(BTE 483) - Anti-HIV Using Nanotechnology: Seminar Report on Innovative Approaches for HIV Treatment
VerifiedAdded on 2023/06/01
|25
|5098
|455
Assignment
AI Summary
In this assignment we will discuss about anti-HIV using nanotechnology and below are the summaries point:-
The seminar report titled "Anti-HIV Using Nanotechnology" by Igwe Ifeoma Modesta
Submitted to the Department of Biotechnology, Ebonyi State University, Abakaliki
Explores the use of nanotechnology in combating HIV and its potential applications in drug delivery
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

i
ANTI-HIV USING NANOTECHNOLOGY
BY
IGWE IFEOMA MODESTA
EBSU/2019/98309
A SEMINAR REPORT SUBMITTED TO THE DEPARTMENT OF
BIOTECHNOLOGY FACULTY OF BIOLOGICAL SCINECE, EBONYI STATE
UNIVERSITY, ABAKALIKI .
COURSE CODE: BTE 483
FEBRUARY, 2023
ANTI-HIV USING NANOTECHNOLOGY
BY
IGWE IFEOMA MODESTA
EBSU/2019/98309
A SEMINAR REPORT SUBMITTED TO THE DEPARTMENT OF
BIOTECHNOLOGY FACULTY OF BIOLOGICAL SCINECE, EBONYI STATE
UNIVERSITY, ABAKALIKI .
COURSE CODE: BTE 483
FEBRUARY, 2023
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

ii
ANTI-HIV USING NANOTECHNOLOGY
BY
IGWE IFEOMA MODESTA
EBSU/2019/98309
A SEMINAR REPORT SUBMITTED TO THE DEPARTMENT OF
BIOTECHNOLOGY FACULTY OF BIOLOGICAL SCINECE, EBONYI STATE
UNIVERSITY, ABAKALIKI .
IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE AWARD OF
BACHELOR OF SCIENCE ( B. Sc.)
DEGREE IN BIOTECHNOLOGY
COURSE CODE: BTE 483
SUPERVISOR: PROF. E. I. UGWUJA
FEBRUARY, 2023
ANTI-HIV USING NANOTECHNOLOGY
BY
IGWE IFEOMA MODESTA
EBSU/2019/98309
A SEMINAR REPORT SUBMITTED TO THE DEPARTMENT OF
BIOTECHNOLOGY FACULTY OF BIOLOGICAL SCINECE, EBONYI STATE
UNIVERSITY, ABAKALIKI .
IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE AWARD OF
BACHELOR OF SCIENCE ( B. Sc.)
DEGREE IN BIOTECHNOLOGY
COURSE CODE: BTE 483
SUPERVISOR: PROF. E. I. UGWUJA
FEBRUARY, 2023

iii
APPROVAL
This seminar report titled Anti-HIV Using Nanotechnology carried out by Igwe Ifeoma
Modesta with the registration number EBSU/2019/98309 and presented to the Department of
Biotechnology, Faculty of Science, Ebonyi State University Abakaliki.
______________________ ____________________
PROF. E. I. UGWUJA Date
Seminar Supervisor
______________________ ____________________
DR. ALI. FEDRICK Date
Seminar Coordinator
______________________ ____________________
PROF. E. I. UGWUJA Date
Head of department
APPROVAL
This seminar report titled Anti-HIV Using Nanotechnology carried out by Igwe Ifeoma
Modesta with the registration number EBSU/2019/98309 and presented to the Department of
Biotechnology, Faculty of Science, Ebonyi State University Abakaliki.
______________________ ____________________
PROF. E. I. UGWUJA Date
Seminar Supervisor
______________________ ____________________
DR. ALI. FEDRICK Date
Seminar Coordinator
______________________ ____________________
PROF. E. I. UGWUJA Date
Head of department

iv
CERTIFICATION
This is to certify that the seminar report titled Anti-HIV Using Nanotechnology carried out
by Igwe Ifeoma Modesta with the registration number EBSU/2019/98309 and presented to
the Department of Biotechnology, Faculty of Science, Ebonyi State University Abakaliki.
______________________ ____________________
PROF. E. I. UGWUJA Date
Seminar Supervisor
______________________ ____________________
DR. ALI. FEDRICK Date
Seminar Coordinator
______________________ ____________________
PROF. E. I. UGWUJA Date
Head of department
CERTIFICATION
This is to certify that the seminar report titled Anti-HIV Using Nanotechnology carried out
by Igwe Ifeoma Modesta with the registration number EBSU/2019/98309 and presented to
the Department of Biotechnology, Faculty of Science, Ebonyi State University Abakaliki.
______________________ ____________________
PROF. E. I. UGWUJA Date
Seminar Supervisor
______________________ ____________________
DR. ALI. FEDRICK Date
Seminar Coordinator
______________________ ____________________
PROF. E. I. UGWUJA Date
Head of department
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

v
ACKNOWLEDGEMENT
I wish to register my profound gratitude to god almighty for the guidance and Grace
throughout my life.
I'm grateful to the entire staff of Biotechnology department for making my learning
interesting educative and Worthwhile.
My special gratitude to my HOD Prof. E. I. Ugwuja for his effort to see that this works or the
light of the day. I appreciate all my amazing lecturers in the department, my wonderful
supervisor, prof. I. E. Ugwuja for his seasoned lectures, to them all, I say we bless Amen.
My regards to my amazing parents Mr and Mrs Francis Igwe, my guidance Mr and Mrs
Chigozie who financially supported my education pursuit, I said amen blessed by God
Almighty and to my beloved siblings, love you all, you are the best.
ACKNOWLEDGEMENT
I wish to register my profound gratitude to god almighty for the guidance and Grace
throughout my life.
I'm grateful to the entire staff of Biotechnology department for making my learning
interesting educative and Worthwhile.
My special gratitude to my HOD Prof. E. I. Ugwuja for his effort to see that this works or the
light of the day. I appreciate all my amazing lecturers in the department, my wonderful
supervisor, prof. I. E. Ugwuja for his seasoned lectures, to them all, I say we bless Amen.
My regards to my amazing parents Mr and Mrs Francis Igwe, my guidance Mr and Mrs
Chigozie who financially supported my education pursuit, I said amen blessed by God
Almighty and to my beloved siblings, love you all, you are the best.

vi
Table of contents
Title page i
Approval ii
Acknowledgment iii
Table of content iv
Abstract v
CHAPTER ONE: INTRODUCTION
1.1 Background of the Study 1
CHAPTER TWO: DISCUSSIONS
2.1 Edification of Nanotechnology In Field Of Drug Delivery 6
2.2 Liposomes 7
2.3 Liposomal ARV Drug Formulation For Anti-HIV Effect 8
2.4 Dendrimer 9
2.4.1 Dendrimer formulation for targeting HIV-AIDS 10
2.4.2 FDA approved dendrimer of AIDS 11
2.5 Nanoparticle 12
2.5.1 Polymeric nanoparticles 12
2.5.2 Solid lipid nanoparticles (SLN) 12
2.5.3 Nano-structured lipid carries (NCL) 13
2.5.4 Inorganic nanoparticles 14
2.6 Polymeric Micelles 14
2.7 Nanocrystal 15
CHAPTER THREE: CONCLUSION
4.1 Conclusion 16
REFERENCE 17
Table of contents
Title page i
Approval ii
Acknowledgment iii
Table of content iv
Abstract v
CHAPTER ONE: INTRODUCTION
1.1 Background of the Study 1
CHAPTER TWO: DISCUSSIONS
2.1 Edification of Nanotechnology In Field Of Drug Delivery 6
2.2 Liposomes 7
2.3 Liposomal ARV Drug Formulation For Anti-HIV Effect 8
2.4 Dendrimer 9
2.4.1 Dendrimer formulation for targeting HIV-AIDS 10
2.4.2 FDA approved dendrimer of AIDS 11
2.5 Nanoparticle 12
2.5.1 Polymeric nanoparticles 12
2.5.2 Solid lipid nanoparticles (SLN) 12
2.5.3 Nano-structured lipid carries (NCL) 13
2.5.4 Inorganic nanoparticles 14
2.6 Polymeric Micelles 14
2.7 Nanocrystal 15
CHAPTER THREE: CONCLUSION
4.1 Conclusion 16
REFERENCE 17

vii
ABSTRACT
The biggest challenges of the world in this 21st century is to cure HIV-AIDS .
Nanotechnology is an emerging multidisciplinary field that has the potential to advance the
treatment and prevention of HIV/AIDS radically. The use of nanotechnology for numerous
biomedical applications has become an area of intense research over the last decade.1–10 The
potential advantages of using nanomedicine over conventional HIV therapies include the
capacity to incorporate, encapsulate, or conjugate a variety of drugs to target specific cell
populations and to offer tunable and site-specific drug release .In Present scenario different
antiviral drugs are available in the market to reduce the worse condition and managed
improved survivial rate . In this scenario Nanotechnology based antiretroviral drugs delivery
holds drug and will provide to cure AIDS. Nanotechnology based deliver system
Nanocarriers like Liposomes, dendrimers, Nanoparticles, Polymeric Micelles, Nanovesicles,
Nanoemulsion provide the way to deliver drug to targeting tissue. Nanobased carriers
revolutionized the field of Pharmaceutics and Pharmaco Kinetic’s in target drug delivery. The
present study depicts nano based ARV drug provides increase efficiency with less adverse
effects to control HIV. Like same way we can provide and increase nanobased drug delivery
capacity to other available HIV drugs.
ABSTRACT
The biggest challenges of the world in this 21st century is to cure HIV-AIDS .
Nanotechnology is an emerging multidisciplinary field that has the potential to advance the
treatment and prevention of HIV/AIDS radically. The use of nanotechnology for numerous
biomedical applications has become an area of intense research over the last decade.1–10 The
potential advantages of using nanomedicine over conventional HIV therapies include the
capacity to incorporate, encapsulate, or conjugate a variety of drugs to target specific cell
populations and to offer tunable and site-specific drug release .In Present scenario different
antiviral drugs are available in the market to reduce the worse condition and managed
improved survivial rate . In this scenario Nanotechnology based antiretroviral drugs delivery
holds drug and will provide to cure AIDS. Nanotechnology based deliver system
Nanocarriers like Liposomes, dendrimers, Nanoparticles, Polymeric Micelles, Nanovesicles,
Nanoemulsion provide the way to deliver drug to targeting tissue. Nanobased carriers
revolutionized the field of Pharmaceutics and Pharmaco Kinetic’s in target drug delivery. The
present study depicts nano based ARV drug provides increase efficiency with less adverse
effects to control HIV. Like same way we can provide and increase nanobased drug delivery
capacity to other available HIV drugs.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1
CHAPTER ONE
INTRODUCTION
1.1 Background of the Study
Human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) is a
global pandemic and is the leading infectious disease resulting in significant morbidity and
mortality and consequently devastating socioeconomic effects. With the advent of multidrug,
highly active antiretroviral therapy (HAART), the prognosis for HIV-infected patients has
significantly improved; however, it has not eradicated HIV infection, particularly in
sequestered, anatomically privileged sites, such as the brain, testes, gut, liver, kidney, and
secondary lymphoid tissue. HIV most often enters the body via mucosal surfaces and is
transported by dendritic cells to lymphoid organs, where it is then delivered to activated
CD4+ T cells . Productive infection of CD4+ T cells leads to viremia and dissemination of
the virus to other sites in the body. Untreated HIV infection is usually associated with high
plasma viral loads and progressive decline in CD4+ T cells. Antiretroviral drugs inhibit HIV
replication, and treatment with Highly Active Antiretroviral Therapy (HAART), with a
regimen consisting of at least three drugs, from at least two classes of antiretroviral agents,
will suppress plasma viral load to undetectable levels, and lead to recovery of CD4+ T cell
counts. One of the key sources of entry through the mucosal surfaces is the sexual
transmission. The primary path of heterosexual HIV transmission is the female genital tract .
Sexual transmission via the rectal route is also a major issue that, due to its physiology,
renders it more vulnerable to HIV infection (McGowan, 2008). Immune cells, i.e.
macrophages and dendritic cells found in the sub-epithelial layer of the vagina or cervix
mucosa are the main targets of HIV infection. HIV establishes anatomical reservoirs in
lymphoid tissue, the reticuloendothelial system and other sites not shown here. Antiretroviral
drugs do not penetrate these sites adequately. Macrophages and latently infected CD4+ T
cells constitute cellular reservoirs, because antiretroviral drugs do not achieve satisfactory
intracellular concentration within macrophages and antiretrovirals are ineffective against
latent virus, respectively. Potential means of using nanotechnology to combat viral reservoirs
are:( A) Targeted delivery of antiretroviral drugs to the reticuloendothelial system, including
lymphatic tissues ; (B) Targeting the brain; (C) Targeting latently infected CD4+ T cells ; (D)
Achieving optimal intracellular concentration of antiretroviral drugs within macrophages.
CHAPTER ONE
INTRODUCTION
1.1 Background of the Study
Human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) is a
global pandemic and is the leading infectious disease resulting in significant morbidity and
mortality and consequently devastating socioeconomic effects. With the advent of multidrug,
highly active antiretroviral therapy (HAART), the prognosis for HIV-infected patients has
significantly improved; however, it has not eradicated HIV infection, particularly in
sequestered, anatomically privileged sites, such as the brain, testes, gut, liver, kidney, and
secondary lymphoid tissue. HIV most often enters the body via mucosal surfaces and is
transported by dendritic cells to lymphoid organs, where it is then delivered to activated
CD4+ T cells . Productive infection of CD4+ T cells leads to viremia and dissemination of
the virus to other sites in the body. Untreated HIV infection is usually associated with high
plasma viral loads and progressive decline in CD4+ T cells. Antiretroviral drugs inhibit HIV
replication, and treatment with Highly Active Antiretroviral Therapy (HAART), with a
regimen consisting of at least three drugs, from at least two classes of antiretroviral agents,
will suppress plasma viral load to undetectable levels, and lead to recovery of CD4+ T cell
counts. One of the key sources of entry through the mucosal surfaces is the sexual
transmission. The primary path of heterosexual HIV transmission is the female genital tract .
Sexual transmission via the rectal route is also a major issue that, due to its physiology,
renders it more vulnerable to HIV infection (McGowan, 2008). Immune cells, i.e.
macrophages and dendritic cells found in the sub-epithelial layer of the vagina or cervix
mucosa are the main targets of HIV infection. HIV establishes anatomical reservoirs in
lymphoid tissue, the reticuloendothelial system and other sites not shown here. Antiretroviral
drugs do not penetrate these sites adequately. Macrophages and latently infected CD4+ T
cells constitute cellular reservoirs, because antiretroviral drugs do not achieve satisfactory
intracellular concentration within macrophages and antiretrovirals are ineffective against
latent virus, respectively. Potential means of using nanotechnology to combat viral reservoirs
are:( A) Targeted delivery of antiretroviral drugs to the reticuloendothelial system, including
lymphatic tissues ; (B) Targeting the brain; (C) Targeting latently infected CD4+ T cells ; (D)
Achieving optimal intracellular concentration of antiretroviral drugs within macrophages.
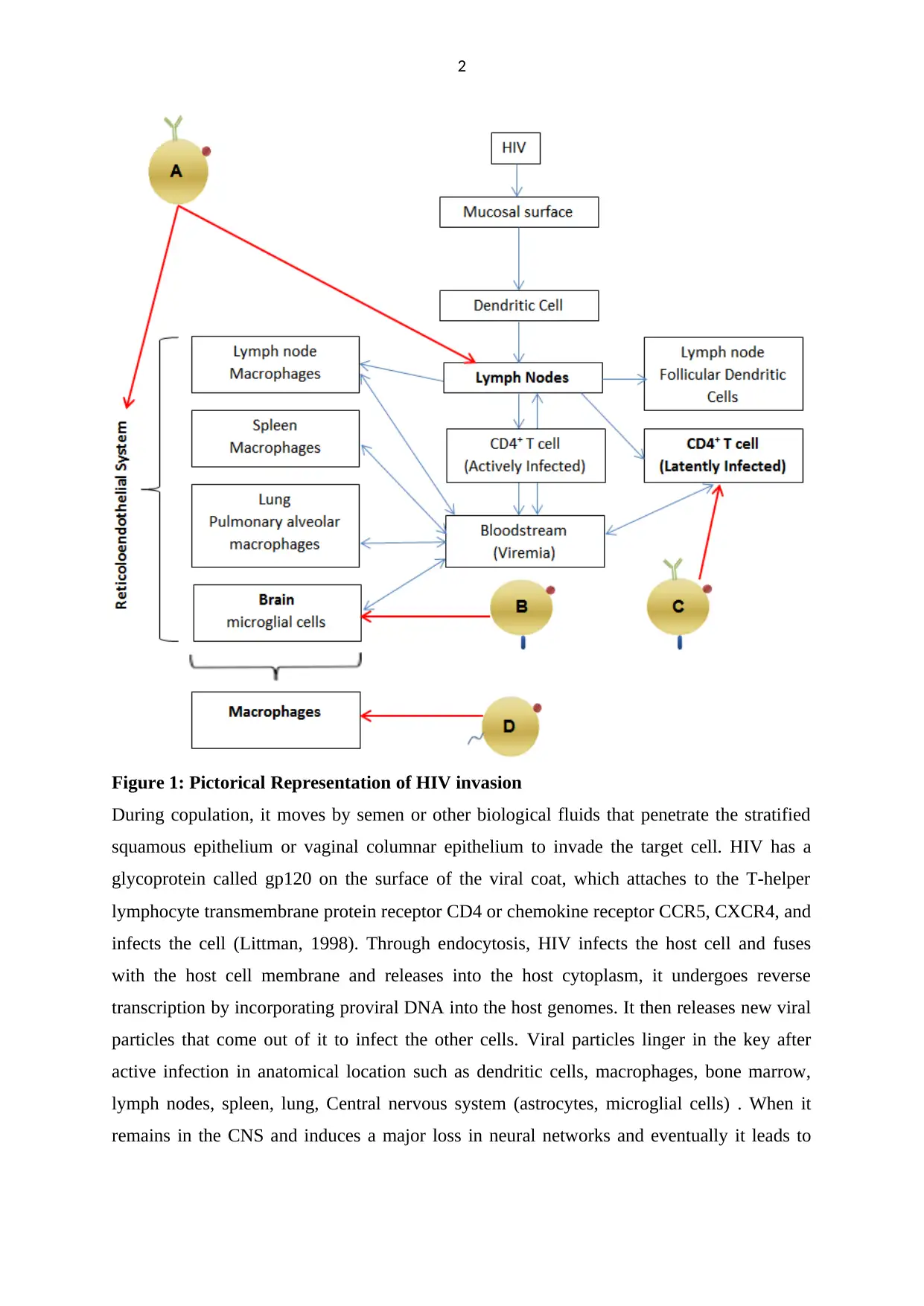
2
Figure 1: Pictorical Representation of HIV invasion
During copulation, it moves by semen or other biological fluids that penetrate the stratified
squamous epithelium or vaginal columnar epithelium to invade the target cell. HIV has a
glycoprotein called gp120 on the surface of the viral coat, which attaches to the T-helper
lymphocyte transmembrane protein receptor CD4 or chemokine receptor CCR5, CXCR4, and
infects the cell (Littman, 1998). Through endocytosis, HIV infects the host cell and fuses
with the host cell membrane and releases into the host cytoplasm, it undergoes reverse
transcription by incorporating proviral DNA into the host genomes. It then releases new viral
particles that come out of it to infect the other cells. Viral particles linger in the key after
active infection in anatomical location such as dendritic cells, macrophages, bone marrow,
lymph nodes, spleen, lung, Central nervous system (astrocytes, microglial cells) . When it
remains in the CNS and induces a major loss in neural networks and eventually it leads to
Figure 1: Pictorical Representation of HIV invasion
During copulation, it moves by semen or other biological fluids that penetrate the stratified
squamous epithelium or vaginal columnar epithelium to invade the target cell. HIV has a
glycoprotein called gp120 on the surface of the viral coat, which attaches to the T-helper
lymphocyte transmembrane protein receptor CD4 or chemokine receptor CCR5, CXCR4, and
infects the cell (Littman, 1998). Through endocytosis, HIV infects the host cell and fuses
with the host cell membrane and releases into the host cytoplasm, it undergoes reverse
transcription by incorporating proviral DNA into the host genomes. It then releases new viral
particles that come out of it to infect the other cells. Viral particles linger in the key after
active infection in anatomical location such as dendritic cells, macrophages, bone marrow,
lymph nodes, spleen, lung, Central nervous system (astrocytes, microglial cells) . When it
remains in the CNS and induces a major loss in neural networks and eventually it leads to
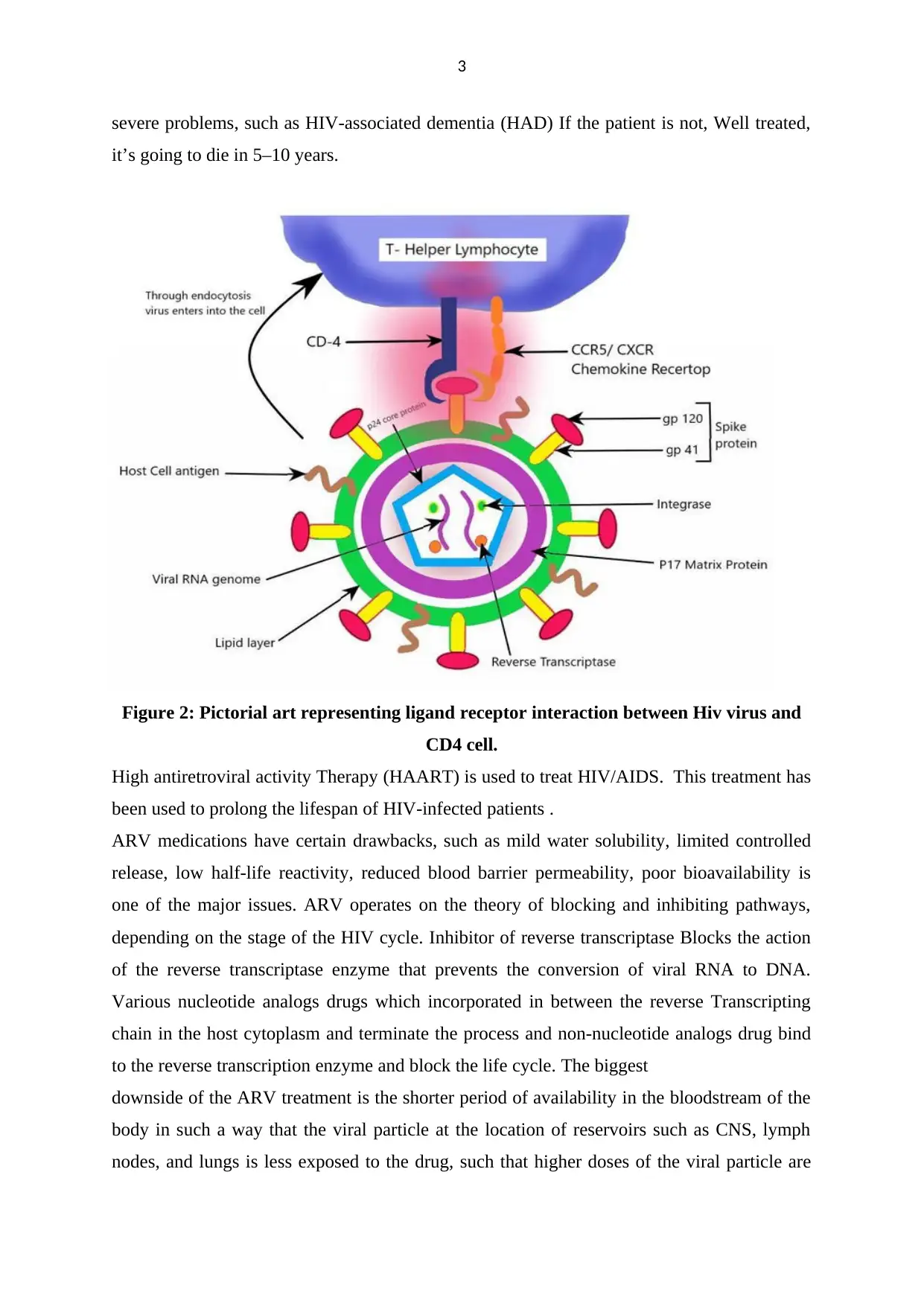
3
severe problems, such as HIV-associated dementia (HAD) If the patient is not, Well treated,
it’s going to die in 5–10 years.
Figure 2: Pictorial art representing ligand receptor interaction between Hiv virus and
CD4 cell.
High antiretroviral activity Therapy (HAART) is used to treat HIV/AIDS. This treatment has
been used to prolong the lifespan of HIV-infected patients .
ARV medications have certain drawbacks, such as mild water solubility, limited controlled
release, low half-life reactivity, reduced blood barrier permeability, poor bioavailability is
one of the major issues. ARV operates on the theory of blocking and inhibiting pathways,
depending on the stage of the HIV cycle. Inhibitor of reverse transcriptase Blocks the action
of the reverse transcriptase enzyme that prevents the conversion of viral RNA to DNA.
Various nucleotide analogs drugs which incorporated in between the reverse Transcripting
chain in the host cytoplasm and terminate the process and non-nucleotide analogs drug bind
to the reverse transcription enzyme and block the life cycle. The biggest
downside of the ARV treatment is the shorter period of availability in the bloodstream of the
body in such a way that the viral particle at the location of reservoirs such as CNS, lymph
nodes, and lungs is less exposed to the drug, such that higher doses of the viral particle are
severe problems, such as HIV-associated dementia (HAD) If the patient is not, Well treated,
it’s going to die in 5–10 years.
Figure 2: Pictorial art representing ligand receptor interaction between Hiv virus and
CD4 cell.
High antiretroviral activity Therapy (HAART) is used to treat HIV/AIDS. This treatment has
been used to prolong the lifespan of HIV-infected patients .
ARV medications have certain drawbacks, such as mild water solubility, limited controlled
release, low half-life reactivity, reduced blood barrier permeability, poor bioavailability is
one of the major issues. ARV operates on the theory of blocking and inhibiting pathways,
depending on the stage of the HIV cycle. Inhibitor of reverse transcriptase Blocks the action
of the reverse transcriptase enzyme that prevents the conversion of viral RNA to DNA.
Various nucleotide analogs drugs which incorporated in between the reverse Transcripting
chain in the host cytoplasm and terminate the process and non-nucleotide analogs drug bind
to the reverse transcription enzyme and block the life cycle. The biggest
downside of the ARV treatment is the shorter period of availability in the bloodstream of the
body in such a way that the viral particle at the location of reservoirs such as CNS, lymph
nodes, and lungs is less exposed to the drug, such that higher doses of the viral particle are
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

4
needed for a sustained period of time that develops resistance to the HIV strain. The reservoir
also includes latently infected cells, including CD4+ T-cells, Monocytes, macrophage lineage
carrying incorporated transcription of the provirus silencing within the genome that might
also re-infect the patient due to activation of the proviral genome . In order to resolve such
problems and drawbacks, nano-based drug delivery technologies, nano-medicines, and other
nano-based strategies play a key role in drug effectiveness, drug reactivity, drug target
accuracy, minimizing drug toxicity and negative impacts, and various major challenges
currently facing ARV drugs in the present context.
Summary of antiretroviral drugs used in Hiv nanotherapeutics (table1):
Antiretroviral drug Nanoparticle type
Stavudine (D4T), Methylmethacrylate -sulfoproplyme
Delavirdine (DLV) (MMA-SPM) nanoparticle with grafted RMP-7
Saquinavir (SQV) (RMP-7/MMA-SPMnanopracticles)
Ampenavir Transferrin (Tf)-conjugated
quantum dots
Dapivirine Poly(ε-caprolactone) nanoparticles
Ritonavir Tat-peptide-conjugated pitonavir
nanoparticles
I
Indinavir, ritonavir, Monocyte-derived macrophages-
nanoparticle
atazanavir, and efavirenz interactions
D4T, DLV, SQV Lipids: Compritol 888 ATO,
tripalmitin, and cacao butter
needed for a sustained period of time that develops resistance to the HIV strain. The reservoir
also includes latently infected cells, including CD4+ T-cells, Monocytes, macrophage lineage
carrying incorporated transcription of the provirus silencing within the genome that might
also re-infect the patient due to activation of the proviral genome . In order to resolve such
problems and drawbacks, nano-based drug delivery technologies, nano-medicines, and other
nano-based strategies play a key role in drug effectiveness, drug reactivity, drug target
accuracy, minimizing drug toxicity and negative impacts, and various major challenges
currently facing ARV drugs in the present context.
Summary of antiretroviral drugs used in Hiv nanotherapeutics (table1):
Antiretroviral drug Nanoparticle type
Stavudine (D4T), Methylmethacrylate -sulfoproplyme
Delavirdine (DLV) (MMA-SPM) nanoparticle with grafted RMP-7
Saquinavir (SQV) (RMP-7/MMA-SPMnanopracticles)
Ampenavir Transferrin (Tf)-conjugated
quantum dots
Dapivirine Poly(ε-caprolactone) nanoparticles
Ritonavir Tat-peptide-conjugated pitonavir
nanoparticles
I
Indinavir, ritonavir, Monocyte-derived macrophages-
nanoparticle
atazanavir, and efavirenz interactions
D4T, DLV, SQV Lipids: Compritol 888 ATO,
tripalmitin, and cacao butter

5
stabilized by L-α-
phospatidylcholine, cholesteryl hemisuccinate,
and taurocholate to form
solid lipid nanoparticles
SQV
Nanoparticles with ternary
components of polyethyleneimine,
poly(γ-glutamic acid), and
poly(lactide-co-glycolide acid) (PLGA)
d4T – nucleoside reverse
transcriptase inhibitor
Chitosan-O-isopropyl-5′- O-d4T
monophosphate conjugate
with a phosphoramidate linkage
SQV Tf-conjugated quantum rods
Ritonavir, lopinavir, and efavirenz PLGA nanoparticles
Stavudine Mannose- and galactose-targeted liposome
Efavirenz Mannose-targeted dendrimer
Lamivudine Mannose-targeted dendrimer
Zidovudine Mannose-targeted liposome
Indinavir Liposome-laden macrophages
stabilized by L-α-
phospatidylcholine, cholesteryl hemisuccinate,
and taurocholate to form
solid lipid nanoparticles
SQV
Nanoparticles with ternary
components of polyethyleneimine,
poly(γ-glutamic acid), and
poly(lactide-co-glycolide acid) (PLGA)
d4T – nucleoside reverse
transcriptase inhibitor
Chitosan-O-isopropyl-5′- O-d4T
monophosphate conjugate
with a phosphoramidate linkage
SQV Tf-conjugated quantum rods
Ritonavir, lopinavir, and efavirenz PLGA nanoparticles
Stavudine Mannose- and galactose-targeted liposome
Efavirenz Mannose-targeted dendrimer
Lamivudine Mannose-targeted dendrimer
Zidovudine Mannose-targeted liposome
Indinavir Liposome-laden macrophages

6
CHAPTER TWO
DISCUSSIONS
2.1 Edification of Nanotechnology In Field Of Drug Delivery
Targeted delivery of antiretrovirals to HIV-1-infected T-cells and macrophages would
improve the efficacy of antiviral drugs, reduce toxicity, reduce HIV-resistance frequency, and
decrease viral production. the field of Nanobiotechnology that emerges with the great
modern manufacturing for higher performance of drug due the scope of the Nanoscale
process, The fundamental theory is to modulate the pharmacokinetics of the chemical
molecule that has deserved to eliminate HIV from the body without damaging the body. It
also increases the bio-distribution and bioavailability of the drug to expose the virus particle
for a longer duration with a higher goal precision. Application of nanotechnology to the
delivery of ARV drugs Holds the potential to treat AIDS and it could be beneficial Drugs at
the anatomical reservoir site and also raise the half-life of drugs Nano-carriers give a range of
advantages, such as control of drugs degradation, drug specificity and delivery of biological
products molecules, such as proteins, peptides, oligopeptides, oligonucleotides, etc.
Nanocarriers are now using it to solve the limitation of therapeutic uses, such as drug
delivery, bioavailability of drugs, drug conformation stability, physicochemical stability,
improved transmission permeability, drug clearance, cellular absorption, reduction of
immunogenic reaction. Inorganic solid lipid nanoparticles liposomes, polymeric micelles,
dendrimers, cyclodextrins, and cell-based nanoformulations have been studied for delivery of
drugs intended for HIV prevention or therapy.43 For anti-HIV drugs to be effective, adequate
distribution to specific sites in the body must be achieved, and effective drug concentrations
must be maintained at those sites for the required period of time. For effective delivery of anti
HIV-1 nanotherapy, an optimal drug delivery nanocarrier vehicle must be generated that
should be of a precise geometry, whose surface (ie, zeta potential, stealth ligands),
drug/biomolecule (antiretrovirals, oligonucleotides, proteins, small interfering RNA [siRNA],
RNA, imaging agents) encapsulation efficiency and release, surface chemistries (targeting
antibodies, PEG chains, metal chelators), and spatial distribution of ligands must be well
engineered. Targeted nanocarrier delivery involves (1) the recognition of HIV-infectable
target cells and tissues; (2) the ability to reach these sites; and (3) the ability to deliver
multiple therapeutic agents. Nowacek and Gendelman have shown that a single intravenous
dose of the nano-ART can elicit high-sustained tissue and plasma drug levels of antiretroviral
drugs in the reticuloendothelial system and brain.
CHAPTER TWO
DISCUSSIONS
2.1 Edification of Nanotechnology In Field Of Drug Delivery
Targeted delivery of antiretrovirals to HIV-1-infected T-cells and macrophages would
improve the efficacy of antiviral drugs, reduce toxicity, reduce HIV-resistance frequency, and
decrease viral production. the field of Nanobiotechnology that emerges with the great
modern manufacturing for higher performance of drug due the scope of the Nanoscale
process, The fundamental theory is to modulate the pharmacokinetics of the chemical
molecule that has deserved to eliminate HIV from the body without damaging the body. It
also increases the bio-distribution and bioavailability of the drug to expose the virus particle
for a longer duration with a higher goal precision. Application of nanotechnology to the
delivery of ARV drugs Holds the potential to treat AIDS and it could be beneficial Drugs at
the anatomical reservoir site and also raise the half-life of drugs Nano-carriers give a range of
advantages, such as control of drugs degradation, drug specificity and delivery of biological
products molecules, such as proteins, peptides, oligopeptides, oligonucleotides, etc.
Nanocarriers are now using it to solve the limitation of therapeutic uses, such as drug
delivery, bioavailability of drugs, drug conformation stability, physicochemical stability,
improved transmission permeability, drug clearance, cellular absorption, reduction of
immunogenic reaction. Inorganic solid lipid nanoparticles liposomes, polymeric micelles,
dendrimers, cyclodextrins, and cell-based nanoformulations have been studied for delivery of
drugs intended for HIV prevention or therapy.43 For anti-HIV drugs to be effective, adequate
distribution to specific sites in the body must be achieved, and effective drug concentrations
must be maintained at those sites for the required period of time. For effective delivery of anti
HIV-1 nanotherapy, an optimal drug delivery nanocarrier vehicle must be generated that
should be of a precise geometry, whose surface (ie, zeta potential, stealth ligands),
drug/biomolecule (antiretrovirals, oligonucleotides, proteins, small interfering RNA [siRNA],
RNA, imaging agents) encapsulation efficiency and release, surface chemistries (targeting
antibodies, PEG chains, metal chelators), and spatial distribution of ligands must be well
engineered. Targeted nanocarrier delivery involves (1) the recognition of HIV-infectable
target cells and tissues; (2) the ability to reach these sites; and (3) the ability to deliver
multiple therapeutic agents. Nowacek and Gendelman have shown that a single intravenous
dose of the nano-ART can elicit high-sustained tissue and plasma drug levels of antiretroviral
drugs in the reticuloendothelial system and brain.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
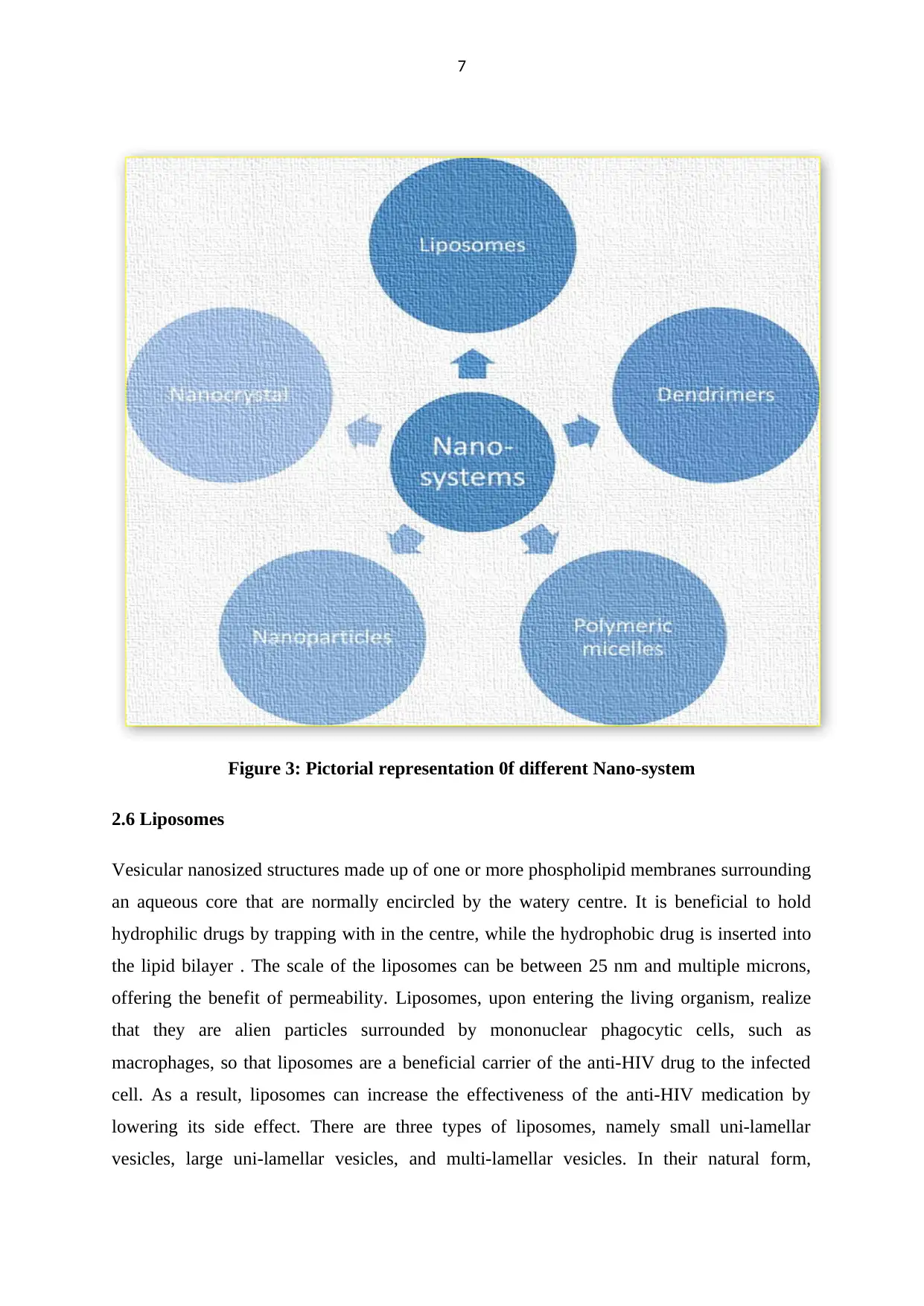
7
Figure 3: Pictorial representation 0f different Nano-system
2.6 Liposomes
Vesicular nanosized structures made up of one or more phospholipid membranes surrounding
an aqueous core that are normally encircled by the watery centre. It is beneficial to hold
hydrophilic drugs by trapping with in the centre, while the hydrophobic drug is inserted into
the lipid bilayer . The scale of the liposomes can be between 25 nm and multiple microns,
offering the benefit of permeability. Liposomes, upon entering the living organism, realize
that they are alien particles surrounded by mononuclear phagocytic cells, such as
macrophages, so that liposomes are a beneficial carrier of the anti-HIV drug to the infected
cell. As a result, liposomes can increase the effectiveness of the anti-HIV medication by
lowering its side effect. There are three types of liposomes, namely small uni-lamellar
vesicles, large uni-lamellar vesicles, and multi-lamellar vesicles. In their natural form,
Figure 3: Pictorial representation 0f different Nano-system
2.6 Liposomes
Vesicular nanosized structures made up of one or more phospholipid membranes surrounding
an aqueous core that are normally encircled by the watery centre. It is beneficial to hold
hydrophilic drugs by trapping with in the centre, while the hydrophobic drug is inserted into
the lipid bilayer . The scale of the liposomes can be between 25 nm and multiple microns,
offering the benefit of permeability. Liposomes, upon entering the living organism, realize
that they are alien particles surrounded by mononuclear phagocytic cells, such as
macrophages, so that liposomes are a beneficial carrier of the anti-HIV drug to the infected
cell. As a result, liposomes can increase the effectiveness of the anti-HIV medication by
lowering its side effect. There are three types of liposomes, namely small uni-lamellar
vesicles, large uni-lamellar vesicles, and multi-lamellar vesicles. In their natural form,
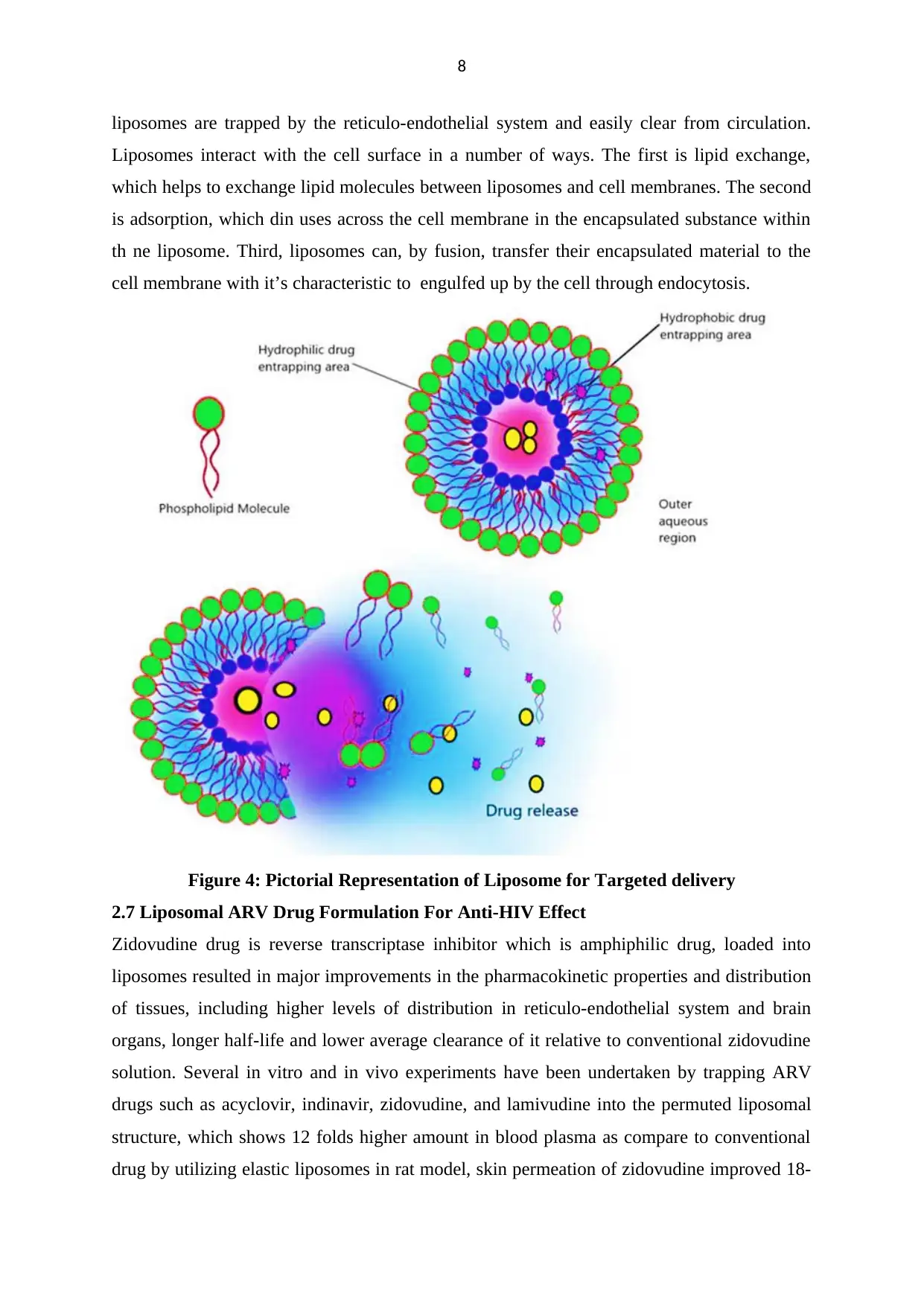
8
liposomes are trapped by the reticulo-endothelial system and easily clear from circulation.
Liposomes interact with the cell surface in a number of ways. The first is lipid exchange,
which helps to exchange lipid molecules between liposomes and cell membranes. The second
is adsorption, which din uses across the cell membrane in the encapsulated substance within
th ne liposome. Third, liposomes can, by fusion, transfer their encapsulated material to the
cell membrane with it’s characteristic to engulfed up by the cell through endocytosis.
Figure 4: Pictorial Representation of Liposome for Targeted delivery
2.7 Liposomal ARV Drug Formulation For Anti-HIV Effect
Zidovudine drug is reverse transcriptase inhibitor which is amphiphilic drug, loaded into
liposomes resulted in major improvements in the pharmacokinetic properties and distribution
of tissues, including higher levels of distribution in reticulo-endothelial system and brain
organs, longer half-life and lower average clearance of it relative to conventional zidovudine
solution. Several in vitro and in vivo experiments have been undertaken by trapping ARV
drugs such as acyclovir, indinavir, zidovudine, and lamivudine into the permuted liposomal
structure, which shows 12 folds higher amount in blood plasma as compare to conventional
drug by utilizing elastic liposomes in rat model, skin permeation of zidovudine improved 18-
liposomes are trapped by the reticulo-endothelial system and easily clear from circulation.
Liposomes interact with the cell surface in a number of ways. The first is lipid exchange,
which helps to exchange lipid molecules between liposomes and cell membranes. The second
is adsorption, which din uses across the cell membrane in the encapsulated substance within
th ne liposome. Third, liposomes can, by fusion, transfer their encapsulated material to the
cell membrane with it’s characteristic to engulfed up by the cell through endocytosis.
Figure 4: Pictorial Representation of Liposome for Targeted delivery
2.7 Liposomal ARV Drug Formulation For Anti-HIV Effect
Zidovudine drug is reverse transcriptase inhibitor which is amphiphilic drug, loaded into
liposomes resulted in major improvements in the pharmacokinetic properties and distribution
of tissues, including higher levels of distribution in reticulo-endothelial system and brain
organs, longer half-life and lower average clearance of it relative to conventional zidovudine
solution. Several in vitro and in vivo experiments have been undertaken by trapping ARV
drugs such as acyclovir, indinavir, zidovudine, and lamivudine into the permuted liposomal
structure, which shows 12 folds higher amount in blood plasma as compare to conventional
drug by utilizing elastic liposomes in rat model, skin permeation of zidovudine improved 18-

9
fold relative to simple drugs, this indicates a high effectiveness of transdermal flux relative to
free drugs and higher deposition in the reticulo-endothelial organ system following the launch
of zidovudine-loaded elastic liposomes trans-dermal. This suggests a greater permeability of
the liposomal composition in rat model. For lymphatic’s targeting, the surface of liposomes
was coordinated by charges and site-specific ligands to facilitate lymphatic, prominently
lymph node and spleen localization. The particle-charged liposomes were formed using
stearylamine, dicetyphosphate, and mannose conjugate. Liposomes are quickly phagocytised
by macrophages, in order to improve the extended circulation time and bioavailability of the
drug the surface is changed by hydrophilic molecules such as polyethene glycol, PEGylated
liposomes with targeting ligand derived from HIV gp120 guided monoclonal antibody F10
and seen as novel approaches to the battle against HIV-1. These Nano-immuno-liposomes
display greater and longer antiviral efficacy than free drugs or drugs that encapsulate non-
targeted liposomes. PEGylated liposomal transmission to mammalian cells in culture
demonstrated sustained release with encapsulation efficiency of approximately 33 per cent. In
cell viability tests of Jurkat T- cell, lower cytotoxicity was found relative to non-PEGylated
liposomes . Immune-liposomes filled with heparin active serine Antithrombin III (hep-AIII)
protease inhibitor injected into the non-human primate system model. The outcome indicates
a steady decrease of more than 1(10) log in plasma viral load that concludes hep-AIII as a
rescue or replacement agent for HIV strain immune to standard ARV drugs . Glycan-
Modifified HIV NFL Envelope Trimer-Liposome vaccine formulation showed broad
generation of neutralizing Antibody in modal, which hold proof for immunogenic vaccine
development to combat AIDS . The structure of the mixture consisted of liposomes that
acted as decoys allowing the HIV virus to bind to liposomes instead of host cells.
2.8 Dendrimer
The dendrimer consists of Dendron’s, a small branching unit that includes an internal and a
periphery end group, a polymeric nanostructure consisting of multiple branching units in a
layer by layer pattern that characterizes the size, growth and microenvironment within it.
Dendrimer with signifificant numbers of peripheral groups and inner cavities are possible
vectors for chemical drugs, peptides and HIV inhibition genes. These compounds are either
capable of interacting with peripheral groups or are encased in dendrimer cavities with
hydrogen bonds, electrostatic and hydrophobic interactions . Dendrimers can improve the
stability of chemical drugs and encourage cellular absorption by functional end-groups. In the
case of gene therapy, Dendrimers can take the place of viruses to transfer interference genes
to target cells to suppress replication of HIV. The kernel can be synthesized by ammonia and
fold relative to simple drugs, this indicates a high effectiveness of transdermal flux relative to
free drugs and higher deposition in the reticulo-endothelial organ system following the launch
of zidovudine-loaded elastic liposomes trans-dermal. This suggests a greater permeability of
the liposomal composition in rat model. For lymphatic’s targeting, the surface of liposomes
was coordinated by charges and site-specific ligands to facilitate lymphatic, prominently
lymph node and spleen localization. The particle-charged liposomes were formed using
stearylamine, dicetyphosphate, and mannose conjugate. Liposomes are quickly phagocytised
by macrophages, in order to improve the extended circulation time and bioavailability of the
drug the surface is changed by hydrophilic molecules such as polyethene glycol, PEGylated
liposomes with targeting ligand derived from HIV gp120 guided monoclonal antibody F10
and seen as novel approaches to the battle against HIV-1. These Nano-immuno-liposomes
display greater and longer antiviral efficacy than free drugs or drugs that encapsulate non-
targeted liposomes. PEGylated liposomal transmission to mammalian cells in culture
demonstrated sustained release with encapsulation efficiency of approximately 33 per cent. In
cell viability tests of Jurkat T- cell, lower cytotoxicity was found relative to non-PEGylated
liposomes . Immune-liposomes filled with heparin active serine Antithrombin III (hep-AIII)
protease inhibitor injected into the non-human primate system model. The outcome indicates
a steady decrease of more than 1(10) log in plasma viral load that concludes hep-AIII as a
rescue or replacement agent for HIV strain immune to standard ARV drugs . Glycan-
Modifified HIV NFL Envelope Trimer-Liposome vaccine formulation showed broad
generation of neutralizing Antibody in modal, which hold proof for immunogenic vaccine
development to combat AIDS . The structure of the mixture consisted of liposomes that
acted as decoys allowing the HIV virus to bind to liposomes instead of host cells.
2.8 Dendrimer
The dendrimer consists of Dendron’s, a small branching unit that includes an internal and a
periphery end group, a polymeric nanostructure consisting of multiple branching units in a
layer by layer pattern that characterizes the size, growth and microenvironment within it.
Dendrimer with signifificant numbers of peripheral groups and inner cavities are possible
vectors for chemical drugs, peptides and HIV inhibition genes. These compounds are either
capable of interacting with peripheral groups or are encased in dendrimer cavities with
hydrogen bonds, electrostatic and hydrophobic interactions . Dendrimers can improve the
stability of chemical drugs and encourage cellular absorption by functional end-groups. In the
case of gene therapy, Dendrimers can take the place of viruses to transfer interference genes
to target cells to suppress replication of HIV. The kernel can be synthesized by ammonia and
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
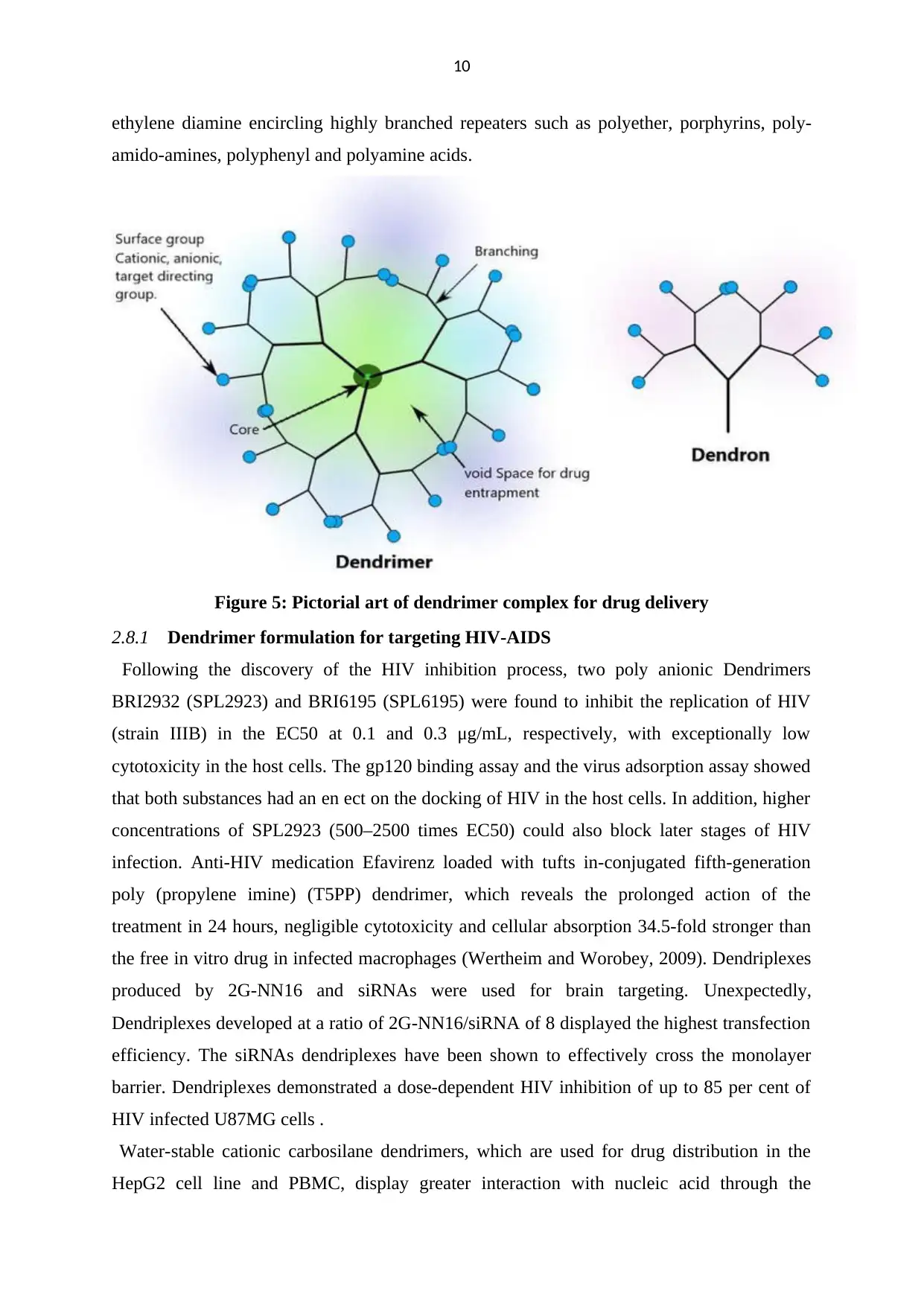
10
ethylene diamine encircling highly branched repeaters such as polyether, porphyrins, poly-
amido-amines, polyphenyl and polyamine acids.
Figure 5: Pictorial art of dendrimer complex for drug delivery
2.8.1 Dendrimer formulation for targeting HIV-AIDS
Following the discovery of the HIV inhibition process, two poly anionic Dendrimers
BRI2932 (SPL2923) and BRI6195 (SPL6195) were found to inhibit the replication of HIV
(strain IIIB) in the EC50 at 0.1 and 0.3 μg/mL, respectively, with exceptionally low
cytotoxicity in the host cells. The gp120 binding assay and the virus adsorption assay showed
that both substances had an en ect on the docking of HIV in the host cells. In addition, higher
concentrations of SPL2923 (500–2500 times EC50) could also block later stages of HIV
infection. Anti-HIV medication Efavirenz loaded with tufts in-conjugated fifth-generation
poly (propylene imine) (T5PP) dendrimer, which reveals the prolonged action of the
treatment in 24 hours, negligible cytotoxicity and cellular absorption 34.5-fold stronger than
the free in vitro drug in infected macrophages (Wertheim and Worobey, 2009). Dendriplexes
produced by 2G-NN16 and siRNAs were used for brain targeting. Unexpectedly,
Dendriplexes developed at a ratio of 2G-NN16/siRNA of 8 displayed the highest transfection
efficiency. The siRNAs dendriplexes have been shown to effectively cross the monolayer
barrier. Dendriplexes demonstrated a dose-dependent HIV inhibition of up to 85 per cent of
HIV infected U87MG cells .
Water-stable cationic carbosilane dendrimers, which are used for drug distribution in the
HepG2 cell line and PBMC, display greater interaction with nucleic acid through the
ethylene diamine encircling highly branched repeaters such as polyether, porphyrins, poly-
amido-amines, polyphenyl and polyamine acids.
Figure 5: Pictorial art of dendrimer complex for drug delivery
2.8.1 Dendrimer formulation for targeting HIV-AIDS
Following the discovery of the HIV inhibition process, two poly anionic Dendrimers
BRI2932 (SPL2923) and BRI6195 (SPL6195) were found to inhibit the replication of HIV
(strain IIIB) in the EC50 at 0.1 and 0.3 μg/mL, respectively, with exceptionally low
cytotoxicity in the host cells. The gp120 binding assay and the virus adsorption assay showed
that both substances had an en ect on the docking of HIV in the host cells. In addition, higher
concentrations of SPL2923 (500–2500 times EC50) could also block later stages of HIV
infection. Anti-HIV medication Efavirenz loaded with tufts in-conjugated fifth-generation
poly (propylene imine) (T5PP) dendrimer, which reveals the prolonged action of the
treatment in 24 hours, negligible cytotoxicity and cellular absorption 34.5-fold stronger than
the free in vitro drug in infected macrophages (Wertheim and Worobey, 2009). Dendriplexes
produced by 2G-NN16 and siRNAs were used for brain targeting. Unexpectedly,
Dendriplexes developed at a ratio of 2G-NN16/siRNA of 8 displayed the highest transfection
efficiency. The siRNAs dendriplexes have been shown to effectively cross the monolayer
barrier. Dendriplexes demonstrated a dose-dependent HIV inhibition of up to 85 per cent of
HIV infected U87MG cells .
Water-stable cationic carbosilane dendrimers, which are used for drug distribution in the
HepG2 cell line and PBMC, display greater interaction with nucleic acid through the
11
development of nanoconjugates in different stable pH ranges. SPL7013 is one of the anionic
dendrimers that contains the divalent benzhydryl amide of L- lysine as the nucleus of
naphthalene sulfonic acid. Multivalent phosphorous-containing catanionic dendrimers with
galactosyl ceramide analogs have a significant affinity to the V3 loop of the HIV-1 viral
envelope protein gp120, which inhibits viral fusion with the plasma membrane and thus
serves as an entry inhibitor.
2.8.2 FDA approved dendrimer of AIDS
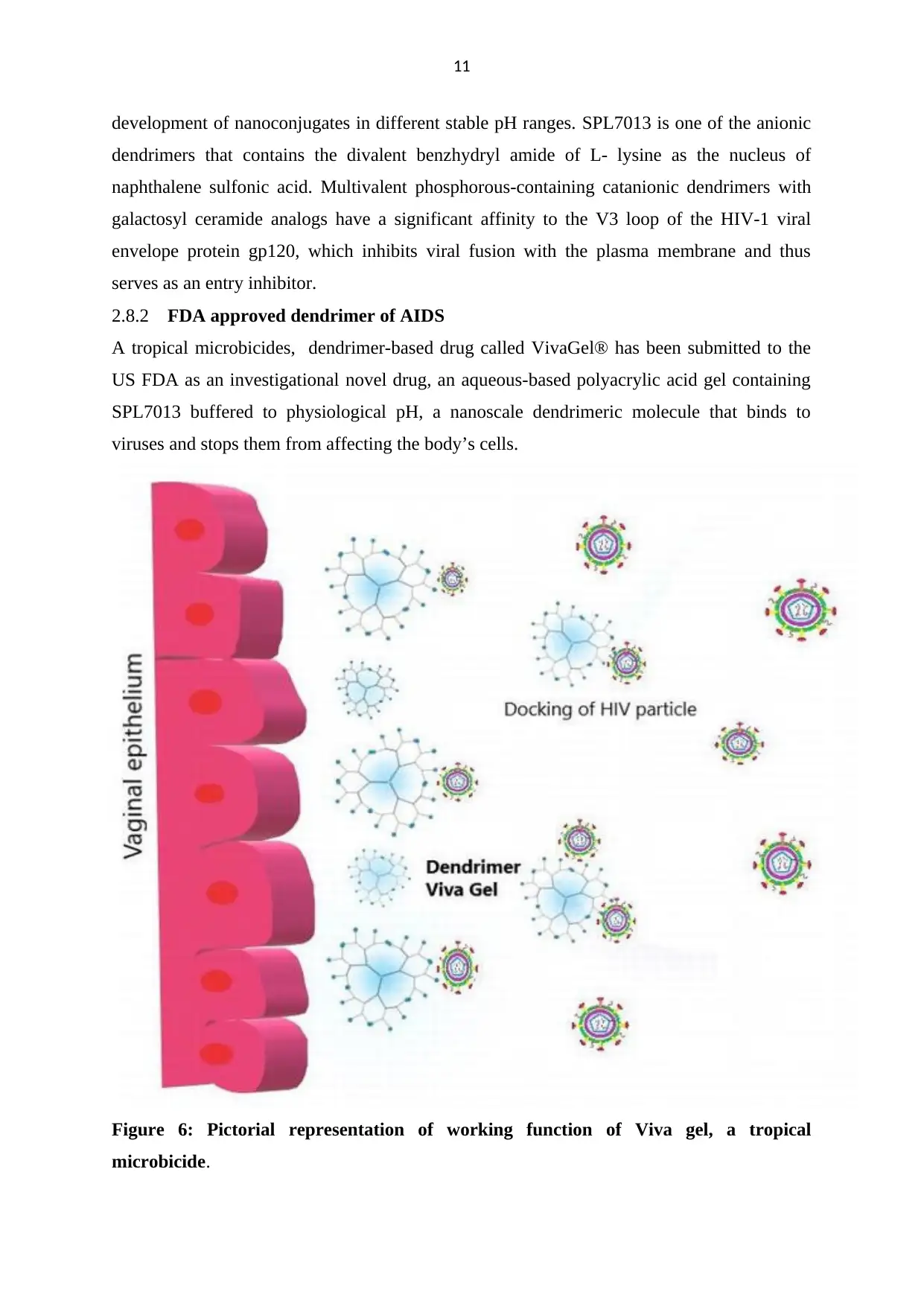
A tropical microbicides, dendrimer-based drug called VivaGel® has been submitted to the
US FDA as an investigational novel drug, an aqueous-based polyacrylic acid gel containing
SPL7013 buffered to physiological pH, a nanoscale dendrimeric molecule that binds to
viruses and stops them from affecting the body’s cells.
Figure 6: Pictorial representation of working function of Viva gel, a tropical
microbicide.
development of nanoconjugates in different stable pH ranges. SPL7013 is one of the anionic
dendrimers that contains the divalent benzhydryl amide of L- lysine as the nucleus of
naphthalene sulfonic acid. Multivalent phosphorous-containing catanionic dendrimers with
galactosyl ceramide analogs have a significant affinity to the V3 loop of the HIV-1 viral
envelope protein gp120, which inhibits viral fusion with the plasma membrane and thus
serves as an entry inhibitor.
2.8.2 FDA approved dendrimer of AIDS
A tropical microbicides, dendrimer-based drug called VivaGel® has been submitted to the
US FDA as an investigational novel drug, an aqueous-based polyacrylic acid gel containing
SPL7013 buffered to physiological pH, a nanoscale dendrimeric molecule that binds to
viruses and stops them from affecting the body’s cells.
Figure 6: Pictorial representation of working function of Viva gel, a tropical
microbicide.

12
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
13
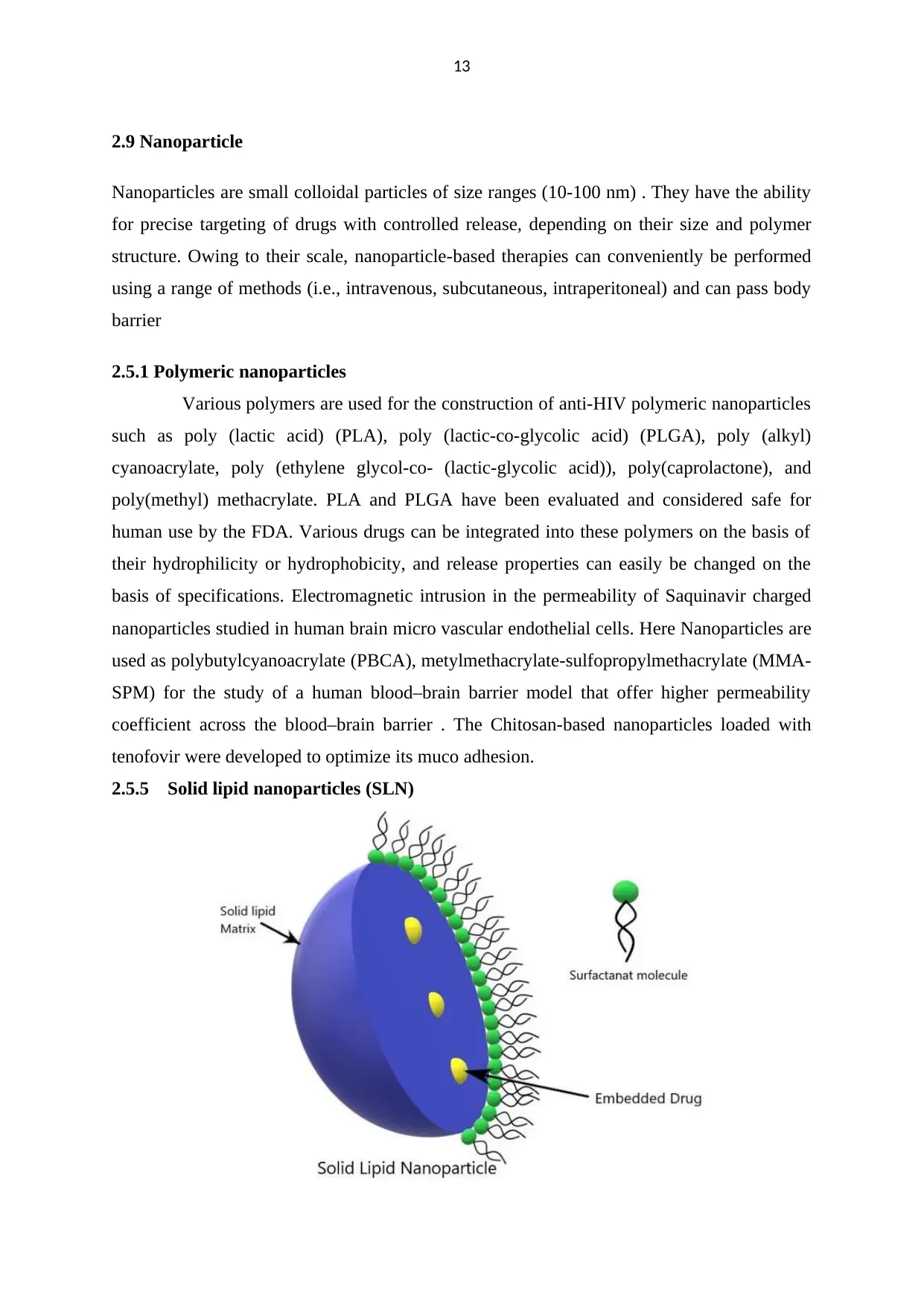
2.9 Nanoparticle
Nanoparticles are small colloidal particles of size ranges (10-100 nm) . They have the ability
for precise targeting of drugs with controlled release, depending on their size and polymer
structure. Owing to their scale, nanoparticle-based therapies can conveniently be performed
using a range of methods (i.e., intravenous, subcutaneous, intraperitoneal) and can pass body
barrier
2.5.1 Polymeric nanoparticles
Various polymers are used for the construction of anti-HIV polymeric nanoparticles
such as poly (lactic acid) (PLA), poly (lactic-co-glycolic acid) (PLGA), poly (alkyl)
cyanoacrylate, poly (ethylene glycol-co- (lactic-glycolic acid)), poly(caprolactone), and
poly(methyl) methacrylate. PLA and PLGA have been evaluated and considered safe for
human use by the FDA. Various drugs can be integrated into these polymers on the basis of
their hydrophilicity or hydrophobicity, and release properties can easily be changed on the
basis of specifications. Electromagnetic intrusion in the permeability of Saquinavir charged
nanoparticles studied in human brain micro vascular endothelial cells. Here Nanoparticles are
used as polybutylcyanoacrylate (PBCA), metylmethacrylate-sulfopropylmethacrylate (MMA-
SPM) for the study of a human blood–brain barrier model that offer higher permeability
coefficient across the blood–brain barrier . The Chitosan-based nanoparticles loaded with
tenofovir were developed to optimize its muco adhesion.
2.5.5 Solid lipid nanoparticles (SLN)
2.9 Nanoparticle
Nanoparticles are small colloidal particles of size ranges (10-100 nm) . They have the ability
for precise targeting of drugs with controlled release, depending on their size and polymer
structure. Owing to their scale, nanoparticle-based therapies can conveniently be performed
using a range of methods (i.e., intravenous, subcutaneous, intraperitoneal) and can pass body
barrier
2.5.1 Polymeric nanoparticles
Various polymers are used for the construction of anti-HIV polymeric nanoparticles
such as poly (lactic acid) (PLA), poly (lactic-co-glycolic acid) (PLGA), poly (alkyl)
cyanoacrylate, poly (ethylene glycol-co- (lactic-glycolic acid)), poly(caprolactone), and
poly(methyl) methacrylate. PLA and PLGA have been evaluated and considered safe for
human use by the FDA. Various drugs can be integrated into these polymers on the basis of
their hydrophilicity or hydrophobicity, and release properties can easily be changed on the
basis of specifications. Electromagnetic intrusion in the permeability of Saquinavir charged
nanoparticles studied in human brain micro vascular endothelial cells. Here Nanoparticles are
used as polybutylcyanoacrylate (PBCA), metylmethacrylate-sulfopropylmethacrylate (MMA-
SPM) for the study of a human blood–brain barrier model that offer higher permeability
coefficient across the blood–brain barrier . The Chitosan-based nanoparticles loaded with
tenofovir were developed to optimize its muco adhesion.
2.5.5 Solid lipid nanoparticles (SLN)
14
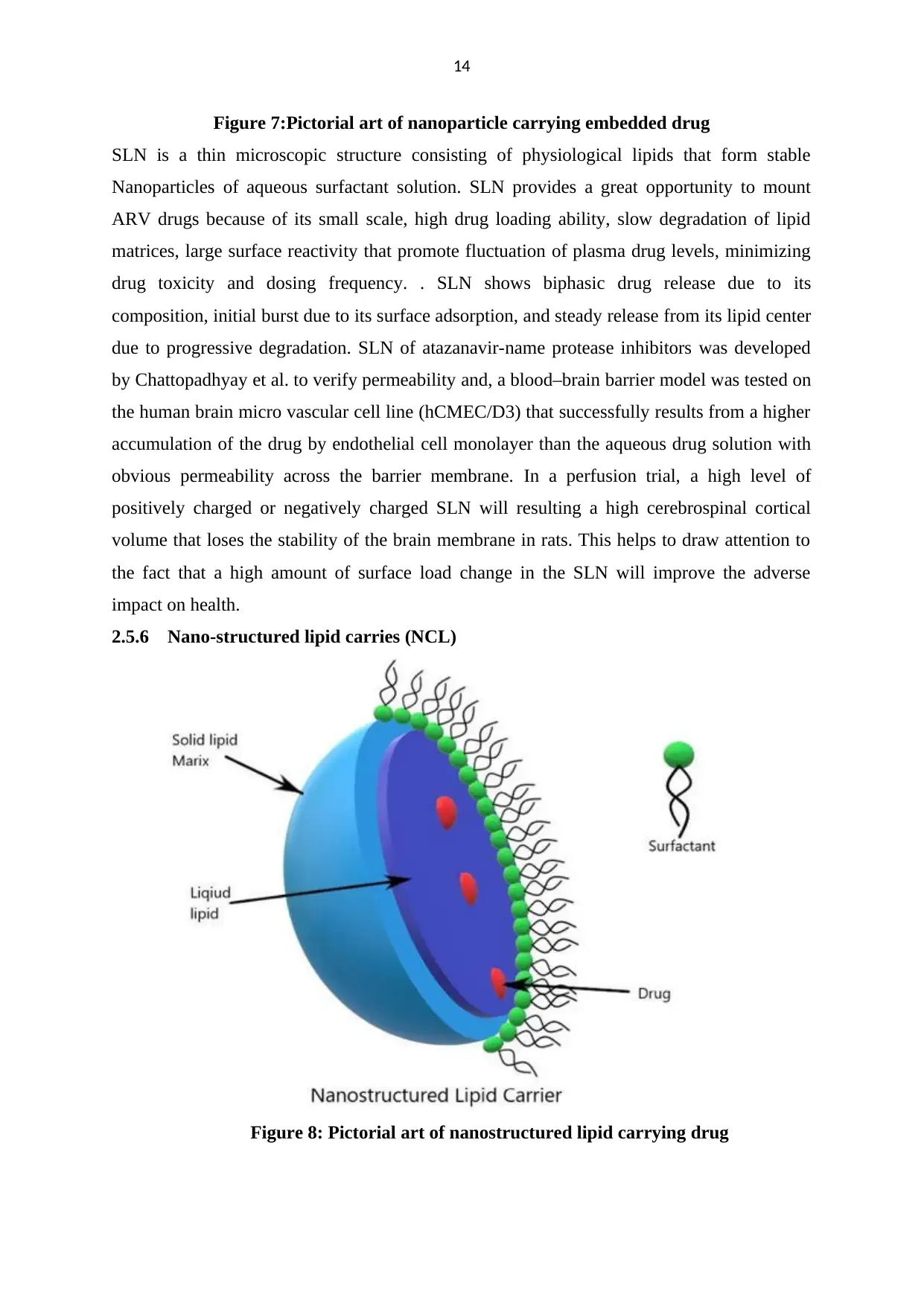
Figure 7:Pictorial art of nanoparticle carrying embedded drug
SLN is a thin microscopic structure consisting of physiological lipids that form stable
Nanoparticles of aqueous surfactant solution. SLN provides a great opportunity to mount
ARV drugs because of its small scale, high drug loading ability, slow degradation of lipid
matrices, large surface reactivity that promote fluctuation of plasma drug levels, minimizing
drug toxicity and dosing frequency. . SLN shows biphasic drug release due to its
composition, initial burst due to its surface adsorption, and steady release from its lipid center
due to progressive degradation. SLN of atazanavir-name protease inhibitors was developed
by Chattopadhyay et al. to verify permeability and, a blood–brain barrier model was tested on
the human brain micro vascular cell line (hCMEC/D3) that successfully results from a higher
accumulation of the drug by endothelial cell monolayer than the aqueous drug solution with
obvious permeability across the barrier membrane. In a perfusion trial, a high level of
positively charged or negatively charged SLN will resulting a high cerebrospinal cortical
volume that loses the stability of the brain membrane in rats. This helps to draw attention to
the fact that a high amount of surface load change in the SLN will improve the adverse
impact on health.
2.5.6 Nano-structured lipid carries (NCL)
Figure 8: Pictorial art of nanostructured lipid carrying drug
Figure 7:Pictorial art of nanoparticle carrying embedded drug
SLN is a thin microscopic structure consisting of physiological lipids that form stable
Nanoparticles of aqueous surfactant solution. SLN provides a great opportunity to mount
ARV drugs because of its small scale, high drug loading ability, slow degradation of lipid
matrices, large surface reactivity that promote fluctuation of plasma drug levels, minimizing
drug toxicity and dosing frequency. . SLN shows biphasic drug release due to its
composition, initial burst due to its surface adsorption, and steady release from its lipid center
due to progressive degradation. SLN of atazanavir-name protease inhibitors was developed
by Chattopadhyay et al. to verify permeability and, a blood–brain barrier model was tested on
the human brain micro vascular cell line (hCMEC/D3) that successfully results from a higher
accumulation of the drug by endothelial cell monolayer than the aqueous drug solution with
obvious permeability across the barrier membrane. In a perfusion trial, a high level of
positively charged or negatively charged SLN will resulting a high cerebrospinal cortical
volume that loses the stability of the brain membrane in rats. This helps to draw attention to
the fact that a high amount of surface load change in the SLN will improve the adverse
impact on health.
2.5.6 Nano-structured lipid carries (NCL)
Figure 8: Pictorial art of nanostructured lipid carrying drug

15
NCL is a fashioned or customized SLN with a solid lipid matrix incorporated with liquid
lipids with different fatty acid chains in a compromised ordered crystalline form that provides
higher drug capacity. NCL consists of low-toxic lipid molecules that have hydrolytic and
oxidative stability. It also indicates the biphasic drug release potential for a liquid lipophilic
surface containing a drug and a solid center with a higher melting point for drug release
through diffusion and matrix erosion.
2.5.7 Inorganic nanoparticles
This class of Nanoparticles contains metal elements such as iron, gold, silver, titanium and
silica that are currently used in anti-cancer treatment, molecular labeling of biomarkers,
clinical methods, bioimaging, biosensors. Which are becoming more common due to their
antimicrobial and antiviral effects through the formulation techniques like chemical bio-
reduction, rough mold, solution-phase synthesis, gas-phase deposition, and sol–gel. . Silver
nanoparticles can bind to the gp120 protein and prevent viral entry, inhibit CD4-mediated
viral fusion, and interfere with post-invasion phases of the HIV life cycle. Conjugated gold
nanoparticle with TAK-779 and SDC-1721 which allow for better anti-HIV activity than its
aqueous solution. Inorganic nanoparticles have limitations such as cytotoxicity, DNA
damage, cellular apoptosis triggered by membrane leakage assay and LDH assay.
2.7 Polymeric Micelles
Polymer micelles are nano-engineered block polymer materials that have core shells
much like surfactant-based micelles and have been used to enhance permeability, aqueous
solubility, chemical corrosion safety, controlled drug release, provide hydrophobic surface
modification. Polymeric micelles are engineered as a hydrophobic heart and a hydrophilic
shell that allows anti-HIV drugs to be trapped depending on their polarity.
NCL is a fashioned or customized SLN with a solid lipid matrix incorporated with liquid
lipids with different fatty acid chains in a compromised ordered crystalline form that provides
higher drug capacity. NCL consists of low-toxic lipid molecules that have hydrolytic and
oxidative stability. It also indicates the biphasic drug release potential for a liquid lipophilic
surface containing a drug and a solid center with a higher melting point for drug release
through diffusion and matrix erosion.
2.5.7 Inorganic nanoparticles
This class of Nanoparticles contains metal elements such as iron, gold, silver, titanium and
silica that are currently used in anti-cancer treatment, molecular labeling of biomarkers,
clinical methods, bioimaging, biosensors. Which are becoming more common due to their
antimicrobial and antiviral effects through the formulation techniques like chemical bio-
reduction, rough mold, solution-phase synthesis, gas-phase deposition, and sol–gel. . Silver
nanoparticles can bind to the gp120 protein and prevent viral entry, inhibit CD4-mediated
viral fusion, and interfere with post-invasion phases of the HIV life cycle. Conjugated gold
nanoparticle with TAK-779 and SDC-1721 which allow for better anti-HIV activity than its
aqueous solution. Inorganic nanoparticles have limitations such as cytotoxicity, DNA
damage, cellular apoptosis triggered by membrane leakage assay and LDH assay.
2.7 Polymeric Micelles
Polymer micelles are nano-engineered block polymer materials that have core shells
much like surfactant-based micelles and have been used to enhance permeability, aqueous
solubility, chemical corrosion safety, controlled drug release, provide hydrophobic surface
modification. Polymeric micelles are engineered as a hydrophobic heart and a hydrophilic
shell that allows anti-HIV drugs to be trapped depending on their polarity.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

16
Figure 9: Pictorial art of polymeric micelles
A number of pharmaceutical scientists have formulated polymeric ARV-loaded mice, such as
lamivudine conjugated with stearic acid-g-chitosan oligosaccharide mice, by esterification
process that results in pH-dependent drug release, low cytotoxic activity. Copolymer: Poly
(ethylene glycol) monomethyl ether and poly (ethylene phosphoric acid) (mPEG-b-PEPA)
uses tenofovir, which varies in the length of the poly (ethylene phosphoric acid) chains and
the degree of their saturation with tenofovir. Both adducts were found to be more active than
conventional tenofovir against HIV-1IIIB in MT-4 cells.
2.7 Nanocrystal
Nanocrystal drug itself may be a nano-sized drug particle that could be dispersed in
aqueous or non-aqueous media. Drug Nanocrystals are mostly developed using approaches
that promote a top-down approach or a bottom-up approach. Top-down techniques, such as
media milling and high-pressure homogenization, are the most favoured methods for the
generation of nanocrystals because they are ideal for large-scale processing. Nanocrystal
medication has longer colloidal stability, prolonged and continuous targeting due to expanded
surface area. Nanoscale pure drug engineering is produced by means of an extremely
hydrophobic drug that is strenuous to administer as an intravenous solution or by means of
drugs with a rate of dissolution-limited oral bioavailability. Nanocrystal technology was used
to formulate a long-acting, parenteral form of the poorly water-soluble antiretroviral
rilpivirine, a next-generation human immunodeficiency virus type 1 (HIV-1) nonnucleoside
reverse transcriptase inhibitor . The half-life of conventional rilpivirine is 38 hours .
Nanocrystals injected into the venous circulation are opsonized by plasma proteins and are
phagocytosed rapidly and predominantly by the Kuppfer cells of the liver, which serves as a
depot for the accumulation and slow release of the drug. This phenomenon may be an
advantage (if reticuloendothelial accumulation and slow release is desired) or a disadvantage
(if the drug is toxic to liver cells, and if high plasma levels are required). These so called
“smartCrystals” avoid phagocytosis and furthermore, due to their large surface area to
volume ratio, undergo rapid dissolution in the bloodstream, resulting in a “bolus” effect post-
injection. Alternatively, a “stealth” particle may be formulated, which is a nanocrystal coated
with polyethyleneglycol, which prevents opsonization, thus promoting prolonged circulation.
A “homing” molecule (which mediates its attachment to the target cell) may additionally be
Figure 9: Pictorial art of polymeric micelles
A number of pharmaceutical scientists have formulated polymeric ARV-loaded mice, such as
lamivudine conjugated with stearic acid-g-chitosan oligosaccharide mice, by esterification
process that results in pH-dependent drug release, low cytotoxic activity. Copolymer: Poly
(ethylene glycol) monomethyl ether and poly (ethylene phosphoric acid) (mPEG-b-PEPA)
uses tenofovir, which varies in the length of the poly (ethylene phosphoric acid) chains and
the degree of their saturation with tenofovir. Both adducts were found to be more active than
conventional tenofovir against HIV-1IIIB in MT-4 cells.
2.7 Nanocrystal
Nanocrystal drug itself may be a nano-sized drug particle that could be dispersed in
aqueous or non-aqueous media. Drug Nanocrystals are mostly developed using approaches
that promote a top-down approach or a bottom-up approach. Top-down techniques, such as
media milling and high-pressure homogenization, are the most favoured methods for the
generation of nanocrystals because they are ideal for large-scale processing. Nanocrystal
medication has longer colloidal stability, prolonged and continuous targeting due to expanded
surface area. Nanoscale pure drug engineering is produced by means of an extremely
hydrophobic drug that is strenuous to administer as an intravenous solution or by means of
drugs with a rate of dissolution-limited oral bioavailability. Nanocrystal technology was used
to formulate a long-acting, parenteral form of the poorly water-soluble antiretroviral
rilpivirine, a next-generation human immunodeficiency virus type 1 (HIV-1) nonnucleoside
reverse transcriptase inhibitor . The half-life of conventional rilpivirine is 38 hours .
Nanocrystals injected into the venous circulation are opsonized by plasma proteins and are
phagocytosed rapidly and predominantly by the Kuppfer cells of the liver, which serves as a
depot for the accumulation and slow release of the drug. This phenomenon may be an
advantage (if reticuloendothelial accumulation and slow release is desired) or a disadvantage
(if the drug is toxic to liver cells, and if high plasma levels are required). These so called
“smartCrystals” avoid phagocytosis and furthermore, due to their large surface area to
volume ratio, undergo rapid dissolution in the bloodstream, resulting in a “bolus” effect post-
injection. Alternatively, a “stealth” particle may be formulated, which is a nanocrystal coated
with polyethyleneglycol, which prevents opsonization, thus promoting prolonged circulation.
A “homing” molecule (which mediates its attachment to the target cell) may additionally be

17
used for targeted delivery of the nanocrystal, which may be preferred to non-specific
accumulation in the reticulendothelial system or rapid dissolution in plasma.
CHAPTER THREE
CONCLUSION
4.1 Conclusion
An empirical application for anti-HIV therapy in drug delivery system lies in the potentiality
of a nanotechnology. Development of ARV drugs through nanotechnology- based system
such as Liposomes, dendrimers, Nanoparticles, Polymeric Micelles, Nanovesicles,
Nanoemulsion offers efficient & wide targeted drug delivery with modulated
pharmacokinetics, a higher therapeutic index. These nano-systems provide prolong drug
circulation, high bioavailability, drug stability, better permeability, bioaccumulation in
known reservoir sites for HIV-AIDS. It also demonstrated the application of ARV
nanocarriers to deliver drugs across the blood– brain barrier and other impermeable tissue to
kill HIV virus. On the basis of HIV lifecycle, diverse nanocarriers are surface modified with
different moiety to prevent viral fusion with intended ARV drug delivery. The majority of
works done in the field of nanocarrier ARV drug delivery system incorporate a single ARV
agent.
used for targeted delivery of the nanocrystal, which may be preferred to non-specific
accumulation in the reticulendothelial system or rapid dissolution in plasma.
CHAPTER THREE
CONCLUSION
4.1 Conclusion
An empirical application for anti-HIV therapy in drug delivery system lies in the potentiality
of a nanotechnology. Development of ARV drugs through nanotechnology- based system
such as Liposomes, dendrimers, Nanoparticles, Polymeric Micelles, Nanovesicles,
Nanoemulsion offers efficient & wide targeted drug delivery with modulated
pharmacokinetics, a higher therapeutic index. These nano-systems provide prolong drug
circulation, high bioavailability, drug stability, better permeability, bioaccumulation in
known reservoir sites for HIV-AIDS. It also demonstrated the application of ARV
nanocarriers to deliver drugs across the blood– brain barrier and other impermeable tissue to
kill HIV virus. On the basis of HIV lifecycle, diverse nanocarriers are surface modified with
different moiety to prevent viral fusion with intended ARV drug delivery. The majority of
works done in the field of nanocarrier ARV drug delivery system incorporate a single ARV
agent.

18
REFERENCE
Boggiano, C., & Littman, D. R. HIV’s Vagina Travelogue. Immunity, 2007; 26(2), 145- 147.
Littman D. R. Chemokine receptors: keys to AIDS pathogenesis. Cell, 1998; 93(5), 677- 680.
McGowan, I. Rectal microbicides: a new focus for HIV prevention. Sexually
Pauwels, R., & De Clercq, E. Development of vaginal microbicides for the prevention of
heterosexual transmission of HIV. Journal of acquired immune deficiency syndromes
and human retrovirology: official publication of the International Retrovirology
Association, 1996; 11(3), 211-221.
Transmitted Infections, 2008; 84(6), 413-417.
Wertheim, J. O., & Worobey, M. (2009). Dating the Age of the SIV Lineages That Gave Rise
to HIV-1 and HIV-2. PLoS Computational Biology, 5(5), e1000377. doi:
10.1371/journal.pcbi.1000377
WHO (Global Health Observatory Data Repository 2019).
REFERENCE
Boggiano, C., & Littman, D. R. HIV’s Vagina Travelogue. Immunity, 2007; 26(2), 145- 147.
Littman D. R. Chemokine receptors: keys to AIDS pathogenesis. Cell, 1998; 93(5), 677- 680.
McGowan, I. Rectal microbicides: a new focus for HIV prevention. Sexually
Pauwels, R., & De Clercq, E. Development of vaginal microbicides for the prevention of
heterosexual transmission of HIV. Journal of acquired immune deficiency syndromes
and human retrovirology: official publication of the International Retrovirology
Association, 1996; 11(3), 211-221.
Transmitted Infections, 2008; 84(6), 413-417.
Wertheim, J. O., & Worobey, M. (2009). Dating the Age of the SIV Lineages That Gave Rise
to HIV-1 and HIV-2. PLoS Computational Biology, 5(5), e1000377. doi:
10.1371/journal.pcbi.1000377
WHO (Global Health Observatory Data Repository 2019).
1 out of 25
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.