Thermodynamics and Chemistry Assignment: Engine and Chemical Problems
VerifiedAdded on 2020/05/11
|9
|532
|378
Homework Assignment
AI Summary
This assignment solution covers fundamental concepts in thermodynamics and basic chemistry, primarily focusing on the analysis of a diesel engine's operation and related calculations. The document begins with an analysis of the engine's thermal efficiency, incorporating calculations based on temperature and specific heat capacity. It then delves into chemical problems, calculating molar masses, reaction stoichiometry, and equilibrium constants. The solutions also involve the use of MATLAB code for simulations and analysis. The assignment includes calculations involving the molar mass of compounds, reaction stoichiometry, and equilibrium constants. Furthermore, it explores the impact of temperature changes on reaction rates. The assignment concludes with a reflection on the concepts and their practical application in mechanical engineering.
Introduction
The assignment focuses on thermodynamics and basic chemistry. Especially, the calculations
based on the operation cycle of a diesel engine.
Part A: Thermodynamics and Engines”
Question 1
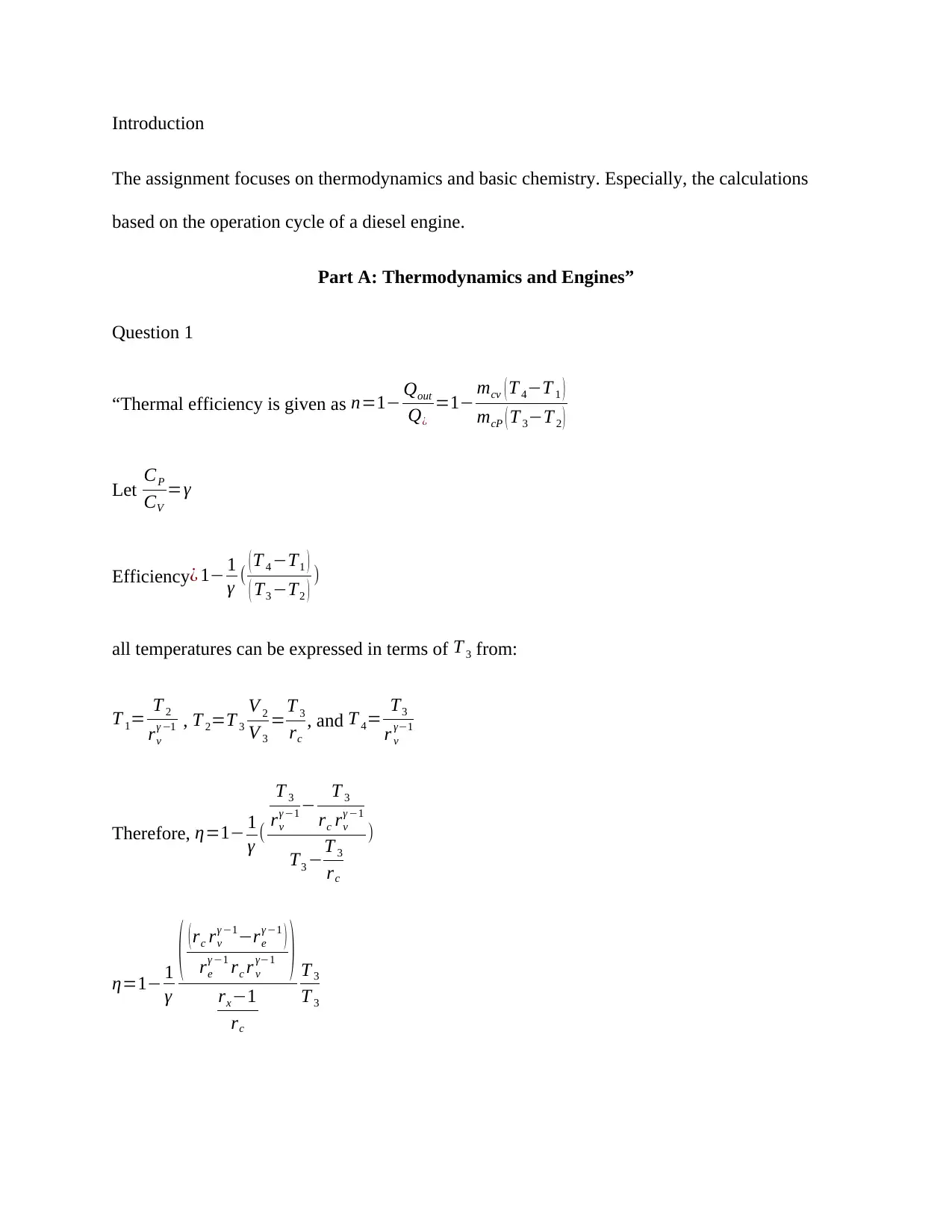
“Thermal efficiency is given as n=1− Qout
Q¿
=1− mcv ( T 4−T 1 )
mcP ( T 3−T 2 )
Let CP
CV
=γ
Efficiency¿ 1− 1
γ ( ( T 4 −T1 )
( T3 −T2 ) )
all temperatures can be expressed in terms of T 3 from:
T 1= T 2
rv
γ −1 , T 2=T 3
V 2
V 3
=T 3
rc
, and T 4= T3
r v
γ−1
Therefore, η=1− 1
γ (
T 3
rv
γ −1 − T 3
rc rv
γ −1
T3 −T 3
rc
)
η=1− 1
γ
( (rc rv
γ −1−re
γ −1 )
re
γ −1 rc r v
γ−1 )
rx−1
rc
T 3
T 3
The assignment focuses on thermodynamics and basic chemistry. Especially, the calculations
based on the operation cycle of a diesel engine.
Part A: Thermodynamics and Engines”
Question 1
“Thermal efficiency is given as n=1− Qout
Q¿
=1− mcv ( T 4−T 1 )
mcP ( T 3−T 2 )
Let CP
CV
=γ
Efficiency¿ 1− 1
γ ( ( T 4 −T1 )
( T3 −T2 ) )
all temperatures can be expressed in terms of T 3 from:
T 1= T 2
rv
γ −1 , T 2=T 3
V 2
V 3
=T 3
rc
, and T 4= T3
r v
γ−1
Therefore, η=1− 1
γ (
T 3
rv
γ −1 − T 3
rc rv
γ −1
T3 −T 3
rc
)
η=1− 1
γ
( (rc rv
γ −1−re
γ −1 )
re
γ −1 rc r v
γ−1 )
rx−1
rc
T 3
T 3
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

η=1− 1
γ
1
re
γ −1 rv
γ −1 [ rc ( re
γ −1 r c
γ−1 ) −re
γ −1
rc−1 ]
Hence, the thermal efficiency, η=1− 1
γ
1
rv
γ −1 [ r c
γ −1
r c−1 ]
But V 3
V 2
=rc=α and V 1
V 2
=rv=Ψ
Replacing these in the thermal efficiency equation yields:
η=1− 1
γ
1
Ψ γ −1 [ αγ −1
α −1 ]
Question 2
V 1=1949.56 litres, V 2=140 Litres , V 3
V 2
=1.86 , V 3=1.86 ×140=260.4 litres ,
V 4 =V 1=1949.56 litres
T 4=50 ° C , T3 =T 4 rv
γ −1 =50× 13.925(¿1.3 −1 )=110.18 ° C ¿
T 2=T 3
V 2
V 3
=110.18× 1
1.86 =59.24 ° C, T 1= T 2
rv
γ −1 = 59.24
13.925(¿1.3 −1)=26.88 ° C ¿
P2=P3 =150¯, P1=P4= P2
rv
γ −1 = 150
13.925(¿1.3−1)=68.07¯¿ ¿
Question 3
The specific heat capacity ratio¿ CP
CV
=γ =1.3
Swept volume ¿ V 1−V 2
γ
1
re
γ −1 rv
γ −1 [ rc ( re
γ −1 r c
γ−1 ) −re
γ −1
rc−1 ]
Hence, the thermal efficiency, η=1− 1
γ
1
rv
γ −1 [ r c
γ −1
r c−1 ]
But V 3
V 2
=rc=α and V 1
V 2
=rv=Ψ
Replacing these in the thermal efficiency equation yields:
η=1− 1
γ
1
Ψ γ −1 [ αγ −1
α −1 ]
Question 2
V 1=1949.56 litres, V 2=140 Litres , V 3
V 2
=1.86 , V 3=1.86 ×140=260.4 litres ,
V 4 =V 1=1949.56 litres
T 4=50 ° C , T3 =T 4 rv
γ −1 =50× 13.925(¿1.3 −1 )=110.18 ° C ¿
T 2=T 3
V 2
V 3
=110.18× 1
1.86 =59.24 ° C, T 1= T 2
rv
γ −1 = 59.24
13.925(¿1.3 −1)=26.88 ° C ¿
P2=P3 =150¯, P1=P4= P2
rv
γ −1 = 150
13.925(¿1.3−1)=68.07¯¿ ¿
Question 3
The specific heat capacity ratio¿ CP
CV
=γ =1.3
Swept volume ¿ V 1−V 2
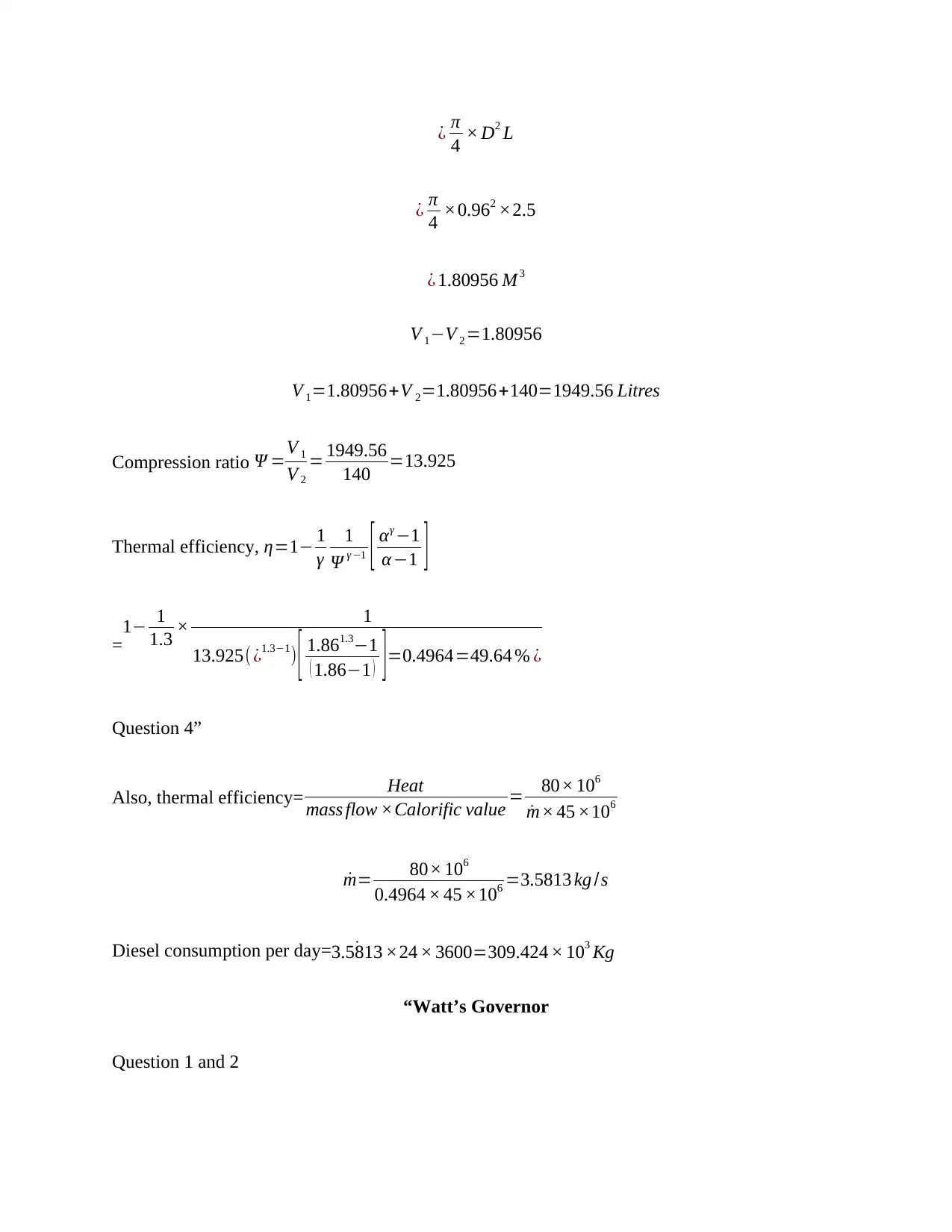
¿ π
4 × D2 L
¿ π
4 ×0.962 ×2.5
¿ 1.80956 M3
V 1−V 2 =1.80956
V 1=1.80956+V 2=1.80956+140=1949.56 Litres
Compression ratio Ψ =V 1
V 2
= 1949.56
140 =13.925
Thermal efficiency, η=1− 1
γ
1
Ψ γ −1 [ αγ −1
α −1 ]
=1− 1
1.3 × 1
13.925( ¿1.3−1) [ 1.861.3−1
( 1.86−1 ) ]=0.4964=49.64 % ¿
Question 4”
Also, thermal efficiency= Heat
mass flow ×Calorific value = 80× 106
˙m× 45 ×106
˙m= 80× 106
0.4964 × 45 ×106 =3.5813 kg /s
Diesel consumption per day= ˙3.5813 ×24 × 3600=309.424 × 103 Kg
“Watt’s Governor
Question 1 and 2
4 × D2 L
¿ π
4 ×0.962 ×2.5
¿ 1.80956 M3
V 1−V 2 =1.80956
V 1=1.80956+V 2=1.80956+140=1949.56 Litres
Compression ratio Ψ =V 1
V 2
= 1949.56
140 =13.925
Thermal efficiency, η=1− 1
γ
1
Ψ γ −1 [ αγ −1
α −1 ]
=1− 1
1.3 × 1
13.925( ¿1.3−1) [ 1.861.3−1
( 1.86−1 ) ]=0.4964=49.64 % ¿
Question 4”
Also, thermal efficiency= Heat
mass flow ×Calorific value = 80× 106
˙m× 45 ×106
˙m= 80× 106
0.4964 × 45 ×106 =3.5813 kg /s
Diesel consumption per day= ˙3.5813 ×24 × 3600=309.424 × 103 Kg
“Watt’s Governor
Question 1 and 2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

If the Q ( l
s ) is the volume flow rate, V fuel= 0.001 Q
π
4 ×0.022
=3.1831Q m
s
ω= V fuel
0.05 = vω
0.15 , vω=9.5493 Q m
s
ωgear = vω
0.03 =318.31 Q rad
s , Q=0.0031416 ωgear
Q=0.031416× 30
π ωgear ∈rpm=0.03 ωgear (¿ rpm∧l
s )
“The MATLAB code used to implement the same is shown in figure 1 and the corresponding
plot is as shown:
Figure 1: MATLAB code”
Figure 2: “MATLAB PLOT”
s ) is the volume flow rate, V fuel= 0.001 Q
π
4 ×0.022
=3.1831Q m
s
ω= V fuel
0.05 = vω
0.15 , vω=9.5493 Q m
s
ωgear = vω
0.03 =318.31 Q rad
s , Q=0.0031416 ωgear
Q=0.031416× 30
π ωgear ∈rpm=0.03 ωgear (¿ rpm∧l
s )
“The MATLAB code used to implement the same is shown in figure 1 and the corresponding
plot is as shown:
Figure 1: MATLAB code”
Figure 2: “MATLAB PLOT”
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

“Question 3
To change to another working point, change the volume flow rate of the fuel.
Basic Chemistry”
“Question 1
The molar mass of CuS O4 is (63.5+32+16 × 4)=159.5 g
Question 2
Potassium oxide = K2 O
Molar mass=39 ×2+16=94 g
100g of K2 O has 100
94 Moles
To change to another working point, change the volume flow rate of the fuel.
Basic Chemistry”
“Question 1
The molar mass of CuS O4 is (63.5+32+16 × 4)=159.5 g
Question 2
Potassium oxide = K2 O
Molar mass=39 ×2+16=94 g
100g of K2 O has 100
94 Moles

But, each mole of K2 O produces 2 moles of potassium cations according to the dissociation
equation: K2 O(aq)→2 K+¿ ( aq ) + O2−¿( aq)¿ ¿
Therefore, the number of moles of potassium cations equals 100
94 ×2=200
94 Moles
But 1mole has 6.022 ×1023 ions
Hence, 200
94 Moles of potassiumhas 200
94 ×6.022 ×1023 ions=1.2813 ×1024 ions
Question 3
2 carats of diamond= ( 2 ×0.2 ) g=0.4 g
1 mole carbon=12g
Therefore, 0.4g carbon has 0.4
12 =0.03333 moles
The number of carbon atoms=0.03333 ×6.022 ×1023=2.00733× 1022 atoms
Question 4
3 Fe+4 H2 O → Fe3 O4 +4 H2
1 mole of water reacts with 1
4 ×3=0.75 moles of Fe
Therefore, 6 moles of water will react with 6 × 0.75moles=4.5 moles of Fe and the chemical
reaction becomes:
4.5 Fe+6 H2 O→ 1.5 Fe3 O4 +4 .5 H2
equation: K2 O(aq)→2 K+¿ ( aq ) + O2−¿( aq)¿ ¿
Therefore, the number of moles of potassium cations equals 100
94 ×2=200
94 Moles
But 1mole has 6.022 ×1023 ions
Hence, 200
94 Moles of potassiumhas 200
94 ×6.022 ×1023 ions=1.2813 ×1024 ions
Question 3
2 carats of diamond= ( 2 ×0.2 ) g=0.4 g
1 mole carbon=12g
Therefore, 0.4g carbon has 0.4
12 =0.03333 moles
The number of carbon atoms=0.03333 ×6.022 ×1023=2.00733× 1022 atoms
Question 4
3 Fe+4 H2 O → Fe3 O4 +4 H2
1 mole of water reacts with 1
4 ×3=0.75 moles of Fe
Therefore, 6 moles of water will react with 6 × 0.75moles=4.5 moles of Fe and the chemical
reaction becomes:
4.5 Fe+6 H2 O→ 1.5 Fe3 O4 +4 .5 H2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

After the reaction 0.5 moles of Fe remain.
Question 5
H2 S O4 + 2 NaOH → Na2 S O4 +2 H2 O
Number of moles of H2 S O4 =Molarity× Volume= 0.3 × 50
1000 =0.015 moles
But, 1mole of H2 S O4 requires 2 moles of NaOH
0.015 moles of H2 S O4 will require 2 ×0.015=0.03 moles of NaOH
Volume of NaOH ( ml ) = No of Moles
Molarity × 1000= 0.3
0.75 × 1000=400 ml
Question 6
MnO2(s)+4HCl (aq)→MnCl2 (aq)+Cl2 (g) +2H2O (l)
1g of MnO2= 1
87 =0.01149 moles
Mole ratio Mn O2 :Cl2=1 :1
Cl2 moles = 0.01149
But 1 mole of Cl2 has a mass of 70.9g
Therefore, 0.01149 moles gives ( 0.01149 ×70.9 ) g=0.8149 g
But the density of Cl2 is 3.17g/L
Volume of Cl2= Mass
Density = 0.8149
3.17 ×1000=257 ml
Question 5
H2 S O4 + 2 NaOH → Na2 S O4 +2 H2 O
Number of moles of H2 S O4 =Molarity× Volume= 0.3 × 50
1000 =0.015 moles
But, 1mole of H2 S O4 requires 2 moles of NaOH
0.015 moles of H2 S O4 will require 2 ×0.015=0.03 moles of NaOH
Volume of NaOH ( ml ) = No of Moles
Molarity × 1000= 0.3
0.75 × 1000=400 ml
Question 6
MnO2(s)+4HCl (aq)→MnCl2 (aq)+Cl2 (g) +2H2O (l)
1g of MnO2= 1
87 =0.01149 moles
Mole ratio Mn O2 :Cl2=1 :1
Cl2 moles = 0.01149
But 1 mole of Cl2 has a mass of 70.9g
Therefore, 0.01149 moles gives ( 0.01149 ×70.9 ) g=0.8149 g
But the density of Cl2 is 3.17g/L
Volume of Cl2= Mass
Density = 0.8149
3.17 ×1000=257 ml
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

In case 50g of Mn O2 are added:
50g of MnO2= 50
87 =0.5747 moles
Therefore, 0.5747 moles gives ( 0.5747 ×70.9 ) g=40.747 g
Volume of Cl2= Mass
Density = 40.747
3.17 =12.850 l
Question 7
2 ONCl ( g ) ⇄2 NO ( g ) +Cl2 (g)
“[ ONCl ]=0.09 ×1=0.09 M
2 ONCl ( g ) ⇄ 2 NO ( g ) +Cl2 (g)
Initial concentration” 1M 0 0
Change -0.09 +0.18 0.09
Equilibrium 0.91 0.18 0.09
Kc= [NO ]2 [Cl2 ]
[ONCl ]2 =[0.18]2 [0.09]
[ 0.91]2 =3.5213 ×10−3”
Question 8
“First, we make a table as follows
H2 ( g ) + CO2 ( g ) ⇄ H2 O ( g ) +CO (g)
Initial
concentration
0.01M 0.01M 0 0
Change -y -y +y +y
50g of MnO2= 50
87 =0.5747 moles
Therefore, 0.5747 moles gives ( 0.5747 ×70.9 ) g=40.747 g
Volume of Cl2= Mass
Density = 40.747
3.17 =12.850 l
Question 7
2 ONCl ( g ) ⇄2 NO ( g ) +Cl2 (g)
“[ ONCl ]=0.09 ×1=0.09 M
2 ONCl ( g ) ⇄ 2 NO ( g ) +Cl2 (g)
Initial concentration” 1M 0 0
Change -0.09 +0.18 0.09
Equilibrium 0.91 0.18 0.09
Kc= [NO ]2 [Cl2 ]
[ONCl ]2 =[0.18]2 [0.09]
[ 0.91]2 =3.5213 ×10−3”
Question 8
“First, we make a table as follows
H2 ( g ) + CO2 ( g ) ⇄ H2 O ( g ) +CO (g)
Initial
concentration
0.01M 0.01M 0 0
Change -y -y +y +y

Equilibrium 0.01-y 0.01-y y y
The equilibrium constant, K= [ H2 O ] [CO ]
[ H 2 ] [CO2 ] = y ( y )
(0.01− y)(0.01− y ) =0.771
Substituting the numbers yields, y2
(0.01− y)2 =0.771
Finding the square roots of both sides
¿ y
0.01− y =0.878066, 0.878066 ( 0.01− y )= y
Rearranging and solving for y yields, y=4.675 ×10−3
So H2=CO=0.004675 M and H2=CO2=0.01−0.004675=0.005325 M
Question 9
The reaction is an endothermic process meaning that heat energy is absorbed. If you increase the
temperature, the rate of the reaction will increase and favor the production of more CO∧H2 O
Question 10”
{Ca} rsub {3} {(P {O} rsub {4} )} rsub {2} + {3H} rsub {2} S {O} rsub {4} → {2H} rsub {3} P {O} rsub {4}
Therefore, a=1 , b=3 , c=2 ,∧d=3
Conclusion
“The task helped me recall the thermodynamics and basic chemistry concepts taught in class.
Notably, the operation of the four-stroke gasoline engine and the calculations involved.
The equilibrium constant, K= [ H2 O ] [CO ]
[ H 2 ] [CO2 ] = y ( y )
(0.01− y)(0.01− y ) =0.771
Substituting the numbers yields, y2
(0.01− y)2 =0.771
Finding the square roots of both sides
¿ y
0.01− y =0.878066, 0.878066 ( 0.01− y )= y
Rearranging and solving for y yields, y=4.675 ×10−3
So H2=CO=0.004675 M and H2=CO2=0.01−0.004675=0.005325 M
Question 9
The reaction is an endothermic process meaning that heat energy is absorbed. If you increase the
temperature, the rate of the reaction will increase and favor the production of more CO∧H2 O
Question 10”
{Ca} rsub {3} {(P {O} rsub {4} )} rsub {2} + {3H} rsub {2} S {O} rsub {4} → {2H} rsub {3} P {O} rsub {4}
Therefore, a=1 , b=3 , c=2 ,∧d=3
Conclusion
“The task helped me recall the thermodynamics and basic chemistry concepts taught in class.
Notably, the operation of the four-stroke gasoline engine and the calculations involved.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.