CHEM 101 - Atoms and Elements (II) Homework Assignment Solution
VerifiedAdded on 2022/10/01
|7
|1125
|379
Homework Assignment
AI Summary
This document presents a comprehensive solution to a chemistry homework assignment focused on atoms and elements. The assignment delves into the fundamental concepts defining elements, including their symbols and the number of protons. It explores the relationship between an element's name and its symbol, proper capitalization conventions, and how to identify elements using the periodic table. The solution also examines the properties of metals, nonmetals, and metalloids, including their positions on the periodic table and specific examples. Furthermore, the assignment analyzes the families of elements, such as noble gases, alkali metals, and halogens, highlighting their shared characteristics and chemical reactivities. Critical thinking questions throughout the assignment challenge the student to apply their knowledge and understanding of atomic structure and the periodic table.

ATOMS AND ELEMENTS (II)
CHEMACTIVITY 4B
MODEL 1: WHAT DEFINES AN ELEMENT?
Element Symbol Number of Protons
silicon Si 14
phosphorus P 15
potassium K 19
gold Au 79
Antimony Sb 51
CRITICAL THINKING QUESTIONS
1. Write the symbol for the element silicon.
Si
2. Write the symbol for the element phosphorus.
P
3. Write the symbols for the element potassium.
K
4. Describe the relationship between the name of an element and its symbol.
Chemical symbols of some elements such as phosphorus and nitrogen are the first symbol of
their names. For some elements, it the first two letters of their names with the first one being
capital and the second being small letter such as silicon. For elements with Latin names such as
potassium and silver, the chemical symbols are derived from Latin names in a similar manner
(Zumdahl & DeCoste, 2010).
5. When the name of an element is written in a sentence, is it capitalized? How do you know?
When a name of element is written in full in a sentence, it should not be capitalized. This is
known from the fact in this case, name of elements simply forms a part of the system.
6. Describe the proper use of capitalization for element symbols.
For elements with a single letter as a chemical symbol, the letter is always capitalized. For
elements with two letters forming the chemical symbol, only the first letter is capitalized (West,
2013).
7. Refer to a periodic table of the elements. What is the name of the element with the symbol
Sb? Add it to the table.
Antimony
8. How many protons are there in silicon? Gold?
Silicon=14 protons
CHEMACTIVITY 4B
MODEL 1: WHAT DEFINES AN ELEMENT?
Element Symbol Number of Protons
silicon Si 14
phosphorus P 15
potassium K 19
gold Au 79
Antimony Sb 51
CRITICAL THINKING QUESTIONS
1. Write the symbol for the element silicon.
Si
2. Write the symbol for the element phosphorus.
P
3. Write the symbols for the element potassium.
K
4. Describe the relationship between the name of an element and its symbol.
Chemical symbols of some elements such as phosphorus and nitrogen are the first symbol of
their names. For some elements, it the first two letters of their names with the first one being
capital and the second being small letter such as silicon. For elements with Latin names such as
potassium and silver, the chemical symbols are derived from Latin names in a similar manner
(Zumdahl & DeCoste, 2010).
5. When the name of an element is written in a sentence, is it capitalized? How do you know?
When a name of element is written in full in a sentence, it should not be capitalized. This is
known from the fact in this case, name of elements simply forms a part of the system.
6. Describe the proper use of capitalization for element symbols.
For elements with a single letter as a chemical symbol, the letter is always capitalized. For
elements with two letters forming the chemical symbol, only the first letter is capitalized (West,
2013).
7. Refer to a periodic table of the elements. What is the name of the element with the symbol
Sb? Add it to the table.
Antimony
8. How many protons are there in silicon? Gold?
Silicon=14 protons
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Gold =79 protons
9. Refer to a periodic table of the elements. Using a complete sentence, describe how to find
the number of protons for an element.
The number of protons of an element is equivalent to the atomic number Z in the Periodic
table (Scerri, 2011)
10. How many protons are there in potassium? Antimony? Add them to the table.
Potassium=19 protons
Antimony=51 protons
11. A certain element has eight protons. What element is it?
The element is oxygen
9. Refer to a periodic table of the elements. Using a complete sentence, describe how to find
the number of protons for an element.
The number of protons of an element is equivalent to the atomic number Z in the Periodic
table (Scerri, 2011)
10. How many protons are there in potassium? Antimony? Add them to the table.
Potassium=19 protons
Antimony=51 protons
11. A certain element has eight protons. What element is it?
The element is oxygen

12. Which particle determines the identity of an element: electron, proton, or neutron?
Protons are the particles that determines the identity of an element (Levi & Posenthal, 2012)
13. A mad scientist claims to have discovered a new form of carbon that has seven protons.
How would you respond?
An element with 7 protons is Nitrogen not carbon. All elements have unique number of
protons.
MODEL 2: WHICH ONES ARE THE METALS?
Protons are the particles that determines the identity of an element (Levi & Posenthal, 2012)
13. A mad scientist claims to have discovered a new form of carbon that has seven protons.
How would you respond?
An element with 7 protons is Nitrogen not carbon. All elements have unique number of
protons.
MODEL 2: WHICH ONES ARE THE METALS?
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

CRITICAL THINKING QUESTIONS
14. B, Si, Ge, As, Sb, Te, and At are all metalloids. Give the name of each element and compare and
contrast metalloids.
B=Boron
Si=Silicon
Ge=Germanium
As=Arsenic
Sb=Antimony
Te=Tellurium
At=Astatine
Comparison
Contrast
Metalloids have properties between nonmetals and metals. The metallic character for metalloids
is strongest for leftmost elements in the periodic table and decreases to the right for any period.
Within any group, the metallic character increases from top to bottom. The ionization energy
increases down a group. Therefore, polonium has a higher ionization energy compared to
Tellurium (Zumdahl & DeCoste, 2010).
15. According to your daily experience, are copper (Cu), chromium (Cr), and lead (Pb)metals or
nonmetals (circle one)? Do they appear to the right or left of the metalloid line.
They are metals
They appear to the left of the metalloid line
16. Which metalloid has the lowest atomic number? Highest?
Boron has the lowest atomic number while Astatine has the highest atomic number
17. Hydrogen is exceptional in several ways. List all the ways hydrogen is exceptional. In
particular, why is hydrogen in the same group has metals?
Hydrogen has only one electron. It can thus lose one electron and remain as just a
positive charge like metals or gain one electron and achieve a duplet state. This makes
it behave like halogens
Hydrogen can be placed in the same group as metals because it can lose one electron
as the alkali metals (Zumdahl & DeCoste, 2010).
14. B, Si, Ge, As, Sb, Te, and At are all metalloids. Give the name of each element and compare and
contrast metalloids.
B=Boron
Si=Silicon
Ge=Germanium
As=Arsenic
Sb=Antimony
Te=Tellurium
At=Astatine
Comparison
Contrast
Metalloids have properties between nonmetals and metals. The metallic character for metalloids
is strongest for leftmost elements in the periodic table and decreases to the right for any period.
Within any group, the metallic character increases from top to bottom. The ionization energy
increases down a group. Therefore, polonium has a higher ionization energy compared to
Tellurium (Zumdahl & DeCoste, 2010).
15. According to your daily experience, are copper (Cu), chromium (Cr), and lead (Pb)metals or
nonmetals (circle one)? Do they appear to the right or left of the metalloid line.
They are metals
They appear to the left of the metalloid line
16. Which metalloid has the lowest atomic number? Highest?
Boron has the lowest atomic number while Astatine has the highest atomic number
17. Hydrogen is exceptional in several ways. List all the ways hydrogen is exceptional. In
particular, why is hydrogen in the same group has metals?
Hydrogen has only one electron. It can thus lose one electron and remain as just a
positive charge like metals or gain one electron and achieve a duplet state. This makes
it behave like halogens
Hydrogen can be placed in the same group as metals because it can lose one electron
as the alkali metals (Zumdahl & DeCoste, 2010).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
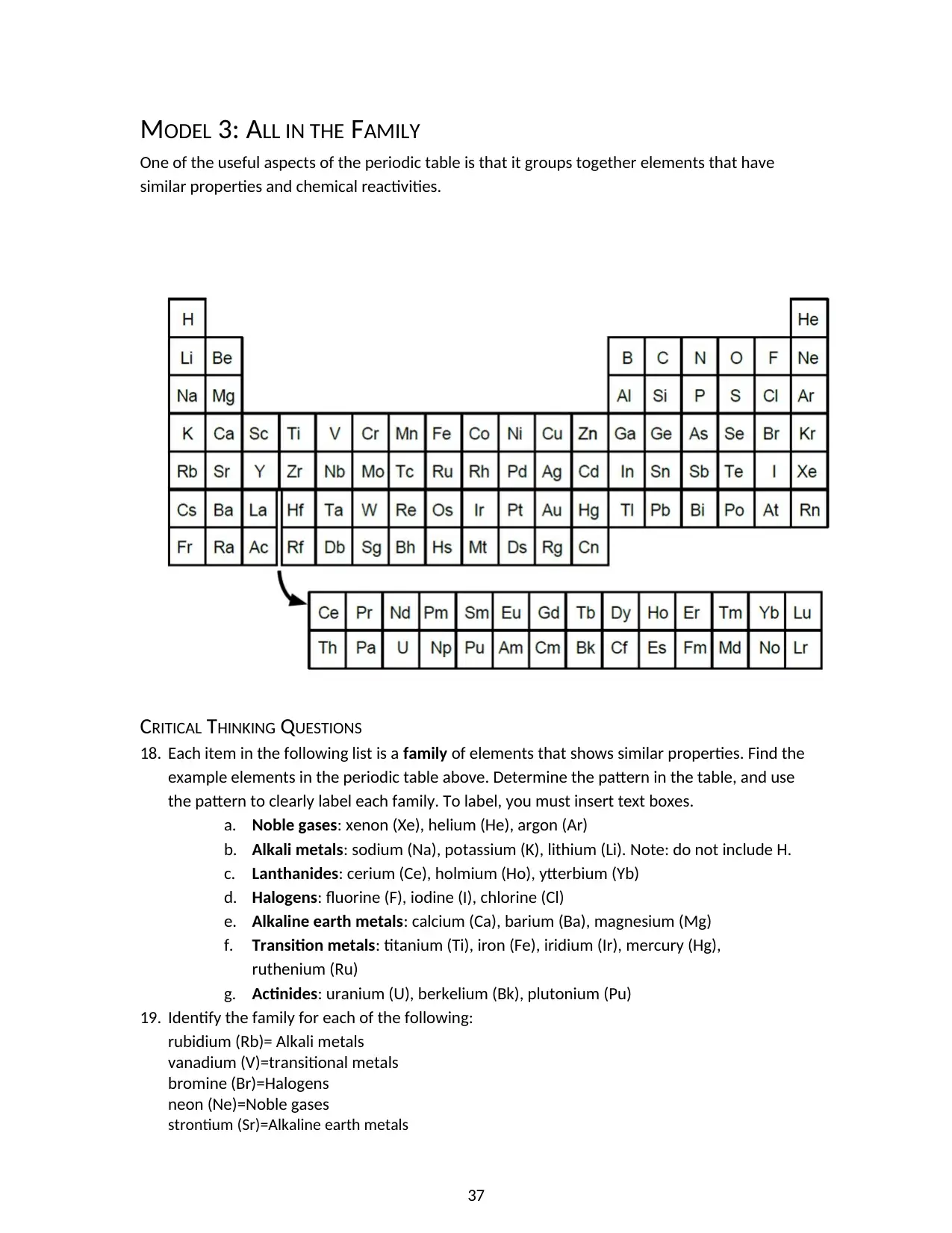
MODEL 3: ALL IN THE FAMILY
One of the useful aspects of the periodic table is that it groups together elements that have
similar properties and chemical reactivities.
CRITICAL THINKING QUESTIONS
18. Each item in the following list is a family of elements that shows similar properties. Find the
example elements in the periodic table above. Determine the pattern in the table, and use
the pattern to clearly label each family. To label, you must insert text boxes.
a. Noble gases: xenon (Xe), helium (He), argon (Ar)
b. Alkali metals: sodium (Na), potassium (K), lithium (Li). Note: do not include H.
c. Lanthanides: cerium (Ce), holmium (Ho), ytterbium (Yb)
d. Halogens: fluorine (F), iodine (I), chlorine (Cl)
e. Alkaline earth metals: calcium (Ca), barium (Ba), magnesium (Mg)
f. Transition metals: titanium (Ti), iron (Fe), iridium (Ir), mercury (Hg),
ruthenium (Ru)
g. Actinides: uranium (U), berkelium (Bk), plutonium (Pu)
19. Identify the family for each of the following:
rubidium (Rb)= Alkali metals
vanadium (V)=transitional metals
bromine (Br)=Halogens
neon (Ne)=Noble gases
strontium (Sr)=Alkaline earth metals
37
One of the useful aspects of the periodic table is that it groups together elements that have
similar properties and chemical reactivities.
CRITICAL THINKING QUESTIONS
18. Each item in the following list is a family of elements that shows similar properties. Find the
example elements in the periodic table above. Determine the pattern in the table, and use
the pattern to clearly label each family. To label, you must insert text boxes.
a. Noble gases: xenon (Xe), helium (He), argon (Ar)
b. Alkali metals: sodium (Na), potassium (K), lithium (Li). Note: do not include H.
c. Lanthanides: cerium (Ce), holmium (Ho), ytterbium (Yb)
d. Halogens: fluorine (F), iodine (I), chlorine (Cl)
e. Alkaline earth metals: calcium (Ca), barium (Ba), magnesium (Mg)
f. Transition metals: titanium (Ti), iron (Fe), iridium (Ir), mercury (Hg),
ruthenium (Ru)
g. Actinides: uranium (U), berkelium (Bk), plutonium (Pu)
19. Identify the family for each of the following:
rubidium (Rb)= Alkali metals
vanadium (V)=transitional metals
bromine (Br)=Halogens
neon (Ne)=Noble gases
strontium (Sr)=Alkaline earth metals
37

Copyright © 2015 Pearson Education,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

References
Levi, P., & Posenthal, R. (2012). The Periodic Table. New York, NY: Viking.
Scerri, E. R. (2011). The Periodic Table: A Very Short Introduction. New York, NY: Oxford
University Press.
West, K. (2013). The Basics of Metals and Metalloids. New York, NY: The Rosen Publishing
Group.
Zumdahl, S. S., & DeCoste, D. J. (2010). Introductory Chemistry: A Foundation. Cengage
Learning.
Levi, P., & Posenthal, R. (2012). The Periodic Table. New York, NY: Viking.
Scerri, E. R. (2011). The Periodic Table: A Very Short Introduction. New York, NY: Oxford
University Press.
West, K. (2013). The Basics of Metals and Metalloids. New York, NY: The Rosen Publishing
Group.
Zumdahl, S. S., & DeCoste, D. J. (2010). Introductory Chemistry: A Foundation. Cengage
Learning.
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.