Assay of Alkaline Phosphatase in Serum Samples - Practical Schedule
VerifiedAdded on 2023/05/30
|7
|1578
|270
AI Summary
This practical schedule explains how to measure the activity of alkaline phosphatase and determine the presence of elevated levels of alkaline phosphatase in serum samples. It includes essential reading, introduction, methods, and results. The practical will be assessed and contributes 10% of the grade for the module.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

SCIU1LS Practical Schedule for practical 6
The laboratory assay II: Alkaline phosphatase in serum
Registration Number
Lab Session - Tues pm
The laboratory assay II: Alkaline phosphatase in serum
Registration Number
Lab Session - Tues pm
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Practical 6 SCIU1LS
Assay of the activity of the enzyme alkaline phosphatase present in
serum samples
NOTE- this practical will be assessed and contributes 10% of the grade
for the module. This sheet must be completed, together with
embedded Excel graphs (instructions for this are included in the
materials on Canvas) and must be submitted to Canvas by 12:00 noon
on 26/11/2018
Aims of practical
(i) measure activity of alkaline phosphatase
(ii) determine presence of elevated levels of alkaline phosphatase in serum samples
Essential reading
(i) Campbell (9th Edition), Chapter 8, pp198-203 ( Enzymes)
(ii) Practical Skills in Biological & Environmental Sciences (2011)
Chapter 21 Working with liquids
Chapter 22 Basic laboratory procedures
Chapter 50 Calibration
Page 247-248 Types of assay
Chapter 59 Basic Spectroscopy
(iii) Foundation Mathematics for Biosciences (Bryson)
Chapters on Enzyme kinetics and Graphs, Trendlines and Equations
Introduction
Alkaline phosphatase is a very widely distributed enzyme. As the name implies it catalyses
(speeds up) the hydrolysis of phosphate esters (e.g. glucose phosphate, ATP etc.) under alkaline
conditions, i.e. at pH values above 7. Although the physiological function of the enzyme is not
properly understood, the assay of the enzyme in samples of serum is very commonly performed in
biochemistry laboratories in hospitals. This is because the presence of substantial amounts of the
enzyme in serum indicates certain types of disease (including jaundice and a number of bone
diseases).
In this experiment you will assay (measure) the activity (rate of reaction) of the alkaline
phosphatase towards the substrate 4-nitrophenylphosphate. The reaction products are 4-
nitrophenol and phosphate.
1
Assay of the activity of the enzyme alkaline phosphatase present in
serum samples
NOTE- this practical will be assessed and contributes 10% of the grade
for the module. This sheet must be completed, together with
embedded Excel graphs (instructions for this are included in the
materials on Canvas) and must be submitted to Canvas by 12:00 noon
on 26/11/2018
Aims of practical
(i) measure activity of alkaline phosphatase
(ii) determine presence of elevated levels of alkaline phosphatase in serum samples
Essential reading
(i) Campbell (9th Edition), Chapter 8, pp198-203 ( Enzymes)
(ii) Practical Skills in Biological & Environmental Sciences (2011)
Chapter 21 Working with liquids
Chapter 22 Basic laboratory procedures
Chapter 50 Calibration
Page 247-248 Types of assay
Chapter 59 Basic Spectroscopy
(iii) Foundation Mathematics for Biosciences (Bryson)
Chapters on Enzyme kinetics and Graphs, Trendlines and Equations
Introduction
Alkaline phosphatase is a very widely distributed enzyme. As the name implies it catalyses
(speeds up) the hydrolysis of phosphate esters (e.g. glucose phosphate, ATP etc.) under alkaline
conditions, i.e. at pH values above 7. Although the physiological function of the enzyme is not
properly understood, the assay of the enzyme in samples of serum is very commonly performed in
biochemistry laboratories in hospitals. This is because the presence of substantial amounts of the
enzyme in serum indicates certain types of disease (including jaundice and a number of bone
diseases).
In this experiment you will assay (measure) the activity (rate of reaction) of the alkaline
phosphatase towards the substrate 4-nitrophenylphosphate. The reaction products are 4-
nitrophenol and phosphate.
1
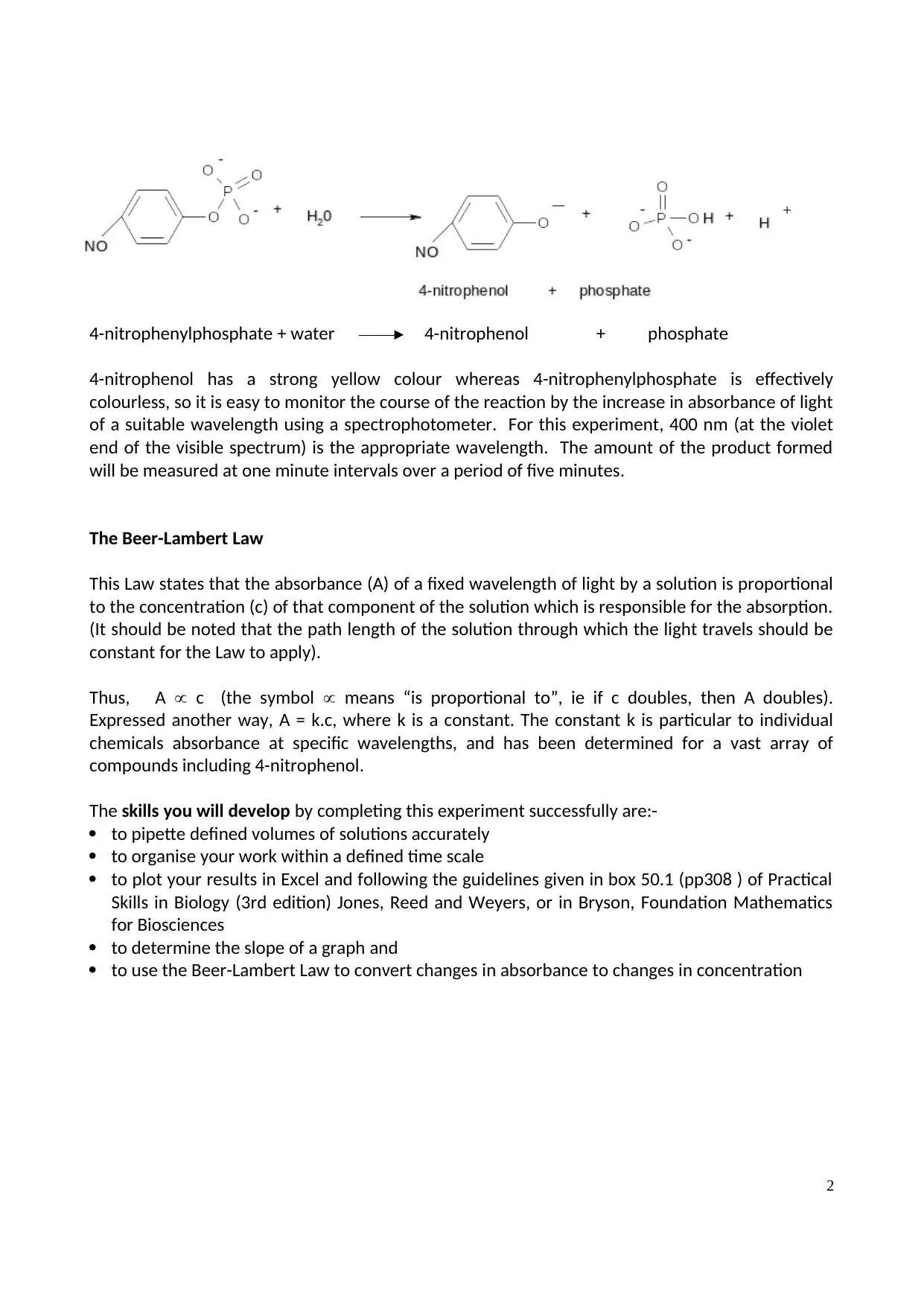
4-nitrophenylphosphate + water 4-nitrophenol + phosphate
4-nitrophenol has a strong yellow colour whereas 4-nitrophenylphosphate is effectively
colourless, so it is easy to monitor the course of the reaction by the increase in absorbance of light
of a suitable wavelength using a spectrophotometer. For this experiment, 400 nm (at the violet
end of the visible spectrum) is the appropriate wavelength. The amount of the product formed
will be measured at one minute intervals over a period of five minutes.
The Beer-Lambert Law
This Law states that the absorbance (A) of a fixed wavelength of light by a solution is proportional
to the concentration (c) of that component of the solution which is responsible for the absorption.
(It should be noted that the path length of the solution through which the light travels should be
constant for the Law to apply).
Thus, A c (the symbol means “is proportional to”, ie if c doubles, then A doubles).
Expressed another way, A = k.c, where k is a constant. The constant k is particular to individual
chemicals absorbance at specific wavelengths, and has been determined for a vast array of
compounds including 4-nitrophenol.
The skills you will develop by completing this experiment successfully are:-
to pipette defined volumes of solutions accurately
to organise your work within a defined time scale
to plot your results in Excel and following the guidelines given in box 50.1 (pp308 ) of Practical
Skills in Biology (3rd edition) Jones, Reed and Weyers, or in Bryson, Foundation Mathematics
for Biosciences
to determine the slope of a graph and
to use the Beer-Lambert Law to convert changes in absorbance to changes in concentration
+
2
4-nitrophenol has a strong yellow colour whereas 4-nitrophenylphosphate is effectively
colourless, so it is easy to monitor the course of the reaction by the increase in absorbance of light
of a suitable wavelength using a spectrophotometer. For this experiment, 400 nm (at the violet
end of the visible spectrum) is the appropriate wavelength. The amount of the product formed
will be measured at one minute intervals over a period of five minutes.
The Beer-Lambert Law
This Law states that the absorbance (A) of a fixed wavelength of light by a solution is proportional
to the concentration (c) of that component of the solution which is responsible for the absorption.
(It should be noted that the path length of the solution through which the light travels should be
constant for the Law to apply).
Thus, A c (the symbol means “is proportional to”, ie if c doubles, then A doubles).
Expressed another way, A = k.c, where k is a constant. The constant k is particular to individual
chemicals absorbance at specific wavelengths, and has been determined for a vast array of
compounds including 4-nitrophenol.
The skills you will develop by completing this experiment successfully are:-
to pipette defined volumes of solutions accurately
to organise your work within a defined time scale
to plot your results in Excel and following the guidelines given in box 50.1 (pp308 ) of Practical
Skills in Biology (3rd edition) Jones, Reed and Weyers, or in Bryson, Foundation Mathematics
for Biosciences
to determine the slope of a graph and
to use the Beer-Lambert Law to convert changes in absorbance to changes in concentration
+
2
Methods
(i) Measuring alkaline phosphatase activity
a) Set up 'quench tubes'
Pipette 500 L of sodium carbonate solution (15 %, w/v) in each of 5 eppendorf tubes; mark
these Q1, Q2, Q3, Q4 and Q5.
b) Set up reaction tube
Pipette 3 mL of substrate solution (2 mM 4-nitrophenylphosphate in 50 mM glycine buffer, pH 9.5)
into a test tube.
c) Start reaction
Add 25 L enzyme solution (0.2 mg/mL) to this substrate solution, mix the contents quickly by
inversion using a piece of parafilm and immediately start the clock.
d) Stop reaction at 1 minute intervals
At times 1, 2, 3, 4 and 5 minutes after starting the reaction withdraw 500 L samples and add in
turn to the quench tubes Q1, Q2, Q3, Q4 and Q5 respectively. Mix the contents thoroughly (close
eppendorf lid and invert 4-5 times) as quickly as you can. The sodium carbonate solution is quite
dense, so vigorous mixing is necessary. The very alkaline nature (high pH) of the sodium
carbonate denatures the enzyme and thus stops the reaction at the time the sample has been
withdrawn and quenched.
e) Finally read the absorbances of all the tubes at 400 nm relative to a water blank.
Results
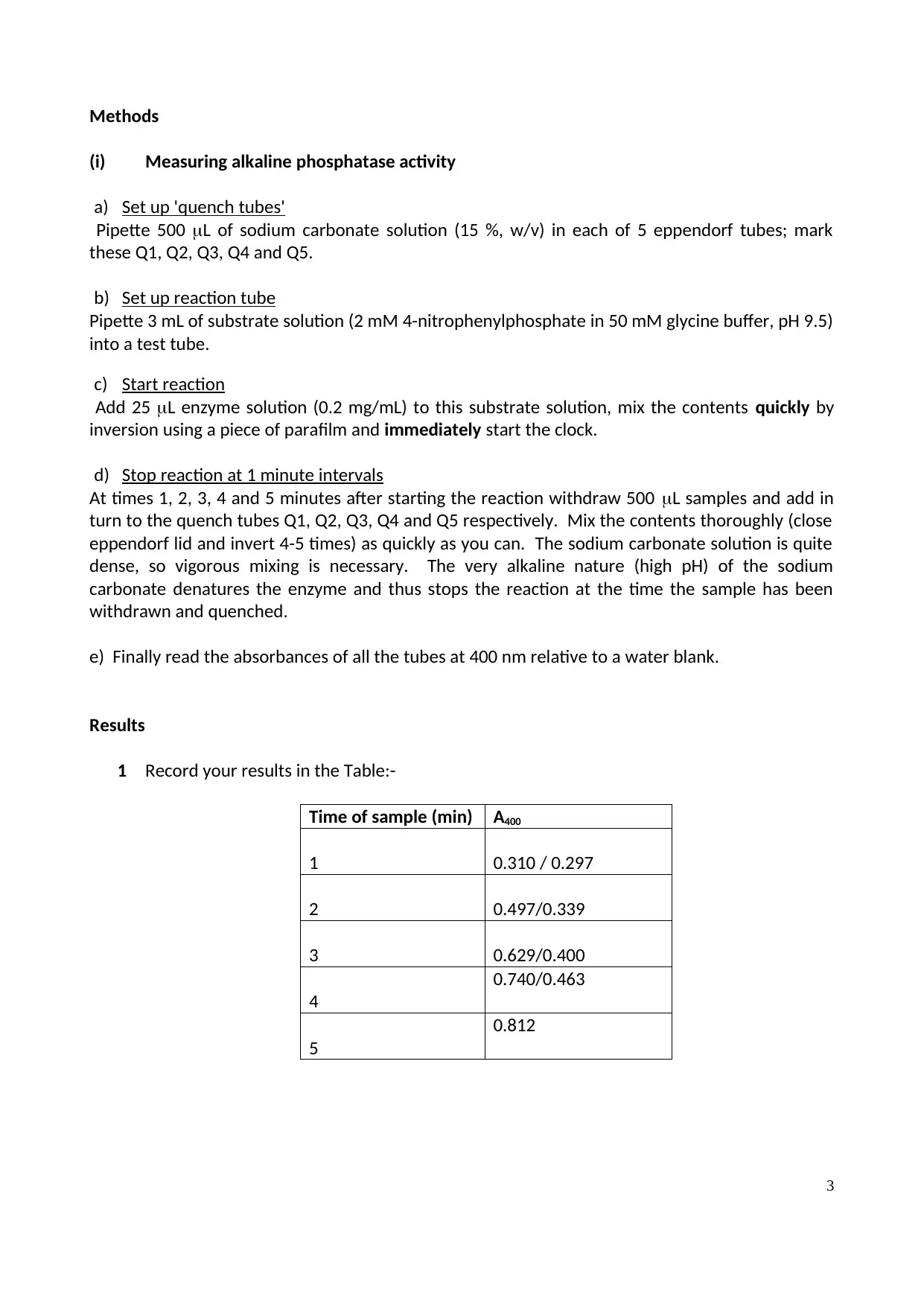
1 Record your results in the Table:-
Time of sample (min) A400
1 0.310 / 0.297
2 0.497/0.339
3 0.629/0.400
4
0.740/0.463
5
0.812
3
(i) Measuring alkaline phosphatase activity
a) Set up 'quench tubes'
Pipette 500 L of sodium carbonate solution (15 %, w/v) in each of 5 eppendorf tubes; mark
these Q1, Q2, Q3, Q4 and Q5.
b) Set up reaction tube
Pipette 3 mL of substrate solution (2 mM 4-nitrophenylphosphate in 50 mM glycine buffer, pH 9.5)
into a test tube.
c) Start reaction
Add 25 L enzyme solution (0.2 mg/mL) to this substrate solution, mix the contents quickly by
inversion using a piece of parafilm and immediately start the clock.
d) Stop reaction at 1 minute intervals
At times 1, 2, 3, 4 and 5 minutes after starting the reaction withdraw 500 L samples and add in
turn to the quench tubes Q1, Q2, Q3, Q4 and Q5 respectively. Mix the contents thoroughly (close
eppendorf lid and invert 4-5 times) as quickly as you can. The sodium carbonate solution is quite
dense, so vigorous mixing is necessary. The very alkaline nature (high pH) of the sodium
carbonate denatures the enzyme and thus stops the reaction at the time the sample has been
withdrawn and quenched.
e) Finally read the absorbances of all the tubes at 400 nm relative to a water blank.
Results
1 Record your results in the Table:-
Time of sample (min) A400
1 0.310 / 0.297
2 0.497/0.339
3 0.629/0.400
4
0.740/0.463
5
0.812
3
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
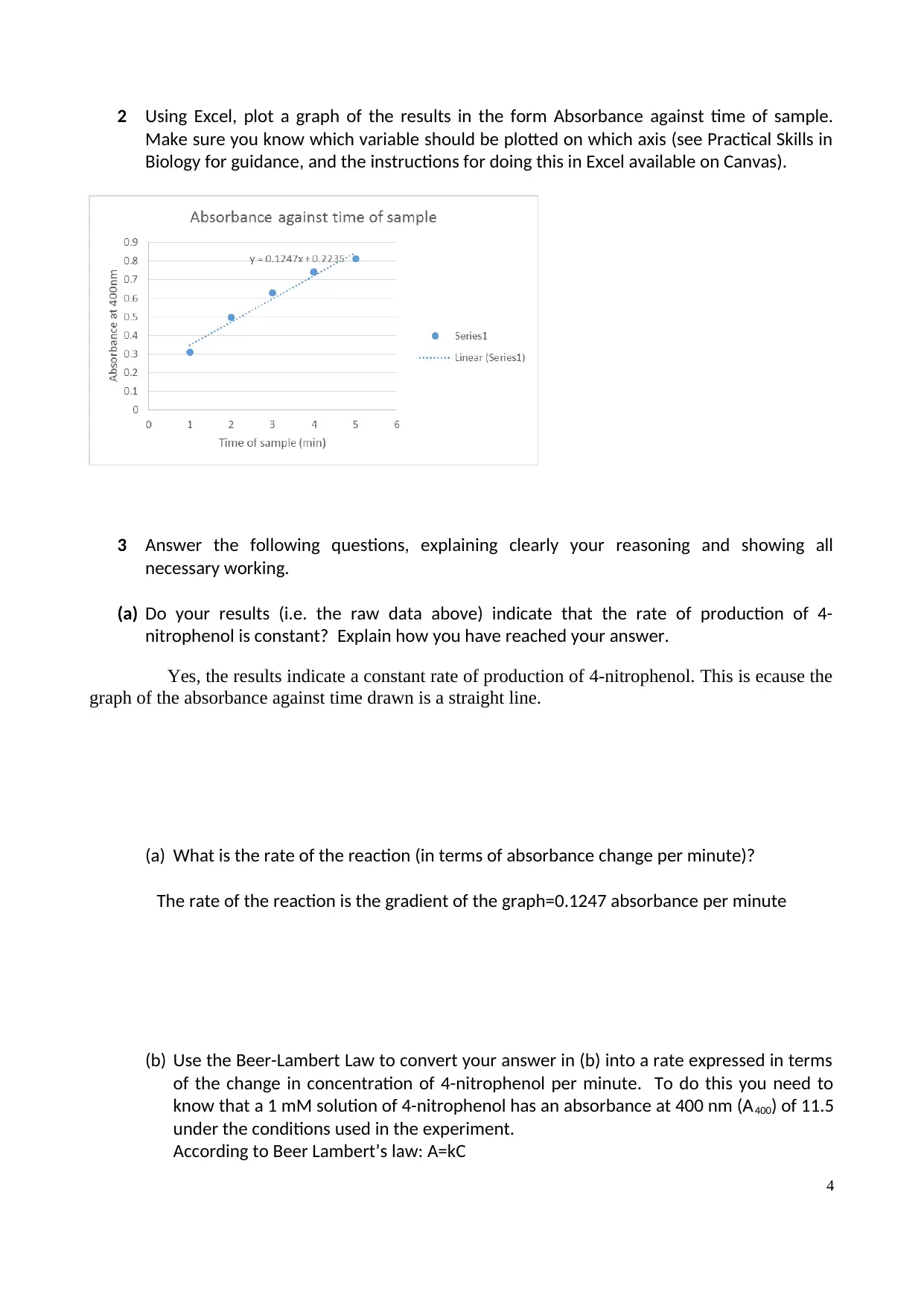
2 Using Excel, plot a graph of the results in the form Absorbance against time of sample.
Make sure you know which variable should be plotted on which axis (see Practical Skills in
Biology for guidance, and the instructions for doing this in Excel available on Canvas).
3 Answer the following questions, explaining clearly your reasoning and showing all
necessary working.
(a) Do your results (i.e. the raw data above) indicate that the rate of production of 4-
nitrophenol is constant? Explain how you have reached your answer.
Yes, the results indicate a constant rate of production of 4-nitrophenol. This is ecause the
graph of the absorbance against time drawn is a straight line.
(a) What is the rate of the reaction (in terms of absorbance change per minute)?
The rate of the reaction is the gradient of the graph=0.1247 absorbance per minute
(b) Use the Beer-Lambert Law to convert your answer in (b) into a rate expressed in terms
of the change in concentration of 4-nitrophenol per minute. To do this you need to
know that a 1 mM solution of 4-nitrophenol has an absorbance at 400 nm (A400) of 11.5
under the conditions used in the experiment.
According to Beer Lambert’s law: A=kC
4
Make sure you know which variable should be plotted on which axis (see Practical Skills in
Biology for guidance, and the instructions for doing this in Excel available on Canvas).
3 Answer the following questions, explaining clearly your reasoning and showing all
necessary working.
(a) Do your results (i.e. the raw data above) indicate that the rate of production of 4-
nitrophenol is constant? Explain how you have reached your answer.
Yes, the results indicate a constant rate of production of 4-nitrophenol. This is ecause the
graph of the absorbance against time drawn is a straight line.
(a) What is the rate of the reaction (in terms of absorbance change per minute)?
The rate of the reaction is the gradient of the graph=0.1247 absorbance per minute
(b) Use the Beer-Lambert Law to convert your answer in (b) into a rate expressed in terms
of the change in concentration of 4-nitrophenol per minute. To do this you need to
know that a 1 mM solution of 4-nitrophenol has an absorbance at 400 nm (A400) of 11.5
under the conditions used in the experiment.
According to Beer Lambert’s law: A=kC
4

Where A=absorbance
k= constant
C=concentration
1Mm=11.5A(for k)
But we used 2mM= 2x11.5= 23A
Our rate in terms of absorbance change per minute =
our rate in absorbance change per minute
k
=0.1247/23=5.4217x10-3 Mm per minute
Determination of elevated alkaline phosphatase activity in patient serum samples
Your demonstrator will supply you with a sample of serum from a patient (A, B or C) suspected of
suffering from a liver disorder. Elevated levels of alkaline phosphatase activity in serum are used as an
indicator of a number of clinical disorders, including liver disorders. You will measure the alkaline
phosphatase activity present in the serum of patient A, B or C and determine the clinical significance.
Method
Repeat steps 1-4 for measuring alkaline phosphatase activity detailed in the first part of the
practical, however, rather than adding enzyme solution in step 3, add 25 L of patient's serum ( A,
B or C).
IMPORTANT: ensure that you use fresh cuvettes, or thoroughly wash out the previous cuvettes in
water
Sample A, B or C?
Record your results
Time of
sample (min)
A400
1 0.158
2 0.148
3 0.156
4
0.164
5
0.155
1 Insert an Excel graph of these results as previously and from this, calculate the rate of the
alkaline phosphatase reaction (in absorbance change per minute).
A
5
k= constant
C=concentration
1Mm=11.5A(for k)
But we used 2mM= 2x11.5= 23A
Our rate in terms of absorbance change per minute =
our rate in absorbance change per minute
k
=0.1247/23=5.4217x10-3 Mm per minute
Determination of elevated alkaline phosphatase activity in patient serum samples
Your demonstrator will supply you with a sample of serum from a patient (A, B or C) suspected of
suffering from a liver disorder. Elevated levels of alkaline phosphatase activity in serum are used as an
indicator of a number of clinical disorders, including liver disorders. You will measure the alkaline
phosphatase activity present in the serum of patient A, B or C and determine the clinical significance.
Method
Repeat steps 1-4 for measuring alkaline phosphatase activity detailed in the first part of the
practical, however, rather than adding enzyme solution in step 3, add 25 L of patient's serum ( A,
B or C).
IMPORTANT: ensure that you use fresh cuvettes, or thoroughly wash out the previous cuvettes in
water
Sample A, B or C?
Record your results
Time of
sample (min)
A400
1 0.158
2 0.148
3 0.156
4
0.164
5
0.155
1 Insert an Excel graph of these results as previously and from this, calculate the rate of the
alkaline phosphatase reaction (in absorbance change per minute).
A
5

2 Express rate in terms of the change in concentration of 4-nitrophenol per minute.
Rate= gradient =0.001 absorbance per minute
According to Beer Lambert’s law: A=kC
Where A=absorbance
k= constant
C=concentration
Remember our k=23A
Rate in terms of concentration=0.001/23=4.3479x10-5 mM per minute
3 Under the assay conditions detailed here, the change in concentration of 4-nitrophenol per
minute is 0.5 M for the serum from a healthy patient. Do your results indicate that your
patient is suffering from a possible liver disorder? Explain clearly.
Change in concentration from the assay results= 5x10-4 mM per minute(from a healthy person)
Change in concentration from my experimental results= 4.3479x10-5 mM (from patient)
From the results above, i conclude that the person is suffering from liver disorder this clinical
significance arises because the reduced rate of concentration of the 4-nitrophenol in the patient
which depicts a lower absorbance value rate than that of a healthy person. Hence the person is
possibly suffering from Liver disorder.
6
Rate= gradient =0.001 absorbance per minute
According to Beer Lambert’s law: A=kC
Where A=absorbance
k= constant
C=concentration
Remember our k=23A
Rate in terms of concentration=0.001/23=4.3479x10-5 mM per minute
3 Under the assay conditions detailed here, the change in concentration of 4-nitrophenol per
minute is 0.5 M for the serum from a healthy patient. Do your results indicate that your
patient is suffering from a possible liver disorder? Explain clearly.
Change in concentration from the assay results= 5x10-4 mM per minute(from a healthy person)
Change in concentration from my experimental results= 4.3479x10-5 mM (from patient)
From the results above, i conclude that the person is suffering from liver disorder this clinical
significance arises because the reduced rate of concentration of the 4-nitrophenol in the patient
which depicts a lower absorbance value rate than that of a healthy person. Hence the person is
possibly suffering from Liver disorder.
6
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
