Evidence-Based Protocols for Acute Stroke: A Critical Article Review
VerifiedAdded on 2023/06/11
|12
|1611
|394
Report
AI Summary
This report presents a detailed review and critique of a research article focusing on the implementation of evidence-based treatment protocols for managing fever, hyperglycaemia, and swallowing dysfunction in acute stroke patients. The reviewed study, the Quality in Acute Stroke Care (QASC) trial, is a single-blind cluster randomised controlled trial conducted in New South Wales, Australia, assessing patient outcomes 90 days post-hospital admission following a multidisciplinary intervention. The review includes an overview of the study's background, objectives, methodology, and key findings, emphasizing the significance of multidisciplinary teamwork and nursing-led protocols in improving patient outcomes. The report also incorporates a CASP checklist assessment, evaluating the study's validity, results, and applicability, alongside a discussion on the rigour of the reported evidence and its clinical implications. The review concludes by highlighting the importance of the FeSS intervention in enhancing stroke unit care and improving patient outcomes, noting its limitations and generalizability.

Running head: ARTICLE REVIEW
ARTICLE REVIEW
Name of the Student:
Name of the University:
Author Note:
ARTICLE REVIEW
Name of the Student:
Name of the University:
Author Note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1ARTICLE REVIEW
Title
A cluster randomised controlled trial of implementation of evidence-based treatment
protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke.
Abstract
A single-blind cluster randomised controlled trail was performed using randomised ASUs in
New South Wales, Australia. Patient outcomes were assessed after 90 days of hospital
admission, followed by a multidisciplinary intervention. The study results report that,
intervention ASU patients were significantly likely to be dead or dependent at a lesser rate
(mRS ≥2) at 90 days compared to control ASU patients which was 236 [42%] of 558
patients in the intervention group compared to 259 individuals [58%] of 449 in the control
group. This was irrespective of the stroke. Multidisciplinary supported evidence-based
protocols initiated by nurses was implemented for the management of fever, hyperglycaemia,
and swallowing dysfunction. This was successful in delivering better patient outcomes after
getting discharge from stroke units.
Introduction
Background and objectives
The responsibility of the multidisciplinary team is high in acute stroke patients hence the
Quality in Acute Stroke Care (QASC) study was designed. It assessed the effect of the team
in conducting an interactive education programme to implement evidence-based treatment
protocols for the management of fever, hyperglycaemia, and swallowing dysfunction on
patient outcomes 90 days after admission for stroke
Methods
Title
A cluster randomised controlled trial of implementation of evidence-based treatment
protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke.
Abstract
A single-blind cluster randomised controlled trail was performed using randomised ASUs in
New South Wales, Australia. Patient outcomes were assessed after 90 days of hospital
admission, followed by a multidisciplinary intervention. The study results report that,
intervention ASU patients were significantly likely to be dead or dependent at a lesser rate
(mRS ≥2) at 90 days compared to control ASU patients which was 236 [42%] of 558
patients in the intervention group compared to 259 individuals [58%] of 449 in the control
group. This was irrespective of the stroke. Multidisciplinary supported evidence-based
protocols initiated by nurses was implemented for the management of fever, hyperglycaemia,
and swallowing dysfunction. This was successful in delivering better patient outcomes after
getting discharge from stroke units.
Introduction
Background and objectives
The responsibility of the multidisciplinary team is high in acute stroke patients hence the
Quality in Acute Stroke Care (QASC) study was designed. It assessed the effect of the team
in conducting an interactive education programme to implement evidence-based treatment
protocols for the management of fever, hyperglycaemia, and swallowing dysfunction on
patient outcomes 90 days after admission for stroke
Methods

2ARTICLE REVIEW
Trial design
A single-blind cluster randomised controlled trail was performed. Prior to allocation a pre-
intervention patient cohort was recruited. After implementation d a second post-intervention
patient cohort to provide a follow-up sample after intervention implementation.
Participants
The location of was the large and tertiary referral centres in the region of New South Wales
(NSW), Australia, which provided care for stroke patients who are in a geographically
defined location with immediate CT access and on-site high dependency units. Eligibility
criteria included English speaking ability and age group of 18 and more. The patients should
have been previously diagnosed with ischaemic stroke or intracerebral haemorrhage. They
must be presented to the Acute Stoke Units within 48 hours of onset of symptoms. Possession
of telephone required and the patients should not be admitted to palliative care.
Interventions
Fever, Sugar, Swallowing (FeSS) intervention was conducted, which was for the ASU
clinicians. It addressed the barriers, reinforcing the multidisciplinary teamwork, adapting to
local problems and using site champions. Using help from Australia’s national clinical
guidelines for stroke, management protocols developed for fever, hyperglycaemia and
dysphagia for first 72 hours after admission to ASU. Care was given to nursing assessment
and bedside care. Site based interactive and didactic educational interventions conducted for
protocol discussions by clinicians. It was administered from May 15, 2007 to August 25,
2010.
Trial design
A single-blind cluster randomised controlled trail was performed. Prior to allocation a pre-
intervention patient cohort was recruited. After implementation d a second post-intervention
patient cohort to provide a follow-up sample after intervention implementation.
Participants
The location of was the large and tertiary referral centres in the region of New South Wales
(NSW), Australia, which provided care for stroke patients who are in a geographically
defined location with immediate CT access and on-site high dependency units. Eligibility
criteria included English speaking ability and age group of 18 and more. The patients should
have been previously diagnosed with ischaemic stroke or intracerebral haemorrhage. They
must be presented to the Acute Stoke Units within 48 hours of onset of symptoms. Possession
of telephone required and the patients should not be admitted to palliative care.
Interventions
Fever, Sugar, Swallowing (FeSS) intervention was conducted, which was for the ASU
clinicians. It addressed the barriers, reinforcing the multidisciplinary teamwork, adapting to
local problems and using site champions. Using help from Australia’s national clinical
guidelines for stroke, management protocols developed for fever, hyperglycaemia and
dysphagia for first 72 hours after admission to ASU. Care was given to nursing assessment
and bedside care. Site based interactive and didactic educational interventions conducted for
protocol discussions by clinicians. It was administered from May 15, 2007 to August 25,
2010.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3ARTICLE REVIEW
Outcomes
Primary outcomes
Death or dependency (dependency: modified Rankin Scale (mRS) ≥2)17, functional
dependency, mean SF-36 mental component summary (MCS) and mean physical component
summary (PCS)
Conducted after 90 days of hospital admission
Secondary outcomes
Mean temperature for the first 72 h after acute stroke unit (ASU) admission, the mean of
finger-prick blood glucose for the first 72 h after ASU admission, proportion of swallowing
screening undertaken within the first 24 h of ASU admission, discharge diagnosis of
aspiration pneumonia and length of hospital stay
Included processes of care
Sample size
The initial sample size consisted of 20 clusters
Outcomes
Primary outcomes
Death or dependency (dependency: modified Rankin Scale (mRS) ≥2)17, functional
dependency, mean SF-36 mental component summary (MCS) and mean physical component
summary (PCS)
Conducted after 90 days of hospital admission
Secondary outcomes
Mean temperature for the first 72 h after acute stroke unit (ASU) admission, the mean of
finger-prick blood glucose for the first 72 h after ASU admission, proportion of swallowing
screening undertaken within the first 24 h of ASU admission, discharge diagnosis of
aspiration pneumonia and length of hospital stay
Included processes of care
Sample size
The initial sample size consisted of 20 clusters
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4ARTICLE REVIEW
Randomisation:
Sequence generation
Random allocation sequence was generated by stratifying into categories in which pre- -intervention
cohort patients were recruited.
Allocation concealment
High recruiters resented more than two patients per month and low recruiters desired to or less patients.
Random number generating software used to randomise within the strata. To conceal allocation
de-identified stratification details were provided.
Implementation
Random allocation sequence was generated by independent statisticians. Participants were enrolled by
the clinical research assistants and the research assistants assigned participants to interventions.
Blinding
The research assistants were blinded to the trial aims, design, and group allocation, those who were
involved in telephonic interviews and group allocation. The trial statisticians were also blinded to the
group allocations.
Statistical methods
Randomisation:
Sequence generation
Random allocation sequence was generated by stratifying into categories in which pre- -intervention
cohort patients were recruited.
Allocation concealment
High recruiters resented more than two patients per month and low recruiters desired to or less patients.
Random number generating software used to randomise within the strata. To conceal allocation
de-identified stratification details were provided.
Implementation
Random allocation sequence was generated by independent statisticians. Participants were enrolled by
the clinical research assistants and the research assistants assigned participants to interventions.
Blinding
The research assistants were blinded to the trial aims, design, and group allocation, those who were
involved in telephonic interviews and group allocation. The trial statisticians were also blinded to the
group allocations.
Statistical methods
5ARTICLE REVIEW
SAS v9·2 software was used to compare groups for primary and secondary outcomes.
Conventional descriptive statistics used for subgroup analyses. A logistic regression model was implemented for
dichotomous outcomes and a random intercept linear regression model for continuous out comes.
Results
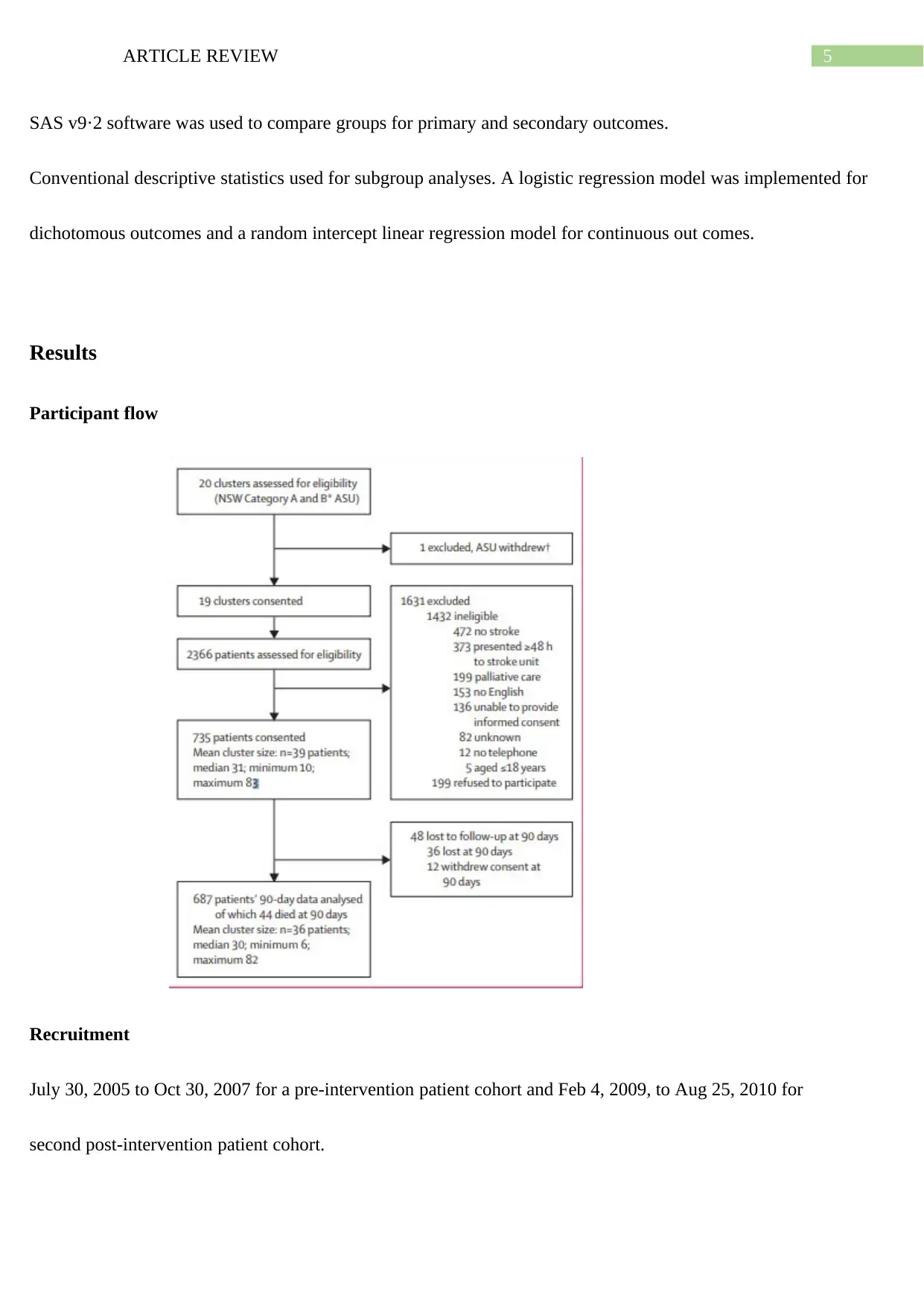
Participant flow
Recruitment
July 30, 2005 to Oct 30, 2007 for a pre-intervention patient cohort and Feb 4, 2009, to Aug 25, 2010 for
second post-intervention patient cohort.
SAS v9·2 software was used to compare groups for primary and secondary outcomes.
Conventional descriptive statistics used for subgroup analyses. A logistic regression model was implemented for
dichotomous outcomes and a random intercept linear regression model for continuous out comes.
Results
Participant flow
Recruitment
July 30, 2005 to Oct 30, 2007 for a pre-intervention patient cohort and Feb 4, 2009, to Aug 25, 2010 for
second post-intervention patient cohort.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
6ARTICLE REVIEW
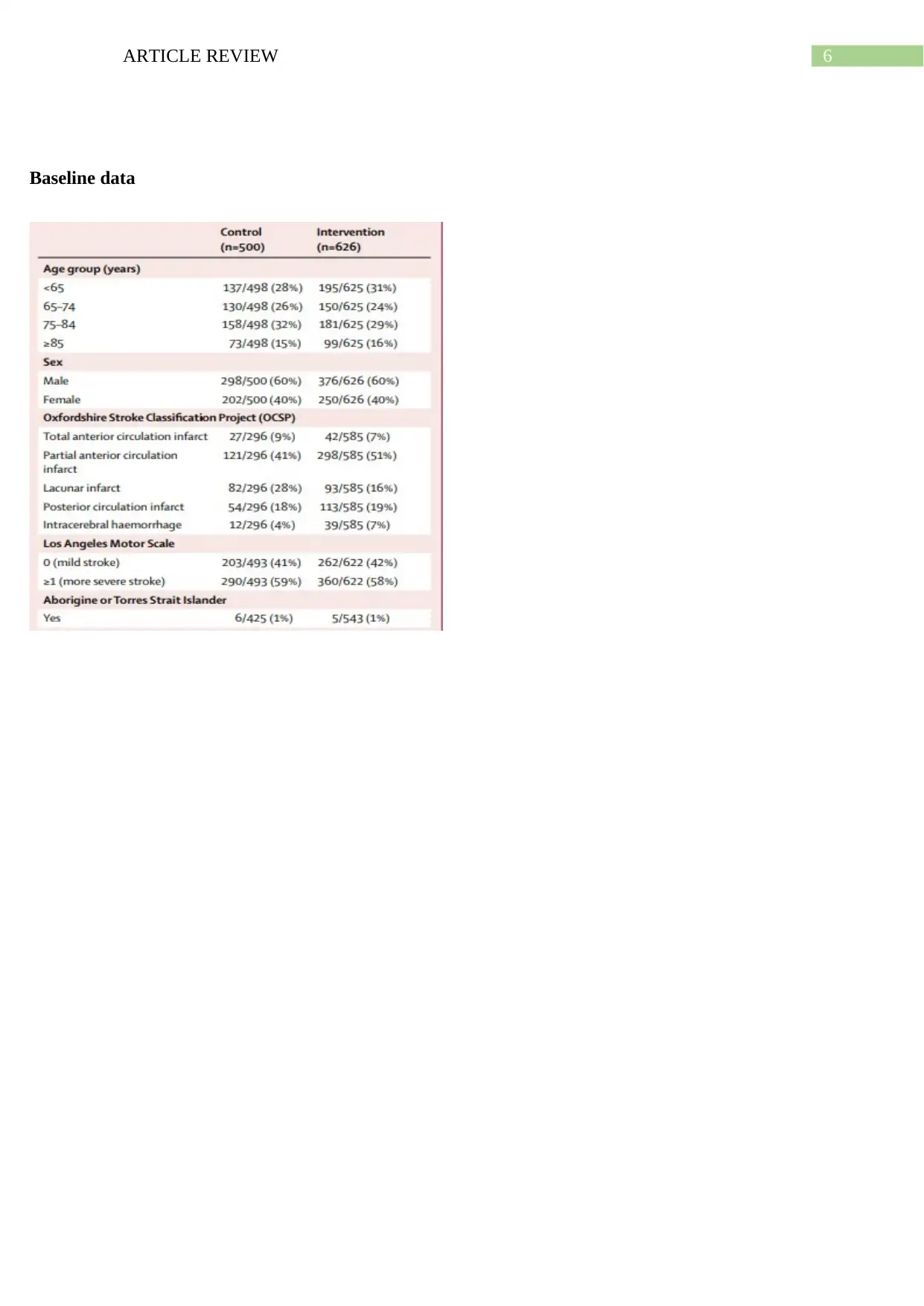
Baseline data
Baseline data
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
7ARTICLE REVIEW
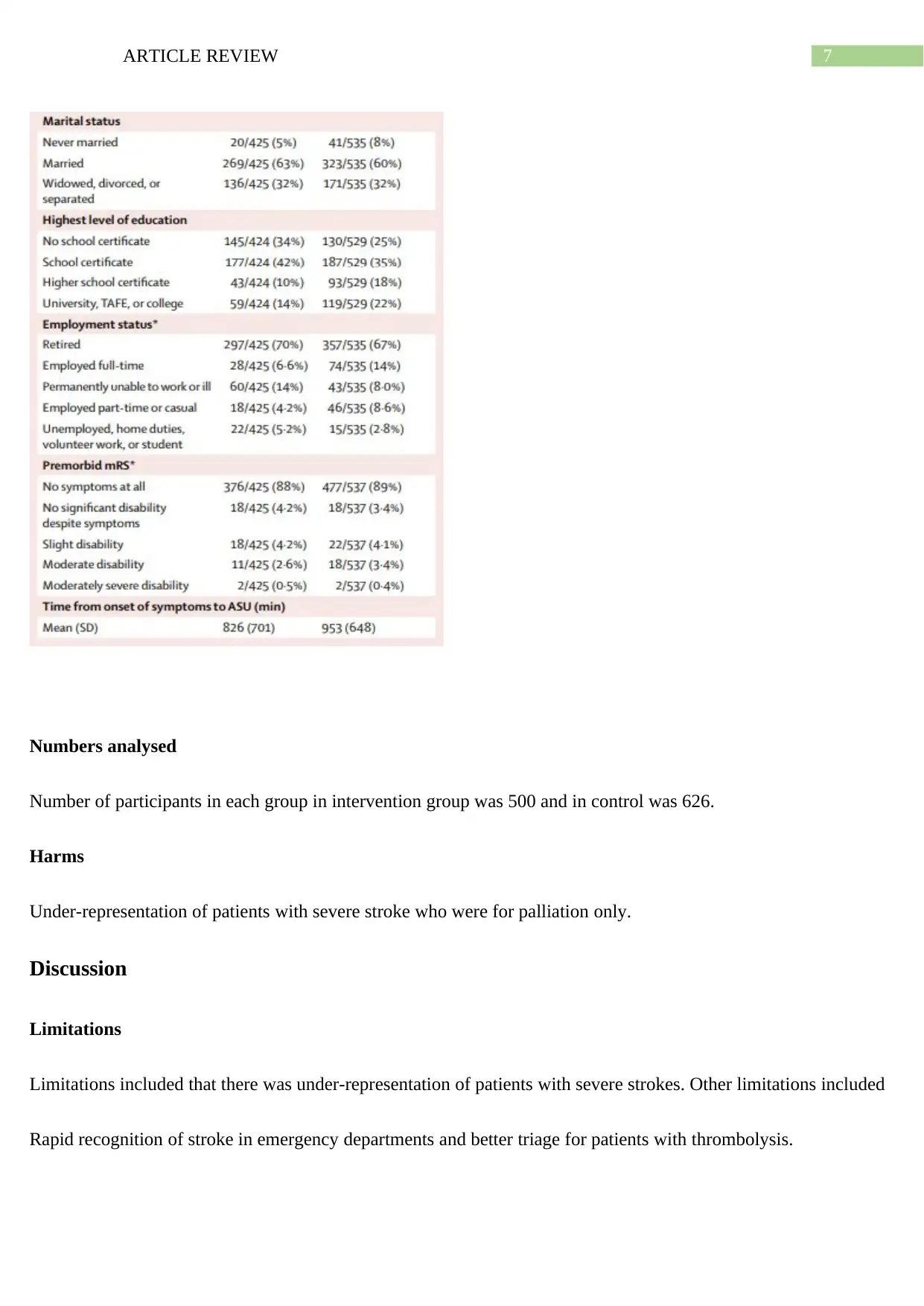
Numbers analysed
Number of participants in each group in intervention group was 500 and in control was 626.
Harms
Under-representation of patients with severe stroke who were for palliation only.
Discussion
Limitations
Limitations included that there was under-representation of patients with severe strokes. Other limitations included
Rapid recognition of stroke in emergency departments and better triage for patients with thrombolysis.
Numbers analysed
Number of participants in each group in intervention group was 500 and in control was 626.
Harms
Under-representation of patients with severe stroke who were for palliation only.
Discussion
Limitations
Limitations included that there was under-representation of patients with severe strokes. Other limitations included
Rapid recognition of stroke in emergency departments and better triage for patients with thrombolysis.

8ARTICLE REVIEW
Generalisability
This study was focused on the ASU patients hence cannot be applicable for the stoke patients in
the general medical wards.
Interpretation
From the paper it was interpreted that with better management of fever, hyperglycaemia, and swallowing
in acute stroke patients during the initial 72 h of admission to an ASU, can lead to decreased rates of death,
dependency, and improved processes of care. This paper was one of first paper to discuss that good nursing
care effected death and dependency. It also showed that implementation of trials was helpful in addressing the
acute stroke in the stroke unit network in Australia using a multidisciplinary team. The intervention was important
since it augmented the benefits of a stroke unit care.
Other information
Protocol
The protocol can be accessed using the link http:// www.acu.edu.au/qasc
Funding
The study was funded by the National Health & Medical Research Council, St Vincent’s Clinic Foundation,
the Curran Foundation, Australian Diabetes Society-Servicer, the College of Nursing, and Australian Catholic
University. Additional support was provided by f the Stroke Unit Directors, Stroke Clinical Nurse Consultants,
Clinical Nurse Educators, Stroke Liaison Nurses, Stroke Unit Coordinators, Clinical Research Assistants.
The funders had no role in designing the study or data collection and analysis.
Generalisability
This study was focused on the ASU patients hence cannot be applicable for the stoke patients in
the general medical wards.
Interpretation
From the paper it was interpreted that with better management of fever, hyperglycaemia, and swallowing
in acute stroke patients during the initial 72 h of admission to an ASU, can lead to decreased rates of death,
dependency, and improved processes of care. This paper was one of first paper to discuss that good nursing
care effected death and dependency. It also showed that implementation of trials was helpful in addressing the
acute stroke in the stroke unit network in Australia using a multidisciplinary team. The intervention was important
since it augmented the benefits of a stroke unit care.
Other information
Protocol
The protocol can be accessed using the link http:// www.acu.edu.au/qasc
Funding
The study was funded by the National Health & Medical Research Council, St Vincent’s Clinic Foundation,
the Curran Foundation, Australian Diabetes Society-Servicer, the College of Nursing, and Australian Catholic
University. Additional support was provided by f the Stroke Unit Directors, Stroke Clinical Nurse Consultants,
Clinical Nurse Educators, Stroke Liaison Nurses, Stroke Unit Coordinators, Clinical Research Assistants.
The funders had no role in designing the study or data collection and analysis.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9ARTICLE REVIEW
CASP Checklist
1. The trail successfully addressed a clearly focussed issue in terms of sample population, given intervention,
comparator given and the considered outcomes.
2. The assignment was randomised as the allocation sequence was concealed from the researchers and patients.
3. All of the patients who entered the trial were accounted properly
4. The patients, health workers and study personnel were blinded to the treatment.
5. All the groups were similar.
6. All the groups were treated equally.
7. Both primary and secondary outcomes were measured.
8. The estimates were very precise.
9. The results cannot be applied to local population since the results are not generalised and is applicable
only for acute stroke unit patients. It is not applicable for the local patients of the general medical wards.
10. Clinically important outcomes were considered however more care could have given to prompt recognition
of stroke in emergency departments.
11. The benefits are worth the harms and costs since it is beneficial to the stroke patients who are unable
to access immediate stroke unit care.
Rigour of result
CASP Checklist
1. The trail successfully addressed a clearly focussed issue in terms of sample population, given intervention,
comparator given and the considered outcomes.
2. The assignment was randomised as the allocation sequence was concealed from the researchers and patients.
3. All of the patients who entered the trial were accounted properly
4. The patients, health workers and study personnel were blinded to the treatment.
5. All the groups were similar.
6. All the groups were treated equally.
7. Both primary and secondary outcomes were measured.
8. The estimates were very precise.
9. The results cannot be applied to local population since the results are not generalised and is applicable
only for acute stroke unit patients. It is not applicable for the local patients of the general medical wards.
10. Clinically important outcomes were considered however more care could have given to prompt recognition
of stroke in emergency departments.
11. The benefits are worth the harms and costs since it is beneficial to the stroke patients who are unable
to access immediate stroke unit care.
Rigour of result
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10ARTICLE REVIEW
The result of the research successfully demonstrated that multidisciplinary intervention was required for supporting
The evidence based management of fever, hyper glycaemia, and swallowing. The results clearly showed
significance for a period of 90 days after admission to ASU. The clinical significance is appreciable
especially when compared to other established clinical and organisational interventions. It was highly
evident from the study that 15.7 % improvement occurred after the implementation of FeSS intervention,
which proved to be of immediate importance to the clinicians, patients, and the carers of the patients.
The methods applied were also significant since large units of patients were considered for the study.
While a sensitive analysis was undertaken the death and dependency results remained significant.
Both 90 days patient outcomes and processes of care was considered which proved to be exceptional in
stroke research.
Bibliography
CASP Checklists - CASP - Critical Appraisal Skills Programme. (2018).
Retrieved from https://casp-uk.net/casp-tools-checklists/
Consort - Welcome to the CONSORT Website. (2018). Retrieved from http://www.consort-statement.org/
University, A. (2018). Arizona State University | Ranked #1 university in the US for innovation | ASU.
Retrieved from https://www.asu.edu/
Quality in Acute Stroke Care (QASC) Trial - ACU (Australian Catholic University). (2018).
Retrieved from https://www.acu.edu.au/about_acu/faculties,_institutes_and_centres/health_sciences/research/
The result of the research successfully demonstrated that multidisciplinary intervention was required for supporting
The evidence based management of fever, hyper glycaemia, and swallowing. The results clearly showed
significance for a period of 90 days after admission to ASU. The clinical significance is appreciable
especially when compared to other established clinical and organisational interventions. It was highly
evident from the study that 15.7 % improvement occurred after the implementation of FeSS intervention,
which proved to be of immediate importance to the clinicians, patients, and the carers of the patients.
The methods applied were also significant since large units of patients were considered for the study.
While a sensitive analysis was undertaken the death and dependency results remained significant.
Both 90 days patient outcomes and processes of care was considered which proved to be exceptional in
stroke research.
Bibliography
CASP Checklists - CASP - Critical Appraisal Skills Programme. (2018).
Retrieved from https://casp-uk.net/casp-tools-checklists/
Consort - Welcome to the CONSORT Website. (2018). Retrieved from http://www.consort-statement.org/
University, A. (2018). Arizona State University | Ranked #1 university in the US for innovation | ASU.
Retrieved from https://www.asu.edu/
Quality in Acute Stroke Care (QASC) Trial - ACU (Australian Catholic University). (2018).
Retrieved from https://www.acu.edu.au/about_acu/faculties,_institutes_and_centres/health_sciences/research/

11ARTICLE REVIEW
quality_in_acute_stroke_care
quality_in_acute_stroke_care
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 12
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
