Addition of bromine to transcinnamic acid.
VerifiedAdded on 2023/01/16
|9
|2050
|61
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

1
DATE \@ "MMMM d" \*
MERGEFORMAT April 8
Farah Darwiche
17801863
Addition of
bromine to trans-
cinnamic acid
DATE \@ "MMMM d" \*
MERGEFORMAT April 8
Farah Darwiche
17801863
Addition of
bromine to trans-
cinnamic acid
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
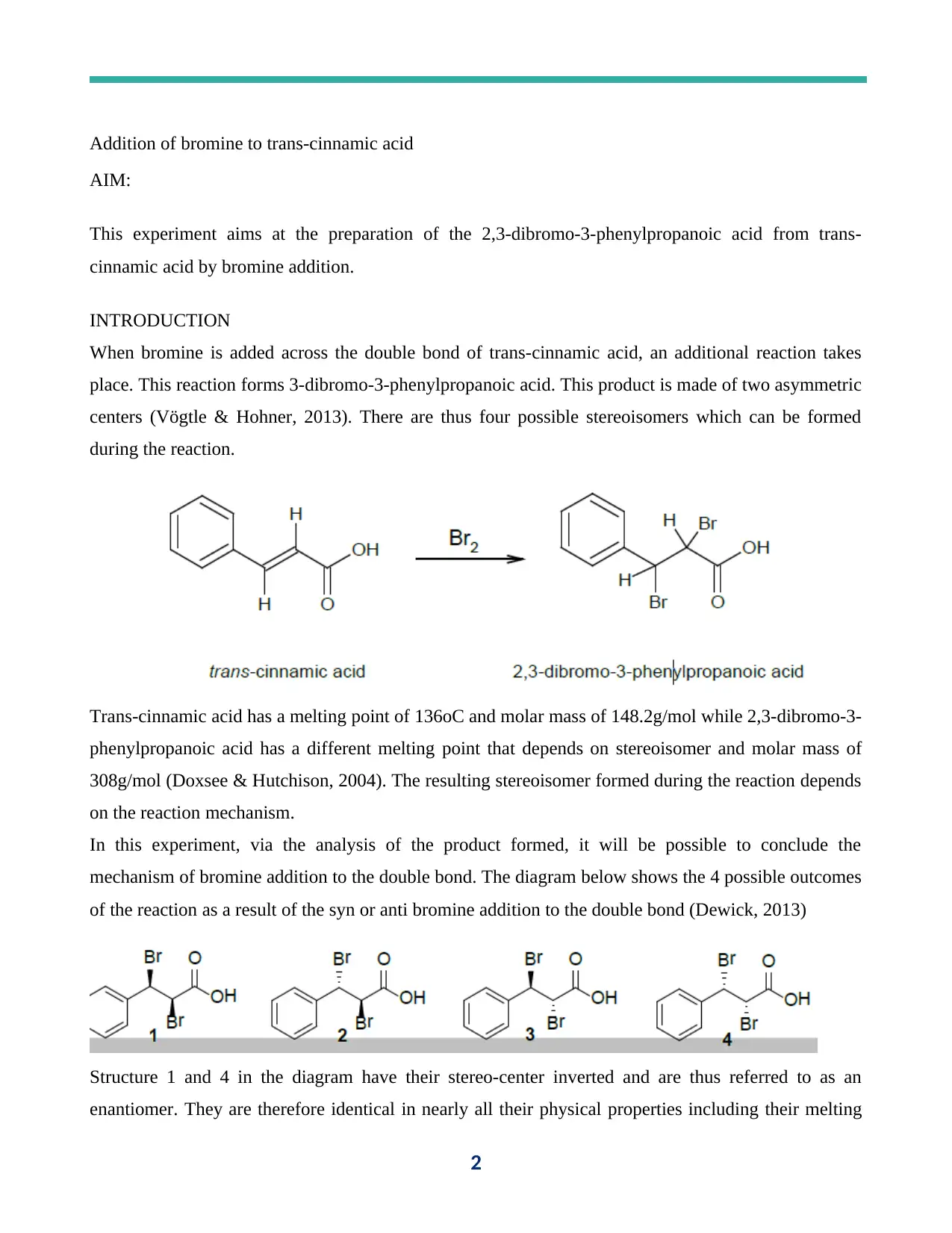
Addition of bromine to trans-cinnamic acid
AIM:
This experiment aims at the preparation of the 2,3-dibromo-3-phenylpropanoic acid from trans-
cinnamic acid by bromine addition.
INTRODUCTION
When bromine is added across the double bond of trans-cinnamic acid, an additional reaction takes
place. This reaction forms 3-dibromo-3-phenylpropanoic acid. This product is made of two asymmetric
centers (Vögtle & Hohner, 2013). There are thus four possible stereoisomers which can be formed
during the reaction.
Trans-cinnamic acid has a melting point of 136oC and molar mass of 148.2g/mol while 2,3-dibromo-3-
phenylpropanoic acid has a different melting point that depends on stereoisomer and molar mass of
308g/mol (Doxsee & Hutchison, 2004). The resulting stereoisomer formed during the reaction depends
on the reaction mechanism.
In this experiment, via the analysis of the product formed, it will be possible to conclude the
mechanism of bromine addition to the double bond. The diagram below shows the 4 possible outcomes
of the reaction as a result of the syn or anti bromine addition to the double bond (Dewick, 2013)
Structure 1 and 4 in the diagram have their stereo-center inverted and are thus referred to as an
enantiomer. They are therefore identical in nearly all their physical properties including their melting
2
AIM:
This experiment aims at the preparation of the 2,3-dibromo-3-phenylpropanoic acid from trans-
cinnamic acid by bromine addition.
INTRODUCTION
When bromine is added across the double bond of trans-cinnamic acid, an additional reaction takes
place. This reaction forms 3-dibromo-3-phenylpropanoic acid. This product is made of two asymmetric
centers (Vögtle & Hohner, 2013). There are thus four possible stereoisomers which can be formed
during the reaction.
Trans-cinnamic acid has a melting point of 136oC and molar mass of 148.2g/mol while 2,3-dibromo-3-
phenylpropanoic acid has a different melting point that depends on stereoisomer and molar mass of
308g/mol (Doxsee & Hutchison, 2004). The resulting stereoisomer formed during the reaction depends
on the reaction mechanism.
In this experiment, via the analysis of the product formed, it will be possible to conclude the
mechanism of bromine addition to the double bond. The diagram below shows the 4 possible outcomes
of the reaction as a result of the syn or anti bromine addition to the double bond (Dewick, 2013)
Structure 1 and 4 in the diagram have their stereo-center inverted and are thus referred to as an
enantiomer. They are therefore identical in nearly all their physical properties including their melting
2

point. Similarly, structures 2 and 3 are also enantiomers and have similar properties (Barton, Ollis, &
Sammes, 2009). Structure 1, 2 and 3 are diastereomers which have different physical properties.
EXPERIMENTAL PROCEDURE
1.2g of trans-cinnamic acid was placed in a 50 ml round bottom flask. 10 ml of dichloromethane and
4.0mL of 10% bromine were then added to the dichloromethane solution. A condenser was then
attached to the top of the flask. The condenser and flask were then clamped such that the flask sat on
the water bath whose temperature was between 45-50oC because no water was needed to re-condense
dichloromethane because of its low boiling point. The reaction mixture was then heated for 30
minutes. The product began to precipitate as the reaction proceeded. 10% bromine was added to
dichloromethane solution dropwise via the top of the condenser until the light orange color persisted
when bromine color disappeared during this period.
The reaction flask was then cooled to room temperature and then cooled further in an ice-water for 10
minutes. The crude product was then collected via vacuum filtration in a Buckner funnel. The crystals
were then washed 3 times with a 5ml portion of ice cold dichloromethane by disconnecting the
vacuum, pouring the solvent over the crystal and then reconnecting the vacuum. The product was then
air dried for 5 minutes. The yield and the melting point of the final product were then measured. The
IR spectrum of the final product was also recorded and then analyzed using the spectral data.
RESULTS AND OBSERVATIONS
Theoretical yield: (1.2g trans-cinnamic acid) (1 mol reactant 148.1586g/mol) (1 mol product/1
mol reactant) (307.696g/ 1 Mol product)
Number of mole of trans-cinnamic acid= 1.2g/148.1586g/mol
= 0.008099 mol
mass of product = moles of limiting reagent x MW
= 0.008099 X 307.696
M= 2.49g
1.98g was collected from the vacuum filtration.
Percent yield: 1.98g actual / 2.49 theoretical * 100%
= 79.5%
The melting point of my product was 200°C.
3
Sammes, 2009). Structure 1, 2 and 3 are diastereomers which have different physical properties.
EXPERIMENTAL PROCEDURE
1.2g of trans-cinnamic acid was placed in a 50 ml round bottom flask. 10 ml of dichloromethane and
4.0mL of 10% bromine were then added to the dichloromethane solution. A condenser was then
attached to the top of the flask. The condenser and flask were then clamped such that the flask sat on
the water bath whose temperature was between 45-50oC because no water was needed to re-condense
dichloromethane because of its low boiling point. The reaction mixture was then heated for 30
minutes. The product began to precipitate as the reaction proceeded. 10% bromine was added to
dichloromethane solution dropwise via the top of the condenser until the light orange color persisted
when bromine color disappeared during this period.
The reaction flask was then cooled to room temperature and then cooled further in an ice-water for 10
minutes. The crude product was then collected via vacuum filtration in a Buckner funnel. The crystals
were then washed 3 times with a 5ml portion of ice cold dichloromethane by disconnecting the
vacuum, pouring the solvent over the crystal and then reconnecting the vacuum. The product was then
air dried for 5 minutes. The yield and the melting point of the final product were then measured. The
IR spectrum of the final product was also recorded and then analyzed using the spectral data.
RESULTS AND OBSERVATIONS
Theoretical yield: (1.2g trans-cinnamic acid) (1 mol reactant 148.1586g/mol) (1 mol product/1
mol reactant) (307.696g/ 1 Mol product)
Number of mole of trans-cinnamic acid= 1.2g/148.1586g/mol
= 0.008099 mol
mass of product = moles of limiting reagent x MW
= 0.008099 X 307.696
M= 2.49g
1.98g was collected from the vacuum filtration.
Percent yield: 1.98g actual / 2.49 theoretical * 100%
= 79.5%
The melting point of my product was 200°C.
3
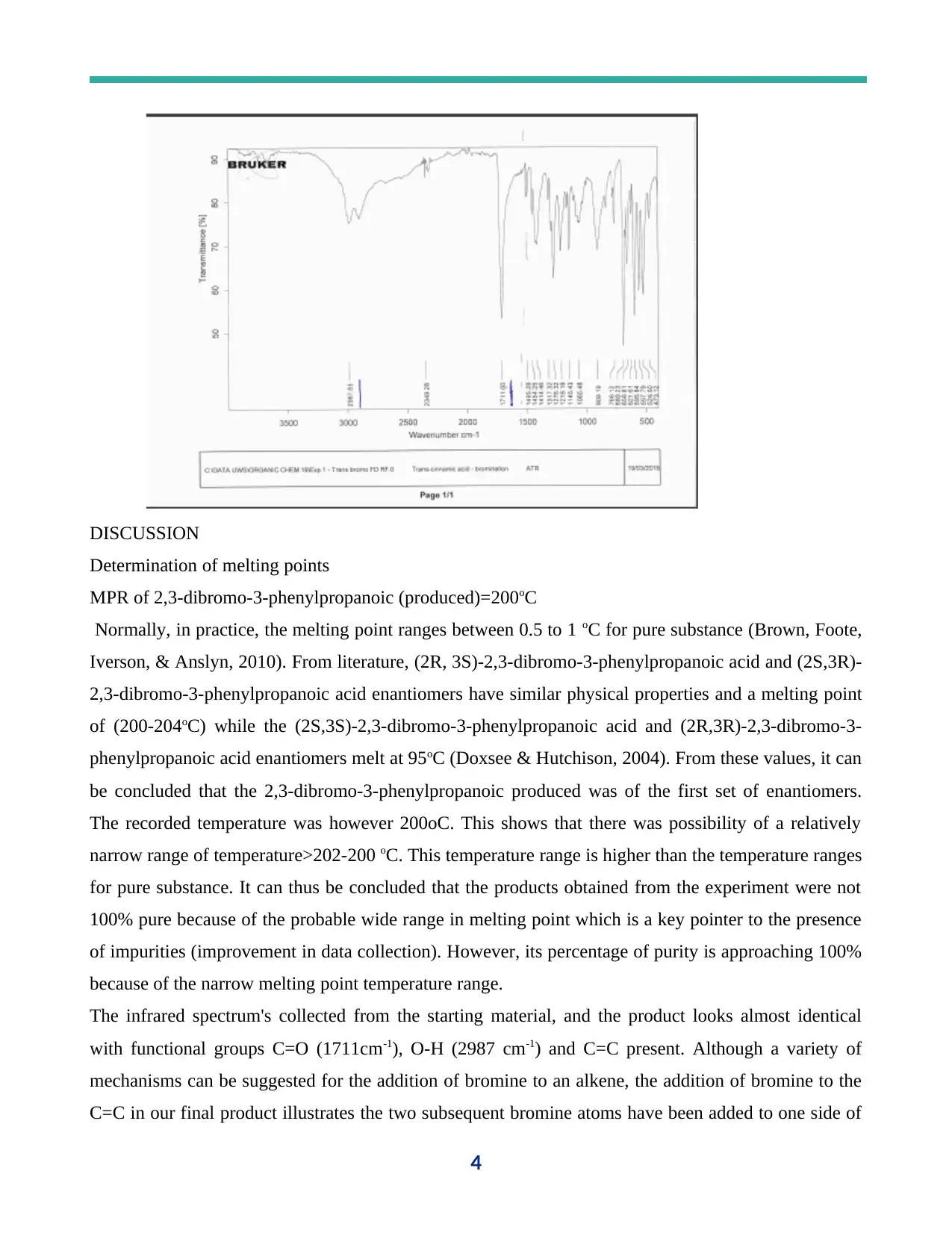
DISCUSSION
Determination of melting points
MPR of 2,3-dibromo-3-phenylpropanoic (produced)=200oC
Normally, in practice, the melting point ranges between 0.5 to 1 oC for pure substance (Brown, Foote,
Iverson, & Anslyn, 2010). From literature, (2R, 3S)-2,3-dibromo-3-phenylpropanoic acid and (2S,3R)-
2,3-dibromo-3-phenylpropanoic acid enantiomers have similar physical properties and a melting point
of (200-204oC) while the (2S,3S)-2,3-dibromo-3-phenylpropanoic acid and (2R,3R)-2,3-dibromo-3-
phenylpropanoic acid enantiomers melt at 95oC (Doxsee & Hutchison, 2004). From these values, it can
be concluded that the 2,3-dibromo-3-phenylpropanoic produced was of the first set of enantiomers.
The recorded temperature was however 200oC. This shows that there was possibility of a relatively
narrow range of temperature>202-200 oC. This temperature range is higher than the temperature ranges
for pure substance. It can thus be concluded that the products obtained from the experiment were not
100% pure because of the probable wide range in melting point which is a key pointer to the presence
of impurities (improvement in data collection). However, its percentage of purity is approaching 100%
because of the narrow melting point temperature range.
The infrared spectrum's collected from the starting material, and the product looks almost identical
with functional groups C=O (1711cm-1), O-H (2987 cm-1) and C=C present. Although a variety of
mechanisms can be suggested for the addition of bromine to an alkene, the addition of bromine to the
C=C in our final product illustrates the two subsequent bromine atoms have been added to one side of
4
Determination of melting points
MPR of 2,3-dibromo-3-phenylpropanoic (produced)=200oC
Normally, in practice, the melting point ranges between 0.5 to 1 oC for pure substance (Brown, Foote,
Iverson, & Anslyn, 2010). From literature, (2R, 3S)-2,3-dibromo-3-phenylpropanoic acid and (2S,3R)-
2,3-dibromo-3-phenylpropanoic acid enantiomers have similar physical properties and a melting point
of (200-204oC) while the (2S,3S)-2,3-dibromo-3-phenylpropanoic acid and (2R,3R)-2,3-dibromo-3-
phenylpropanoic acid enantiomers melt at 95oC (Doxsee & Hutchison, 2004). From these values, it can
be concluded that the 2,3-dibromo-3-phenylpropanoic produced was of the first set of enantiomers.
The recorded temperature was however 200oC. This shows that there was possibility of a relatively
narrow range of temperature>202-200 oC. This temperature range is higher than the temperature ranges
for pure substance. It can thus be concluded that the products obtained from the experiment were not
100% pure because of the probable wide range in melting point which is a key pointer to the presence
of impurities (improvement in data collection). However, its percentage of purity is approaching 100%
because of the narrow melting point temperature range.
The infrared spectrum's collected from the starting material, and the product looks almost identical
with functional groups C=O (1711cm-1), O-H (2987 cm-1) and C=C present. Although a variety of
mechanisms can be suggested for the addition of bromine to an alkene, the addition of bromine to the
C=C in our final product illustrates the two subsequent bromine atoms have been added to one side of
4
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
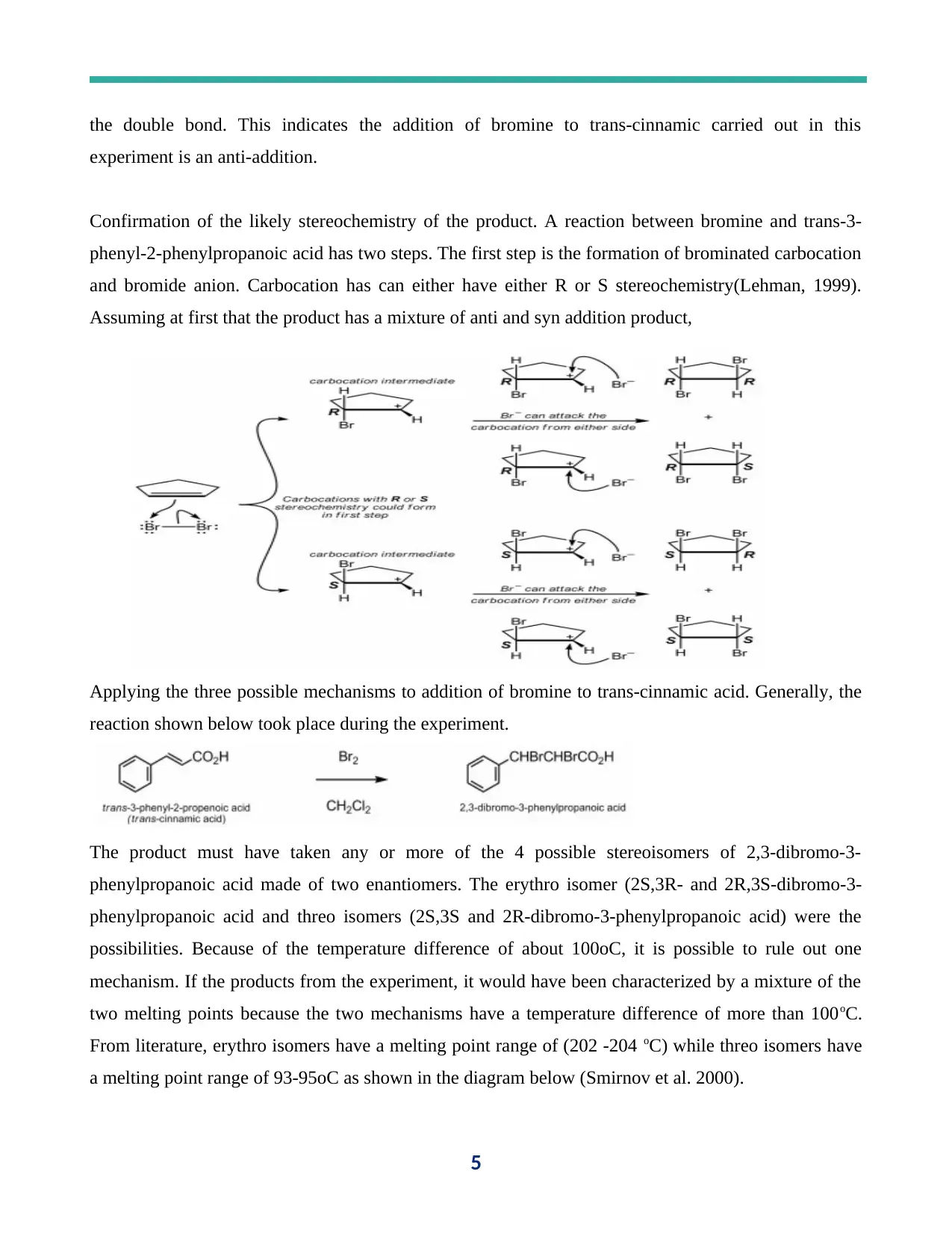
the double bond. This indicates the addition of bromine to trans-cinnamic carried out in this
experiment is an anti-addition.
Confirmation of the likely stereochemistry of the product. A reaction between bromine and trans-3-
phenyl-2-phenylpropanoic acid has two steps. The first step is the formation of brominated carbocation
and bromide anion. Carbocation has can either have either R or S stereochemistry(Lehman, 1999).
Assuming at first that the product has a mixture of anti and syn addition product,
Applying the three possible mechanisms to addition of bromine to trans-cinnamic acid. Generally, the
reaction shown below took place during the experiment.
The product must have taken any or more of the 4 possible stereoisomers of 2,3-dibromo-3-
phenylpropanoic acid made of two enantiomers. The erythro isomer (2S,3R- and 2R,3S-dibromo-3-
phenylpropanoic acid and threo isomers (2S,3S and 2R-dibromo-3-phenylpropanoic acid) were the
possibilities. Because of the temperature difference of about 100oC, it is possible to rule out one
mechanism. If the products from the experiment, it would have been characterized by a mixture of the
two melting points because the two mechanisms have a temperature difference of more than 100oC.
From literature, erythro isomers have a melting point range of (202 -204 oC) while threo isomers have
a melting point range of 93-95oC as shown in the diagram below (Smirnov et al. 2000).
5
experiment is an anti-addition.
Confirmation of the likely stereochemistry of the product. A reaction between bromine and trans-3-
phenyl-2-phenylpropanoic acid has two steps. The first step is the formation of brominated carbocation
and bromide anion. Carbocation has can either have either R or S stereochemistry(Lehman, 1999).
Assuming at first that the product has a mixture of anti and syn addition product,
Applying the three possible mechanisms to addition of bromine to trans-cinnamic acid. Generally, the
reaction shown below took place during the experiment.
The product must have taken any or more of the 4 possible stereoisomers of 2,3-dibromo-3-
phenylpropanoic acid made of two enantiomers. The erythro isomer (2S,3R- and 2R,3S-dibromo-3-
phenylpropanoic acid and threo isomers (2S,3S and 2R-dibromo-3-phenylpropanoic acid) were the
possibilities. Because of the temperature difference of about 100oC, it is possible to rule out one
mechanism. If the products from the experiment, it would have been characterized by a mixture of the
two melting points because the two mechanisms have a temperature difference of more than 100oC.
From literature, erythro isomers have a melting point range of (202 -204 oC) while threo isomers have
a melting point range of 93-95oC as shown in the diagram below (Smirnov et al. 2000).
5

Since the melting point of 3-dibromo-3-phenylpropanoic acid formed in the experiment is 200oC, it is
conclusive to say that our product was (2S,3R) and (2R,3S) stereochemistry.
Percentage yield of 79.5% was realized in this experiment. This a relatively low amount of yield
compared to the theoretical expectation. This low yield could be attributed to various factors such as
loss of product during the procedure. The following activities would have led to loss 2,3-dibromo-3-
phenylpropanoic produced in the experiment; loss of crystals during glass transfers on the filter paper
during filtration, competition among the various sides of reaction, failure of the recrystallization
process to be done in time before the filtration process. Cases of mass loss in the purification of crude
material is also another source of error in the experiment.
Failure to let reaction complete is also another possible reason for such as low yield. There was a
possibility of collecting the crude product before all the 1.2g of trans-cinnamic acid reacted. Some
products might have also been lost when the products was being washed with an ice-cold CH2Cl2. Ice
cold CH2Cl2 was used because at this temperature, the solubility of the product is extremely low and
nearly none of the product is dissolved in CH2Cl2.
In order to recover the products in the filtrate, the procedure can be improved using a re-crystallization
process. In order to enhance the entire procedure and have a higher percentage yield, the crystals
should be recovered from the crude product using recrystallization using substances such as ascorbic
acid.
The melting point was obtained as 200oC. From the literature, this temperature corresponds to (2R,2S)
and (2S,3R) 2,3-dibromo-3-phenylpropanoic acid whose temperature ranges from 202 to 204oC
CONCLUSION
In a nutshell, we were able to preparation 2,3-dibromo-3-phenylpropanoic acid from trans-cinnamic
acid by bromine addition. Compared to the theoretical expectation, the prepared product had a percent
6
conclusive to say that our product was (2S,3R) and (2R,3S) stereochemistry.
Percentage yield of 79.5% was realized in this experiment. This a relatively low amount of yield
compared to the theoretical expectation. This low yield could be attributed to various factors such as
loss of product during the procedure. The following activities would have led to loss 2,3-dibromo-3-
phenylpropanoic produced in the experiment; loss of crystals during glass transfers on the filter paper
during filtration, competition among the various sides of reaction, failure of the recrystallization
process to be done in time before the filtration process. Cases of mass loss in the purification of crude
material is also another source of error in the experiment.
Failure to let reaction complete is also another possible reason for such as low yield. There was a
possibility of collecting the crude product before all the 1.2g of trans-cinnamic acid reacted. Some
products might have also been lost when the products was being washed with an ice-cold CH2Cl2. Ice
cold CH2Cl2 was used because at this temperature, the solubility of the product is extremely low and
nearly none of the product is dissolved in CH2Cl2.
In order to recover the products in the filtrate, the procedure can be improved using a re-crystallization
process. In order to enhance the entire procedure and have a higher percentage yield, the crystals
should be recovered from the crude product using recrystallization using substances such as ascorbic
acid.
The melting point was obtained as 200oC. From the literature, this temperature corresponds to (2R,2S)
and (2S,3R) 2,3-dibromo-3-phenylpropanoic acid whose temperature ranges from 202 to 204oC
CONCLUSION
In a nutshell, we were able to preparation 2,3-dibromo-3-phenylpropanoic acid from trans-cinnamic
acid by bromine addition. Compared to the theoretical expectation, the prepared product had a percent
6

yield of 79.5 %. The discrepancy from the theoretical expectation was attributed to the sources of mass
wastage mentioned in the discussion section and probable termination of the experiment before the
competition of the reaction. Analysis of the reactant and product IR spectrum showed the reactant and
products had nearly similar bond characteristics in the infrared spectrum. The product had a relatively
narrow melting point temperature indicating that our product was almost pure. Stereochemistry
analysis of the 3-dibromo-3-phenylpropanoic acid formed suggest that the product was (2S, 3R) and
(2R, 3S) stereochemistry. The experiment was thus a success because the objective was fully met.
References
Barton, D., Ollis, W. D., & Sammes, P. G. (2009). Comprehensive Organic Chemistry: The Synthesis
and Reactions of Organic Compounds. Heterocyclic compounds. Vol. 4.
Brown, W. H., Foote, C. S., Iverson, B. L., & Anslyn, E. (2011). Organic Chemistry. Boston, MA:
Cengage Learning.
Dewick, P. M. (2013). Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal
Chemistry and Biological Chemistry. Hoboken, NJ: John Wiley & Sons.
Doxsee, K. M., & Hutchison, J. E. (2004). Bromination of an Alkene: Preparation of Stilbene
Dibromide In Green Organic Chemistry – Strategies, Tools, and Laboratory Experiments;
Thomson Brooks/Cole. Pacific Grove.
Lehman, J. W. (1999). Operational Organic Chemistry: A Problem-Solving Approach to the
Laboratory Course, 3rd ed; Upper Saddle River pp 175-181. Prentice Hall.
Smirnov, V. V., Zelikman, V. M., Beletskaya, I. P., Levitskii, M. M., & Kazankova, M. A. (2000).
Selective bromination of alkanes and arylalkanes with CBr4. Mendeleev Communications,
10(5), 175-176.
Smit, W. A., Bochkov, A. F., & Caple, R. (1998). Organic Synthesis: The Science Behind the Art.
London, England: Royal Society of Chemistry.
Vögtle, F., & Hohner, G. (2013). Stereochemistry of multibridge, multilayered, and multi-stepped
aromatic compounds — Transannular steric and electronic effects. Organic Compounds, 1-29.
7
wastage mentioned in the discussion section and probable termination of the experiment before the
competition of the reaction. Analysis of the reactant and product IR spectrum showed the reactant and
products had nearly similar bond characteristics in the infrared spectrum. The product had a relatively
narrow melting point temperature indicating that our product was almost pure. Stereochemistry
analysis of the 3-dibromo-3-phenylpropanoic acid formed suggest that the product was (2S, 3R) and
(2R, 3S) stereochemistry. The experiment was thus a success because the objective was fully met.
References
Barton, D., Ollis, W. D., & Sammes, P. G. (2009). Comprehensive Organic Chemistry: The Synthesis
and Reactions of Organic Compounds. Heterocyclic compounds. Vol. 4.
Brown, W. H., Foote, C. S., Iverson, B. L., & Anslyn, E. (2011). Organic Chemistry. Boston, MA:
Cengage Learning.
Dewick, P. M. (2013). Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal
Chemistry and Biological Chemistry. Hoboken, NJ: John Wiley & Sons.
Doxsee, K. M., & Hutchison, J. E. (2004). Bromination of an Alkene: Preparation of Stilbene
Dibromide In Green Organic Chemistry – Strategies, Tools, and Laboratory Experiments;
Thomson Brooks/Cole. Pacific Grove.
Lehman, J. W. (1999). Operational Organic Chemistry: A Problem-Solving Approach to the
Laboratory Course, 3rd ed; Upper Saddle River pp 175-181. Prentice Hall.
Smirnov, V. V., Zelikman, V. M., Beletskaya, I. P., Levitskii, M. M., & Kazankova, M. A. (2000).
Selective bromination of alkanes and arylalkanes with CBr4. Mendeleev Communications,
10(5), 175-176.
Smit, W. A., Bochkov, A. F., & Caple, R. (1998). Organic Synthesis: The Science Behind the Art.
London, England: Royal Society of Chemistry.
Vögtle, F., & Hohner, G. (2013). Stereochemistry of multibridge, multilayered, and multi-stepped
aromatic compounds — Transannular steric and electronic effects. Organic Compounds, 1-29.
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Prelab questions
1.
F
The mechanism in part C is anti=addition because the solvent approaches the chloride ion with
a backside orientation to produce the additional product. The interactoion can thus either occur
from either below or above and there is no stereochemical control for the reaction and the
enantiomers that are produced. In this case regioselectivity in a nucleophilic solvent is
comparable to oxymercuration-demerucuration pathway where the Cl-takes the role of
eletrophile that accepts pi electrons form the alkenes while simultaneously forming a bond with
vinyl carbon to form a chlorium ion
8
1.
F
The mechanism in part C is anti=addition because the solvent approaches the chloride ion with
a backside orientation to produce the additional product. The interactoion can thus either occur
from either below or above and there is no stereochemical control for the reaction and the
enantiomers that are produced. In this case regioselectivity in a nucleophilic solvent is
comparable to oxymercuration-demerucuration pathway where the Cl-takes the role of
eletrophile that accepts pi electrons form the alkenes while simultaneously forming a bond with
vinyl carbon to form a chlorium ion
8

2. The mass of trans-cinnamic acid used=1.2g.
Molar mass of trans-cinnamic acid=148.1586 g/mol
Mole ratio of reactant and product=1:1
Moles of trans-cinnamic acid =1.2g/148.1586g/mol =0.008099 mol =moles of 2,3-dibromo-3-
phenylpropanoic acid obtained.
Mass=moles* molar mass
Molar mass of 2,3-dibromo-3-phenylpropanoic acid=307.696g/mol
Masss of 2,3-dibromo-3-phenylpropanoic acid =307.696*0.008099=2.49g
9
Molar mass of trans-cinnamic acid=148.1586 g/mol
Mole ratio of reactant and product=1:1
Moles of trans-cinnamic acid =1.2g/148.1586g/mol =0.008099 mol =moles of 2,3-dibromo-3-
phenylpropanoic acid obtained.
Mass=moles* molar mass
Molar mass of 2,3-dibromo-3-phenylpropanoic acid=307.696g/mol
Masss of 2,3-dibromo-3-phenylpropanoic acid =307.696*0.008099=2.49g
9
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.

