Medicine (US) and National Research Council (US) Committee
on Technologies for the Early Detection of Breast Cancer, pp. 1-267, 2001.
[61] Alex Krizhevsky, Ilya Sutskever, Geoffrey E. Hinton, “ImageNet
Classification with Deep Convolutional Neural Networks,”, pp. 1-9, 2012.
Appendix
NOMENCLATURES
Nomenclature
CAD Computer-Aided Diagnosis
BC Breast Cancer
DNN Deep Neural Network
CNN Convolutional Neural Network
GMM Gaussian Mixture Model
PCA Principle Component Analysis
LDA Linear Discriminant Analysis
IARC International Agency for Research on Cancer
WHO World Health Organization
CAD Computer Aided Diagnosis
MRI Magnetic Resonance Imagining
ROI Region of Interest
SVM Support Vector Machine
SIFT Spatial Invariant Feature Transform
15
within mammography images using deep learning,” in Bioinformatics and
Biomedicine (BIBM), 2015 IEEE International Conference on, pp. 1310–
1315, Nov 2015.
[33] J. Arevalo, F. A. Gonz´alez, R. Ramos-Poll´an, J. L. Oliveira, and M. A. G.
Lopez, “Convolutional neural networks for mammography mass lesion
classification,” in 2015 37th Annual International Conference of the IEEE
Engineering in Medicine and Biology Society (EMBC), pp. 797–800, Aug
2015.
[34] Z. Jiao, X. Gao, Y. Wang, and J. Li, “A deep feature based framework for
breast masses classification,” Neurocomputing, Vol. 197, pp. 221 – 231,
2016.
[35] O. Russakovsky, J. Deng, H. Su, J. Krause, S. Satheesh, S. Ma, Z. Huang,
A. Karpathy, A. Khosla, M. Bernstein, A. C. Berg, and L. Fei-Fei,
“ImageNet Large Scale Visual Recognition Challenge,” International
Journal of Computer Vision (IJCV), vol. 115, no. 3, pp. 211–252, 2015.
[36] A. M. Abdel-Zaher and A. M. Eldeib, “Breast cancer classification using
deep belief networks,” Expert Systems with Applications, vol. 46, pp. 139 –
144, 2016.
[37] E. Olfati, H. Zarabadipour and M. A. Shoorehdeli, "Feature subset selection
and parameters optimization for support vector machine in breast cancer
diagnosis," 2014 Iranian Conference on Intelligent Systems (ICIS), Bam, pp.
1-6, 2014.
[38] R. Nateghi, H. Danyali, M. SadeghHelfroush and F. P. Pour, "Automatic
detection of mitosis cell in breast cancer histopathology images using
genetic algorithm," 2014 21th Iranian Conference on Biomedical
Engineering (ICBME), Tehran, pp. 1-6, 2014.
[39] http://web.inf.ufpr.br/vri/breast-cancer-database
[40] K. Zuiderveld, “Contrast Limited Adaptive Histogram Equalization”,
Graphics gems, Vol IV, pp. 474-485, 1994.
[41] Z. Wang, G. Yu, Y. Kang, Y. Zhao, and Q. Qu, “Breast tumor detection in
digital mammography based on extreme learning machine,”
Neurocomputing, vol. 128, pp. 175 – 184, 2014.
[42] Wentao Zhu, Qi Lou, Yeeleng Scott Vang, and Xiaohui Xie “Deep Multi-
instance Networks with Sparse Label Assignment for Whole Mammogram
Classification” Deep MIL with Sparse Label Assignment for Whole Mamm.
Class.,pp 1-12, 18 Dec 2016.
[43] C. Stauffer and W. E. L. Grimson, “Adaptive background mixture models
for real-time tracking,” in Proc. of IEEE Conference on Computer Vision
and Pattern Recognition, vol. 2, pp. 246–252, 1999.
[44] D. S. Lee, “Effective gaussian mixture learning for video background
subtraction,” IEEE Trans. on Pattern Analysis and Machine Intelligence,
vol. 27, no. 5, pp. 827–832, 2005.
[45] A. Krizhevsky, I. Sutskever, and G. E. Hinton, “Imagenet classification
with deep convolutional neural networks,” in Advances in neural
information processing systems, pp. 1097–1105, 2012.
[46] J. Yosinski, J. Clune, Y. Bengio, and H. Lipson, “How transferable are
features in deep neural networks?” in Advances in Neural Information
Processing Systems, pp. 3320–3328, 2014.
[47] Y. Jia, et al., “Caffe: Convolutional architecture for fast feature
embedding,” in Proceedings of the ACM International Conference on
Multimedia. ACM, pp. 675–678, 2014.
[48] A. Krizhevsky, I. Sutskever, and G. E. Hinton, “Imagenet classification
with deep convolutional neural networks,” in Proceedings of 26th Annual
Conference on Neural Information Processing Systems 2012 (NIPS), P. L.
Bartlett, F. C. N. Pereira, C. J. C. Burges, L. Bottou, and K. Q. Weinberger,
Eds., pp. 1106–1114, Dec. 2012.
[49] Fabio A. Spanhol, Luiz S. Oliveira, Caroline Petitjean, and Laurent Heutte
“Breast Cancer Histopathological Image Classification using Convolutional
Neural Networks” 2016 International Joint Conference on Neural Networks
(IJCNN), Vancouver, BC, pp. 2560-2567, 2016.
[50] Neslihan Bayramoglu and Janne Heikkil “Transfer Learning for Cell Nuclei
Classi_cation in Histopathology Images” The 14th European Conference on
Computer Vision, pp.1-8, 2016.
[51] Henry Zhou ,Yuki Zaninovich, “Mammogram Classification Using
Convolutional Neural Networks”, pp. 1-8.
http://chrisgregory.me/projects/papers/cnn-mammogram.pdf
[52] Dan C. Cires¸an, Alessandro Giusti, Luca M. Gambardella, J¨urgen
Schmidhuber “Mitosis Detection in Breast Cancer Histology Images with
Deep Neural Networks” Medical Image Computing and Computer-Assisted
Intervention – MICCAI 2013, pp 411-418, 2013.
[53] Micha l Z_ ejmo, Marek Kowal, Jo_zef Korbicz and Roman Monczak
“Classiffication of breast cancer cytological specimen using convolutional
neural network” IOP Conf. Series: Journal of Physics: Conf. Series 783, pp.
1-11, 2017.
[54] Wentao Zhu, Qi Lou, Yeeleng Scott Vang, and Xiaohui Xie “Deep Multi-
instance Networks with Sparse Label Assignment for Whole Mammogram
Classification” Deep MIL with Sparse Label Assignment for Whole Mamm
Class, pp. 1-12, 2016.
[55] Baris Gecer, Ozge Yalcinkaya, Onur Tasar and Selim Aksoy “Evaluation of
Joint Multi-Instance Multi-Label Learning For Breast Cancer Diagnosis”,
Computer Vision and Pattern Recognition, pp. 1-4, 2015.
[56] Daniel Lévy, Arzav Jain “Breast Mass Classification from Mammograms
using Deep Convolutional Neural Networks” 30th Conference on Neural
Information Processing Systems (NIPS 2016), Barcelona, Spain, pp. 1-6,
2016.
[57] Rekha Chakravarthi, NM Nandhitha, S Emalda Roslin, N Selvarasu,
“Tumour extraction from breast mammographs through hough transform
and DNN hybrid segmentation technique” Biomedical Research 2016; 27
(4): pp. 1188-1193, 2016.
[58] Vibhu Agarwal, Clayton Carson, “Using Deep Convolutional Neural
Networks to Predict Semantic Feature of Lesions in Mammograms”,
semanticscholar, pp. 1-6, 2015.
[59] Neeraj Dhungel, Gustavo Carneiro, Andrew P. Bradley, “The Automated
Learning of Deep Features for Breast Mass Classification from
Mammograms” Medical Image Computing and Computer-Assisted
Intervention – MICCAI 2016, pp.106-114, 2016.
[60] S. J. Nass, I. C. Henderson, J. C. Lashof, “Mammography and Beyond:
Developing Technologies for the Early Detection of Breast Cancer,”
Institute of Medicine (US) and National Research Council (US) Committee
on Technologies for the Early Detection of Breast Cancer, pp. 1-267, 2001.
[61] Alex Krizhevsky, Ilya Sutskever, Geoffrey E. Hinton, “ImageNet
Classification with Deep Convolutional Neural Networks,”, pp. 1-9, 2012.
Appendix
NOMENCLATURES
Nomenclature
CAD Computer-Aided Diagnosis
BC Breast Cancer
DNN Deep Neural Network
CNN Convolutional Neural Network
GMM Gaussian Mixture Model
PCA Principle Component Analysis
LDA Linear Discriminant Analysis
IARC International Agency for Research on Cancer
WHO World Health Organization
CAD Computer Aided Diagnosis
MRI Magnetic Resonance Imagining
ROI Region of Interest
SVM Support Vector Machine
SIFT Spatial Invariant Feature Transform
15

IFI Initial Feature Point Image
ANN Artificial Neural Network
PNN Probabilistic Neural Network
CLBP Completed Local Binary Pattern
NS Neutrosophic Sets
F-FCM Fast Fuzzy C-Mean
MIL Multiple Instance Learning
ROC Receiver Operating Characteristics
DBN Deep Belief Network
GA Genetic Algorithm
CCA Connected Component Analysis
GPU Graphical Processing Unit
PDF Probability Density Function
CONV Convlutional (Layer)
FC Fully Connected
MEF Most Expressive Features
MDF Most Discriminating Features
RBF Radial Basis Function
Weight Factor
Value of a Pixel at an Instant
Gaussian Mixture
Rate of Learning
Threshold
Lowest Standard Deviation
Fixed Sized Learning Rate
Average Density
Adaptive Learning Rate
Feature Vector
Covariance Matrix
Intra-Class Scatter Matrix
Inter-Class Scatter Matrix
Total Number of Classes
Mean of the Average Vector of a Class
Total Number of Samples in Class
Eigenvectors of
Increasing a Factor
Output of the Neuron
Level of Error Tolerance
Training Vector
16
ANN Artificial Neural Network
PNN Probabilistic Neural Network
CLBP Completed Local Binary Pattern
NS Neutrosophic Sets
F-FCM Fast Fuzzy C-Mean
MIL Multiple Instance Learning
ROC Receiver Operating Characteristics
DBN Deep Belief Network
GA Genetic Algorithm
CCA Connected Component Analysis
GPU Graphical Processing Unit
PDF Probability Density Function
CONV Convlutional (Layer)
FC Fully Connected
MEF Most Expressive Features
MDF Most Discriminating Features
RBF Radial Basis Function
Weight Factor
Value of a Pixel at an Instant
Gaussian Mixture
Rate of Learning
Threshold
Lowest Standard Deviation
Fixed Sized Learning Rate
Average Density
Adaptive Learning Rate
Feature Vector
Covariance Matrix
Intra-Class Scatter Matrix
Inter-Class Scatter Matrix
Total Number of Classes
Mean of the Average Vector of a Class
Total Number of Samples in Class
Eigenvectors of
Increasing a Factor
Output of the Neuron
Level of Error Tolerance
Training Vector
16
1 out of 16
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
