CENG 4710 Environmental Control: Benzene Adsorption and BET Surface
VerifiedAdded on 2023/06/04
|6
|583
|313
Homework Assignment
AI Summary
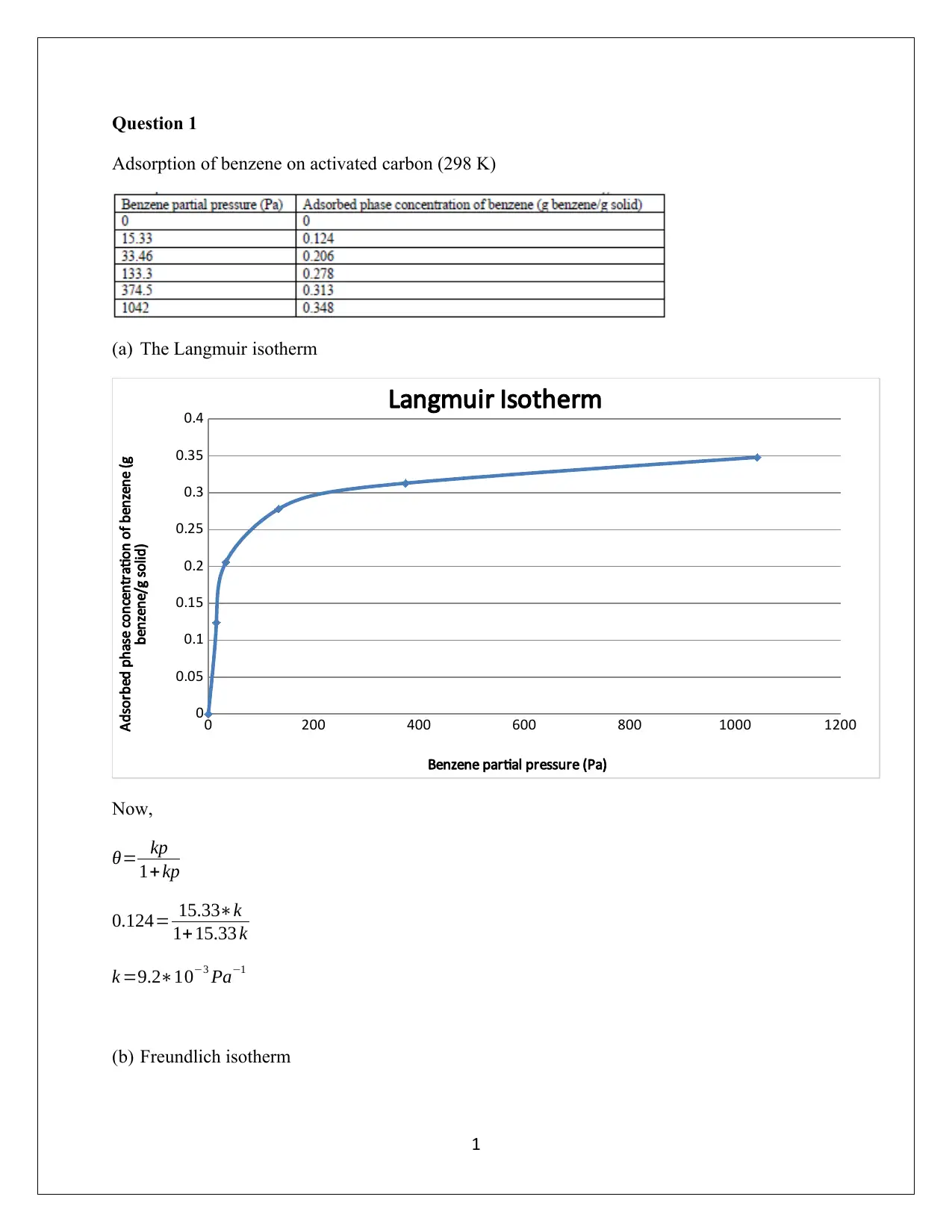
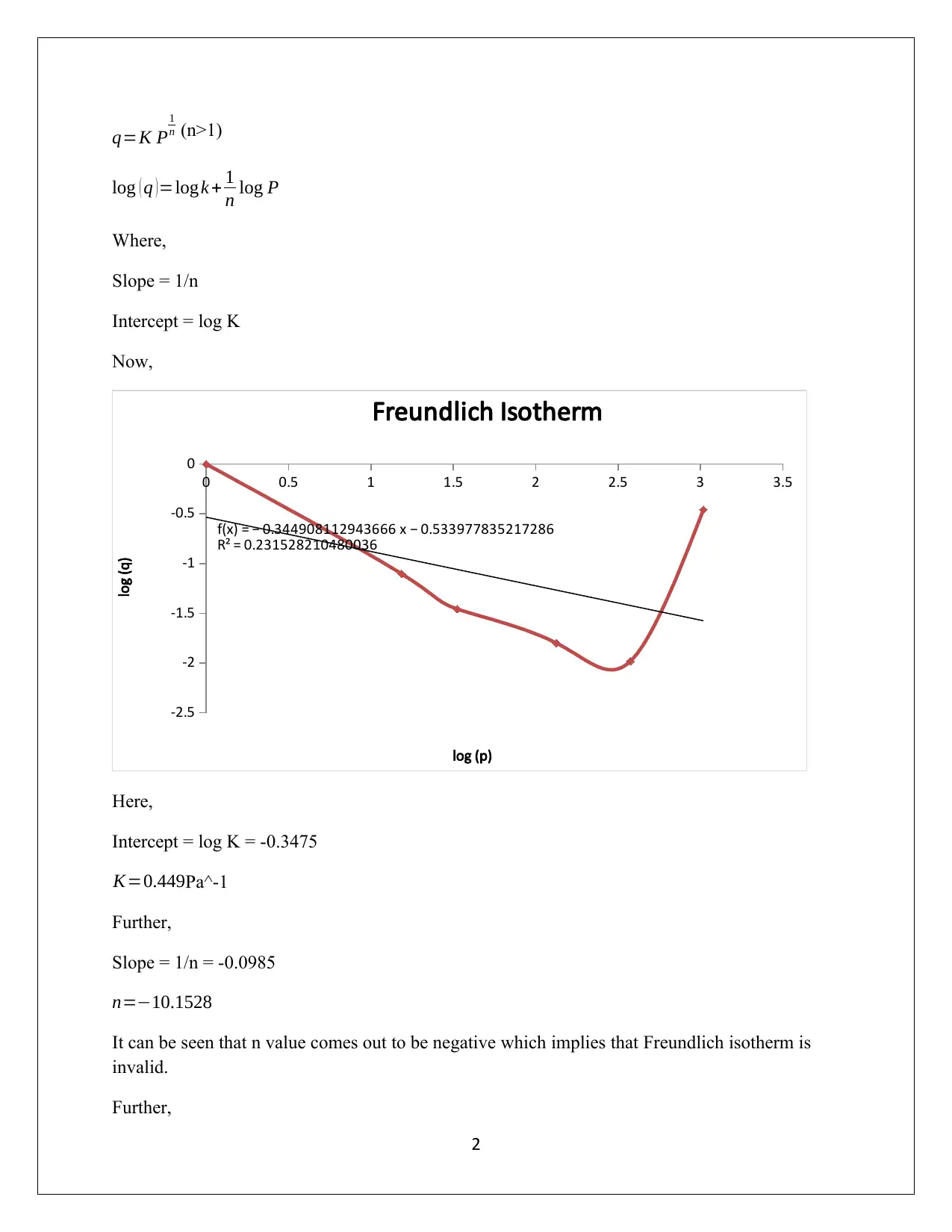
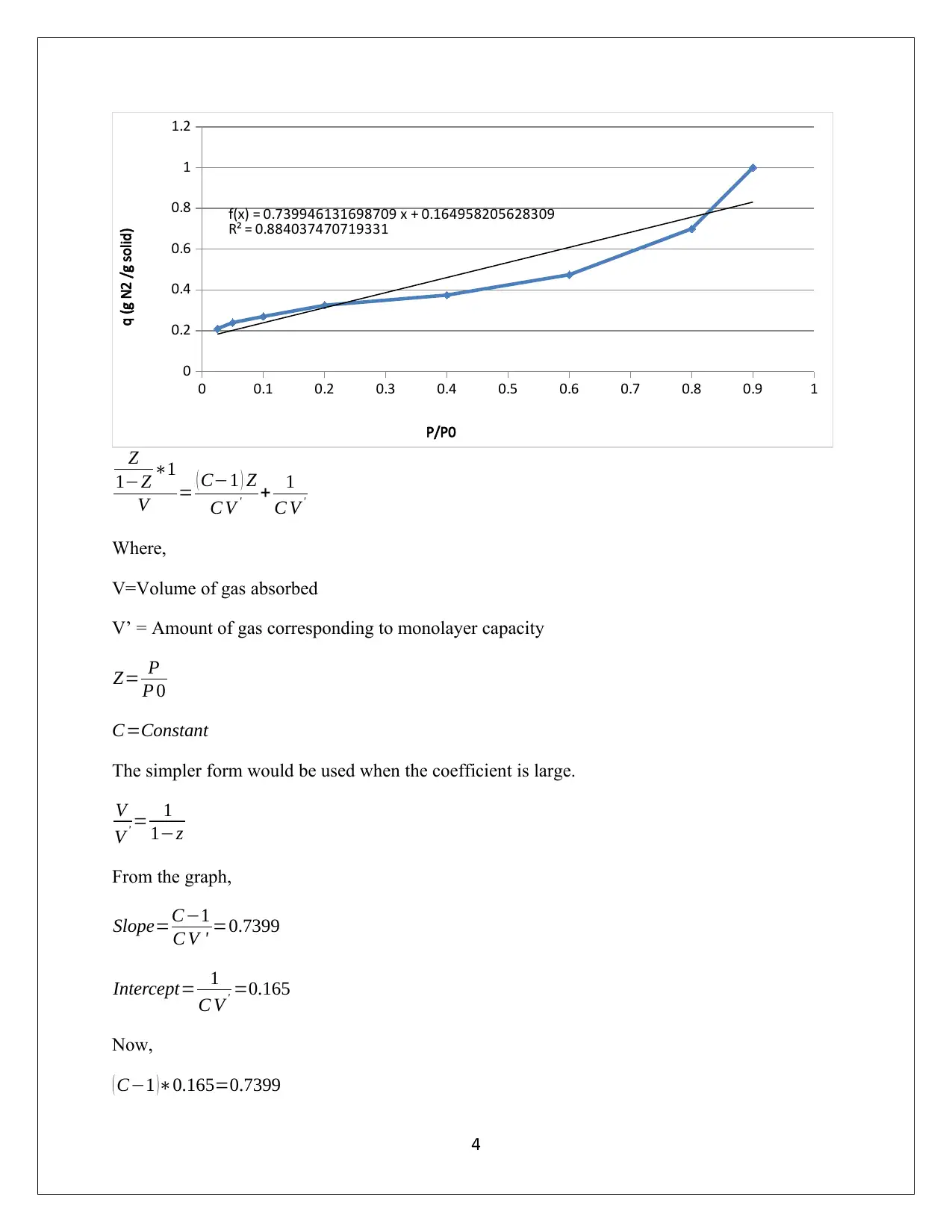
This assignment solution delves into the adsorption of benzene on activated carbon at varying conditions. It begins by fitting the Langmuir isotherm to experimental data at 298 K, estimating the parameters, followed by fitting the Freundlich isotherm and extracting its parameters, ultimately comparing the models. The analysis reveals the limitations of the Freundlich isotherm in this context. Further, the assignment explores the determination of the surface area of activated carbon using the BET equation based on nitrogen adsorption data at 77 K, discussing the limitations of the assumed isotherm model and the accuracy of the BET method under specific pressure conditions. The student provides detailed calculations and explanations for each part, highlighting the importance of understanding adsorption phenomena in environmental control.
1 out of 6




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)