Acetyl CoA Acetyltransferase 2 Deficiency: Detailed Report
VerifiedAdded on 2023/06/03
|9
|2841
|285
Report
AI Summary
This report provides a comprehensive overview of Acetyl CoA acetyltransferase 2 deficiency (ACAT2D), a metabolic disorder characterized by low levels of the cytosolic acetoacetyl-CoA thiolase (ACAT2) enzyme in the liver. The report details the biochemical pathways involved in lipid metabolism, highlighting the role of the ACAT2 gene and its location on chromosome 6. It discusses the genetic mutations, including deletion and substitution, that lead to the deficiency and explores diagnostic techniques like RT-PCR and Western blotting. The report examines the impact of ACAT2 deficiency on mental development, muscle tone, and cholesterol metabolism, referencing relevant research studies. Furthermore, it presents information on treatment approaches, such as the use of lipid-lowering drugs, and policy considerations related to the management of the disorder. The report also includes references to supporting literature, providing a detailed understanding of ACAT2D.
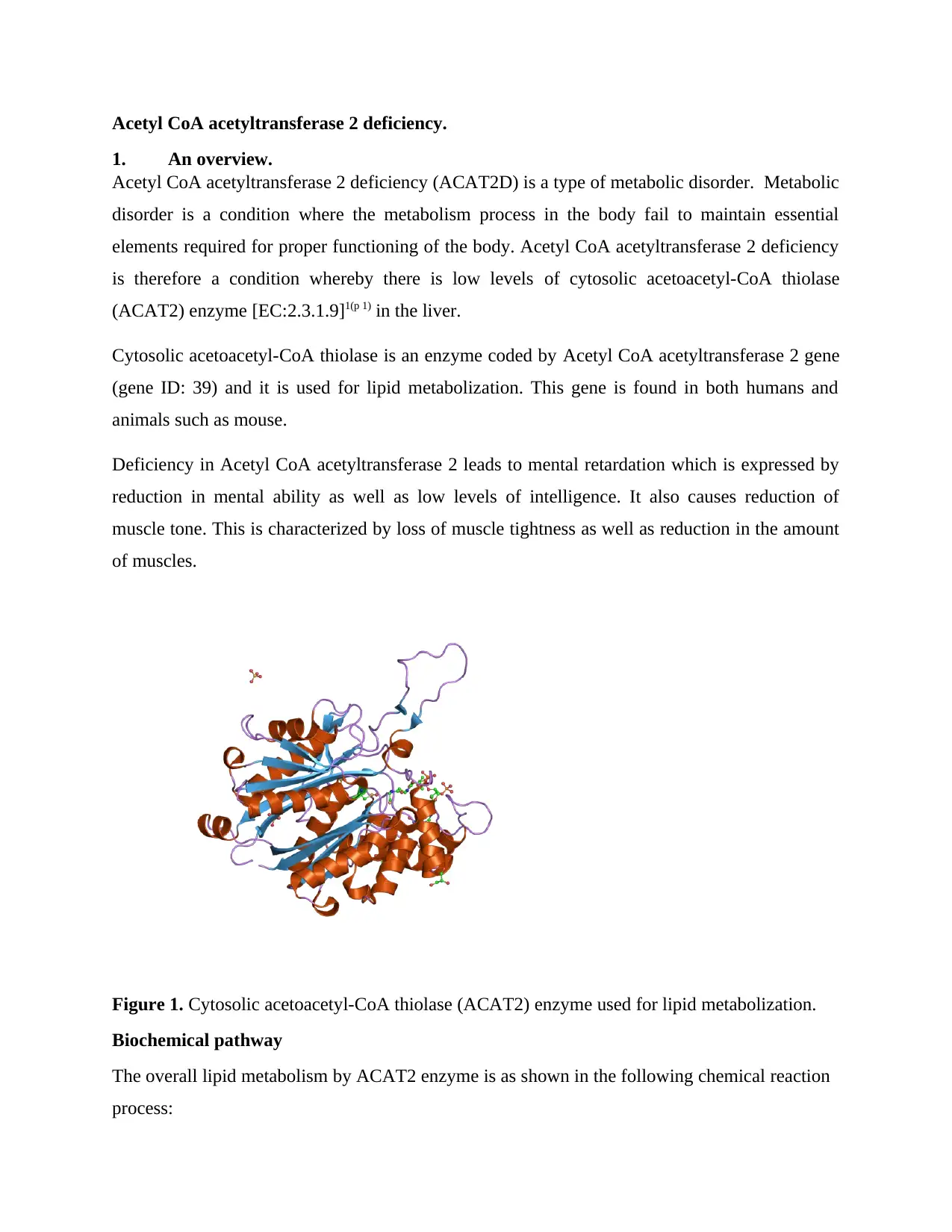
Acetyl CoA acetyltransferase 2 deficiency.
1. An overview.
Acetyl CoA acetyltransferase 2 deficiency (ACAT2D) is a type of metabolic disorder. Metabolic
disorder is a condition where the metabolism process in the body fail to maintain essential
elements required for proper functioning of the body. Acetyl CoA acetyltransferase 2 deficiency
is therefore a condition whereby there is low levels of cytosolic acetoacetyl-CoA thiolase
(ACAT2) enzyme [EC:2.3.1.9]1(p 1) in the liver.
Cytosolic acetoacetyl-CoA thiolase is an enzyme coded by Acetyl CoA acetyltransferase 2 gene
(gene ID: 39) and it is used for lipid metabolization. This gene is found in both humans and
animals such as mouse.
Deficiency in Acetyl CoA acetyltransferase 2 leads to mental retardation which is expressed by
reduction in mental ability as well as low levels of intelligence. It also causes reduction of
muscle tone. This is characterized by loss of muscle tightness as well as reduction in the amount
of muscles.
Figure 1. Cytosolic acetoacetyl-CoA thiolase (ACAT2) enzyme used for lipid metabolization.
Biochemical pathway
The overall lipid metabolism by ACAT2 enzyme is as shown in the following chemical reaction
process:
1. An overview.
Acetyl CoA acetyltransferase 2 deficiency (ACAT2D) is a type of metabolic disorder. Metabolic
disorder is a condition where the metabolism process in the body fail to maintain essential
elements required for proper functioning of the body. Acetyl CoA acetyltransferase 2 deficiency
is therefore a condition whereby there is low levels of cytosolic acetoacetyl-CoA thiolase
(ACAT2) enzyme [EC:2.3.1.9]1(p 1) in the liver.
Cytosolic acetoacetyl-CoA thiolase is an enzyme coded by Acetyl CoA acetyltransferase 2 gene
(gene ID: 39) and it is used for lipid metabolization. This gene is found in both humans and
animals such as mouse.
Deficiency in Acetyl CoA acetyltransferase 2 leads to mental retardation which is expressed by
reduction in mental ability as well as low levels of intelligence. It also causes reduction of
muscle tone. This is characterized by loss of muscle tightness as well as reduction in the amount
of muscles.
Figure 1. Cytosolic acetoacetyl-CoA thiolase (ACAT2) enzyme used for lipid metabolization.
Biochemical pathway
The overall lipid metabolism by ACAT2 enzyme is as shown in the following chemical reaction
process:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

7 FAD + 7 H2O + + 7 CoASH +7 NAD + H(CH2CH2)7CH2CO-SCoA → 7 NADH +7 FADH2 +8
CH3CO-SCoA+ 7 H+
Lipid metabolism is the process whereby the cells break down lipids for energy production or for
storage in form of fats.
The process occurs in the liver. The lipids in the body are in various forms which include fatty
acids, cholesterol, triglycerides and membrane lipids. Both originate from foods consisting of
fats ingested. Hydrolysis by lingual lipase, gastric lipase, pancreatic lipase, bile salt-dependent
lipase enzymes, is the first stage of lipid metabolism in the mouth, stomach and pancreas
respectively. This involves breakdown of triglycerides into monoglycerides. The continuous
digestion of lipids results into fatty acids and glycerol which are then absorbed in the small
intestine via epithelial cells. The absorbed fatty acids and glycerol are packaged into
chilomicrons and get transported to adipose tissue by blood. The chilomicrons are lipoproteins
produced by the liver. These proteins act as transport medium for digested lipids. Since the lipids
are hydrophobic, they cannot be transported in blood, therefore, chilomicrons are required. Once
chilomicrons reach to the target cells, they are broken down to release triglycerides by
lipoprotein lipase. The released tryglycerides before entering into the cells are digested into
glycerol and fatty acids. The glycerol absorbed into the cell cytoplasm is converted into
glyceraldehyde 3-phosphate. This is oxidized further ton produce energy. The fatty acids are
converted into fatty acyl-CoA in the mitochondria. This involves addition of coenzyme A and
the produced Acyl CoA undergoes beta oxidation to produce Acetyl-CoA with the help of 3-
ketoacyl-CoA thiolase, Acyl CoA dehydrogenase, 3-hydroxyacyl-CoA dehydrogenase and
, Enoyl-CoA hydratase enzymes.
Acetyl CoA acetyltransferase 2 gene is located on chromosome number six in human.
CH3CO-SCoA+ 7 H+
Lipid metabolism is the process whereby the cells break down lipids for energy production or for
storage in form of fats.
The process occurs in the liver. The lipids in the body are in various forms which include fatty
acids, cholesterol, triglycerides and membrane lipids. Both originate from foods consisting of
fats ingested. Hydrolysis by lingual lipase, gastric lipase, pancreatic lipase, bile salt-dependent
lipase enzymes, is the first stage of lipid metabolism in the mouth, stomach and pancreas
respectively. This involves breakdown of triglycerides into monoglycerides. The continuous
digestion of lipids results into fatty acids and glycerol which are then absorbed in the small
intestine via epithelial cells. The absorbed fatty acids and glycerol are packaged into
chilomicrons and get transported to adipose tissue by blood. The chilomicrons are lipoproteins
produced by the liver. These proteins act as transport medium for digested lipids. Since the lipids
are hydrophobic, they cannot be transported in blood, therefore, chilomicrons are required. Once
chilomicrons reach to the target cells, they are broken down to release triglycerides by
lipoprotein lipase. The released tryglycerides before entering into the cells are digested into
glycerol and fatty acids. The glycerol absorbed into the cell cytoplasm is converted into
glyceraldehyde 3-phosphate. This is oxidized further ton produce energy. The fatty acids are
converted into fatty acyl-CoA in the mitochondria. This involves addition of coenzyme A and
the produced Acyl CoA undergoes beta oxidation to produce Acetyl-CoA with the help of 3-
ketoacyl-CoA thiolase, Acyl CoA dehydrogenase, 3-hydroxyacyl-CoA dehydrogenase and
, Enoyl-CoA hydratase enzymes.
Acetyl CoA acetyltransferase 2 gene is located on chromosome number six in human.
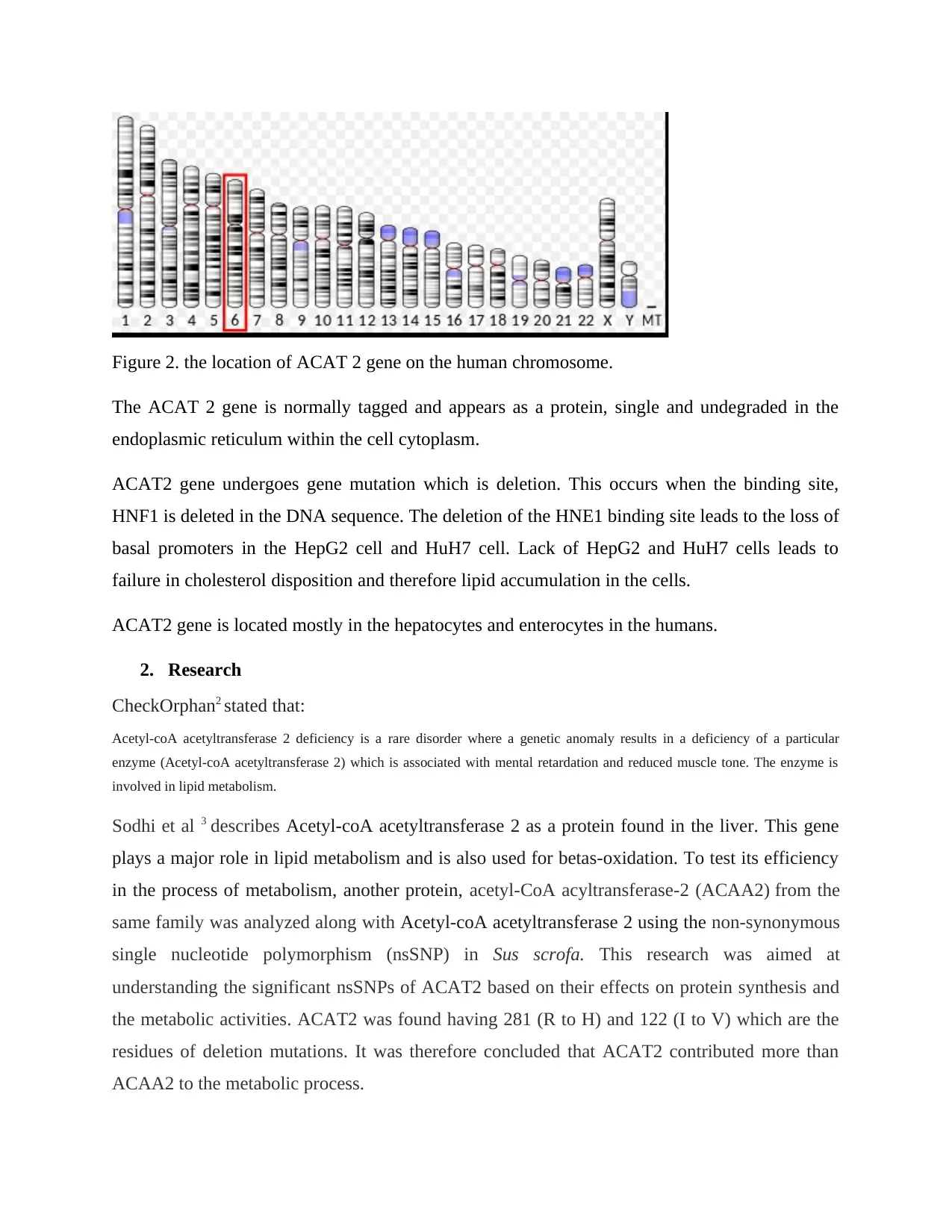
Figure 2. the location of ACAT 2 gene on the human chromosome.
The ACAT 2 gene is normally tagged and appears as a protein, single and undegraded in the
endoplasmic reticulum within the cell cytoplasm.
ACAT2 gene undergoes gene mutation which is deletion. This occurs when the binding site,
HNF1 is deleted in the DNA sequence. The deletion of the HNE1 binding site leads to the loss of
basal promoters in the HepG2 cell and HuH7 cell. Lack of HepG2 and HuH7 cells leads to
failure in cholesterol disposition and therefore lipid accumulation in the cells.
ACAT2 gene is located mostly in the hepatocytes and enterocytes in the humans.
2. Research
CheckOrphan2 stated that:
Acetyl-coA acetyltransferase 2 deficiency is a rare disorder where a genetic anomaly results in a deficiency of a particular
enzyme (Acetyl-coA acetyltransferase 2) which is associated with mental retardation and reduced muscle tone. The enzyme is
involved in lipid metabolism.
Sodhi et al 3 describes Acetyl-coA acetyltransferase 2 as a protein found in the liver. This gene
plays a major role in lipid metabolism and is also used for betas-oxidation. To test its efficiency
in the process of metabolism, another protein, acetyl-CoA acyltransferase-2 (ACAA2) from the
same family was analyzed along with Acetyl-coA acetyltransferase 2 using the non-synonymous
single nucleotide polymorphism (nsSNP) in Sus scrofa. This research was aimed at
understanding the significant nsSNPs of ACAT2 based on their effects on protein synthesis and
the metabolic activities. ACAT2 was found having 281 (R to H) and 122 (I to V) which are the
residues of deletion mutations. It was therefore concluded that ACAT2 contributed more than
ACAA2 to the metabolic process.
The ACAT 2 gene is normally tagged and appears as a protein, single and undegraded in the
endoplasmic reticulum within the cell cytoplasm.
ACAT2 gene undergoes gene mutation which is deletion. This occurs when the binding site,
HNF1 is deleted in the DNA sequence. The deletion of the HNE1 binding site leads to the loss of
basal promoters in the HepG2 cell and HuH7 cell. Lack of HepG2 and HuH7 cells leads to
failure in cholesterol disposition and therefore lipid accumulation in the cells.
ACAT2 gene is located mostly in the hepatocytes and enterocytes in the humans.
2. Research
CheckOrphan2 stated that:
Acetyl-coA acetyltransferase 2 deficiency is a rare disorder where a genetic anomaly results in a deficiency of a particular
enzyme (Acetyl-coA acetyltransferase 2) which is associated with mental retardation and reduced muscle tone. The enzyme is
involved in lipid metabolism.
Sodhi et al 3 describes Acetyl-coA acetyltransferase 2 as a protein found in the liver. This gene
plays a major role in lipid metabolism and is also used for betas-oxidation. To test its efficiency
in the process of metabolism, another protein, acetyl-CoA acyltransferase-2 (ACAA2) from the
same family was analyzed along with Acetyl-coA acetyltransferase 2 using the non-synonymous
single nucleotide polymorphism (nsSNP) in Sus scrofa. This research was aimed at
understanding the significant nsSNPs of ACAT2 based on their effects on protein synthesis and
the metabolic activities. ACAT2 was found having 281 (R to H) and 122 (I to V) which are the
residues of deletion mutations. It was therefore concluded that ACAT2 contributed more than
ACAA2 to the metabolic process.

Wang 4 in his study on the functions of ACAT2 in liver using broiler chicken concluded that
ACAT2 gene works by either promoting or by suppressing abdominal fat accumulation. The
gene as well plays a role in controlling the enzymes which are used during lipogenesis and
lipolysis processes5.
The efficiency in the performance of ACAT2 depends on many factors. Among the factors
include the structure and stability of the gene. In the study carried out by Xuelian 6 on the effects
of deletion mutation on the structure of ACAT2, he identified some SNPs and examined them
using SIFT to establish the effect of their substitution. He states that:
It was found that the two nsSNPs, rs321479200 and rs342236888 of ACAT2 were categorised as borderline
(0.101–0.20) and tolerable (0.201–1.00) on the basis of their index score i.e. 0.15 and 0.35 respectively. In terms of
the nucleotide variations, out of nsSNPs, one nsSNP involved a T>C change, another involved a T>G change, and
three nsSNPs involved C>T changes.
The change in structure of the gene affected its functionality either by increasing or decreasing.
Furthermore, the stability of the protein was examined by the use of SRide tool. It was
discovered that nsSNP-rs321479200 (R281H) contributed to the protein stability which
subscribes to its efficiency in metabolism.
ACAT2 has a significant role to play during absorption of cholesterol and metabolism of
lipoprotein7-8. There is a close relationship between cholesterol intake and muscle tone.
Riechman9 stated that:
It is also believed that inflammation plays a significant role in muscle building. Cholesterol plays a direct role in inflammation
and inflammation plays a significant role in muscle building.
The above claim is furthermore supported by another study by Jiang10 which reported that:
There is an important function of cholesterol in the muscle building under the dose dependent manner. Therefore, it
has been concluded that cholesterol levels are dose-dependently associated with increased muscle size and strength.
Esterification of cholesterol and oxysterols in human is carried out by ACAT2. The lipid rafts
which are used in formation pathways for signaling are formed by the cholesterol11. This is
achieved by arrangement of proteins and building of the signaling complexes in the skeletal
muscles. As a result of this complex process, there is muscle development and proper muscle
tone12-14.
ACAT2 gene works by either promoting or by suppressing abdominal fat accumulation. The
gene as well plays a role in controlling the enzymes which are used during lipogenesis and
lipolysis processes5.
The efficiency in the performance of ACAT2 depends on many factors. Among the factors
include the structure and stability of the gene. In the study carried out by Xuelian 6 on the effects
of deletion mutation on the structure of ACAT2, he identified some SNPs and examined them
using SIFT to establish the effect of their substitution. He states that:
It was found that the two nsSNPs, rs321479200 and rs342236888 of ACAT2 were categorised as borderline
(0.101–0.20) and tolerable (0.201–1.00) on the basis of their index score i.e. 0.15 and 0.35 respectively. In terms of
the nucleotide variations, out of nsSNPs, one nsSNP involved a T>C change, another involved a T>G change, and
three nsSNPs involved C>T changes.
The change in structure of the gene affected its functionality either by increasing or decreasing.
Furthermore, the stability of the protein was examined by the use of SRide tool. It was
discovered that nsSNP-rs321479200 (R281H) contributed to the protein stability which
subscribes to its efficiency in metabolism.
ACAT2 has a significant role to play during absorption of cholesterol and metabolism of
lipoprotein7-8. There is a close relationship between cholesterol intake and muscle tone.
Riechman9 stated that:
It is also believed that inflammation plays a significant role in muscle building. Cholesterol plays a direct role in inflammation
and inflammation plays a significant role in muscle building.
The above claim is furthermore supported by another study by Jiang10 which reported that:
There is an important function of cholesterol in the muscle building under the dose dependent manner. Therefore, it
has been concluded that cholesterol levels are dose-dependently associated with increased muscle size and strength.
Esterification of cholesterol and oxysterols in human is carried out by ACAT2. The lipid rafts
which are used in formation pathways for signaling are formed by the cholesterol11. This is
achieved by arrangement of proteins and building of the signaling complexes in the skeletal
muscles. As a result of this complex process, there is muscle development and proper muscle
tone12-14.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

3. Diagnosis.
Acetyl CoA acetyltransferase 2 deficiency is associated to gene mutation and gene instability.
The major types of mutations in the ACAT2 are deletion and substitution15.
The gene mutation in Acetyl CoA acetyltransferase 2 can be determined using various
techniques. The techniques include:
RT-PCR and quantitative real-time PCR technique.
The ACAT2 is extracted from the liver tissue using Radio Immuno Precipitation Assay (RIPA)
buffer and primers are separated. This procedure involves placing the target gene into Eppendorf
and subjecting it to specific conditions in order to replicate the targeted gene part to check for the
presence or absence of a particular nucleotide. Van17 stated that:
An Eppendorf Mastercycler gradient instrument was used to conduct RT-PCR under the following conditions: an initial 5 min at
94°C; 35 cycles of 30 seconds at 94°C, 30 seconds at the freezing temperature and 1 min at 72°C; followed by a single extension
period of 5 min at 72°C. Each individual sample was quantified in triplicate under the following amplification conditions: 95°C
for 10 min initially, and then 40 cycles of 95°C for 15 sec and 60°C for 1 min.
Western blotting analysis technique.
This technique uses the intensity at which the protein is expressed to determine its mutation
status. To achieve this, another gene from the same source as ACAT2 is used in order to check
the effect of mutation on gene expression. ACAA2 is normally used. Hussain 18(p188) explains the
procedure as follows:
Sixty micrograms of protein extract was diluted 1∶1 with 2× loading buffer (4% sodium dodecyl sulfate (SDS), 20% glycerol,
0.004% bromophenol blue, 25% 0.5 M Tris, and 5% β-mercaptoethanol). Protein extracts were denatured by boiling for 5 min
before loading onto a 12% SDS-PAGE gel. Proteins were transferred to nitrocellulose membrane after electrophoresis. The
membrane was blocked for two hours at room temperature and incubated with primary and secondary antibodies specific for
ACAA2, ACAT2, and β-actin.
Stability.
Acetyl CoA acetyltransferase 2 stability also affects its structure and functionality. The stability
is determined using I-Mutant and SRide tool. The tools detect the presence or absence of the
stabilizing residues I122V and R281H. the presence of R281H residue means that the ACAT2
gene is more stable and has a good structure for proper functioning19.
Acetyl CoA acetyltransferase 2 deficiency is associated to gene mutation and gene instability.
The major types of mutations in the ACAT2 are deletion and substitution15.
The gene mutation in Acetyl CoA acetyltransferase 2 can be determined using various
techniques. The techniques include:
RT-PCR and quantitative real-time PCR technique.
The ACAT2 is extracted from the liver tissue using Radio Immuno Precipitation Assay (RIPA)
buffer and primers are separated. This procedure involves placing the target gene into Eppendorf
and subjecting it to specific conditions in order to replicate the targeted gene part to check for the
presence or absence of a particular nucleotide. Van17 stated that:
An Eppendorf Mastercycler gradient instrument was used to conduct RT-PCR under the following conditions: an initial 5 min at
94°C; 35 cycles of 30 seconds at 94°C, 30 seconds at the freezing temperature and 1 min at 72°C; followed by a single extension
period of 5 min at 72°C. Each individual sample was quantified in triplicate under the following amplification conditions: 95°C
for 10 min initially, and then 40 cycles of 95°C for 15 sec and 60°C for 1 min.
Western blotting analysis technique.
This technique uses the intensity at which the protein is expressed to determine its mutation
status. To achieve this, another gene from the same source as ACAT2 is used in order to check
the effect of mutation on gene expression. ACAA2 is normally used. Hussain 18(p188) explains the
procedure as follows:
Sixty micrograms of protein extract was diluted 1∶1 with 2× loading buffer (4% sodium dodecyl sulfate (SDS), 20% glycerol,
0.004% bromophenol blue, 25% 0.5 M Tris, and 5% β-mercaptoethanol). Protein extracts were denatured by boiling for 5 min
before loading onto a 12% SDS-PAGE gel. Proteins were transferred to nitrocellulose membrane after electrophoresis. The
membrane was blocked for two hours at room temperature and incubated with primary and secondary antibodies specific for
ACAA2, ACAT2, and β-actin.
Stability.
Acetyl CoA acetyltransferase 2 stability also affects its structure and functionality. The stability
is determined using I-Mutant and SRide tool. The tools detect the presence or absence of the
stabilizing residues I122V and R281H. the presence of R281H residue means that the ACAT2
gene is more stable and has a good structure for proper functioning19.

Finally, low intelligence and underdeveloped muscles in an individual is a sign of Acetyl CoA
acetyltransferase 2 deficiency2.
4. Treatment
The Acetyl CoA acetyltransferase 2 deficiency is controlled by the use of lipid-lowering drugs.in
a study carried out in Xinjiang Medical university on 2010 July to 2014 May under the standards
of the Declaration of Helsinki. In the study by Zhu20(1047-1054.). he states describes the procedure as:
A total of 759 (male: 452 and female: 307) Han Chinese subjects all randomly selected from the First Affiliated Hospital of
Xinjiang Medical University from July 2010 to May 2014. We have conducted a case-control study including 233 patients and
526 control subjects who had passed the eligibility criteria and had complete data on ACAT2 genotype for the current study.
Hyperlipidemia was defined as a total plasma cholesterol >6.22 mmol or low density lipoprotein cholesterol >4.14 mmol/L or
plasma triglycerides >2.26 mmol and /or the current use of lipid-lowering drugs with an established diagnosis of hyperlipidemia.
Further, all subjects live in Xinjiang Uighur Autonomous Region of China and free from thyroid disease, or any history of taking
lipid-lowering drugs.
Having carried out the research and concluded on results, Mayila21(p 1) states that:
First of all, we have found that the incidence of smoking, alcohol use and the frequency of the elementary education were higher
in the case subjects than the controls and this outcome may suggest that people who had lower level of education may lack of the
cognition regarding the diseases. Further, the frequency of never or rarely walk and 1-2 days per week were also higher in the
case group than the controls, which indicates that the less psychical activity in daily life, the more people may have a higher risk
of suffering hyperlipedima.
The statistics are as shown in the table below.
acetyltransferase 2 deficiency2.
4. Treatment
The Acetyl CoA acetyltransferase 2 deficiency is controlled by the use of lipid-lowering drugs.in
a study carried out in Xinjiang Medical university on 2010 July to 2014 May under the standards
of the Declaration of Helsinki. In the study by Zhu20(1047-1054.). he states describes the procedure as:
A total of 759 (male: 452 and female: 307) Han Chinese subjects all randomly selected from the First Affiliated Hospital of
Xinjiang Medical University from July 2010 to May 2014. We have conducted a case-control study including 233 patients and
526 control subjects who had passed the eligibility criteria and had complete data on ACAT2 genotype for the current study.
Hyperlipidemia was defined as a total plasma cholesterol >6.22 mmol or low density lipoprotein cholesterol >4.14 mmol/L or
plasma triglycerides >2.26 mmol and /or the current use of lipid-lowering drugs with an established diagnosis of hyperlipidemia.
Further, all subjects live in Xinjiang Uighur Autonomous Region of China and free from thyroid disease, or any history of taking
lipid-lowering drugs.
Having carried out the research and concluded on results, Mayila21(p 1) states that:
First of all, we have found that the incidence of smoking, alcohol use and the frequency of the elementary education were higher
in the case subjects than the controls and this outcome may suggest that people who had lower level of education may lack of the
cognition regarding the diseases. Further, the frequency of never or rarely walk and 1-2 days per week were also higher in the
case group than the controls, which indicates that the less psychical activity in daily life, the more people may have a higher risk
of suffering hyperlipedima.
The statistics are as shown in the table below.
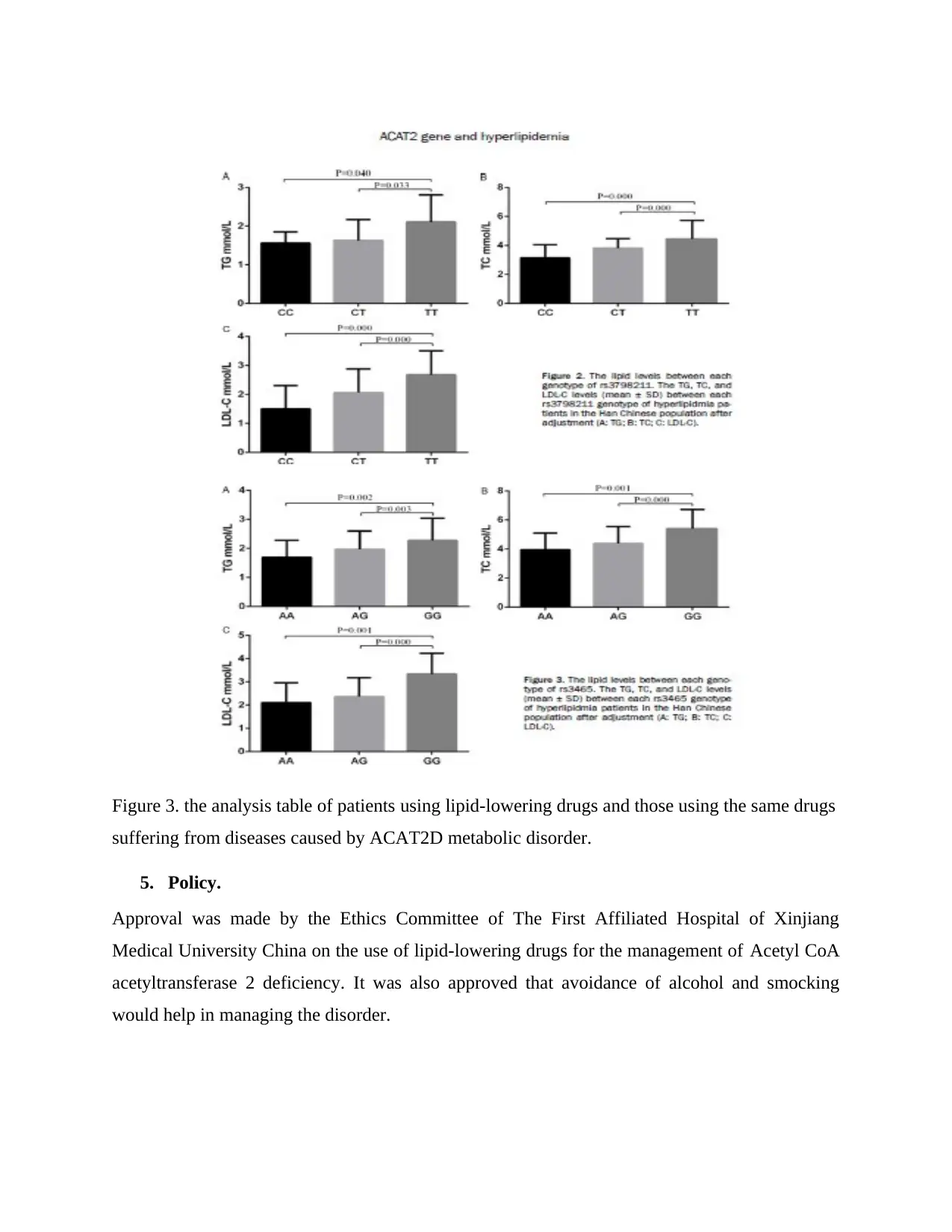
Figure 3. the analysis table of patients using lipid-lowering drugs and those using the same drugs
suffering from diseases caused by ACAT2D metabolic disorder.
5. Policy.
Approval was made by the Ethics Committee of The First Affiliated Hospital of Xinjiang
Medical University China on the use of lipid-lowering drugs for the management of Acetyl CoA
acetyltransferase 2 deficiency. It was also approved that avoidance of alcohol and smocking
would help in managing the disorder.
suffering from diseases caused by ACAT2D metabolic disorder.
5. Policy.
Approval was made by the Ethics Committee of The First Affiliated Hospital of Xinjiang
Medical University China on the use of lipid-lowering drugs for the management of Acetyl CoA
acetyltransferase 2 deficiency. It was also approved that avoidance of alcohol and smocking
would help in managing the disorder.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References.
1. https://www.genome.jp/dbget-bin/www_bget?hsa:39
2.CheckOrphan.Rare, orphan and neglected diseases:Acetyl-coa acetyltransferase 2 deficiency.
[internet]. 2018;http://www.checkorphan.org/diseases/acetyl-coa-acetyltransferase-2-deficiency.
3.Sodhi SS, Ghosh M, Song KD, Sharma N, Kim JH, Kim NE, et al. (2014) An Approach to
Identify SNPs in the Gene Encoding Acetyl-CoA Acetyltransferase-2 (ACAT-2) and Their
Proposed Role in Metabolic Processes in Pig. PLoS ONE 9(7): e102432.
https://doi.org/10.1371/journal.pone.0102432.
4.Wang X, Carré W, Rejto L, Cogburn LA (2015) Transcriptional profiling in liver of
hormonally-manipulated chickens. In: Dawson A, Sharp PJ, editors. Functional avian
endocrinology. Narosa Publishing House, New Delhi, India.
5.Ushio H, Nagasaka R (2013) Utilization of biological responses of fish and shellfish for
improving seafood qualities. Aqua Bio Sci Mono 6: 91–98.
6.Xuelian HE (2012) Studies of common variations in two candidate genes of dyslipidemia and
coronary artery disease. Ph.D. thesis, National University of Singapore.
7.Nguyen TM, Sawyer JK, Kelley KL, Davis MA, Kent CR, et al. (2012) ACAT2 and
ABCG5/G8 are both required for efficient cholesterol absorption in mice: evidence from thoracic
lymph duct cannulation. J Lipid Res 53: 1598–1609.
8.Alger HM, Brown JM, Sawyer JK, Kelley KL, Shah R, et al. (2012) Inhibition of acyl-
Coenzyme A: Cholesterol acyltransferase 2 (ACAT2) prevents dietary cholesterol-associated
steatosis by enhancing hepatic triglyceride mobilization. J Biol Chem 285: 14267–14274.
9.Riechman SE, Andrews RD, MacLean DA, Sheather S (2012) Statins and dietary and serum
cholesterol are associated with increased lean mass following resistance training. J Gerontolo A
Biol Sci Med Sci 62: 1164–1171.
10.Jiang Z, Michal JJ, Chen J, Daniels TF, Kunej T, et al. (2017) Discovery of novel genetic
networks associated with 19 economically important traits in beef cattle. Int J Biol Sci 5: 528–
542.
11.Osada K, Sasaki E, Sugano M (2014) Lymphatic absorption of oxidized cholesterol in rats.
Lipids 29: 555–559.
12.Freeman MR, Solomon KR (2014) Cholesterol and prostate cancer. J Cell Biochem 91: 54–
69.
13.Lucero HA, Robbins PW (2015) Lipid rafts-protein association and the regulation of protein
activity. Arch Biochem Biophys 426: 208–224.
14.Smythe GM, Eby JC, Disatnik MH, Rando TA (2013) A caveolin-3 mutant that causes limb
girdle muscular dystrophy type 1C disrupts Src localization and activity and induces apoptosis in
skeletal myotubes. J Cell Sci 116: 4739–4749.
1. https://www.genome.jp/dbget-bin/www_bget?hsa:39
2.CheckOrphan.Rare, orphan and neglected diseases:Acetyl-coa acetyltransferase 2 deficiency.
[internet]. 2018;http://www.checkorphan.org/diseases/acetyl-coa-acetyltransferase-2-deficiency.
3.Sodhi SS, Ghosh M, Song KD, Sharma N, Kim JH, Kim NE, et al. (2014) An Approach to
Identify SNPs in the Gene Encoding Acetyl-CoA Acetyltransferase-2 (ACAT-2) and Their
Proposed Role in Metabolic Processes in Pig. PLoS ONE 9(7): e102432.
https://doi.org/10.1371/journal.pone.0102432.
4.Wang X, Carré W, Rejto L, Cogburn LA (2015) Transcriptional profiling in liver of
hormonally-manipulated chickens. In: Dawson A, Sharp PJ, editors. Functional avian
endocrinology. Narosa Publishing House, New Delhi, India.
5.Ushio H, Nagasaka R (2013) Utilization of biological responses of fish and shellfish for
improving seafood qualities. Aqua Bio Sci Mono 6: 91–98.
6.Xuelian HE (2012) Studies of common variations in two candidate genes of dyslipidemia and
coronary artery disease. Ph.D. thesis, National University of Singapore.
7.Nguyen TM, Sawyer JK, Kelley KL, Davis MA, Kent CR, et al. (2012) ACAT2 and
ABCG5/G8 are both required for efficient cholesterol absorption in mice: evidence from thoracic
lymph duct cannulation. J Lipid Res 53: 1598–1609.
8.Alger HM, Brown JM, Sawyer JK, Kelley KL, Shah R, et al. (2012) Inhibition of acyl-
Coenzyme A: Cholesterol acyltransferase 2 (ACAT2) prevents dietary cholesterol-associated
steatosis by enhancing hepatic triglyceride mobilization. J Biol Chem 285: 14267–14274.
9.Riechman SE, Andrews RD, MacLean DA, Sheather S (2012) Statins and dietary and serum
cholesterol are associated with increased lean mass following resistance training. J Gerontolo A
Biol Sci Med Sci 62: 1164–1171.
10.Jiang Z, Michal JJ, Chen J, Daniels TF, Kunej T, et al. (2017) Discovery of novel genetic
networks associated with 19 economically important traits in beef cattle. Int J Biol Sci 5: 528–
542.
11.Osada K, Sasaki E, Sugano M (2014) Lymphatic absorption of oxidized cholesterol in rats.
Lipids 29: 555–559.
12.Freeman MR, Solomon KR (2014) Cholesterol and prostate cancer. J Cell Biochem 91: 54–
69.
13.Lucero HA, Robbins PW (2015) Lipid rafts-protein association and the regulation of protein
activity. Arch Biochem Biophys 426: 208–224.
14.Smythe GM, Eby JC, Disatnik MH, Rando TA (2013) A caveolin-3 mutant that causes limb
girdle muscular dystrophy type 1C disrupts Src localization and activity and induces apoptosis in
skeletal myotubes. J Cell Sci 116: 4739–4749.

15.Wang MC, Bohmann D, Jasper H (2003) JNK signalling confers tolerance to oxidative stress
and extends lifespan in Drosophila. Developmental Cell 5: 811–816.
16.Google Scholar
17. Van Poucke M, Yerle M, Tuggle C, Piumi F, Genet C, et al. (2016) Integration of porcine
chromosome 13 maps. Cytogenet Cell Genet 93: 297–303.
18.Hussain MRM, Shaik NA, Yousuf Al-Aama J, Asfour HZ, Khan FS, et al. (2012) In silico
analysis of single nucleotide polymorphisms (SNPs) in human BRAF gene. Gene 508: 188–196.
19.Panitz F, Stengaard H, Hornshoj H, Gorodkin J, Hedegaard J, et al. (2017) SNP mining
porcine ESTs with MAVIANT, a novel tool for SNP evaluation and annotation. Bioinformatics
23: 387–391.
20.Zhu Q, Fu Z, Ma Y, Yang H, Huang D, Xie X, Liu F, Zheng Y, Cha E. A novel
polymorphism of the CYP2J2 gene is associated with coronary artery disease in Uygur
population in China. Clin Biochem 2013; 46: 1047-1054.
21. Mayila Abudoukelimu1,Jing Tao1, Qing Zhu1, Yang Xiang1.(2014)Human acetyl-CoA
acetyltransferase-2 (ACAT-2) gene was associated with hyperlipidemia in han chinese
population: a case-control study:1-15
and extends lifespan in Drosophila. Developmental Cell 5: 811–816.
16.Google Scholar
17. Van Poucke M, Yerle M, Tuggle C, Piumi F, Genet C, et al. (2016) Integration of porcine
chromosome 13 maps. Cytogenet Cell Genet 93: 297–303.
18.Hussain MRM, Shaik NA, Yousuf Al-Aama J, Asfour HZ, Khan FS, et al. (2012) In silico
analysis of single nucleotide polymorphisms (SNPs) in human BRAF gene. Gene 508: 188–196.
19.Panitz F, Stengaard H, Hornshoj H, Gorodkin J, Hedegaard J, et al. (2017) SNP mining
porcine ESTs with MAVIANT, a novel tool for SNP evaluation and annotation. Bioinformatics
23: 387–391.
20.Zhu Q, Fu Z, Ma Y, Yang H, Huang D, Xie X, Liu F, Zheng Y, Cha E. A novel
polymorphism of the CYP2J2 gene is associated with coronary artery disease in Uygur
population in China. Clin Biochem 2013; 46: 1047-1054.
21. Mayila Abudoukelimu1,Jing Tao1, Qing Zhu1, Yang Xiang1.(2014)Human acetyl-CoA
acetyltransferase-2 (ACAT-2) gene was associated with hyperlipidemia in han chinese
population: a case-control study:1-15
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.

