Energetics, Kinetics and Redox: Rate of Reaction Report
VerifiedAdded on 2023/01/18
|11
|2167
|28
Report
AI Summary
This report investigates the rate of reaction between sodium thiosulphate and hydrochloric acid, focusing on the impact of reactant concentration. The experiment involves measuring the time it takes for sulfur to form, making a cross disappear, and analyzing the reaction's order through graphical analysis. The report discusses the background theory, methodology, and results, including the reaction equation and the effect of concentration on reaction rate. The analysis covers the order of the reaction, the impact of catalysts on reaction rates, and the application of Le Chatelier's principle to optimize reaction conditions, along with the rate equation and the rate constant determination for various reactions. The study also covers the effects of temperature and pressure on reaction yield, and the role of catalysts in increasing reaction rates, along with the equilibrium constant and its application.

Access to HE Diploma Unit Title: Energetics, Kinetics and Redox Assignment
Student name
Course name
Professor name
University name
Sate name
Date
Student name
Course name
Professor name
University name
Sate name
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Question 1
Report on Rate of Reaction of Sodium Thiosulphate and Hydrochloric Acid.
Introduction
This demonstration was aimed at investigating the effects of sodium thiosulphate
concentration on a reaction involving 2M hydrochloric acid and sodium thiosulphate. The
products of this reaction include sulfur and being opaque, the reaction time can be predicted by
measuring the time it takes the produced Sulphur to make a cross made on a graph paper
disappear. The obtained results will be graphically analyzed with an objective of evaluating the
reaction’s order. The order of the reaction obtained provides a mathematical relationship
between the reactant’s concentration and reaction rate.
Background theory
Reactant concentration plays a major role in reaction rate. Increasing reactant’s
concentration consequently raises the number of reacting molecules (Leffler & Grunwald, 2013).
This has an effect of increasing the number collisions which increases the rate of reaction.
Reaction between hydrochloric acid and sodium thiosulphate
The impact of concentration on reaction rate is easily studied by examining a reaction
between sodium thiosulfate and HCl. The two reagents react to produce SO2, S and water. SO2
produced is soluble in water. Sulfur on the other hand is insoluble. It forms a precipitate made of
pale yellow or white colloid which forms an opaque vision (Covington, 2012). This property
makes it possible to study reaction rates by considering how long it takes to form amount of
sulfur that block the possibility of seeing the cross.
Aim
This experiment is aimed at finding the rate equation for a reaction between sodium thiosulphate
and hydrochloric acid.
Methodology
Requirements
Report on Rate of Reaction of Sodium Thiosulphate and Hydrochloric Acid.
Introduction
This demonstration was aimed at investigating the effects of sodium thiosulphate
concentration on a reaction involving 2M hydrochloric acid and sodium thiosulphate. The
products of this reaction include sulfur and being opaque, the reaction time can be predicted by
measuring the time it takes the produced Sulphur to make a cross made on a graph paper
disappear. The obtained results will be graphically analyzed with an objective of evaluating the
reaction’s order. The order of the reaction obtained provides a mathematical relationship
between the reactant’s concentration and reaction rate.
Background theory
Reactant concentration plays a major role in reaction rate. Increasing reactant’s
concentration consequently raises the number of reacting molecules (Leffler & Grunwald, 2013).
This has an effect of increasing the number collisions which increases the rate of reaction.
Reaction between hydrochloric acid and sodium thiosulphate
The impact of concentration on reaction rate is easily studied by examining a reaction
between sodium thiosulfate and HCl. The two reagents react to produce SO2, S and water. SO2
produced is soluble in water. Sulfur on the other hand is insoluble. It forms a precipitate made of
pale yellow or white colloid which forms an opaque vision (Covington, 2012). This property
makes it possible to study reaction rates by considering how long it takes to form amount of
sulfur that block the possibility of seeing the cross.
Aim
This experiment is aimed at finding the rate equation for a reaction between sodium thiosulphate
and hydrochloric acid.
Methodology
Requirements
0.4 mol/dm3 sodium thiosulphate Na2SO3 (aq)
2.0 mol/dm3 hydrochloric acid HCl(aq)
De-ionized water
Conical flask
Graduated pipette
Stop clock
Graph paper
Procedure
A cross was marked on a graph paper with a waterproof pen. A 250cm3 conical flask was
erected over the cross. Using a labelled burette, thiosulphate and de-ionized water was added to
the flask using quantities in the table below. The acid was then added to the flask using 10cm3
graduated pipette. Stop clock was started at the same time while the flask was given a swirl to
ensure mixing. The duration it takes the cross to disappear was recorded by looking down at the
cross. The flask was then cleansed and the experiment repeated as shown in the result section.
Results
Experiment 1 2 3 4 5 6 7 8 9
Na2S2O3(cm3) 50 40 30 20 10 40 40 40 40
concentration of
Na2S2O3(mol/dm3) 0.4 0.32 0.24 0.16 0.08 0.32 0.32 0.32 0.32
H2O(cm3) 0 10 20 30 40 12 14 16 18
time(s) 11 12 26.3 32 27 14 21 21 16
1/time(s-1) 0.090909 0.083333 0.038023 0.03125 0.037037 0.071429 0.047619 0.047619 0.0625
Discussion of the Results.
Sodium thiosulfate reacts with HCl to form sulfur IV oxide and sulfur as shown in equation (i)
shown below.
Na2S2O3(aq) + 2HCl(aq) → S(s) + SO2(g) + 2NaCl(aq)
The reaction kinetics in this case is easily analysed by drawing a graph of concentration of
Na2S2O3 against reaction time and 1/time.
2.0 mol/dm3 hydrochloric acid HCl(aq)
De-ionized water
Conical flask
Graduated pipette
Stop clock
Graph paper
Procedure
A cross was marked on a graph paper with a waterproof pen. A 250cm3 conical flask was
erected over the cross. Using a labelled burette, thiosulphate and de-ionized water was added to
the flask using quantities in the table below. The acid was then added to the flask using 10cm3
graduated pipette. Stop clock was started at the same time while the flask was given a swirl to
ensure mixing. The duration it takes the cross to disappear was recorded by looking down at the
cross. The flask was then cleansed and the experiment repeated as shown in the result section.
Results
Experiment 1 2 3 4 5 6 7 8 9
Na2S2O3(cm3) 50 40 30 20 10 40 40 40 40
concentration of
Na2S2O3(mol/dm3) 0.4 0.32 0.24 0.16 0.08 0.32 0.32 0.32 0.32
H2O(cm3) 0 10 20 30 40 12 14 16 18
time(s) 11 12 26.3 32 27 14 21 21 16
1/time(s-1) 0.090909 0.083333 0.038023 0.03125 0.037037 0.071429 0.047619 0.047619 0.0625
Discussion of the Results.
Sodium thiosulfate reacts with HCl to form sulfur IV oxide and sulfur as shown in equation (i)
shown below.
Na2S2O3(aq) + 2HCl(aq) → S(s) + SO2(g) + 2NaCl(aq)
The reaction kinetics in this case is easily analysed by drawing a graph of concentration of
Na2S2O3 against reaction time and 1/time.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
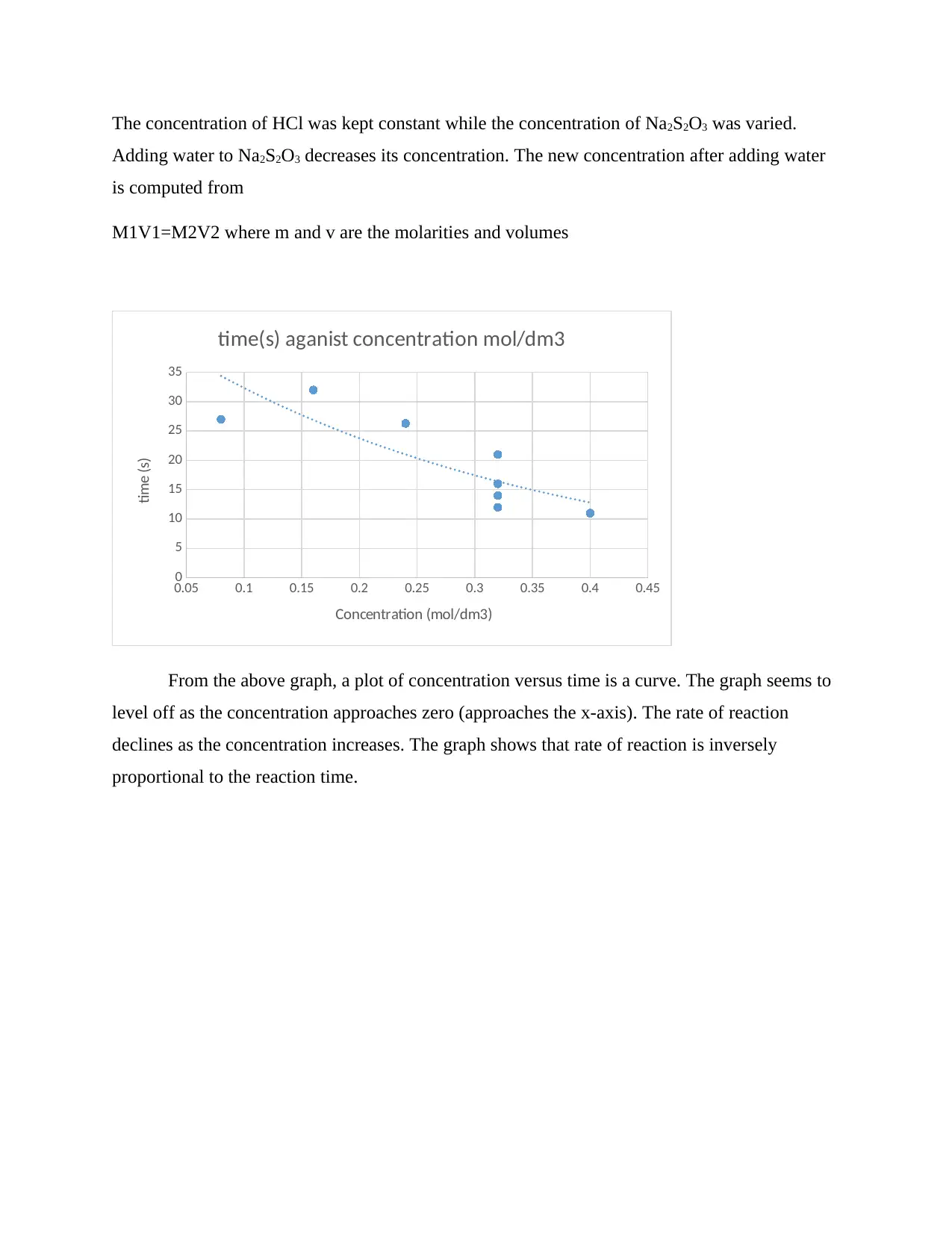
The concentration of HCl was kept constant while the concentration of Na2S2O3 was varied.
Adding water to Na2S2O3 decreases its concentration. The new concentration after adding water
is computed from
M1V1=M2V2 where m and v are the molarities and volumes
0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 0.45
0
5
10
15
20
25
30
35
time(s) aganist concentration mol/dm3
Concentration (mol/dm3)
time (s)
From the above graph, a plot of concentration versus time is a curve. The graph seems to
level off as the concentration approaches zero (approaches the x-axis). The rate of reaction
declines as the concentration increases. The graph shows that rate of reaction is inversely
proportional to the reaction time.
Adding water to Na2S2O3 decreases its concentration. The new concentration after adding water
is computed from
M1V1=M2V2 where m and v are the molarities and volumes
0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 0.45
0
5
10
15
20
25
30
35
time(s) aganist concentration mol/dm3
Concentration (mol/dm3)
time (s)
From the above graph, a plot of concentration versus time is a curve. The graph seems to
level off as the concentration approaches zero (approaches the x-axis). The rate of reaction
declines as the concentration increases. The graph shows that rate of reaction is inversely
proportional to the reaction time.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
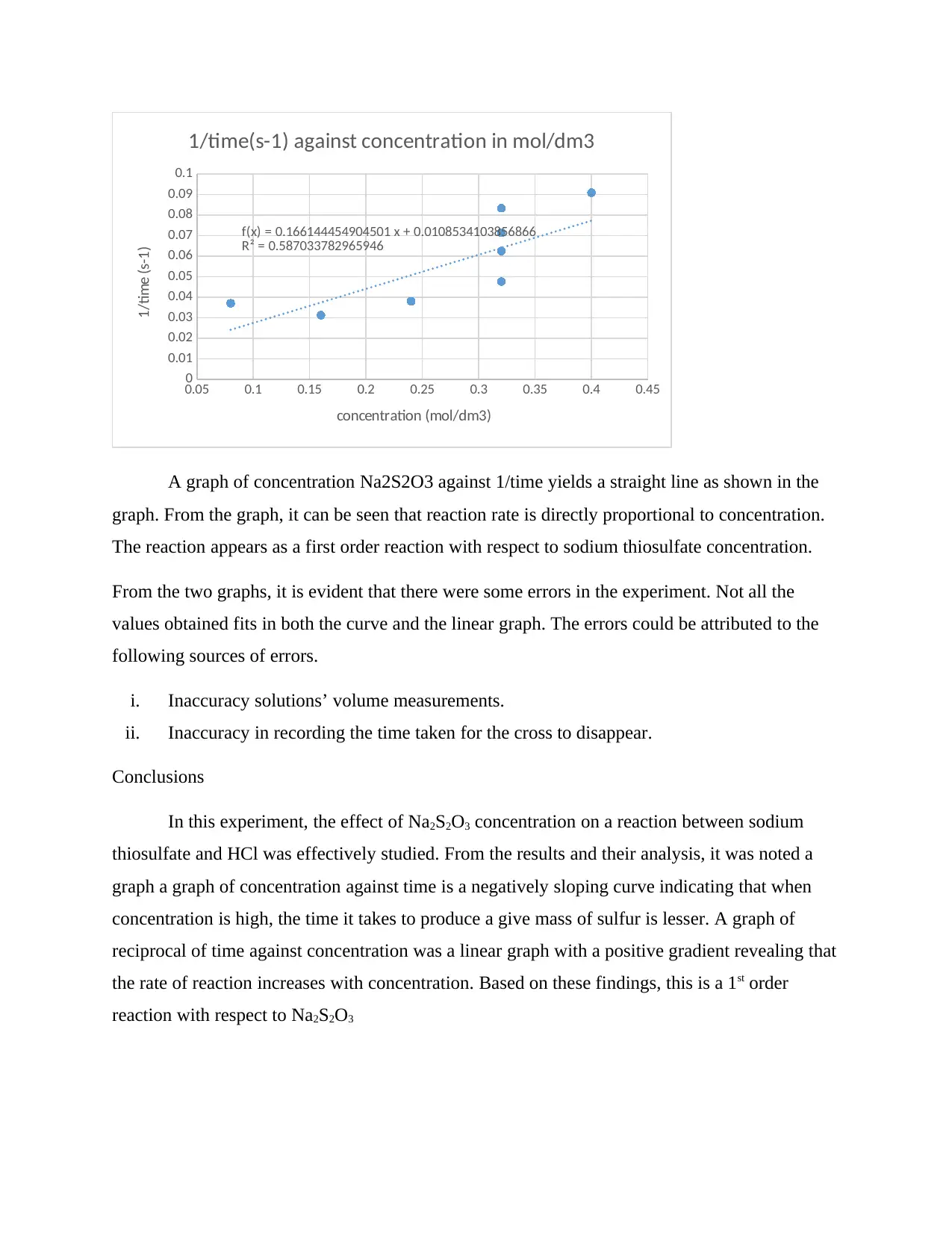
0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 0.45
0
0.01
0.02
0.03
0.04
0.05
0.06
0.07
0.08
0.09
0.1
f(x) = 0.166144454904501 x + 0.0108534103856866
R² = 0.587033782965946
1/time(s-1) against concentration in mol/dm3
concentration (mol/dm3)
1/time (s-1)
A graph of concentration Na2S2O3 against 1/time yields a straight line as shown in the
graph. From the graph, it can be seen that reaction rate is directly proportional to concentration.
The reaction appears as a first order reaction with respect to sodium thiosulfate concentration.
From the two graphs, it is evident that there were some errors in the experiment. Not all the
values obtained fits in both the curve and the linear graph. The errors could be attributed to the
following sources of errors.
i. Inaccuracy solutions’ volume measurements.
ii. Inaccuracy in recording the time taken for the cross to disappear.
Conclusions
In this experiment, the effect of Na2S2O3 concentration on a reaction between sodium
thiosulfate and HCl was effectively studied. From the results and their analysis, it was noted a
graph a graph of concentration against time is a negatively sloping curve indicating that when
concentration is high, the time it takes to produce a give mass of sulfur is lesser. A graph of
reciprocal of time against concentration was a linear graph with a positive gradient revealing that
the rate of reaction increases with concentration. Based on these findings, this is a 1st order
reaction with respect to Na2S2O3
0
0.01
0.02
0.03
0.04
0.05
0.06
0.07
0.08
0.09
0.1
f(x) = 0.166144454904501 x + 0.0108534103856866
R² = 0.587033782965946
1/time(s-1) against concentration in mol/dm3
concentration (mol/dm3)
1/time (s-1)
A graph of concentration Na2S2O3 against 1/time yields a straight line as shown in the
graph. From the graph, it can be seen that reaction rate is directly proportional to concentration.
The reaction appears as a first order reaction with respect to sodium thiosulfate concentration.
From the two graphs, it is evident that there were some errors in the experiment. Not all the
values obtained fits in both the curve and the linear graph. The errors could be attributed to the
following sources of errors.
i. Inaccuracy solutions’ volume measurements.
ii. Inaccuracy in recording the time taken for the cross to disappear.
Conclusions
In this experiment, the effect of Na2S2O3 concentration on a reaction between sodium
thiosulfate and HCl was effectively studied. From the results and their analysis, it was noted a
graph a graph of concentration against time is a negatively sloping curve indicating that when
concentration is high, the time it takes to produce a give mass of sulfur is lesser. A graph of
reciprocal of time against concentration was a linear graph with a positive gradient revealing that
the rate of reaction increases with concentration. Based on these findings, this is a 1st order
reaction with respect to Na2S2O3
Question 2
i.
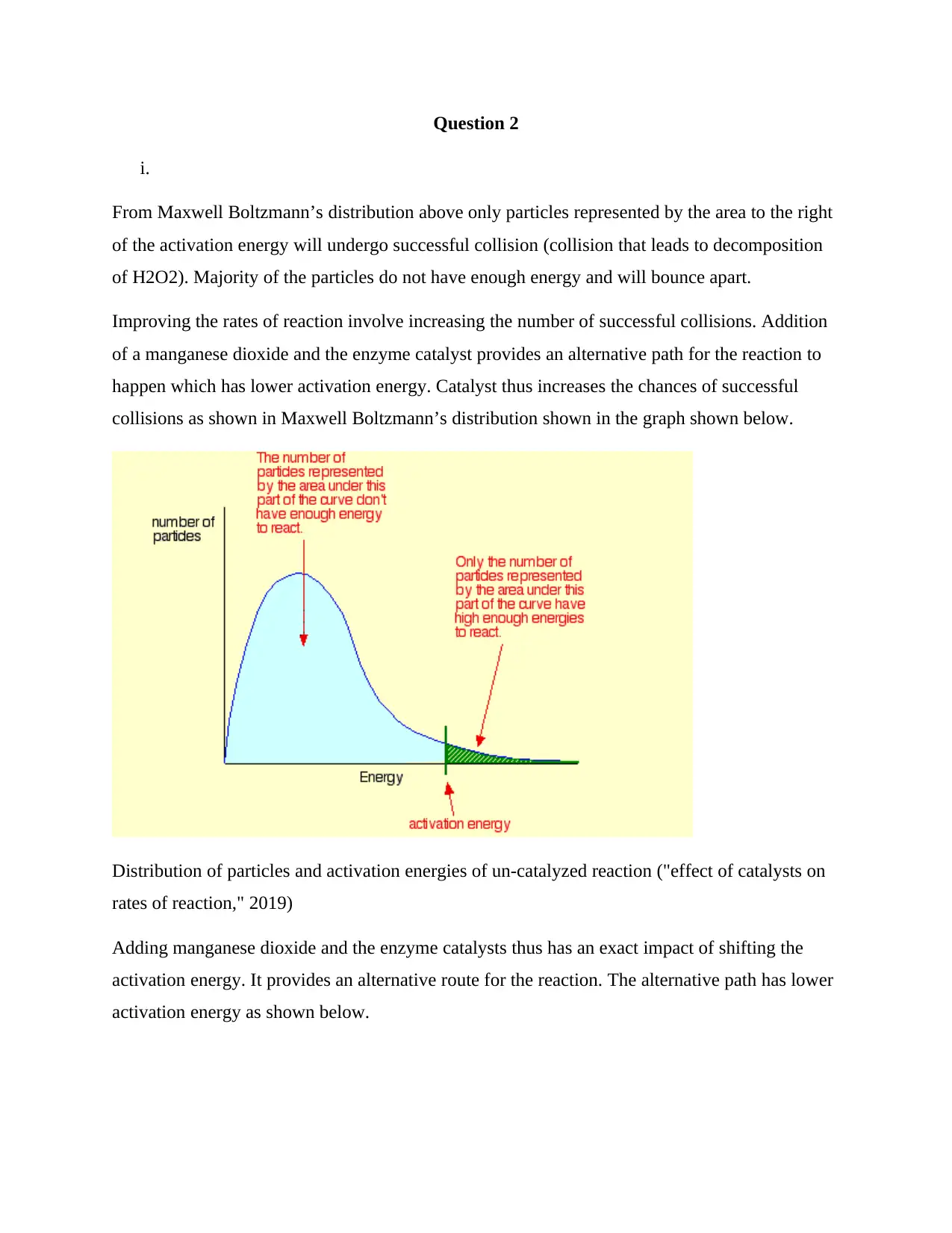
From Maxwell Boltzmann’s distribution above only particles represented by the area to the right
of the activation energy will undergo successful collision (collision that leads to decomposition
of H2O2). Majority of the particles do not have enough energy and will bounce apart.
Improving the rates of reaction involve increasing the number of successful collisions. Addition
of a manganese dioxide and the enzyme catalyst provides an alternative path for the reaction to
happen which has lower activation energy. Catalyst thus increases the chances of successful
collisions as shown in Maxwell Boltzmann’s distribution shown in the graph shown below.
Distribution of particles and activation energies of un-catalyzed reaction ("effect of catalysts on
rates of reaction," 2019)
Adding manganese dioxide and the enzyme catalysts thus has an exact impact of shifting the
activation energy. It provides an alternative route for the reaction. The alternative path has lower
activation energy as shown below.
i.
From Maxwell Boltzmann’s distribution above only particles represented by the area to the right
of the activation energy will undergo successful collision (collision that leads to decomposition
of H2O2). Majority of the particles do not have enough energy and will bounce apart.
Improving the rates of reaction involve increasing the number of successful collisions. Addition
of a manganese dioxide and the enzyme catalyst provides an alternative path for the reaction to
happen which has lower activation energy. Catalyst thus increases the chances of successful
collisions as shown in Maxwell Boltzmann’s distribution shown in the graph shown below.
Distribution of particles and activation energies of un-catalyzed reaction ("effect of catalysts on
rates of reaction," 2019)
Adding manganese dioxide and the enzyme catalysts thus has an exact impact of shifting the
activation energy. It provides an alternative route for the reaction. The alternative path has lower
activation energy as shown below.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
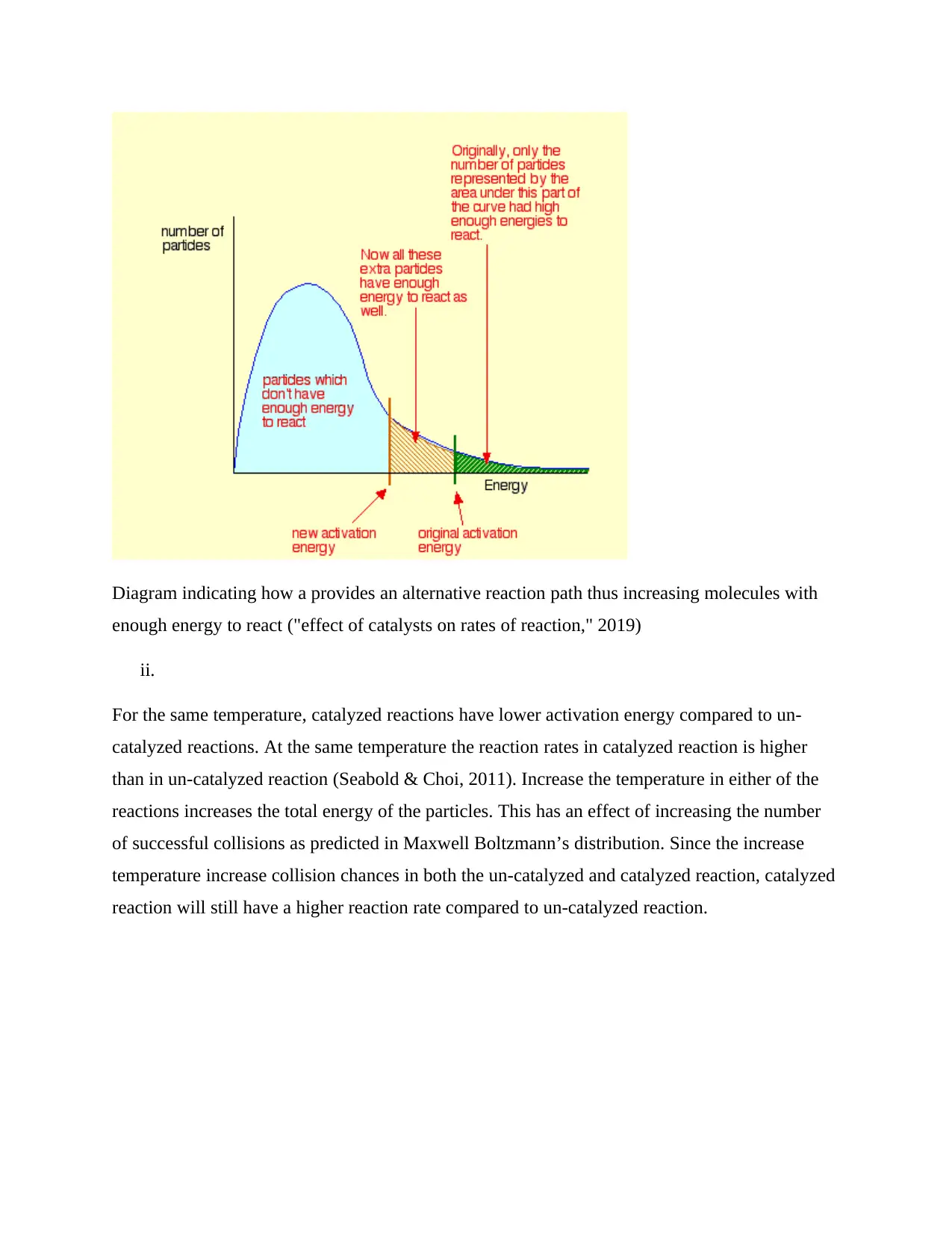
Diagram indicating how a provides an alternative reaction path thus increasing molecules with
enough energy to react ("effect of catalysts on rates of reaction," 2019)
ii.
For the same temperature, catalyzed reactions have lower activation energy compared to un-
catalyzed reactions. At the same temperature the reaction rates in catalyzed reaction is higher
than in un-catalyzed reaction (Seabold & Choi, 2011). Increase the temperature in either of the
reactions increases the total energy of the particles. This has an effect of increasing the number
of successful collisions as predicted in Maxwell Boltzmann’s distribution. Since the increase
temperature increase collision chances in both the un-catalyzed and catalyzed reaction, catalyzed
reaction will still have a higher reaction rate compared to un-catalyzed reaction.
enough energy to react ("effect of catalysts on rates of reaction," 2019)
ii.
For the same temperature, catalyzed reactions have lower activation energy compared to un-
catalyzed reactions. At the same temperature the reaction rates in catalyzed reaction is higher
than in un-catalyzed reaction (Seabold & Choi, 2011). Increase the temperature in either of the
reactions increases the total energy of the particles. This has an effect of increasing the number
of successful collisions as predicted in Maxwell Boltzmann’s distribution. Since the increase
temperature increase collision chances in both the un-catalyzed and catalyzed reaction, catalyzed
reaction will still have a higher reaction rate compared to un-catalyzed reaction.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
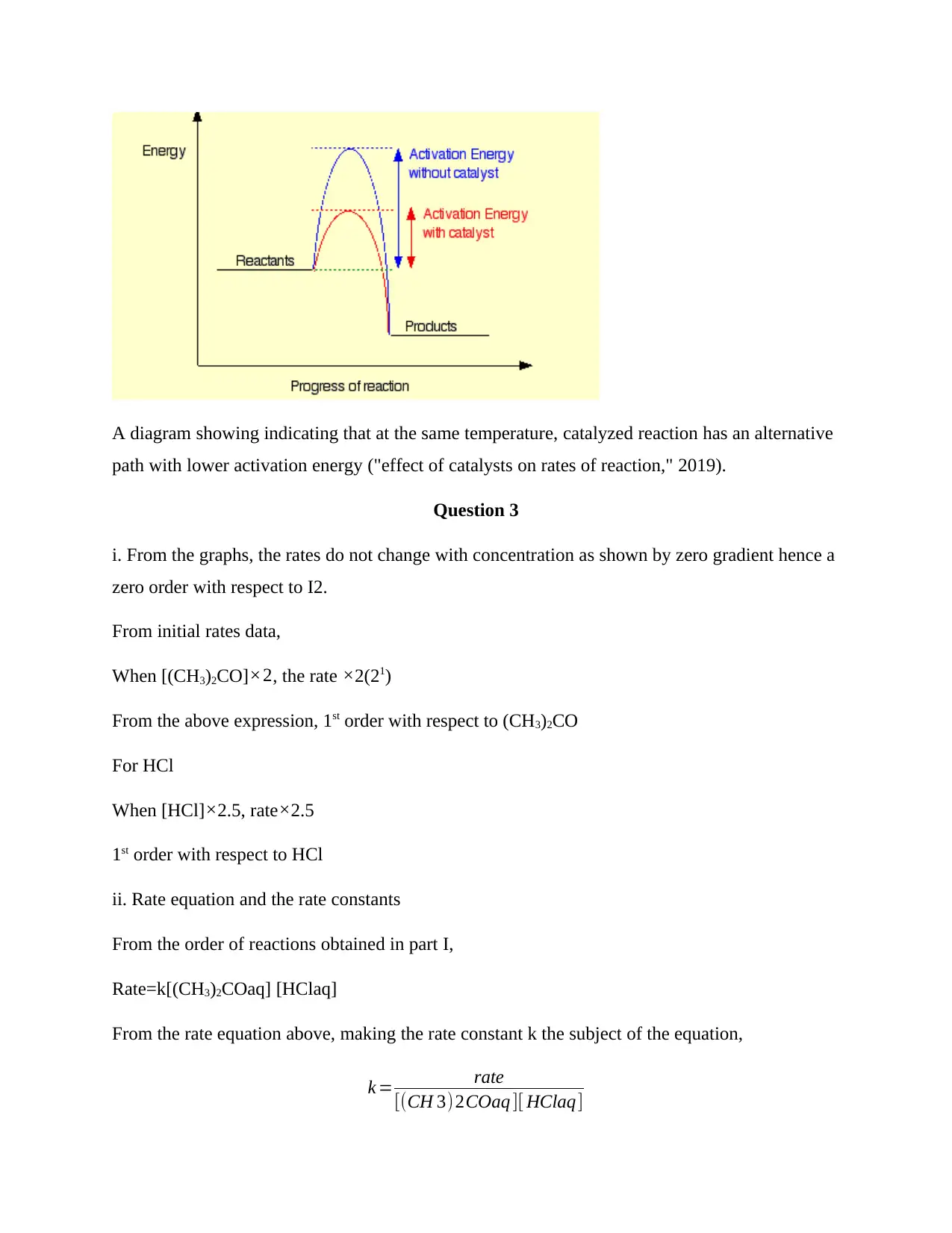
A diagram showing indicating that at the same temperature, catalyzed reaction has an alternative
path with lower activation energy ("effect of catalysts on rates of reaction," 2019).
Question 3
i. From the graphs, the rates do not change with concentration as shown by zero gradient hence a
zero order with respect to I2.
From initial rates data,
When [(CH3)2CO]×2, the rate ×2(21)
From the above expression, 1st order with respect to (CH3)2CO
For HCl
When [HCl]×2.5, rate×2.5
1st order with respect to HCl
ii. Rate equation and the rate constants
From the order of reactions obtained in part I,
Rate=k[(CH3)2COaq] [HClaq]
From the rate equation above, making the rate constant k the subject of the equation,
k = rate
[(CH 3)2COaq ][ HClaq]
path with lower activation energy ("effect of catalysts on rates of reaction," 2019).
Question 3
i. From the graphs, the rates do not change with concentration as shown by zero gradient hence a
zero order with respect to I2.
From initial rates data,
When [(CH3)2CO]×2, the rate ×2(21)
From the above expression, 1st order with respect to (CH3)2CO
For HCl
When [HCl]×2.5, rate×2.5
1st order with respect to HCl
ii. Rate equation and the rate constants
From the order of reactions obtained in part I,
Rate=k[(CH3)2COaq] [HClaq]
From the rate equation above, making the rate constant k the subject of the equation,
k = rate
[(CH 3)2COaq ][ HClaq]

Substituting the values in the table,
k = 2.1 ×10−9
(1.5 ×2.0)× 10−5 =7.0 ×10−5 dm3 mol−1 s−1
Question 4
The reaction in problem statement is
CO(g) + 2H2(g) CH3OH(g) ∆H = -91 kJmol-1
i. Temperature. From the reaction equation, this process is an exothermic reaction.
From Le Chatlier’s Principle, if we desire to realize maximum yield, we must apply a
change that does not favor exothermic reaction. Because forward reaction is
exothermic, decreasing the temperature will favor production of more heat yielding
more methanol. For maximum yield, the temperature should be decreased below
temperature at equilibrium.
Pressure. From the reversible equation, the total gas mole in the left hand side is
(1+2) while gas mole on the left hand-side is (1). To achieve maximum yield, the
process must favor backward reaction. According to Le Chatlier’s principle, in order
to favor formation of methanol, we must apply a change that opposes the change we
desire. For maximum yield, the pressure must thus be increased beyond the pressure
at equilibrium point.
ii. In the industry, the optimum temperature might be higher than equilibrium
temperature and pressure different from the ones predicted using Le Chatlier’s
principle due to the following reasons;
In the industry, cost is of a great importance. The cost of achieving these conditions
might be higher compared to the relative benefit of the maximum yield.
Other determinants of reaction rate are put into place at to complement temperature
and pressure for example according to Barnett (2016) most industrial manufacture of
methanol involves gasification process where excess hydrogen is burnt (effect of
k = 2.1 ×10−9
(1.5 ×2.0)× 10−5 =7.0 ×10−5 dm3 mol−1 s−1
Question 4
The reaction in problem statement is
CO(g) + 2H2(g) CH3OH(g) ∆H = -91 kJmol-1
i. Temperature. From the reaction equation, this process is an exothermic reaction.
From Le Chatlier’s Principle, if we desire to realize maximum yield, we must apply a
change that does not favor exothermic reaction. Because forward reaction is
exothermic, decreasing the temperature will favor production of more heat yielding
more methanol. For maximum yield, the temperature should be decreased below
temperature at equilibrium.
Pressure. From the reversible equation, the total gas mole in the left hand side is
(1+2) while gas mole on the left hand-side is (1). To achieve maximum yield, the
process must favor backward reaction. According to Le Chatlier’s principle, in order
to favor formation of methanol, we must apply a change that opposes the change we
desire. For maximum yield, the pressure must thus be increased beyond the pressure
at equilibrium point.
ii. In the industry, the optimum temperature might be higher than equilibrium
temperature and pressure different from the ones predicted using Le Chatlier’s
principle due to the following reasons;
In the industry, cost is of a great importance. The cost of achieving these conditions
might be higher compared to the relative benefit of the maximum yield.
Other determinants of reaction rate are put into place at to complement temperature
and pressure for example according to Barnett (2016) most industrial manufacture of
methanol involves gasification process where excess hydrogen is burnt (effect of
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
concentration) characterized with partial oxidation reaction in the steam forming
process.
Using catalyst also improves maximum yields different from those predicted by Le-
chatlier’s principle
iii. A catalyst will increase the number of successful collisions between carbon (II) oxide
and hydrogen by providing an alternative reaction path with lower activation energy.
This has an effect of improving the rate of reaction. Introducing a catalyst in this
reaction thus improves increases the rate of reaction.
Question 5
i.
Kc= [ HI ] 2
[ H2 ] ×[ I 2]
ii.
From the expression in 1,
[I2 ]= [ HI ]2
[ H2 ] × Kc
Substituting the variables
[ I2 ]= [ 1.507 ×10−2 ]2
[2.1 ×10−4 ] ×35 =0.0308986 mol /dm3
iii.
From Le Chatelier’s Principle, decreasing the volume (compressing at constant temperature) is
supposed to make the equilibrium to shift towards reaction side that has fewer particles. In this
reaction however, the number of gas particles are the same in both sides of the reaction.
Compressing the gas at a constant temperature thus has no effect on the equilibrium point.
process.
Using catalyst also improves maximum yields different from those predicted by Le-
chatlier’s principle
iii. A catalyst will increase the number of successful collisions between carbon (II) oxide
and hydrogen by providing an alternative reaction path with lower activation energy.
This has an effect of improving the rate of reaction. Introducing a catalyst in this
reaction thus improves increases the rate of reaction.
Question 5
i.
Kc= [ HI ] 2
[ H2 ] ×[ I 2]
ii.
From the expression in 1,
[I2 ]= [ HI ]2
[ H2 ] × Kc
Substituting the variables
[ I2 ]= [ 1.507 ×10−2 ]2
[2.1 ×10−4 ] ×35 =0.0308986 mol /dm3
iii.
From Le Chatelier’s Principle, decreasing the volume (compressing at constant temperature) is
supposed to make the equilibrium to shift towards reaction side that has fewer particles. In this
reaction however, the number of gas particles are the same in both sides of the reaction.
Compressing the gas at a constant temperature thus has no effect on the equilibrium point.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
Barnett, E. D. (2016). PREPARATION OF ORGANIC COMPOUND. Sydney, Australia:
Wentworth Press.
Covington, A. (2012). Physical Chemistry of Organic Solvent Systems. Berlin, Germany:
Springer Science & Business Media.
The effect of catalysts on rates of reaction. (2019). Retrieved from
https://www.chemguide.co.uk/physical/basicrates/catalyst.html
Leffler, J. E., & Grunwald, E. (2013). Rates and equilibria of organic reactions: as treated by
statistical, thermodynamic and extra thermodynamic methods. Courier Corporation.
Seabold, J. A., & Choi, K. (2011). Effect of a Cobalt-Based Oxygen Evolution Catalyst on the
Stability and the Selectivity of Photo-Oxidation Reactions of a WO3Photoanode.
Chemistry of Materials, 23(5), 1105-1112.
Barnett, E. D. (2016). PREPARATION OF ORGANIC COMPOUND. Sydney, Australia:
Wentworth Press.
Covington, A. (2012). Physical Chemistry of Organic Solvent Systems. Berlin, Germany:
Springer Science & Business Media.
The effect of catalysts on rates of reaction. (2019). Retrieved from
https://www.chemguide.co.uk/physical/basicrates/catalyst.html
Leffler, J. E., & Grunwald, E. (2013). Rates and equilibria of organic reactions: as treated by
statistical, thermodynamic and extra thermodynamic methods. Courier Corporation.
Seabold, J. A., & Choi, K. (2011). Effect of a Cobalt-Based Oxygen Evolution Catalyst on the
Stability and the Selectivity of Photo-Oxidation Reactions of a WO3Photoanode.
Chemistry of Materials, 23(5), 1105-1112.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




