Adverse Drug Reaction Case Studies - Pharmacology Assessment
VerifiedAdded on 2022/10/02
|4
|912
|453
Practical Assignment
AI Summary
This assignment involves analyzing four adverse drug reaction (ADR) case studies, focusing on drugs like indomethacin, cyclosporin A, sumatriptan, and sodium aurothiomalate. Students are tasked with explaining how each drug may have produced the observed effects, considering the EIDOS & DoTS classification systems to understand the underlying mechanisms. The analysis requires knowledge of drug distribution, intrinsic and extrinsic factors, outcomes, and potential sequelae. The cases cover various adverse events, including lethargy, stomach pains, changes in blood parameters, and chest pains, prompting students to identify potential drug-induced effects and consider alternative explanations. The assignment aims to enhance understanding of ADR mechanisms and classification, providing a practical application of pharmacological knowledge.

Adverse Drug Reaction Case Studies -
Student Briefing Document
Assessment 1
Description
This will be a group workshop but individually written-up. This work is
assessed.
Learning Objectives
To gain knowledge and understanding of the mechanisms underpinning
clinical cases of adverse drug reactions
To enable students to consolidate their understanding of adverse drug
reaction classification systems.
Student Tasks
You are provided with four (4) adverse drug reaction reports in section
3.1 and an EIDOS &DoTS classification table.
Each of the cases listed below describes an adverse event. Using your
knowledge, and the reference sources available, explain how the drug
may have produced the effects. Include in your answer a completed
EIDOS &DoTS table as this will aid your description of the likely
underlying mechanism. Indicate if there are alternative (non-drug
induced) explanations. The estimated time for this exercise is 60 minutes.
1
Student Briefing Document
Assessment 1
Description
This will be a group workshop but individually written-up. This work is
assessed.
Learning Objectives
To gain knowledge and understanding of the mechanisms underpinning
clinical cases of adverse drug reactions
To enable students to consolidate their understanding of adverse drug
reaction classification systems.
Student Tasks
You are provided with four (4) adverse drug reaction reports in section
3.1 and an EIDOS &DoTS classification table.
Each of the cases listed below describes an adverse event. Using your
knowledge, and the reference sources available, explain how the drug
may have produced the effects. Include in your answer a completed
EIDOS &DoTS table as this will aid your description of the likely
underlying mechanism. Indicate if there are alternative (non-drug
induced) explanations. The estimated time for this exercise is 60 minutes.
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Assessment Information
3.1 Adverse Drug Reaction Case Studies
1. A 65-year-old male subject is given indomethacin (50mg b.d.) for the
treatment of arthritis. After 4 weeks of therapy the subject attends their
regular clinic and complains of lethargy and stomach pains. A blood
test reveals a haemoglobin level of 8.2g/dL, an erythrocyte count of 2.5
x 1012/L and an MCV of 65fL.
2. A patient with psoriasis is treated with UVB (ultraviolet light) and
cyclosporin A (2.5 mg/kg/day). After 1 month of treatment routine
haematology reveals that plasma creatinine has increased from
100μmol/L to 515μmol/L and urea has increased from 70mmol/l to
147mmol/L. Blood lymphocyte count has decreased from 2.19 x 109/L
to 1.39 x 109/L and platelet count from 278x 109/L to 78 x 109/L.
3. A 55-year-old patient with a history of migraine is given sumatriptan
(6mg autoinjector) to use as soon as possible after the onset of a
migraine. One month later the patient is seen in casualty having
developed chest pains following administration of the drug. Their blood
pressure (at rest) is found to be slightly elevated (140/115 mmHg).
4. A 59-year-old lady with severe rheumatoid arthritis is receiving monthly
gold injections. Haematology reveals haemaglobin to be 10.5g/L, white
cell count to be 4 x 109/L (neutrophils 0.2x 109/L) and the platelet count
to be 80x109/L. She is also taking paracetamol for the relief of pain.
Abbreviations
B.d.: Twice Daily
DoTS: Dose-relation, Time-course, susceptibility factors
EIDOS:Extrinsic and Intrinsic moiety, Distribution, Outcome, Sequel
fL: femtoliters (fL, or 10-15L),
MCV: mean corpuscular volume, or "mean cell volume
2
3.1 Adverse Drug Reaction Case Studies
1. A 65-year-old male subject is given indomethacin (50mg b.d.) for the
treatment of arthritis. After 4 weeks of therapy the subject attends their
regular clinic and complains of lethargy and stomach pains. A blood
test reveals a haemoglobin level of 8.2g/dL, an erythrocyte count of 2.5
x 1012/L and an MCV of 65fL.
2. A patient with psoriasis is treated with UVB (ultraviolet light) and
cyclosporin A (2.5 mg/kg/day). After 1 month of treatment routine
haematology reveals that plasma creatinine has increased from
100μmol/L to 515μmol/L and urea has increased from 70mmol/l to
147mmol/L. Blood lymphocyte count has decreased from 2.19 x 109/L
to 1.39 x 109/L and platelet count from 278x 109/L to 78 x 109/L.
3. A 55-year-old patient with a history of migraine is given sumatriptan
(6mg autoinjector) to use as soon as possible after the onset of a
migraine. One month later the patient is seen in casualty having
developed chest pains following administration of the drug. Their blood
pressure (at rest) is found to be slightly elevated (140/115 mmHg).
4. A 59-year-old lady with severe rheumatoid arthritis is receiving monthly
gold injections. Haematology reveals haemaglobin to be 10.5g/L, white
cell count to be 4 x 109/L (neutrophils 0.2x 109/L) and the platelet count
to be 80x109/L. She is also taking paracetamol for the relief of pain.
Abbreviations
B.d.: Twice Daily
DoTS: Dose-relation, Time-course, susceptibility factors
EIDOS:Extrinsic and Intrinsic moiety, Distribution, Outcome, Sequel
fL: femtoliters (fL, or 10-15L),
MCV: mean corpuscular volume, or "mean cell volume
2
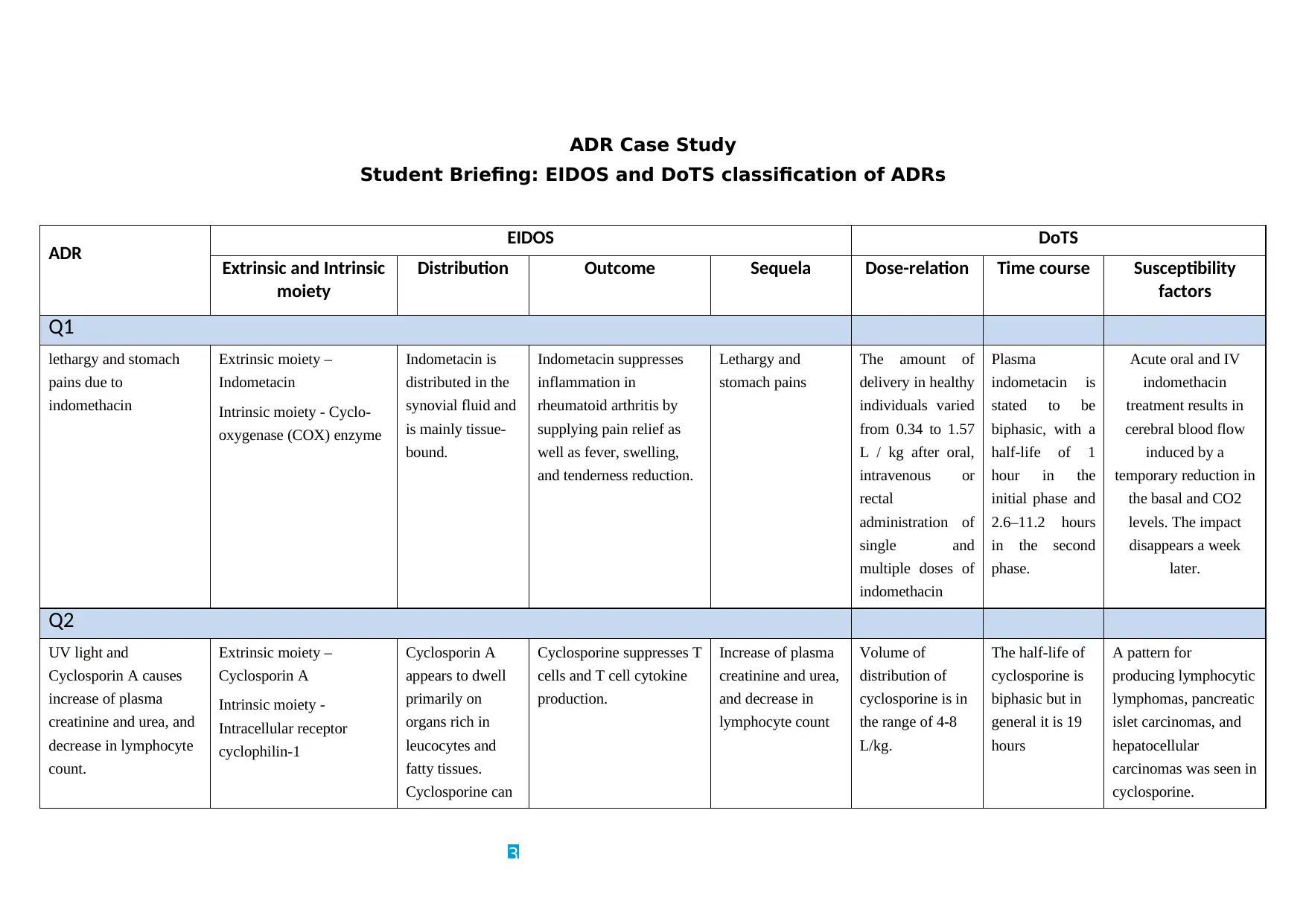
ADR Case Study
Student Briefing: EIDOS and DoTS classification of ADRs
ADR EIDOS DoTS
Extrinsic and Intrinsic
moiety
Distribution Outcome Sequela Dose-relation Time course Susceptibility
factors
Q1
lethargy and stomach
pains due to
indomethacin
Extrinsic moiety –
Indometacin
Intrinsic moiety - Cyclo-
oxygenase (COX) enzyme
Indometacin is
distributed in the
synovial fluid and
is mainly tissue-
bound.
Indometacin suppresses
inflammation in
rheumatoid arthritis by
supplying pain relief as
well as fever, swelling,
and tenderness reduction.
Lethargy and
stomach pains
The amount of
delivery in healthy
individuals varied
from 0.34 to 1.57
L / kg after oral,
intravenous or
rectal
administration of
single and
multiple doses of
indomethacin
Plasma
indometacin is
stated to be
biphasic, with a
half-life of 1
hour in the
initial phase and
2.6–11.2 hours
in the second
phase.
Acute oral and IV
indomethacin
treatment results in
cerebral blood flow
induced by a
temporary reduction in
the basal and CO2
levels. The impact
disappears a week
later.
Q2
UV light and
Cyclosporin A causes
increase of plasma
creatinine and urea, and
decrease in lymphocyte
count.
Extrinsic moiety –
Cyclosporin A
Intrinsic moiety -
Intracellular receptor
cyclophilin-1
Cyclosporin A
appears to dwell
primarily on
organs rich in
leucocytes and
fatty tissues.
Cyclosporine can
Cyclosporine suppresses T
cells and T cell cytokine
production.
Increase of plasma
creatinine and urea,
and decrease in
lymphocyte count
Volume of
distribution of
cyclosporine is in
the range of 4-8
L/kg.
The half-life of
cyclosporine is
biphasic but in
general it is 19
hours
A pattern for
producing lymphocytic
lymphomas, pancreatic
islet carcinomas, and
hepatocellular
carcinomas was seen in
cyclosporine.
3
Student Briefing: EIDOS and DoTS classification of ADRs
ADR EIDOS DoTS
Extrinsic and Intrinsic
moiety
Distribution Outcome Sequela Dose-relation Time course Susceptibility
factors
Q1
lethargy and stomach
pains due to
indomethacin
Extrinsic moiety –
Indometacin
Intrinsic moiety - Cyclo-
oxygenase (COX) enzyme
Indometacin is
distributed in the
synovial fluid and
is mainly tissue-
bound.
Indometacin suppresses
inflammation in
rheumatoid arthritis by
supplying pain relief as
well as fever, swelling,
and tenderness reduction.
Lethargy and
stomach pains
The amount of
delivery in healthy
individuals varied
from 0.34 to 1.57
L / kg after oral,
intravenous or
rectal
administration of
single and
multiple doses of
indomethacin
Plasma
indometacin is
stated to be
biphasic, with a
half-life of 1
hour in the
initial phase and
2.6–11.2 hours
in the second
phase.
Acute oral and IV
indomethacin
treatment results in
cerebral blood flow
induced by a
temporary reduction in
the basal and CO2
levels. The impact
disappears a week
later.
Q2
UV light and
Cyclosporin A causes
increase of plasma
creatinine and urea, and
decrease in lymphocyte
count.
Extrinsic moiety –
Cyclosporin A
Intrinsic moiety -
Intracellular receptor
cyclophilin-1
Cyclosporin A
appears to dwell
primarily on
organs rich in
leucocytes and
fatty tissues.
Cyclosporine can
Cyclosporine suppresses T
cells and T cell cytokine
production.
Increase of plasma
creatinine and urea,
and decrease in
lymphocyte count
Volume of
distribution of
cyclosporine is in
the range of 4-8
L/kg.
The half-life of
cyclosporine is
biphasic but in
general it is 19
hours
A pattern for
producing lymphocytic
lymphomas, pancreatic
islet carcinomas, and
hepatocellular
carcinomas was seen in
cyclosporine.
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
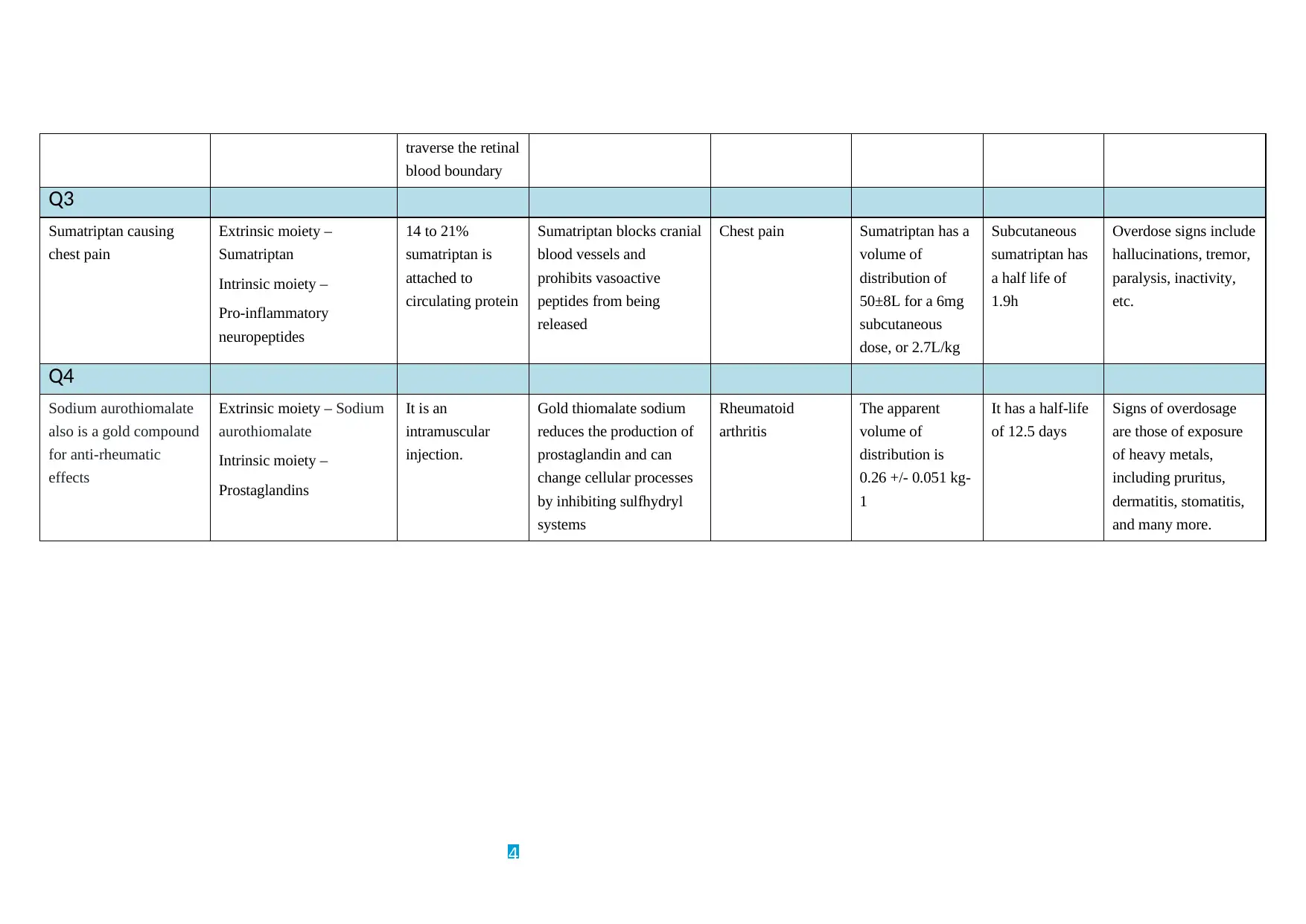
traverse the retinal
blood boundary
Q3
Sumatriptan causing
chest pain
Extrinsic moiety –
Sumatriptan
Intrinsic moiety –
Pro-inflammatory
neuropeptides
14 to 21%
sumatriptan is
attached to
circulating protein
Sumatriptan blocks cranial
blood vessels and
prohibits vasoactive
peptides from being
released
Chest pain Sumatriptan has a
volume of
distribution of
50±8L for a 6mg
subcutaneous
dose, or 2.7L/kg
Subcutaneous
sumatriptan has
a half life of
1.9h
Overdose signs include
hallucinations, tremor,
paralysis, inactivity,
etc.
Q4
Sodium aurothiomalate
also is a gold compound
for anti-rheumatic
effects
Extrinsic moiety – Sodium
aurothiomalate
Intrinsic moiety –
Prostaglandins
It is an
intramuscular
injection.
Gold thiomalate sodium
reduces the production of
prostaglandin and can
change cellular processes
by inhibiting sulfhydryl
systems
Rheumatoid
arthritis
The apparent
volume of
distribution is
0.26 +/- 0.051 kg-
1
It has a half-life
of 12.5 days
Signs of overdosage
are those of exposure
of heavy metals,
including pruritus,
dermatitis, stomatitis,
and many more.
4
blood boundary
Q3
Sumatriptan causing
chest pain
Extrinsic moiety –
Sumatriptan
Intrinsic moiety –
Pro-inflammatory
neuropeptides
14 to 21%
sumatriptan is
attached to
circulating protein
Sumatriptan blocks cranial
blood vessels and
prohibits vasoactive
peptides from being
released
Chest pain Sumatriptan has a
volume of
distribution of
50±8L for a 6mg
subcutaneous
dose, or 2.7L/kg
Subcutaneous
sumatriptan has
a half life of
1.9h
Overdose signs include
hallucinations, tremor,
paralysis, inactivity,
etc.
Q4
Sodium aurothiomalate
also is a gold compound
for anti-rheumatic
effects
Extrinsic moiety – Sodium
aurothiomalate
Intrinsic moiety –
Prostaglandins
It is an
intramuscular
injection.
Gold thiomalate sodium
reduces the production of
prostaglandin and can
change cellular processes
by inhibiting sulfhydryl
systems
Rheumatoid
arthritis
The apparent
volume of
distribution is
0.26 +/- 0.051 kg-
1
It has a half-life
of 12.5 days
Signs of overdosage
are those of exposure
of heavy metals,
including pruritus,
dermatitis, stomatitis,
and many more.
4
1 out of 4
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.