The Efficacy and Safety of Atypical Antipsychotics for the Treatment of Dementia: A Meta-Analysis
Added on 2023-06-08
24 Pages14033 Words117 Views
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/263934324
The Efficacy and Safety of Atypical Antipsychotics for the Treatment of
Dementia: A Meta-Analysis of Randomized Placebo-Controlled Trials
Article in Journal of Alzheimer's disease: JAD · July 2014
DOI: 10.3233/JAD-140579 · Source: PubMed
CITATIONS
31
READS
735
7 authors, including:
Yinglin Huang
China Medical University (PRC)
29 PUBLICATIONS 156 CITATIONS
SEE PROFILE
Gang Zhu
The First Affiliated Hospital of China Medical University (PRC)
75 PUBLICATIONS 1,046 CITATIONS
SEE PROFILE
All content following this page was uploaded by Gang Zhu on 25 September 2014.
The user has requested enhancement of the downloaded file.
The Efficacy and Safety of Atypical Antipsychotics for the Treatment of
Dementia: A Meta-Analysis of Randomized Placebo-Controlled Trials
Article in Journal of Alzheimer's disease: JAD · July 2014
DOI: 10.3233/JAD-140579 · Source: PubMed
CITATIONS
31
READS
735
7 authors, including:
Yinglin Huang
China Medical University (PRC)
29 PUBLICATIONS 156 CITATIONS
SEE PROFILE
Gang Zhu
The First Affiliated Hospital of China Medical University (PRC)
75 PUBLICATIONS 1,046 CITATIONS
SEE PROFILE
All content following this page was uploaded by Gang Zhu on 25 September 2014.
The user has requested enhancement of the downloaded file.
Uncorrected Author Proof
Journal of Alzheimer’s Disease xx (20xx) x–xx
DOI 10.3233/JAD-140579
IOS Press
1
The Efficacy and Safety of Atypical
Antipsychotics for the Treatment of
Dementia: A Meta-Analysis of Randomized
Placebo-Controlled Trials
1
2
3
4
Hui Maa,b , Yinglin Huanga,c , Zhengtu Conga , Yuan Wanga , Wenhai Jiangd , Shuhe Gaod and
Gang Zhua,∗
5
6
aDepartment of Psychiatry, The First Affiliated Hospital of China Medical University, Shenyang, China7
bCenter for Mental Health, Yanshan University, Qinhuangdao, China8
cDepartment of Psychiatry, Shengjing Hospital of China Medical University, Shenyang, China9
dThe Third People’s Hospital of Daqing, Daqing, China10
Accepted 25 April 2014
Abstract.11
Background: The application of atypical antipsychotics (SGAs) for treatment of psychiatric and behavioral symptoms of
dementia is controversial since their efficacy might be offset by their adverse events (AEs).
12
13
Objective: To assess the efficacy, safety, and tolerability of SGAs for treatment of psychological and behavioral symptoms of
dementia.
14
15
Methods: Two researchers searched MEDLINE, PsychINFO, and the Cochrane Central Register of Controlled Trials indepen-
dently for double-blind, placebo-controlled, randomized controlled trials (DB-PC-RCTs) as of June 2013, written in English.
Efficacy was measured using the Brief Psychiatric Rating Scale (BPRS), Cohen-Mansfield Agitation Inventory (CMAI), Neu-
ropsychiatric Inventory (NPI), Clinical Global Impression of Change (CGI-C), and (or) Clinical Global Impression of Severity
(CGI-S). Safety and tolerability were measured by frequencies of drop-outs, adverse events (AEs), and death. In total, 19 treat-
ment comparisons drawn from 16 DB-PC-RCTs were included, and 3,343 patients randomized to the antipsychotic group and
1,707 to the placebo group were assessed.
16
17
18
19
20
21
22
Results: This meta-analysis demonstrated a significant efficacy of atypical antipsychotics on BPRS (MD = −1.58, 95%
CI = −2.52 - −0.65), CMAI (−1.84, −3.01 - −0.61), NPI (−2.81, −4.35 - −1.28), CGI-C (−0.32, −0.44 - −0.20), and CGI-S
(−0.19, −0.30 - −0.09), compared to placebo (p < 0.01 for all). Patients receiving atypical antipsychotics showed no difference
in risk for discontinuation (p > 0.05), significantly higher risks (p < 0.05 for all) for somnolence (OR = 2.95), extrapyramidal
symptoms (1.74), cerebrovascular AEs (2.50), urinary tract infection (1.35), edema (1.80), gait abnormality (3.35), and death
(1.52), and a lower risk for agitation (OR = 0.80, p = 0.03).
23
24
25
26
27
28
Conclusions: The higher risks for AEs and mortality may offset the efficacy of atypical antipsychotics for treatment of dementia.
Efficacy, safety, and tolerability thus should be carefully considered against clinical need.
29
30
Keywords: Antipsychotic, clinical trial, dementia, meta-analysis31
∗Correspondence to: Prof. Gang Zhu, MD, PhD, Department of
Psychiatry, The First Affiliated Hospital of China Medical Univer-
sity, Shenyang 110001, China. Tel./Fax: +86 24 83282184; E-mail:
gzhu@mail.cmu.edu.cn.
ISSN 1387-2877/14/$27.50 © 2014 – IOS Press and the authors. All rights reserved
Journal of Alzheimer’s Disease xx (20xx) x–xx
DOI 10.3233/JAD-140579
IOS Press
1
The Efficacy and Safety of Atypical
Antipsychotics for the Treatment of
Dementia: A Meta-Analysis of Randomized
Placebo-Controlled Trials
1
2
3
4
Hui Maa,b , Yinglin Huanga,c , Zhengtu Conga , Yuan Wanga , Wenhai Jiangd , Shuhe Gaod and
Gang Zhua,∗
5
6
aDepartment of Psychiatry, The First Affiliated Hospital of China Medical University, Shenyang, China7
bCenter for Mental Health, Yanshan University, Qinhuangdao, China8
cDepartment of Psychiatry, Shengjing Hospital of China Medical University, Shenyang, China9
dThe Third People’s Hospital of Daqing, Daqing, China10
Accepted 25 April 2014
Abstract.11
Background: The application of atypical antipsychotics (SGAs) for treatment of psychiatric and behavioral symptoms of
dementia is controversial since their efficacy might be offset by their adverse events (AEs).
12
13
Objective: To assess the efficacy, safety, and tolerability of SGAs for treatment of psychological and behavioral symptoms of
dementia.
14
15
Methods: Two researchers searched MEDLINE, PsychINFO, and the Cochrane Central Register of Controlled Trials indepen-
dently for double-blind, placebo-controlled, randomized controlled trials (DB-PC-RCTs) as of June 2013, written in English.
Efficacy was measured using the Brief Psychiatric Rating Scale (BPRS), Cohen-Mansfield Agitation Inventory (CMAI), Neu-
ropsychiatric Inventory (NPI), Clinical Global Impression of Change (CGI-C), and (or) Clinical Global Impression of Severity
(CGI-S). Safety and tolerability were measured by frequencies of drop-outs, adverse events (AEs), and death. In total, 19 treat-
ment comparisons drawn from 16 DB-PC-RCTs were included, and 3,343 patients randomized to the antipsychotic group and
1,707 to the placebo group were assessed.
16
17
18
19
20
21
22
Results: This meta-analysis demonstrated a significant efficacy of atypical antipsychotics on BPRS (MD = −1.58, 95%
CI = −2.52 - −0.65), CMAI (−1.84, −3.01 - −0.61), NPI (−2.81, −4.35 - −1.28), CGI-C (−0.32, −0.44 - −0.20), and CGI-S
(−0.19, −0.30 - −0.09), compared to placebo (p < 0.01 for all). Patients receiving atypical antipsychotics showed no difference
in risk for discontinuation (p > 0.05), significantly higher risks (p < 0.05 for all) for somnolence (OR = 2.95), extrapyramidal
symptoms (1.74), cerebrovascular AEs (2.50), urinary tract infection (1.35), edema (1.80), gait abnormality (3.35), and death
(1.52), and a lower risk for agitation (OR = 0.80, p = 0.03).
23
24
25
26
27
28
Conclusions: The higher risks for AEs and mortality may offset the efficacy of atypical antipsychotics for treatment of dementia.
Efficacy, safety, and tolerability thus should be carefully considered against clinical need.
29
30
Keywords: Antipsychotic, clinical trial, dementia, meta-analysis31
∗Correspondence to: Prof. Gang Zhu, MD, PhD, Department of
Psychiatry, The First Affiliated Hospital of China Medical Univer-
sity, Shenyang 110001, China. Tel./Fax: +86 24 83282184; E-mail:
gzhu@mail.cmu.edu.cn.
ISSN 1387-2877/14/$27.50 © 2014 – IOS Press and the authors. All rights reserved

Uncorrected Author Proof
2 H. Ma et al. / Effects of Antipsychotics on Dementia
INTRODUCTION32
Dementia is a multidimensional syndrome asso-33
ciated with many progressive brain diseases. About34
6.1% of all people older than 65 years and approxi-35
mately 20% of those older than 80 years show signs of36
dementia [1], characterized by a progressive declined37
cognitive function, behavioral disturbances such as38
agitation and aggression, and psychotic symptoms39
like delusions and hallucinations. These symptoms40
severely impact on the patient quality of life and are a41
major source of caregiver stress.42
Antipsychotic drugs are widely used as a first-43
line pharmacological approach to treat the psychiatric44
and behavioral symptoms of dementia. In the clin-45
ical setting, the atypical antipsychotics, also known46
as the second-generation antipsychotics (SGAs),47
such as quetiapine, aripiprazole, risperidone, and48
olanzapine, have largely replaced the conventional49
antipsychotics (chlorpromazine, sulpiride, haloperi-50
done, perphenazine), due to fewer or less adverse51
events (AEs). However, in dementia patients, even the52
SGAs are associated with an increased risk of severe53
side effects, such as increased cerebrovascular adverse54
events (CVAEs) [2, 3], further cognitive deterioration55
[4], extrapyramidal symptoms (EPs) [2, 5, 6], som-56
nolence [6–11], urinary tract infections [7], edema57
[6], and metabolic dysfunction [12]. The Clinical58
Antipsychotic Trials of Intervention Effectiveness-59
Alzheimer’s disease (CATIE-AD) study is the first60
head-to-head, prospective, randomized, double-blind,61
placebo-controlled flexible-dose effectiveness trial of62
antipsychotic therapy for Alzheimer’s disease (AD).63
The unique design measures outcome associated with64
real-world administration of these medications to treat65
related symptoms. In the CATIE-AD study, Vigen and66
colleagues reported that patients with AD showed sig-67
nificant steady decline over 36 weeks in most cognitive68
metrics including the Mini-Mental State Examination69
(MMSE) (2.4 point) and Alzheimer’s Disease Assess-70
ment Scale-cog (4.4 points), and that patients declined71
more on SGAs (including olanzapine, quetiapine, and72
risperidone) than on placebo on multiple cognitive73
measures, including the MMSE, Brief Psychiatric Rat-74
ing Scale (BPRS) cognitive subscale, and a cognitive75
summary score summarizing change on 18 cogni-76
tive tests [13]. Based on all above findings, the U.S.77
Food and Drug Administration (FDA) issued a medi-78
cal advisory in 2005 warning of the increased mortality79
associated with SGAs for patients with dementia.80
However, numerous studies have found that the inci-81
dence rates of these AEs are no greater than those for82
placebos [6, 9, 10, 14–17]. Moreover, clinicians still 83
prescribe these drugs to patients with dementia, so it 84
is important to evaluate the potential benefits and risks 85
of SGAs in patients with dementia. 86
We conducted a meta-analysis of SGA trials 87
to assess their efficacy and safety for the treat- 88
ment of patients with dementia. The latest available 89
meta-analysis of double-blind, placebo-controlled, 90
randomized controlled trials (DB-PC-RCTs) evaluat- 91
ing the treatment effect of antipsychotics for dementia 92
was based on literature published up to 2005 [18]. 93
Since several new relevant studies have been published, 94
an updated meta-analysis is necessary to guide clini- 95
cal practice. The objective of this meta-analysis is to 96
determine the efficacy, safety and tolerability of SGAs 97
for dementia patients. 98
METHODS 99
Search strategy 100
Our search included MEDLINE, PsychINFO, and 101
the Cochrane Central Register of Controlled Trials. Pri- 102
mary search terms were “dementia”, “psychological, 103
psychiatric, or behavioral symptoms”, “double-blind”, 104
“placebo”, “random”, together with one of the fol- 105
lowing terms: “atypical antipsychotic”, “quetiapine”, 106
“aripiprazole”, “risperidone”, “olanzapine”, “amisul- 107
pride”, or “ziprasidone”. Moreover, the references of 108
the included studies as well as previously-published 109
reviews and meta-analytic papers satisfying our selec- 110
tion criteria (see below) were checked manually for 111
additional relevant articles. All the articles have been 112
published as of June 2013, and written in English. 113
The trials presented at meetings but not published 114
were not included. At the same time, trials, in which 115
drugs were administrated by intramuscular injection 116
and effect evaluations were immediate (several hours 117
post-treatment), were also excluded. 118
Two authors independently conducted the literature 119
search, and then the other one checked their literature 120
search results and communicated with them when their 121
results are inconsistent, to ensure that no relevant stud- 122
ies were missed and that all met the inclusion criteria. 123
We contacted some trial authors to obtain unpublished 124
data or to clarify specific aspects of their studies. 125
Trial selection criteria 126
We selected DB-PC-RCTs comparing SGAs to 127
placebo that used the Neuropsychiatric Inventory 128
(NPI), Clinical Global Impression of Change (CGI- 129
2 H. Ma et al. / Effects of Antipsychotics on Dementia
INTRODUCTION32
Dementia is a multidimensional syndrome asso-33
ciated with many progressive brain diseases. About34
6.1% of all people older than 65 years and approxi-35
mately 20% of those older than 80 years show signs of36
dementia [1], characterized by a progressive declined37
cognitive function, behavioral disturbances such as38
agitation and aggression, and psychotic symptoms39
like delusions and hallucinations. These symptoms40
severely impact on the patient quality of life and are a41
major source of caregiver stress.42
Antipsychotic drugs are widely used as a first-43
line pharmacological approach to treat the psychiatric44
and behavioral symptoms of dementia. In the clin-45
ical setting, the atypical antipsychotics, also known46
as the second-generation antipsychotics (SGAs),47
such as quetiapine, aripiprazole, risperidone, and48
olanzapine, have largely replaced the conventional49
antipsychotics (chlorpromazine, sulpiride, haloperi-50
done, perphenazine), due to fewer or less adverse51
events (AEs). However, in dementia patients, even the52
SGAs are associated with an increased risk of severe53
side effects, such as increased cerebrovascular adverse54
events (CVAEs) [2, 3], further cognitive deterioration55
[4], extrapyramidal symptoms (EPs) [2, 5, 6], som-56
nolence [6–11], urinary tract infections [7], edema57
[6], and metabolic dysfunction [12]. The Clinical58
Antipsychotic Trials of Intervention Effectiveness-59
Alzheimer’s disease (CATIE-AD) study is the first60
head-to-head, prospective, randomized, double-blind,61
placebo-controlled flexible-dose effectiveness trial of62
antipsychotic therapy for Alzheimer’s disease (AD).63
The unique design measures outcome associated with64
real-world administration of these medications to treat65
related symptoms. In the CATIE-AD study, Vigen and66
colleagues reported that patients with AD showed sig-67
nificant steady decline over 36 weeks in most cognitive68
metrics including the Mini-Mental State Examination69
(MMSE) (2.4 point) and Alzheimer’s Disease Assess-70
ment Scale-cog (4.4 points), and that patients declined71
more on SGAs (including olanzapine, quetiapine, and72
risperidone) than on placebo on multiple cognitive73
measures, including the MMSE, Brief Psychiatric Rat-74
ing Scale (BPRS) cognitive subscale, and a cognitive75
summary score summarizing change on 18 cogni-76
tive tests [13]. Based on all above findings, the U.S.77
Food and Drug Administration (FDA) issued a medi-78
cal advisory in 2005 warning of the increased mortality79
associated with SGAs for patients with dementia.80
However, numerous studies have found that the inci-81
dence rates of these AEs are no greater than those for82
placebos [6, 9, 10, 14–17]. Moreover, clinicians still 83
prescribe these drugs to patients with dementia, so it 84
is important to evaluate the potential benefits and risks 85
of SGAs in patients with dementia. 86
We conducted a meta-analysis of SGA trials 87
to assess their efficacy and safety for the treat- 88
ment of patients with dementia. The latest available 89
meta-analysis of double-blind, placebo-controlled, 90
randomized controlled trials (DB-PC-RCTs) evaluat- 91
ing the treatment effect of antipsychotics for dementia 92
was based on literature published up to 2005 [18]. 93
Since several new relevant studies have been published, 94
an updated meta-analysis is necessary to guide clini- 95
cal practice. The objective of this meta-analysis is to 96
determine the efficacy, safety and tolerability of SGAs 97
for dementia patients. 98
METHODS 99
Search strategy 100
Our search included MEDLINE, PsychINFO, and 101
the Cochrane Central Register of Controlled Trials. Pri- 102
mary search terms were “dementia”, “psychological, 103
psychiatric, or behavioral symptoms”, “double-blind”, 104
“placebo”, “random”, together with one of the fol- 105
lowing terms: “atypical antipsychotic”, “quetiapine”, 106
“aripiprazole”, “risperidone”, “olanzapine”, “amisul- 107
pride”, or “ziprasidone”. Moreover, the references of 108
the included studies as well as previously-published 109
reviews and meta-analytic papers satisfying our selec- 110
tion criteria (see below) were checked manually for 111
additional relevant articles. All the articles have been 112
published as of June 2013, and written in English. 113
The trials presented at meetings but not published 114
were not included. At the same time, trials, in which 115
drugs were administrated by intramuscular injection 116
and effect evaluations were immediate (several hours 117
post-treatment), were also excluded. 118
Two authors independently conducted the literature 119
search, and then the other one checked their literature 120
search results and communicated with them when their 121
results are inconsistent, to ensure that no relevant stud- 122
ies were missed and that all met the inclusion criteria. 123
We contacted some trial authors to obtain unpublished 124
data or to clarify specific aspects of their studies. 125
Trial selection criteria 126
We selected DB-PC-RCTs comparing SGAs to 127
placebo that used the Neuropsychiatric Inventory 128
(NPI), Clinical Global Impression of Change (CGI- 129

Uncorrected Author Proof
H. Ma et al. / Effects of Antipsychotics on Dementia 3
C), Clinical Global Impression of Severity (CGI-S),130
BPRS, and (or) Cohen-Mansfield Agitation Inventory131
(CMAI) as outcome measures. We did not distinguish132
between trials with fixed or flexible dosing.133
Quality assessment of included trials134
Since quality score for each studies included in a135
meta- analysis may be useful to ensure that better stud-136
ies receive more weight, many scales and checklists137
have been developed to assess the quality of a trial138
and the quality of its reports [19–22]. The scales of139
Brown [20], Jadad et al. [21], and van Tulder et al.140
[22] were used to evaluate the quality of primary RCTs141
for our meta-analysis by two reviewers independently.142
According to previous related literature [19–24], the143
trial was considered to be high quality when its total144
score of Brown scale was ≥17 points, its total score145
of Jadad scale was ≥3 points, or its total score of van146
Tulder scale was ≥5 points.147
Data extraction148
Trial data extracted included design characteristics,149
key inclusion criteria, subject nationality, mean age,150
gender ratio, study group size, drug dose(s), trial dura-151
tion, baseline rating scores, endpoint outcomes of all152
rating scales, drop-outs, mortality, and main AEs.153
Statistical analysis154
All meta-analytic calculations were per-155
formed with the free software Review Manager156
Version 5.1 (The Cochrane Collaboration,157
http://ims.cochrane.org/revman ).158
If different fixed-dose subgroups were used in a159
trial, both the total mean values and standard devi-160
ations (SDs) for the changes of all scale scores and161
the total amounts for AEs were calculated on behalf162
of the data of this trial based on the data from all163
the subgroups. We also chose the subgroup with the164
most effective dose for the psychological/behavioral165
symptoms of dementia patients to represent the data166
of this trial, or regarded the data of each fixed-dose167
subgroup together with control group in a trial as indi-168
vidual contrast to analyze. The latter two kinds of169
meta-analysis results are included in the Supplemen-170
tary Material. The odds ratios (ORs) with associated171
95% confidence intervals (CIs) were calculated for172
dichotomous data (drop-outs, death, and AEs). Stan-173
dardized mean differences (SMDs) or weighted mean174
differences (WMDs) with the associated 95% CIs175
were calculated for continuous data (NPI, CGI-S, 176
BPRS, CMAI, and CGI-C outcomes at endpoints). 177
Moreover, the Der-Simonian and Laird random-effects 178
model or Mantel-Haenszel fixed-effects model for the 179
dichotomous outcomes of drop-outs, death, and AEs 180
were applied to calculate the pooled safety and tol- 181
erability of SGAs. A fixed-effects or random-effects 182
model for rating scale changes was used to assess 183
the pooled drug efficacies. It generally referred to fix- 184
effects model except when random-effects model was 185
emphasized. The two types of parameters (ORs and 186
SMDs/WMDs) were plotted graphically using forest 187
plots. 188
To ensure that the included studies were combin- 189
able, the level of homogeneity was assessed by the 190
I2 statistic and the χ2 test of homogeneity. A p < 0.05 191
or I2 > 50% was regarded as statistically significant 192
heterogeneity across studies. The likelihood of pub- 193
lication bias in main trial outcomes was investigated 194
using funnel plots. 195
Subgroup analyses 196
After overall efficacy, safety, and tolerability were 197
evaluated, different SGAs (aripiprazole, olanzapine, 198
quetiapine, and risperidone) were examined separately 199
as subgroups. 200
RESULTS 201
The main trial outcomes included efficacy of 202
antipsychotic treatment as measured by BPRS, total 203
NPI, CGI-C, CGI-S, and CMAI, and safety and tolera- 204
bility as estimated by all-cause drop-outs, deaths, and 205
AEs, compared to placebo. 206
General characteristics of the included studies 207
Our search strategies yielded a total of 22 DB- 208
PC-RCTs (Fig. 1). After evaluation, three trials were 209
excluded because their effect evaluations (mainly 210
for agitation) were immediate (4 or 24 hours post- 211
treatment) and the drugs were administrated by 212
intramuscular injection [25–27], while the other 19 tri- 213
als were short- or long-term trials (6–26 weeks) of oral 214
administration. The specific study methods in these 215
trials were not consistent, and the primary and sec- 216
ondary outcomes were also different. We selected the 217
trials with total NPI, CGI-C, CGI-S, BPRS, and (or) 218
CMAI as their primary and secondary outcomes. Three 219
of these studies were excluded for lack of these evalu- 220
ation results [28–30]. The remaining 16 trials included 221
H. Ma et al. / Effects of Antipsychotics on Dementia 3
C), Clinical Global Impression of Severity (CGI-S),130
BPRS, and (or) Cohen-Mansfield Agitation Inventory131
(CMAI) as outcome measures. We did not distinguish132
between trials with fixed or flexible dosing.133
Quality assessment of included trials134
Since quality score for each studies included in a135
meta- analysis may be useful to ensure that better stud-136
ies receive more weight, many scales and checklists137
have been developed to assess the quality of a trial138
and the quality of its reports [19–22]. The scales of139
Brown [20], Jadad et al. [21], and van Tulder et al.140
[22] were used to evaluate the quality of primary RCTs141
for our meta-analysis by two reviewers independently.142
According to previous related literature [19–24], the143
trial was considered to be high quality when its total144
score of Brown scale was ≥17 points, its total score145
of Jadad scale was ≥3 points, or its total score of van146
Tulder scale was ≥5 points.147
Data extraction148
Trial data extracted included design characteristics,149
key inclusion criteria, subject nationality, mean age,150
gender ratio, study group size, drug dose(s), trial dura-151
tion, baseline rating scores, endpoint outcomes of all152
rating scales, drop-outs, mortality, and main AEs.153
Statistical analysis154
All meta-analytic calculations were per-155
formed with the free software Review Manager156
Version 5.1 (The Cochrane Collaboration,157
http://ims.cochrane.org/revman ).158
If different fixed-dose subgroups were used in a159
trial, both the total mean values and standard devi-160
ations (SDs) for the changes of all scale scores and161
the total amounts for AEs were calculated on behalf162
of the data of this trial based on the data from all163
the subgroups. We also chose the subgroup with the164
most effective dose for the psychological/behavioral165
symptoms of dementia patients to represent the data166
of this trial, or regarded the data of each fixed-dose167
subgroup together with control group in a trial as indi-168
vidual contrast to analyze. The latter two kinds of169
meta-analysis results are included in the Supplemen-170
tary Material. The odds ratios (ORs) with associated171
95% confidence intervals (CIs) were calculated for172
dichotomous data (drop-outs, death, and AEs). Stan-173
dardized mean differences (SMDs) or weighted mean174
differences (WMDs) with the associated 95% CIs175
were calculated for continuous data (NPI, CGI-S, 176
BPRS, CMAI, and CGI-C outcomes at endpoints). 177
Moreover, the Der-Simonian and Laird random-effects 178
model or Mantel-Haenszel fixed-effects model for the 179
dichotomous outcomes of drop-outs, death, and AEs 180
were applied to calculate the pooled safety and tol- 181
erability of SGAs. A fixed-effects or random-effects 182
model for rating scale changes was used to assess 183
the pooled drug efficacies. It generally referred to fix- 184
effects model except when random-effects model was 185
emphasized. The two types of parameters (ORs and 186
SMDs/WMDs) were plotted graphically using forest 187
plots. 188
To ensure that the included studies were combin- 189
able, the level of homogeneity was assessed by the 190
I2 statistic and the χ2 test of homogeneity. A p < 0.05 191
or I2 > 50% was regarded as statistically significant 192
heterogeneity across studies. The likelihood of pub- 193
lication bias in main trial outcomes was investigated 194
using funnel plots. 195
Subgroup analyses 196
After overall efficacy, safety, and tolerability were 197
evaluated, different SGAs (aripiprazole, olanzapine, 198
quetiapine, and risperidone) were examined separately 199
as subgroups. 200
RESULTS 201
The main trial outcomes included efficacy of 202
antipsychotic treatment as measured by BPRS, total 203
NPI, CGI-C, CGI-S, and CMAI, and safety and tolera- 204
bility as estimated by all-cause drop-outs, deaths, and 205
AEs, compared to placebo. 206
General characteristics of the included studies 207
Our search strategies yielded a total of 22 DB- 208
PC-RCTs (Fig. 1). After evaluation, three trials were 209
excluded because their effect evaluations (mainly 210
for agitation) were immediate (4 or 24 hours post- 211
treatment) and the drugs were administrated by 212
intramuscular injection [25–27], while the other 19 tri- 213
als were short- or long-term trials (6–26 weeks) of oral 214
administration. The specific study methods in these 215
trials were not consistent, and the primary and sec- 216
ondary outcomes were also different. We selected the 217
trials with total NPI, CGI-C, CGI-S, BPRS, and (or) 218
CMAI as their primary and secondary outcomes. Three 219
of these studies were excluded for lack of these evalu- 220
ation results [28–30]. The remaining 16 trials included 221

Uncorrected Author Proof
4 H. Ma et al. / Effects of Antipsychotics on Dementia
Fig. 1. Flowchart of literature selection process.
19 contrasts of SGAs versus placebo, of which 3 tri-222
als studied the effects of aripiprazole [3, 10, 15], 2223
olanzapine [9, 14], 5 quetiapine [11, 16, 17, 31, 32], 4224
risperidone [6–8,33], 1 olanzapine and risperidone [5],225
and 1 olanzapine, quetiepine, and risperidone [34]. We226
therefore included 19 data sets from 16 trials in our227
meta-analysis encompassing 3,343 drug-treated and228
1,707 placebo-treated dementia patients. The descrip-229
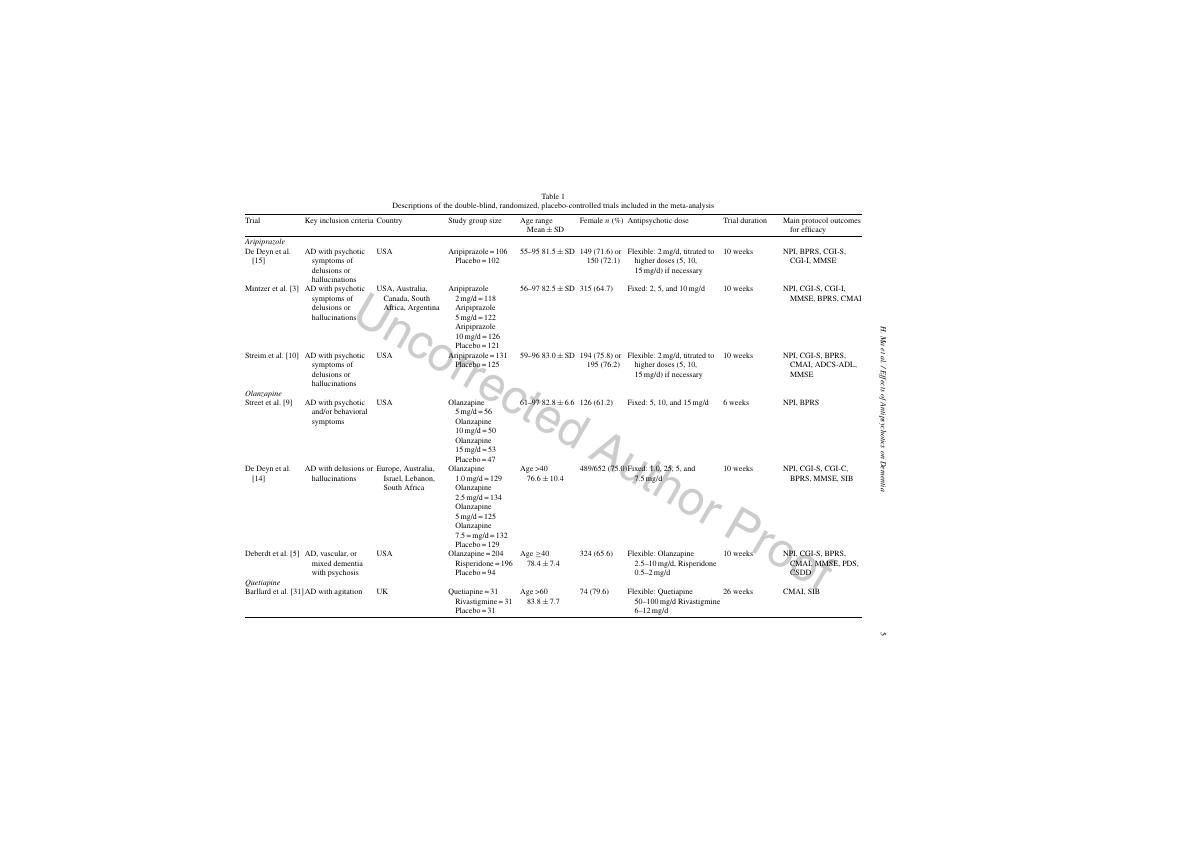
tions of these studies, including key inclusion criteria,230
country, study group size, age, gender, drug dose, trial231
duration, and main outcomes measures, are shown232
in Table 1. All these 16 trials were randomized and233
double-blind, but the concrete methods of randomiza-234
tion were reported in only 5 papers [6, 7, 31, 33, 34],235
and the concrete schemes of blindness were described236
in only 2 papers [31, 33]. Moreover, most of these 16237
studies were multicenter trials.238
Quality assessment results of the included studies239
The Brown, Jadad, and van Tulder scales [22] were240
used to evaluate the quality of all RCTs included241
in our meta-analysis. Table 2 showed the assessment242
scores (mean ± SD) and numbers (percentages) of high243
quality studies for all the studies and 4 subgroups244
by each scale. Totally, the mean scores of Brown,245
Jadad and van Tulder scales for 19 studies from 16246
trials were 18.21 ± 0.98, 3.47 ± 0.70, and 6.21 ± 1.03,247
respectively. Each of them was higher than the related248
cut point for high quality study. On the assessment249
with Brown, Jadad, and van Tulder scales, the per-250
centages of high quality RCTs were 89.5%, 100%,251
and 100%. Therefore, the quality of trials included in252
our meta-analysis is high, and this ensures the qual-253
ity of our meta-analysis and the reliability of our254
results.255
Efficacy 256
The main efficacy outcomes for all included com- 257
parisons are listed in Table 1. Different rating scales 258
were used to evaluate the efficacy of individual antipsy- 259
chotics. In general, BPRS was used for aripiprazole (2 260
out of 3 comparisons) and olanzapine (4 out of 4), and 261
BEHAVE-AD or CMAI for risperidone (4 out of 6 for 262
both drugs). Moreover, the NPI or CGI was also used 263
as main outcome measures for efficacy in all the trials 264
except Ballard et al. [31]. Figures 2A-E show the over- 265
all and subgroup effect sizes and meta-analysis results 266
for all SGAs versus placebo comparisons. 267
Efficacy on NPI 268
Figure 2A shows the overall and subgroup effects of 269
3 contrasts for aripiprazole, 4 for olanzapine, 5 for que- 270
tiapine, and 2 for risperidone on the NPI total scores. 271
There was no evidence of overall heterogeneity across 272
these 14 contrasts from 11 studies (χ2 = 11.93, p = 0.53, 273
I2 = 0%). Patients receiving SGAs exhibited signifi- 274
cantly improved NPI total score compared to placebo 275
(WMD = −2.81, 95% CI: −4.35–1.28, p = 0.0003). 276
Aripiprazole (WMD = −3.81, 95% CI: −6.36–1.26, 277
p = 0.003) and quetiapine (WMD = −3.45, 95% CI: 278
−6.78–0.11, p = 0.04) subgroups showed significant 279
improvements on NPI. 280
Efficacy on BPRS 281
Figure 2B shows the overall and subgroup effects 282
on BPRS scores of 2 contrasts for aripiprazole, 4 for 283
olanzapine, 2 for quetiapine, and 2 for risperidone. 284
There was no evidence of overall heterogeneity across 285
these 10 contrasts from 7 studies (χ2 = 10.83, p = 0.29, 286
I2 = 17%). Patients receiving SGAs showed signifi- 287
cant improvement compared to those receiving placebo 288
(WMD = −1.58, 95% CI: −2.52 - −0.65, p = 0.0009). 289
Moreover, the aripiprazole (WMD = −2.41, 95% CI: 290
−4.24–0.58, p = 0.01) and quetiapine (WMD = −2.70, 291
95% CI: −5.24–0.16, p = 0.04) subgroups showed 292
improvement on BPRS. 293
Efficacy on CMAI 294
Overall and subgroup effects on CMAI scores 295
(Fig. 2C) were calculated from one contrast for arip- 296
iprazole, one for olanzapine, 2 for quetiapine, and 297
4 for risperidone. There was overall heterogeneity 298
across these 8 contrasts from 7 studies (χ2 = 17.07, 299
p = 0.02, I2 = 59%), so random-effects model was 300
used. Patients on SGAs showed significant improve- 301
ment compared to those on placebo (WMD = −1.84, 302
95% CI: −3.01–0.67, p = 0.002). The aripiprazole 303
4 H. Ma et al. / Effects of Antipsychotics on Dementia
Fig. 1. Flowchart of literature selection process.
19 contrasts of SGAs versus placebo, of which 3 tri-222
als studied the effects of aripiprazole [3, 10, 15], 2223
olanzapine [9, 14], 5 quetiapine [11, 16, 17, 31, 32], 4224
risperidone [6–8,33], 1 olanzapine and risperidone [5],225
and 1 olanzapine, quetiepine, and risperidone [34]. We226
therefore included 19 data sets from 16 trials in our227
meta-analysis encompassing 3,343 drug-treated and228
1,707 placebo-treated dementia patients. The descrip-229
tions of these studies, including key inclusion criteria,230
country, study group size, age, gender, drug dose, trial231
duration, and main outcomes measures, are shown232
in Table 1. All these 16 trials were randomized and233
double-blind, but the concrete methods of randomiza-234
tion were reported in only 5 papers [6, 7, 31, 33, 34],235
and the concrete schemes of blindness were described236
in only 2 papers [31, 33]. Moreover, most of these 16237
studies were multicenter trials.238
Quality assessment results of the included studies239
The Brown, Jadad, and van Tulder scales [22] were240
used to evaluate the quality of all RCTs included241
in our meta-analysis. Table 2 showed the assessment242
scores (mean ± SD) and numbers (percentages) of high243
quality studies for all the studies and 4 subgroups244
by each scale. Totally, the mean scores of Brown,245
Jadad and van Tulder scales for 19 studies from 16246
trials were 18.21 ± 0.98, 3.47 ± 0.70, and 6.21 ± 1.03,247
respectively. Each of them was higher than the related248
cut point for high quality study. On the assessment249
with Brown, Jadad, and van Tulder scales, the per-250
centages of high quality RCTs were 89.5%, 100%,251
and 100%. Therefore, the quality of trials included in252
our meta-analysis is high, and this ensures the qual-253
ity of our meta-analysis and the reliability of our254
results.255
Efficacy 256
The main efficacy outcomes for all included com- 257
parisons are listed in Table 1. Different rating scales 258
were used to evaluate the efficacy of individual antipsy- 259
chotics. In general, BPRS was used for aripiprazole (2 260
out of 3 comparisons) and olanzapine (4 out of 4), and 261
BEHAVE-AD or CMAI for risperidone (4 out of 6 for 262
both drugs). Moreover, the NPI or CGI was also used 263
as main outcome measures for efficacy in all the trials 264
except Ballard et al. [31]. Figures 2A-E show the over- 265
all and subgroup effect sizes and meta-analysis results 266
for all SGAs versus placebo comparisons. 267
Efficacy on NPI 268
Figure 2A shows the overall and subgroup effects of 269
3 contrasts for aripiprazole, 4 for olanzapine, 5 for que- 270
tiapine, and 2 for risperidone on the NPI total scores. 271
There was no evidence of overall heterogeneity across 272
these 14 contrasts from 11 studies (χ2 = 11.93, p = 0.53, 273
I2 = 0%). Patients receiving SGAs exhibited signifi- 274
cantly improved NPI total score compared to placebo 275
(WMD = −2.81, 95% CI: −4.35–1.28, p = 0.0003). 276
Aripiprazole (WMD = −3.81, 95% CI: −6.36–1.26, 277
p = 0.003) and quetiapine (WMD = −3.45, 95% CI: 278
−6.78–0.11, p = 0.04) subgroups showed significant 279
improvements on NPI. 280
Efficacy on BPRS 281
Figure 2B shows the overall and subgroup effects 282
on BPRS scores of 2 contrasts for aripiprazole, 4 for 283
olanzapine, 2 for quetiapine, and 2 for risperidone. 284
There was no evidence of overall heterogeneity across 285
these 10 contrasts from 7 studies (χ2 = 10.83, p = 0.29, 286
I2 = 17%). Patients receiving SGAs showed signifi- 287
cant improvement compared to those receiving placebo 288
(WMD = −1.58, 95% CI: −2.52 - −0.65, p = 0.0009). 289
Moreover, the aripiprazole (WMD = −2.41, 95% CI: 290
−4.24–0.58, p = 0.01) and quetiapine (WMD = −2.70, 291
95% CI: −5.24–0.16, p = 0.04) subgroups showed 292
improvement on BPRS. 293
Efficacy on CMAI 294
Overall and subgroup effects on CMAI scores 295
(Fig. 2C) were calculated from one contrast for arip- 296
iprazole, one for olanzapine, 2 for quetiapine, and 297
4 for risperidone. There was overall heterogeneity 298
across these 8 contrasts from 7 studies (χ2 = 17.07, 299
p = 0.02, I2 = 59%), so random-effects model was 300
used. Patients on SGAs showed significant improve- 301
ment compared to those on placebo (WMD = −1.84, 302
95% CI: −3.01–0.67, p = 0.002). The aripiprazole 303

Uncorrected Author Proof
H. Ma et al. / Effects of Antipsychotics on Dementia 5
Table 1
Descriptions of the double-blind, randomized, placebo-controlled trials included in the meta-analysis
Trial Key inclusion criteria Country Study group size Age range
Mean ± SD
Female n (%) Antipsychotic dose Trial duration Main protocol outcomes
for efficacy
Aripiprazole
De Deyn et al.
[15]
AD with psychotic
symptoms of
delusions or
hallucinations
USA Aripiprazole = 106
Placebo = 102
55–95 81.5 ± SD 149 (71.6) or
150 (72.1)
Flexible: 2 mg/d, titrated to
higher doses (5, 10,
15 mg/d) if necessary
10 weeks NPI, BPRS, CGI-S,
CGI-I, MMSE
Mintzer et al. [3] AD with psychotic
symptoms of
delusions or
hallucinations
USA, Australia,
Canada, South
Africa, Argentina
Aripiprazole
2 mg/d = 118
Aripiprazole
5 mg/d = 122
Aripiprazole
10 mg/d = 126
Placebo = 121
56–97 82.5 ± SD 315 (64.7) Fixed: 2, 5, and 10 mg/d 10 weeks NPI, CGI-S, CGI-I,
MMSE, BPRS, CMAI
Streim et al. [10] AD with psychotic
symptoms of
delusions or
hallucinations
USA Aripiprazole = 131
Placebo = 125
59–96 83.0 ± SD 194 (75.8) or
195 (76.2)
Flexible: 2 mg/d, titrated to
higher doses (5, 10,
15 mg/d) if necessary
10 weeks NPI, CGI-S, BPRS,
CMAI, ADCS-ADL,
MMSE
Olanzapine
Street et al. [9] AD with psychotic
and/or behavioral
symptoms
USA Olanzapine
5 mg/d = 56
Olanzapine
10 mg/d = 50
Olanzapine
15 mg/d = 53
Placebo = 47
61–97 82.8 ± 6.6 126 (61.2) Fixed: 5, 10, and 15 mg/d 6 weeks NPI, BPRS
De Deyn et al.
[14]
AD with delusions or
hallucinations
Europe, Australia,
Israel, Lebanon,
South Africa
Olanzapine
1.0 mg/d = 129
Olanzapine
2.5 mg/d = 134
Olanzapine
5 mg/d = 125
Olanzapine
7.5 = mg/d = 132
Placebo = 129
Age >40
76.6 ± 10.4
489/652 (75.0)Fixed: 1.0, 25, 5, and
7.5 mg/d
10 weeks NPI, CGI-S, CGI-C,
BPRS, MMSE, SIB
Deberdt et al. [5] AD, vascular, or
mixed dementia
with psychosis
USA Olanzapine = 204
Risperidone = 196
Placebo = 94
Age ≥40
78.4 ± 7.4
324 (65.6) Flexible: Olanzapine
2.5–10 mg/d, Risperidone
0.5–2 mg/d
10 weeks NPI, CGI-S, BPRS,
CMAI, MMSE, PDS,
CSDD
Quetiapine
Barllard et al. [31] AD with agitation UK Quetiapine = 31
Rivastigmine = 31
Placebo = 31
Age >60
83.8 ± 7.7
74 (79.6) Flexible: Quetiapine
50–100 mg/d Rivastigmine
6–12 mg/d
26 weeks CMAI, SIB
H. Ma et al. / Effects of Antipsychotics on Dementia 5
Table 1
Descriptions of the double-blind, randomized, placebo-controlled trials included in the meta-analysis
Trial Key inclusion criteria Country Study group size Age range
Mean ± SD
Female n (%) Antipsychotic dose Trial duration Main protocol outcomes
for efficacy
Aripiprazole
De Deyn et al.
[15]
AD with psychotic
symptoms of
delusions or
hallucinations
USA Aripiprazole = 106
Placebo = 102
55–95 81.5 ± SD 149 (71.6) or
150 (72.1)
Flexible: 2 mg/d, titrated to
higher doses (5, 10,
15 mg/d) if necessary
10 weeks NPI, BPRS, CGI-S,
CGI-I, MMSE
Mintzer et al. [3] AD with psychotic
symptoms of
delusions or
hallucinations
USA, Australia,
Canada, South
Africa, Argentina
Aripiprazole
2 mg/d = 118
Aripiprazole
5 mg/d = 122
Aripiprazole
10 mg/d = 126
Placebo = 121
56–97 82.5 ± SD 315 (64.7) Fixed: 2, 5, and 10 mg/d 10 weeks NPI, CGI-S, CGI-I,
MMSE, BPRS, CMAI
Streim et al. [10] AD with psychotic
symptoms of
delusions or
hallucinations
USA Aripiprazole = 131
Placebo = 125
59–96 83.0 ± SD 194 (75.8) or
195 (76.2)
Flexible: 2 mg/d, titrated to
higher doses (5, 10,
15 mg/d) if necessary
10 weeks NPI, CGI-S, BPRS,
CMAI, ADCS-ADL,
MMSE
Olanzapine
Street et al. [9] AD with psychotic
and/or behavioral
symptoms
USA Olanzapine
5 mg/d = 56
Olanzapine
10 mg/d = 50
Olanzapine
15 mg/d = 53
Placebo = 47
61–97 82.8 ± 6.6 126 (61.2) Fixed: 5, 10, and 15 mg/d 6 weeks NPI, BPRS
De Deyn et al.
[14]
AD with delusions or
hallucinations
Europe, Australia,
Israel, Lebanon,
South Africa
Olanzapine
1.0 mg/d = 129
Olanzapine
2.5 mg/d = 134
Olanzapine
5 mg/d = 125
Olanzapine
7.5 = mg/d = 132
Placebo = 129
Age >40
76.6 ± 10.4
489/652 (75.0)Fixed: 1.0, 25, 5, and
7.5 mg/d
10 weeks NPI, CGI-S, CGI-C,
BPRS, MMSE, SIB
Deberdt et al. [5] AD, vascular, or
mixed dementia
with psychosis
USA Olanzapine = 204
Risperidone = 196
Placebo = 94
Age ≥40
78.4 ± 7.4
324 (65.6) Flexible: Olanzapine
2.5–10 mg/d, Risperidone
0.5–2 mg/d
10 weeks NPI, CGI-S, BPRS,
CMAI, MMSE, PDS,
CSDD
Quetiapine
Barllard et al. [31] AD with agitation UK Quetiapine = 31
Rivastigmine = 31
Placebo = 31
Age >60
83.8 ± 7.7
74 (79.6) Flexible: Quetiapine
50–100 mg/d Rivastigmine
6–12 mg/d
26 weeks CMAI, SIB
End of preview
Want to access all the pages? Upload your documents or become a member.
