Antimalarial Drugs Research: Analysis of Current Therapies
VerifiedAdded on 2023/06/08
|23
|5280
|360
Report
AI Summary
This report delves into the critical field of antimalarial drug research, addressing the global challenge of malaria. The study begins with an overview of the disease, its prevalence, and the various Plasmodium species responsible, with a specific focus on Plasmodium falciparum, the most lethal species. It examines the history of antimalarial drugs, including the development of quinine and other alkaloids, as well as more effective drugs like sontoquine, mefloquine, and amodiaquine. The report highlights the mechanism of action of aminoquinolines, particularly their interaction with ferriprotoporphyrinIX-FPIX, and the subsequent inhibition of hemozoin formation. It also discusses the development of drug resistance, the emergence of new therapeutics like artemisinin, and the limitations of current treatments. The methodology section details the methods used, including the synthesis of 4-amino-7-chloroquinolines and the heme-targeted antimalarial approach. The results section presents the antimalarial activity of various aminoquinoline compounds against different strains of P. falciparum. The report concludes with a discussion of the findings, highlighting the importance of research into new drugs and the development of therapies that target both blood-stage and liver-stage parasites.

ANTIMALARIAL DRUGS RESEARCH
By Name
Course
Instructor
Institution
Location
Date
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Contents
1.0 ABSTRACT.........................................................................................................................3
2.0INDEX..................................................................................................................................4
2.1 LIST OF TABLES...........................................................................................................4
2.2 LIST OF FIGURES..........................................................................................................4
2.3 ABBREVIATIONS..........................................................................................................5
2.4 ACKNOWLEDGEMENT................................................................................................5
3.0 INTRODUCTION................................................................................................................5
4.0 AIMS OF THE PROJECT...................................................................................................8
5.0METHODS...........................................................................................................................8
6.0RESULTS...........................................................................................................................10
7.0DISCUSSION.....................................................................................................................12
8.0CONCLUSION...................................................................................................................19
9.0REFERENCES....................................................................................................................20
10.0APPENDIX.......................................................................................................................22
11.0LABORATORY CLEARANCE FORM..........................................................................23
1.0 ABSTRACT.........................................................................................................................3
2.0INDEX..................................................................................................................................4
2.1 LIST OF TABLES...........................................................................................................4
2.2 LIST OF FIGURES..........................................................................................................4
2.3 ABBREVIATIONS..........................................................................................................5
2.4 ACKNOWLEDGEMENT................................................................................................5
3.0 INTRODUCTION................................................................................................................5
4.0 AIMS OF THE PROJECT...................................................................................................8
5.0METHODS...........................................................................................................................8
6.0RESULTS...........................................................................................................................10
7.0DISCUSSION.....................................................................................................................12
8.0CONCLUSION...................................................................................................................19
9.0REFERENCES....................................................................................................................20
10.0APPENDIX.......................................................................................................................22
11.0LABORATORY CLEARANCE FORM..........................................................................23

1.0 ABSTRACT
Malaria is considered the most widespread disease in the world today. The disease is
devastating and is very infectious claiming nearly two million per year while those diagnosed
with the disease are roughly 300million.The protozoan parasites of the genus plasmodium
that causes malaria are very many. However, the most lethal one is called plasmodium
falciparum. There have been a number of discoveries of the antimalarial drugs including
quinine and other alkaloids of cinchona.
Apart from the mentioned ones, there exist other drugs that are more effective including
sontoquine, mefloquine, amodiaquine among others. Aminoquinolines are commonly known
for the formation of the complex compound with the ferriprotoporphyrinIX-FPIX.This
compound is normally generated in the food vacuole within the cells of the parasites. This is
necessitated by the process called proteolysis of haemoglobin within the cells of the host
thereby acting as the source of the required amino acids.
This amino acid is much needed during the life stages of the protozoa within the red blood
cells. Studies have indicated that the free FPIX is very much cytotoxic to plasmodium.
Plasmodium however has developed a strategy of reducing the quantity of FPIX which is
achieved through changing it into the crystalline but insoluble compound called hemozoin.
FPIX reactions prevent the conversion of the hematin to hemozoin thereby introducing
detoxification through crystallization process.
The accumulation of the considerable concentration of this compound is probably responsible
for the killings of the plasmodium parasite. There is general acceptance that this 4-
aminoquinoline pharmacophore has very significant duty in the process of complexation to
FPIX that leads to the inhibition of the formation of the hemozoin and subsequent growth of
the parasite. In the present date, several species of plasmodium have developed resistance
against the commonly used antimalarial drugs. Attempts to have this addressed have led to
Malaria is considered the most widespread disease in the world today. The disease is
devastating and is very infectious claiming nearly two million per year while those diagnosed
with the disease are roughly 300million.The protozoan parasites of the genus plasmodium
that causes malaria are very many. However, the most lethal one is called plasmodium
falciparum. There have been a number of discoveries of the antimalarial drugs including
quinine and other alkaloids of cinchona.
Apart from the mentioned ones, there exist other drugs that are more effective including
sontoquine, mefloquine, amodiaquine among others. Aminoquinolines are commonly known
for the formation of the complex compound with the ferriprotoporphyrinIX-FPIX.This
compound is normally generated in the food vacuole within the cells of the parasites. This is
necessitated by the process called proteolysis of haemoglobin within the cells of the host
thereby acting as the source of the required amino acids.
This amino acid is much needed during the life stages of the protozoa within the red blood
cells. Studies have indicated that the free FPIX is very much cytotoxic to plasmodium.
Plasmodium however has developed a strategy of reducing the quantity of FPIX which is
achieved through changing it into the crystalline but insoluble compound called hemozoin.
FPIX reactions prevent the conversion of the hematin to hemozoin thereby introducing
detoxification through crystallization process.
The accumulation of the considerable concentration of this compound is probably responsible
for the killings of the plasmodium parasite. There is general acceptance that this 4-
aminoquinoline pharmacophore has very significant duty in the process of complexation to
FPIX that leads to the inhibition of the formation of the hemozoin and subsequent growth of
the parasite. In the present date, several species of plasmodium have developed resistance
against the commonly used antimalarial drugs. Attempts to have this addressed have led to
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

the development of new therapeutics that includes artemisinin together with other
endoperoxides.
2.0INDEX
Procedure-3
Disease-4
Methods-5
2.1 LIST OF TABLES
Table 1: Samples of drugs, 10
Table 2: mineral water ratio, 12
Table 3: demographic samples, 14
Table 4: ACT samples, 16
Table 5: Summary of effects, 18
2.2 LIST OF FIGURES
Figure1: life cycle of mosquitoes, 5
Figure2: Anopheles mosquito, 6
Figure 3: Samples of injections, 11
Figure 4: Samples of drug effects
2.3 ABBREVIATIONS
A.C.T-Artemisinin combination therapies
C.Q.R-Chloroquine resistance.
HB3-Chloroquine sensitive strain
Dd2-chloroquine resistance strain
endoperoxides.
2.0INDEX
Procedure-3
Disease-4
Methods-5
2.1 LIST OF TABLES
Table 1: Samples of drugs, 10
Table 2: mineral water ratio, 12
Table 3: demographic samples, 14
Table 4: ACT samples, 16
Table 5: Summary of effects, 18
2.2 LIST OF FIGURES
Figure1: life cycle of mosquitoes, 5
Figure2: Anopheles mosquito, 6
Figure 3: Samples of injections, 11
Figure 4: Samples of drug effects
2.3 ABBREVIATIONS
A.C.T-Artemisinin combination therapies
C.Q.R-Chloroquine resistance.
HB3-Chloroquine sensitive strain
Dd2-chloroquine resistance strain
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PQ-Premaquine
2.4 ACKNOWLEDGEMENT
There were individuals who really helped me in the various stages of my project. My sincere
appreciation goes to the technician who made the reagents available for my experiments. To
my course mates I remain thankful to you too for the creation of the conducive environment
for the studies and above all to my lecturers who have been so resourceful in this task
3.0 INTRODUCTION
Several efforts have been put in place to help in the controlling of the malaria and even
possibly have it completely eradicated. Although there has been success in some other parts
of the world including Southern Europe among other countries in the Africa and in the
Middle East, other regions in the world still experience the fatal effects of this particular
disease. The reasons as to why this has been the case is because of the continued resistance of
the parasites of malaria to the drugs available (Koesdjojo et.al 2014).
Figure 1: Life cycle of malaria extracted from (Koesdjojo et.al 2014).
2.4 ACKNOWLEDGEMENT
There were individuals who really helped me in the various stages of my project. My sincere
appreciation goes to the technician who made the reagents available for my experiments. To
my course mates I remain thankful to you too for the creation of the conducive environment
for the studies and above all to my lecturers who have been so resourceful in this task
3.0 INTRODUCTION
Several efforts have been put in place to help in the controlling of the malaria and even
possibly have it completely eradicated. Although there has been success in some other parts
of the world including Southern Europe among other countries in the Africa and in the
Middle East, other regions in the world still experience the fatal effects of this particular
disease. The reasons as to why this has been the case is because of the continued resistance of
the parasites of malaria to the drugs available (Koesdjojo et.al 2014).
Figure 1: Life cycle of malaria extracted from (Koesdjojo et.al 2014).

The complex life cycle of this plasmodium is another reason for the spreading of this disease.
Malaria especially for the human is initiated by infection caused by the plasmodium parasite.
This is transmitted to human host through an infected female mosquito called Anopheles.
After getting into the host, its circulation begins with the phase of sexual life cycle in which
the parasite quickly moves to the liver. This stage is very silent and it is merely described as
the liver infection. The stage is characterised by the formation of thousands of merozoites
that are subsequently released into the bloodstream.
Once in the bloodstream, they invade the red blood cells and this becomes the beginning of
the pathogenic asexual life cycle. In very many countries, the manufacturing operations have
been made easier using lean manufacturing. This task however appears to be very much
challenging. There are very many factors that influence this method of manufacturing of
these factors are complex in their properties and are therefore much challenging. The rules
that have been put in place can actually help in the reduction of the time that is consumed in
the process of production.
This is only possible when these principles are applied accordingly. The purpose of this
project is to shed much light on the manner of the application of these principles and their
resultant effects which may be termed as positive or disadvantages in some cases. The
required steps of the smooth operation are basically laid down in a very systematic
framework. This opera ration frame work also explains the depth of the applications that
either directly or indirectly reduces the throughput time.
Malaria especially for the human is initiated by infection caused by the plasmodium parasite.
This is transmitted to human host through an infected female mosquito called Anopheles.
After getting into the host, its circulation begins with the phase of sexual life cycle in which
the parasite quickly moves to the liver. This stage is very silent and it is merely described as
the liver infection. The stage is characterised by the formation of thousands of merozoites
that are subsequently released into the bloodstream.
Once in the bloodstream, they invade the red blood cells and this becomes the beginning of
the pathogenic asexual life cycle. In very many countries, the manufacturing operations have
been made easier using lean manufacturing. This task however appears to be very much
challenging. There are very many factors that influence this method of manufacturing of
these factors are complex in their properties and are therefore much challenging. The rules
that have been put in place can actually help in the reduction of the time that is consumed in
the process of production.
This is only possible when these principles are applied accordingly. The purpose of this
project is to shed much light on the manner of the application of these principles and their
resultant effects which may be termed as positive or disadvantages in some cases. The
required steps of the smooth operation are basically laid down in a very systematic
framework. This opera ration frame work also explains the depth of the applications that
either directly or indirectly reduces the throughput time.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Figure 2 Anopheles mosquito extracted from (Lelièvre et.al 2012).
The resistance of the malaria parasites to the past versions of the medicines has seriously
undermined the malaria control efforts. During the previous decades, therapies that are based
on the combination of the artemisinin have been developed in the world as the first attempts
of malaria treatment. This has been commonly referred to as ACTs. ACTs include a fast-
acting artemisinin compound and another drug that is meant to improve the efficacy so as to
hinder the emergence of the resistance to artemisinin. Increase in the quantity of the
compound especially in the terms of its bulkiness in the branch R which actually seems to
improve the activity of the vitro.
As had been indicated in the past results, the IC50 values for the series 7 demonstrated a
perfect correlation with all the clog pf P and the parameters of the Charton. Other new series
including series 9 also gave a fair correlation with these very parameters of ACharton but
there was an exception to the values of the clog P. The supporting data as indicated in the
series 10 was actually too small to permit the drawing of the convincing conclusion on the
same subject. The HEFLECINs having the chloroquinoline core was actually found to be
more active as opposed to that of the CQ. The two compounds that are also known as 7C and
7h had their activities meeting the requirement of the selected studies. Lean manufacturing of
drugs helps in the reduction of the crucial step. The processing time is reduced, cost of the
operation minimised and quality of the services and products improved with the application
of this drug manufacturing tool. The errors that are predictable can be foreseen and corrected
in advance using what is called preventive maintenance.
The reliability of the suppliers to the companies doing manufacturing will for sure improve
without any doubt. The initiative has led to the increased profitability within the companies
and even the improved public impression and perception. Among the companies that have
The resistance of the malaria parasites to the past versions of the medicines has seriously
undermined the malaria control efforts. During the previous decades, therapies that are based
on the combination of the artemisinin have been developed in the world as the first attempts
of malaria treatment. This has been commonly referred to as ACTs. ACTs include a fast-
acting artemisinin compound and another drug that is meant to improve the efficacy so as to
hinder the emergence of the resistance to artemisinin. Increase in the quantity of the
compound especially in the terms of its bulkiness in the branch R which actually seems to
improve the activity of the vitro.
As had been indicated in the past results, the IC50 values for the series 7 demonstrated a
perfect correlation with all the clog pf P and the parameters of the Charton. Other new series
including series 9 also gave a fair correlation with these very parameters of ACharton but
there was an exception to the values of the clog P. The supporting data as indicated in the
series 10 was actually too small to permit the drawing of the convincing conclusion on the
same subject. The HEFLECINs having the chloroquinoline core was actually found to be
more active as opposed to that of the CQ. The two compounds that are also known as 7C and
7h had their activities meeting the requirement of the selected studies. Lean manufacturing of
drugs helps in the reduction of the crucial step. The processing time is reduced, cost of the
operation minimised and quality of the services and products improved with the application
of this drug manufacturing tool. The errors that are predictable can be foreseen and corrected
in advance using what is called preventive maintenance.
The reliability of the suppliers to the companies doing manufacturing will for sure improve
without any doubt. The initiative has led to the increased profitability within the companies
and even the improved public impression and perception. Among the companies that have
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

benefited for this initiative include those that are dealing with the perishable goods whose
delivery must be done within the specific duration of the time. The provision of emergency
responses like for the medical services have been realised the generation of descriptive
terminologies, lean manufacturing has been known to be the lean production. In the
production set up, these initiative plays avital role in the production line where it lowers the
production cost using relevant tools within the shortest time possible.
However, there is now even a bigger challenge considering that there are signs indicating
malaria parasite resistance to this artemisinin. Of concern now is the dependence on the new
drug combination on the derivatives of the artemisinin. It is worrying that most of the
antimalarial drugs that are available only target the blood-stage parasites with very few
known to inhibit the activities of the liver stage parasites (Lelièvre et.al 2012).
In the clinical drugs used worldwide, only Primaquine acts against the operations of the
parasites at the liver stage. Due to the concerns of the continued resistance to the antimalarial
drugs along the effects of difficult-to-treat chronic liver stages, the recommended therapy
should be effective enough to act against the multi-resistant strains of the parasite and also to
be active against the two stages of the parasites both for the liver and for the erythrocytes.
4.0 AIMS OF THE PROJECT
The aim of this project is study the existing drugs of the antimalaria and possibly make
improvements on the ones that are already facing resistance from the plasmodium species.
5.0METHODS
The approach that was used in this particular project which was the heme-targeted
antimalarial employed the utilisation of very cheap materials. The step that was used however
was very much high yielding. There was amination of 4,7-dichloroquinoline with the
compound called diaminoalkanes whose concentration was kept between 82-90% yield.
Combination of this compound with N, N diethylamino-3-propionic acid together alongside
delivery must be done within the specific duration of the time. The provision of emergency
responses like for the medical services have been realised the generation of descriptive
terminologies, lean manufacturing has been known to be the lean production. In the
production set up, these initiative plays avital role in the production line where it lowers the
production cost using relevant tools within the shortest time possible.
However, there is now even a bigger challenge considering that there are signs indicating
malaria parasite resistance to this artemisinin. Of concern now is the dependence on the new
drug combination on the derivatives of the artemisinin. It is worrying that most of the
antimalarial drugs that are available only target the blood-stage parasites with very few
known to inhibit the activities of the liver stage parasites (Lelièvre et.al 2012).
In the clinical drugs used worldwide, only Primaquine acts against the operations of the
parasites at the liver stage. Due to the concerns of the continued resistance to the antimalarial
drugs along the effects of difficult-to-treat chronic liver stages, the recommended therapy
should be effective enough to act against the multi-resistant strains of the parasite and also to
be active against the two stages of the parasites both for the liver and for the erythrocytes.
4.0 AIMS OF THE PROJECT
The aim of this project is study the existing drugs of the antimalaria and possibly make
improvements on the ones that are already facing resistance from the plasmodium species.
5.0METHODS
The approach that was used in this particular project which was the heme-targeted
antimalarial employed the utilisation of very cheap materials. The step that was used however
was very much high yielding. There was amination of 4,7-dichloroquinoline with the
compound called diaminoalkanes whose concentration was kept between 82-90% yield.
Combination of this compound with N, N diethylamino-3-propionic acid together alongside

another compound called 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide was done. The
furnished amides were then lowered using borane to the corresponding series of secondary
amines. This was then followed by the preparation of the tertiary amines by doing the
treatment of the precursor using another compound called sodium borohydride but within a
special type of acetic solution. (Peters 2013).
The extended amines marked 6 and 7 could be made using an acid called 4-ketopimelic,8 and
5-oxoacelaic acid marked 9. The obtained results indicated that CDMT and Pybop when
thoroughly combined allows for the efficient formation with other compounds such as
diethylamine and even with diisopropylamine as followed. The corresponding yield of the
diamides that were marked 10 and 11 were between 62-99%. The triamines yields marked 14
and 15 were as a result of the group of ketones that have lowered amination from acetate of
ammonia and cyanoborohydride of sodium (Jover et.al 2012)
The terminal amides were reduced using another important compound called lithium
aluminium hydride. There was very careful but optimised nucleophilic aromatic constitution
used to employ these amines at high temperatures and in the presence of 1. This was done in
a closed vessel and it resulted into the production of chloroquinolines that were marked 6 and
7 respectively. A mixture of these elements of the isomers together with trans-isomers of
diaminocyclohexane which were temporarily marked 18 and 19 together with4,7-
dichlorpoquinoline were used to prepare 1,3-and 1,4-diaminocylohexane. This was obtained
from chloroquinolines that were marked 16a,16b,17a and finally 17b in that order. This
process was then followed by the intermediate treatment of 20 and 21 using acetic acid and
NaBH4.
The activity of the tribasic compound marked 4a-e,5 a-e,7a, b and 6a, b was considered ant
plasmodial. These properties were also shown by the compounds of the dibasic 1,3-and 1,4-
furnished amides were then lowered using borane to the corresponding series of secondary
amines. This was then followed by the preparation of the tertiary amines by doing the
treatment of the precursor using another compound called sodium borohydride but within a
special type of acetic solution. (Peters 2013).
The extended amines marked 6 and 7 could be made using an acid called 4-ketopimelic,8 and
5-oxoacelaic acid marked 9. The obtained results indicated that CDMT and Pybop when
thoroughly combined allows for the efficient formation with other compounds such as
diethylamine and even with diisopropylamine as followed. The corresponding yield of the
diamides that were marked 10 and 11 were between 62-99%. The triamines yields marked 14
and 15 were as a result of the group of ketones that have lowered amination from acetate of
ammonia and cyanoborohydride of sodium (Jover et.al 2012)
The terminal amides were reduced using another important compound called lithium
aluminium hydride. There was very careful but optimised nucleophilic aromatic constitution
used to employ these amines at high temperatures and in the presence of 1. This was done in
a closed vessel and it resulted into the production of chloroquinolines that were marked 6 and
7 respectively. A mixture of these elements of the isomers together with trans-isomers of
diaminocyclohexane which were temporarily marked 18 and 19 together with4,7-
dichlorpoquinoline were used to prepare 1,3-and 1,4-diaminocylohexane. This was obtained
from chloroquinolines that were marked 16a,16b,17a and finally 17b in that order. This
process was then followed by the intermediate treatment of 20 and 21 using acetic acid and
NaBH4.
The activity of the tribasic compound marked 4a-e,5 a-e,7a, b and 6a, b was considered ant
plasmodial. These properties were also shown by the compounds of the dibasic 1,3-and 1,4-
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
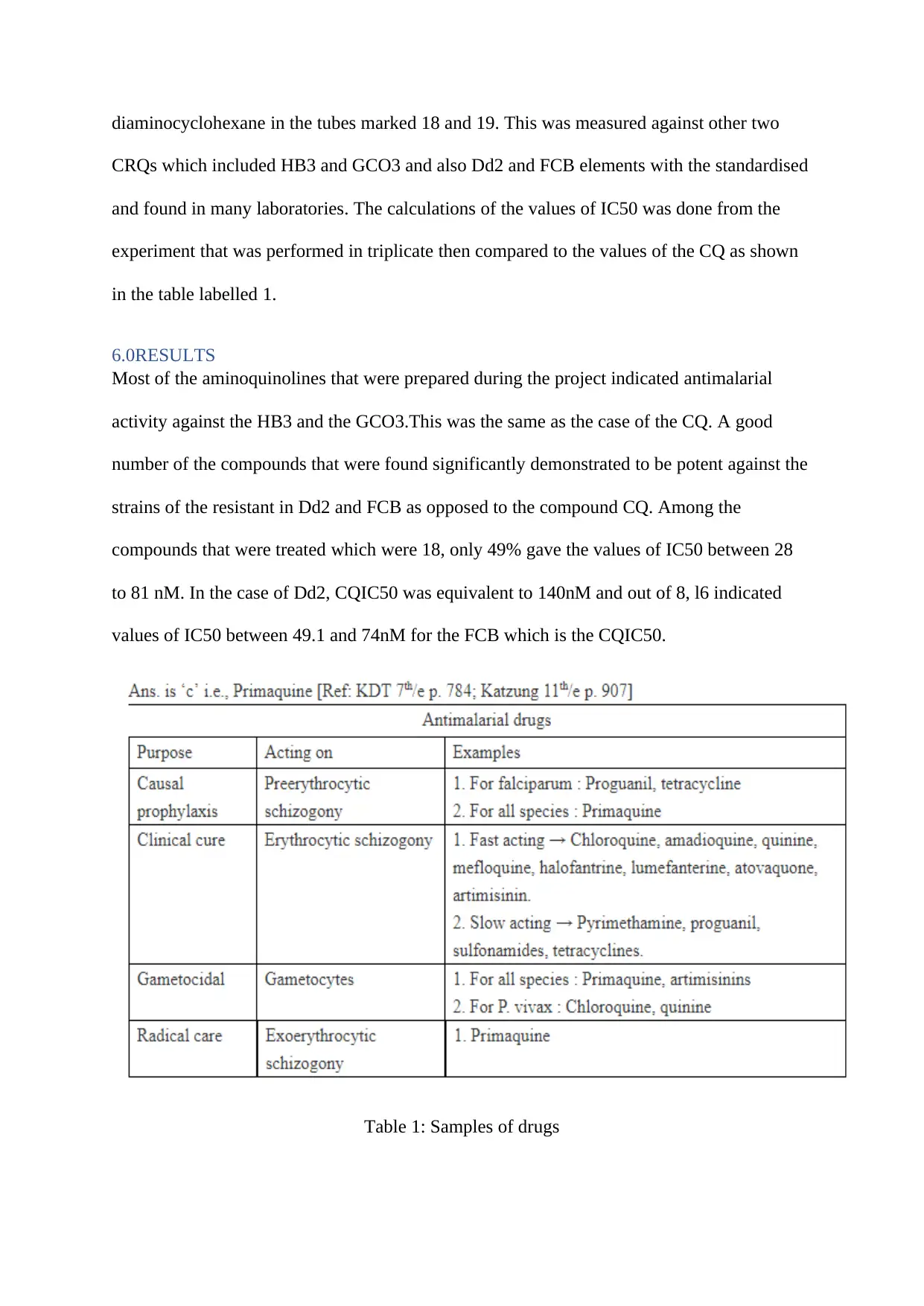
diaminocyclohexane in the tubes marked 18 and 19. This was measured against other two
CRQs which included HB3 and GCO3 and also Dd2 and FCB elements with the standardised
and found in many laboratories. The calculations of the values of IC50 was done from the
experiment that was performed in triplicate then compared to the values of the CQ as shown
in the table labelled 1.
6.0RESULTS
Most of the aminoquinolines that were prepared during the project indicated antimalarial
activity against the HB3 and the GCO3.This was the same as the case of the CQ. A good
number of the compounds that were found significantly demonstrated to be potent against the
strains of the resistant in Dd2 and FCB as opposed to the compound CQ. Among the
compounds that were treated which were 18, only 49% gave the values of IC50 between 28
to 81 nM. In the case of Dd2, CQIC50 was equivalent to 140nM and out of 8, l6 indicated
values of IC50 between 49.1 and 74nM for the FCB which is the CQIC50.
Table 1: Samples of drugs
CRQs which included HB3 and GCO3 and also Dd2 and FCB elements with the standardised
and found in many laboratories. The calculations of the values of IC50 was done from the
experiment that was performed in triplicate then compared to the values of the CQ as shown
in the table labelled 1.
6.0RESULTS
Most of the aminoquinolines that were prepared during the project indicated antimalarial
activity against the HB3 and the GCO3.This was the same as the case of the CQ. A good
number of the compounds that were found significantly demonstrated to be potent against the
strains of the resistant in Dd2 and FCB as opposed to the compound CQ. Among the
compounds that were treated which were 18, only 49% gave the values of IC50 between 28
to 81 nM. In the case of Dd2, CQIC50 was equivalent to 140nM and out of 8, l6 indicated
values of IC50 between 49.1 and 74nM for the FCB which is the CQIC50.
Table 1: Samples of drugs
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

It was however discovered that the activity of the antimalarial of those linear tribasic
aminoquinolines marked 4a up to e and also 5a up to e were generally superior over other
compounds in the tubes marked 6a,7a,6b and finally 7b leading to a systematically side chain
that was branched (Delves et.al 2013). The same antimalarial activity was observed with the
highly branched analogues 16a,17a,16b and 17b which were the branched dibasic of CQ.
These compounds however were characterised by very high index of selectivity. It is
important to note that diastereomeric mixtures of these compound marked 17a and 16a had
been previously been prepared by Drake.
Figure 3: Samples of the injection extracted from (Lelièvre et.al 2012).
Their report indicated higher antimalarial activity against the compounds such as CQS and
CQR elements as opposed to the compound of chloroquine. The quantitative measure was
therefore provided by this selectivity index and this probably indicated the discovery leads of
the drug. The selectivity index of CQ was at 10 while all other compounds that were tested
gave values between a range of 0.68 and 4.43. This implied that there was need to have 5a
and 5b highly combined so as to produce an antimalarial activity that is required against HB3
and GCO3 but this should be vacillated wiry the very low values of the selectivity index. The
recommended range should be between 1.14 and 1.78.
aminoquinolines marked 4a up to e and also 5a up to e were generally superior over other
compounds in the tubes marked 6a,7a,6b and finally 7b leading to a systematically side chain
that was branched (Delves et.al 2013). The same antimalarial activity was observed with the
highly branched analogues 16a,17a,16b and 17b which were the branched dibasic of CQ.
These compounds however were characterised by very high index of selectivity. It is
important to note that diastereomeric mixtures of these compound marked 17a and 16a had
been previously been prepared by Drake.
Figure 3: Samples of the injection extracted from (Lelièvre et.al 2012).
Their report indicated higher antimalarial activity against the compounds such as CQS and
CQR elements as opposed to the compound of chloroquine. The quantitative measure was
therefore provided by this selectivity index and this probably indicated the discovery leads of
the drug. The selectivity index of CQ was at 10 while all other compounds that were tested
gave values between a range of 0.68 and 4.43. This implied that there was need to have 5a
and 5b highly combined so as to produce an antimalarial activity that is required against HB3
and GCO3 but this should be vacillated wiry the very low values of the selectivity index. The
recommended range should be between 1.14 and 1.78.

The present hem targeted antiplasmodial therefore show activity against CQS strains that
were the same as those of CQ. They however retained their potency against the strains of
CQR.When the antimalarial activities of 5a and 5b were compared with the results that were
gotten from the tribasic aminoquinolines 4a and 4b indicates that the presence of this amino
acid is very important for the activity of Dd2, FCB andGCO3.The other compound such as
HB3 was however not affected by this. Also, the effectiveness of 5a and 5b compared with
Dd2 among others diminishes or reduces significantly with increase in the chai length. The
values of the selective index of all the compounds tested illustrate that the differences
witnessed on the CQ chain structure and even the basicity creates the new venues of
overcoming the resistance to the antimalarial drugs.
Table 2: Mineral water ratio
This can be possibly being combined with another third compound of basic amino that will in
turn favour the pilling of the drug inside the cell vacuole of the plasmodium parasite that is
also acidic. The high IC50 that was observed within the compounds of 7a and finally 7b
indicates the structure of the basicity of these chains especially branched ones that cannot be
independently optimised.
were the same as those of CQ. They however retained their potency against the strains of
CQR.When the antimalarial activities of 5a and 5b were compared with the results that were
gotten from the tribasic aminoquinolines 4a and 4b indicates that the presence of this amino
acid is very important for the activity of Dd2, FCB andGCO3.The other compound such as
HB3 was however not affected by this. Also, the effectiveness of 5a and 5b compared with
Dd2 among others diminishes or reduces significantly with increase in the chai length. The
values of the selective index of all the compounds tested illustrate that the differences
witnessed on the CQ chain structure and even the basicity creates the new venues of
overcoming the resistance to the antimalarial drugs.
Table 2: Mineral water ratio
This can be possibly being combined with another third compound of basic amino that will in
turn favour the pilling of the drug inside the cell vacuole of the plasmodium parasite that is
also acidic. The high IC50 that was observed within the compounds of 7a and finally 7b
indicates the structure of the basicity of these chains especially branched ones that cannot be
independently optimised.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 23
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.