University Biochemistry Lab Report: Biuret Assay and Protein Analysis
VerifiedAdded on 2022/09/08
|9
|1678
|17
Report
AI Summary
This Biochemistry lab report details an experiment using the Biuret assay to determine protein concentrations in unknown samples. The report begins with an introduction to proteins, their structures, and the Biuret assay background. The procedure involved preparing BSA standards, reacting them with Biuret reagent, and measuring absorbance using a spectrophotometer at 550nm. Results include a calibration curve and absorbance readings for unknown samples, from which protein concentrations were calculated using the Beer-Lambert law. The discussion analyzes the relationship between absorbance and concentration, while the conclusion highlights the assay's application in medical analysis, such as in urine tests for kidney disorders. The report also includes tables of data and a graph illustrating the calibration curve, along with the calculations for determining the percentage error. It also references relevant scientific articles that support the background information and experimental methods used.

Biochemistry Laboratory Report 1
BIOCHEMISTRY LABORATORY REPORT
Name
Course
Tutor
University
City
Date
BIOCHEMISTRY LABORATORY REPORT
Name
Course
Tutor
University
City
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biochemistry Laboratory Report 2
INTRODUCTION
Protein Background
Proteins have diverse functions since they appear in unique and different structures. They
are macromolecules with a relatively high mass made up of one or more chains of polypeptides
which are composed of different amino acids as the primary building blocks. Cells usually use
twenty amino acids which are available in order to make the polypeptide chains of the proteins.
The amino acids are composed of elements such as nitrogen, carbon, oxygen, hydrogen, and
sulfur. Proteins always share and have similar molecular formula even though most of them
differ in the side groups they possess. Synthesis of proteins involves an enzyme that is located in
the ribosomes of a cell (Piana et al. 2015). It synthesizes proteins by joining the various amino
acids through condensation process where an amino group of an amino acid is joined to an acid
group of the other forming a respective peptide bond linking the carbon and nitrogen atoms of
two amino acids. The structure of a protein and its polypeptide chain is, therefore, very specific
due to the different chemical nature and shape of the amino acids which are involved in bonding
(O'sullivan et al. 2016).
Proteins always have different structural organizations which include the primary
structure which shows the aminoacid sequences in the polypeptide and it basically dictates the
biological function of the protein. This particular sequence is controlled mainly by genes
involved in translation which gives a particular order and the number of a protein’s amino acids,
the second structure level of proteins is the secondary structure which defines the specific shape
that amino acids take in the protein, the third structure level of proteins is the tertiary one which
INTRODUCTION
Protein Background
Proteins have diverse functions since they appear in unique and different structures. They
are macromolecules with a relatively high mass made up of one or more chains of polypeptides
which are composed of different amino acids as the primary building blocks. Cells usually use
twenty amino acids which are available in order to make the polypeptide chains of the proteins.
The amino acids are composed of elements such as nitrogen, carbon, oxygen, hydrogen, and
sulfur. Proteins always share and have similar molecular formula even though most of them
differ in the side groups they possess. Synthesis of proteins involves an enzyme that is located in
the ribosomes of a cell (Piana et al. 2015). It synthesizes proteins by joining the various amino
acids through condensation process where an amino group of an amino acid is joined to an acid
group of the other forming a respective peptide bond linking the carbon and nitrogen atoms of
two amino acids. The structure of a protein and its polypeptide chain is, therefore, very specific
due to the different chemical nature and shape of the amino acids which are involved in bonding
(O'sullivan et al. 2016).
Proteins always have different structural organizations which include the primary
structure which shows the aminoacid sequences in the polypeptide and it basically dictates the
biological function of the protein. This particular sequence is controlled mainly by genes
involved in translation which gives a particular order and the number of a protein’s amino acids,
the second structure level of proteins is the secondary structure which defines the specific shape
that amino acids take in the protein, the third structure level of proteins is the tertiary one which

Biochemistry Laboratory Report 3
defines the 3D structure of the protein which is shown by the bending and folding of the
polypeptide chains in the protein structure, the fourth level of structure of proteins is that of the
quarternary structure which involves the association between two or more polypeptide chains
(Ferreira et al. 2015).
Biuret Assay Background
This is a process that is used in the determination of the concentration of proteins. It is an
important and crucial technique used in most of the processes in protein assay procedures and
analysis including proteomics. Biuret assay reaction is used for both the qualitative and
quantitative analysis of proteins in terms of the peptide bonds which are available in the amino
acid structure bonding in the particular proteins (Liu & Pan 2017).
The principle of this essay revolves around spectrophotometry of color change on copper
ions in solution. A solution containing the proteins is treated with Cu2+ which is the cupric ions
in an alkaline conditioned medium. These ions react with the peptide bonds present in the protein
to form a purple-colored complex which is then measured using a spectrophotometer in a
quantitative analysis of the visible region (Rose, Funk & Neiger, 2016).
Cu2+ - peptide complex formed after the reaction
defines the 3D structure of the protein which is shown by the bending and folding of the
polypeptide chains in the protein structure, the fourth level of structure of proteins is that of the
quarternary structure which involves the association between two or more polypeptide chains
(Ferreira et al. 2015).
Biuret Assay Background
This is a process that is used in the determination of the concentration of proteins. It is an
important and crucial technique used in most of the processes in protein assay procedures and
analysis including proteomics. Biuret assay reaction is used for both the qualitative and
quantitative analysis of proteins in terms of the peptide bonds which are available in the amino
acid structure bonding in the particular proteins (Liu & Pan 2017).
The principle of this essay revolves around spectrophotometry of color change on copper
ions in solution. A solution containing the proteins is treated with Cu2+ which is the cupric ions
in an alkaline conditioned medium. These ions react with the peptide bonds present in the protein
to form a purple-colored complex which is then measured using a spectrophotometer in a
quantitative analysis of the visible region (Rose, Funk & Neiger, 2016).
Cu2+ - peptide complex formed after the reaction
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Biochemistry Laboratory Report 4
Higher absorbance is brought about by a higher intensity of the color and the higher the
peptide bond concentration in the protein which shows the number of molecules of proteins
present. Spectrophotometry is done at 550nm wavelength and concentration is done using the
Beer-Lambert Law (Astrof & Horowitz 2018).
Aims for this practical are:
To plot a calibration graph of standard Biuret assay
To determine the protein concentrations in unknown samples being analyzed using Biuret
Assay
PROCEDURE
Protein series of BSA standards were prepared from the provided 100mg/ml BSA as
shown in the table below. 30ml of 10mg/ml of BSA was prepared and used in the preparation of
other standards as shown in the below table. Another set of standards were prepared using
volumes of 5ml in a proportion equivalent to that of water and BSA in cubic cm as shown below.
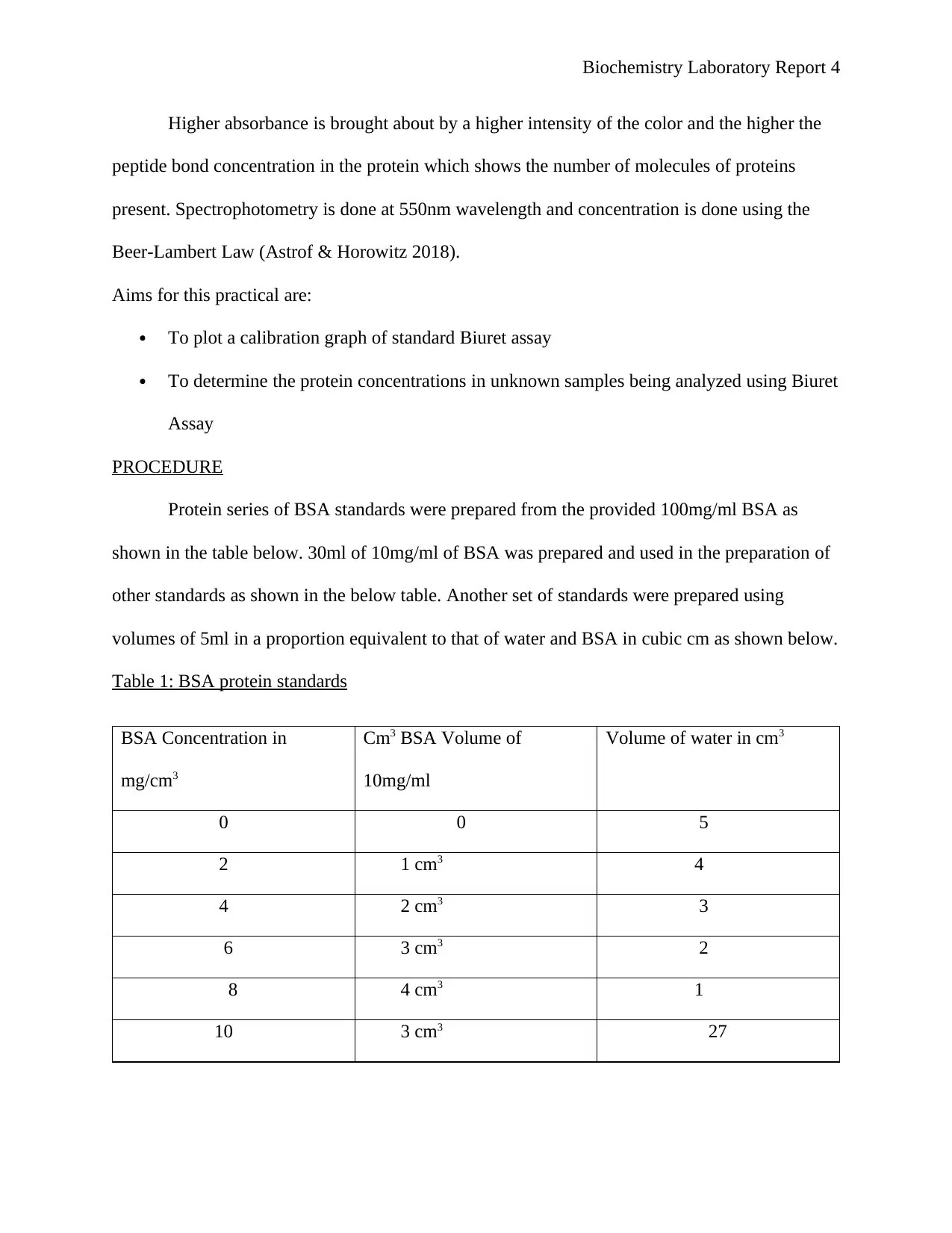
Table 1: BSA protein standards
BSA Concentration in
mg/cm3
Cm3 BSA Volume of
10mg/ml
Volume of water in cm3
0 0 5
2 1 cm3 4
4 2 cm3 3
6 3 cm3 2
8 4 cm3 1
10 3 cm3 27
Higher absorbance is brought about by a higher intensity of the color and the higher the
peptide bond concentration in the protein which shows the number of molecules of proteins
present. Spectrophotometry is done at 550nm wavelength and concentration is done using the
Beer-Lambert Law (Astrof & Horowitz 2018).
Aims for this practical are:
To plot a calibration graph of standard Biuret assay
To determine the protein concentrations in unknown samples being analyzed using Biuret
Assay
PROCEDURE
Protein series of BSA standards were prepared from the provided 100mg/ml BSA as
shown in the table below. 30ml of 10mg/ml of BSA was prepared and used in the preparation of
other standards as shown in the below table. Another set of standards were prepared using
volumes of 5ml in a proportion equivalent to that of water and BSA in cubic cm as shown below.
Table 1: BSA protein standards
BSA Concentration in
mg/cm3
Cm3 BSA Volume of
10mg/ml
Volume of water in cm3
0 0 5
2 1 cm3 4
4 2 cm3 3
6 3 cm3 2
8 4 cm3 1
10 3 cm3 27
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Biochemistry Laboratory Report 5
Sic cuvettes containing the following reagents were prepared:
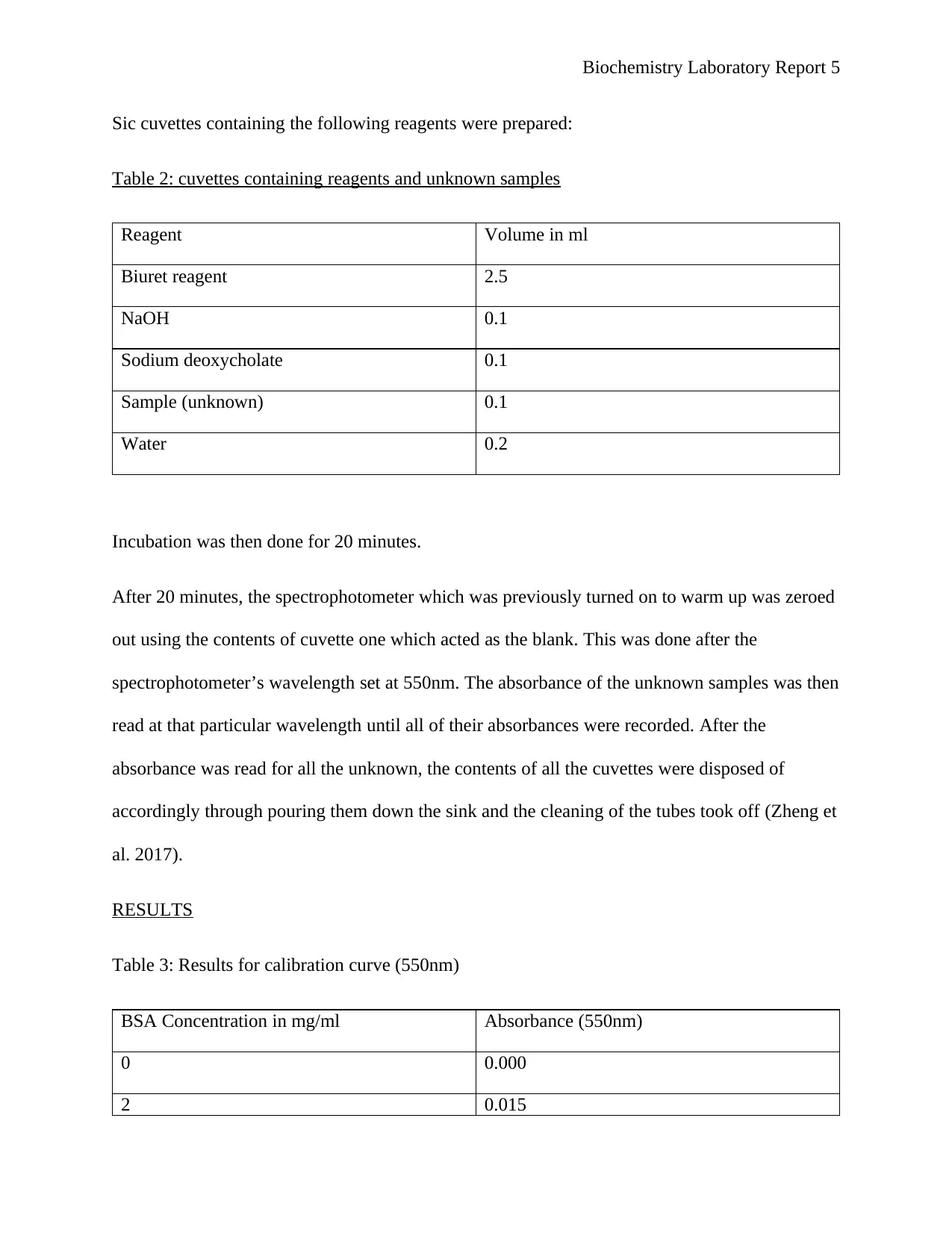
Table 2: cuvettes containing reagents and unknown samples
Reagent Volume in ml
Biuret reagent 2.5
NaOH 0.1
Sodium deoxycholate 0.1
Sample (unknown) 0.1
Water 0.2
Incubation was then done for 20 minutes.
After 20 minutes, the spectrophotometer which was previously turned on to warm up was zeroed
out using the contents of cuvette one which acted as the blank. This was done after the
spectrophotometer’s wavelength set at 550nm. The absorbance of the unknown samples was then
read at that particular wavelength until all of their absorbances were recorded. After the
absorbance was read for all the unknown, the contents of all the cuvettes were disposed of
accordingly through pouring them down the sink and the cleaning of the tubes took off (Zheng et
al. 2017).
RESULTS
Table 3: Results for calibration curve (550nm)
BSA Concentration in mg/ml Absorbance (550nm)
0 0.000
2 0.015
Sic cuvettes containing the following reagents were prepared:
Table 2: cuvettes containing reagents and unknown samples
Reagent Volume in ml
Biuret reagent 2.5
NaOH 0.1
Sodium deoxycholate 0.1
Sample (unknown) 0.1
Water 0.2
Incubation was then done for 20 minutes.
After 20 minutes, the spectrophotometer which was previously turned on to warm up was zeroed
out using the contents of cuvette one which acted as the blank. This was done after the
spectrophotometer’s wavelength set at 550nm. The absorbance of the unknown samples was then
read at that particular wavelength until all of their absorbances were recorded. After the
absorbance was read for all the unknown, the contents of all the cuvettes were disposed of
accordingly through pouring them down the sink and the cleaning of the tubes took off (Zheng et
al. 2017).
RESULTS
Table 3: Results for calibration curve (550nm)
BSA Concentration in mg/ml Absorbance (550nm)
0 0.000
2 0.015
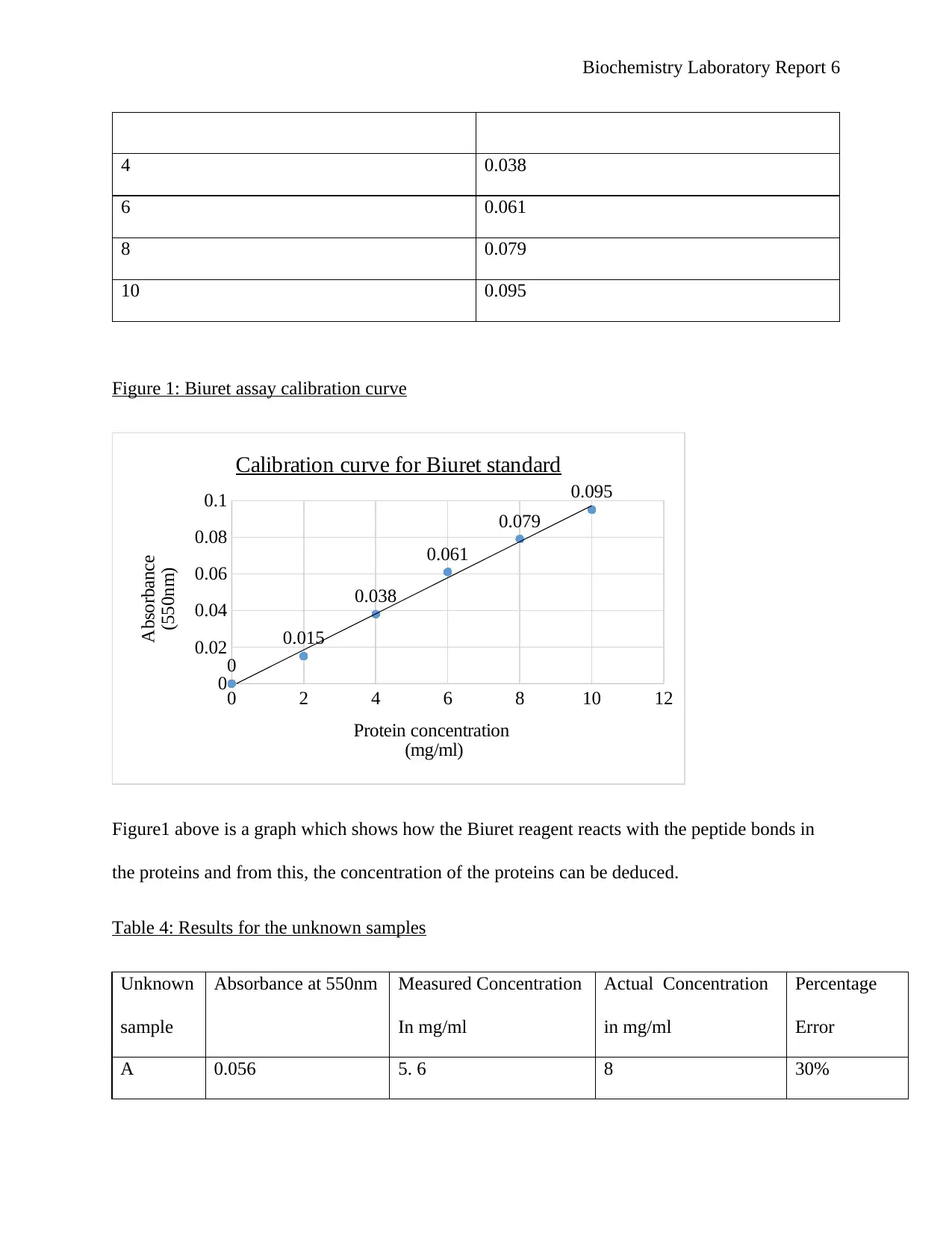
Biochemistry Laboratory Report 6
4 0.038
6 0.061
8 0.079
10 0.095
Figure 1: Biuret assay calibration curve
0 2 4 6 8 10 12
0
0.02
0.04
0.06
0.08
0.1
0
0.015
0.038
0.061
0.079
0.095
Calibration curve for Biuret standard
Protein concentration
(mg/ml)
Absorbance
(550nm)
Figure1 above is a graph which shows how the Biuret reagent reacts with the peptide bonds in
the proteins and from this, the concentration of the proteins can be deduced.
Table 4: Results for the unknown samples
Unknown
sample
Absorbance at 550nm Measured Concentration
In mg/ml
Actual Concentration
in mg/ml
Percentage
Error
A 0.056 5. 6 8 30%
4 0.038
6 0.061
8 0.079
10 0.095
Figure 1: Biuret assay calibration curve
0 2 4 6 8 10 12
0
0.02
0.04
0.06
0.08
0.1
0
0.015
0.038
0.061
0.079
0.095
Calibration curve for Biuret standard
Protein concentration
(mg/ml)
Absorbance
(550nm)
Figure1 above is a graph which shows how the Biuret reagent reacts with the peptide bonds in
the proteins and from this, the concentration of the proteins can be deduced.
Table 4: Results for the unknown samples
Unknown
sample
Absorbance at 550nm Measured Concentration
In mg/ml
Actual Concentration
in mg/ml
Percentage
Error
A 0.056 5. 6 8 30%
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Biochemistry Laboratory Report 7
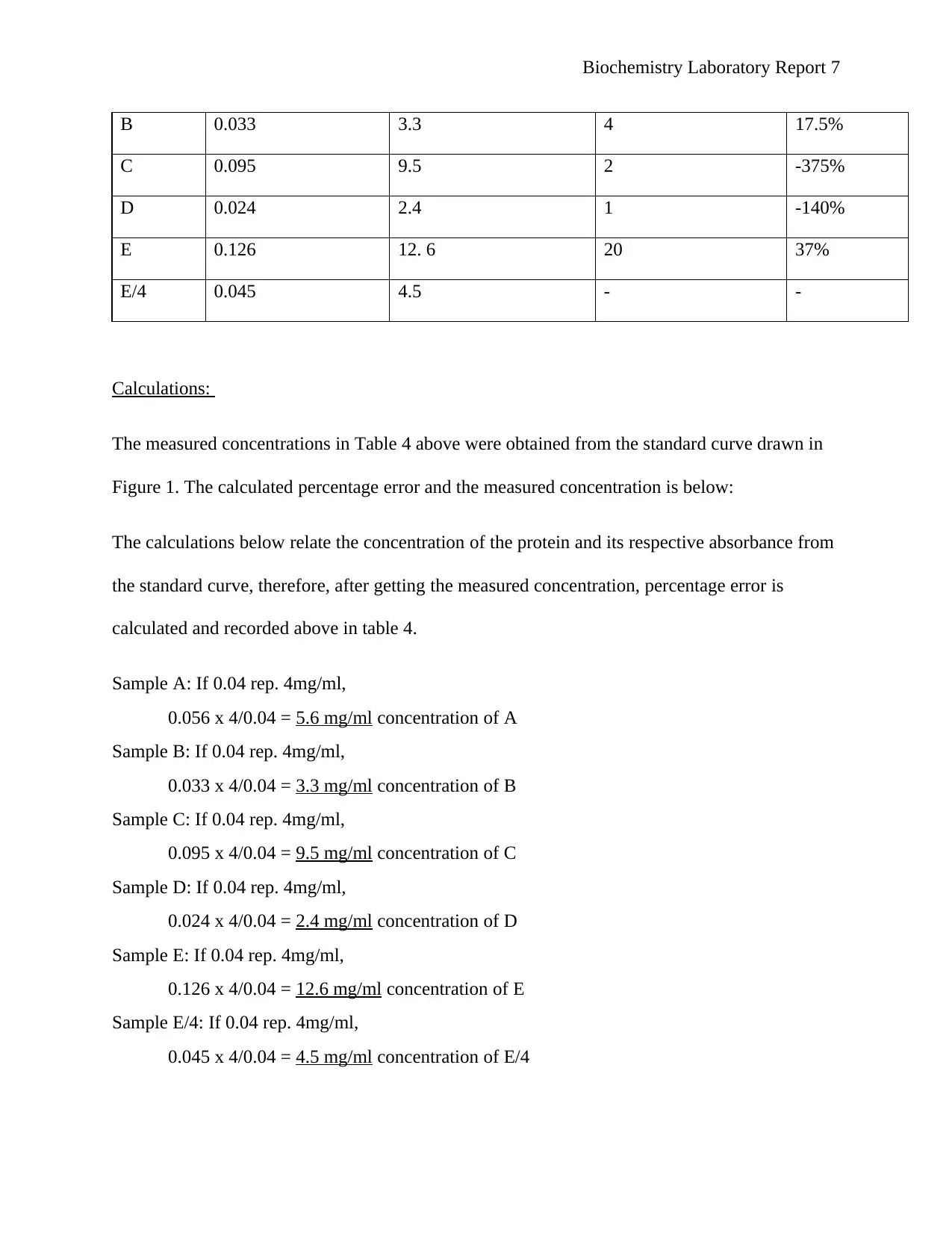
B 0.033 3.3 4 17.5%
C 0.095 9.5 2 -375%
D 0.024 2.4 1 -140%
E 0.126 12. 6 20 37%
E/4 0.045 4.5 - -
Calculations:
The measured concentrations in Table 4 above were obtained from the standard curve drawn in
Figure 1. The calculated percentage error and the measured concentration is below:
The calculations below relate the concentration of the protein and its respective absorbance from
the standard curve, therefore, after getting the measured concentration, percentage error is
calculated and recorded above in table 4.
Sample A: If 0.04 rep. 4mg/ml,
0.056 x 4/0.04 = 5.6 mg/ml concentration of A
Sample B: If 0.04 rep. 4mg/ml,
0.033 x 4/0.04 = 3.3 mg/ml concentration of B
Sample C: If 0.04 rep. 4mg/ml,
0.095 x 4/0.04 = 9.5 mg/ml concentration of C
Sample D: If 0.04 rep. 4mg/ml,
0.024 x 4/0.04 = 2.4 mg/ml concentration of D
Sample E: If 0.04 rep. 4mg/ml,
0.126 x 4/0.04 = 12.6 mg/ml concentration of E
Sample E/4: If 0.04 rep. 4mg/ml,
0.045 x 4/0.04 = 4.5 mg/ml concentration of E/4
B 0.033 3.3 4 17.5%
C 0.095 9.5 2 -375%
D 0.024 2.4 1 -140%
E 0.126 12. 6 20 37%
E/4 0.045 4.5 - -
Calculations:
The measured concentrations in Table 4 above were obtained from the standard curve drawn in
Figure 1. The calculated percentage error and the measured concentration is below:
The calculations below relate the concentration of the protein and its respective absorbance from
the standard curve, therefore, after getting the measured concentration, percentage error is
calculated and recorded above in table 4.
Sample A: If 0.04 rep. 4mg/ml,
0.056 x 4/0.04 = 5.6 mg/ml concentration of A
Sample B: If 0.04 rep. 4mg/ml,
0.033 x 4/0.04 = 3.3 mg/ml concentration of B
Sample C: If 0.04 rep. 4mg/ml,
0.095 x 4/0.04 = 9.5 mg/ml concentration of C
Sample D: If 0.04 rep. 4mg/ml,
0.024 x 4/0.04 = 2.4 mg/ml concentration of D
Sample E: If 0.04 rep. 4mg/ml,
0.126 x 4/0.04 = 12.6 mg/ml concentration of E
Sample E/4: If 0.04 rep. 4mg/ml,
0.045 x 4/0.04 = 4.5 mg/ml concentration of E/4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biochemistry Laboratory Report 8
DISCUSSION
The straight line for the standard curve shows the reaction between the Biuret reagent and the
peptide bonds. Using this, the protein concentrations can be identified for unknown samples. The
more the concentration of BSA, the more the number of proteins present in the sample and from
this relationship, protein concentrations in unknown samples is calculated. As observed from the
curve, the less the concentration, the less the absorbance obtained and the less the number of
proteins obtained (Legendre et al. 2017).
CONCLUSION
Biuret assay of proteins enables the estimation of the number of proteins in unknown samples in
hospitals and health facilities. The number of proteins in urine can be estimated using this
process to help in the urine analysis for kidney examination where a high concentration of
proteins in urine may signify a disorder in the kidneys such as kidney failure.
DISCUSSION
The straight line for the standard curve shows the reaction between the Biuret reagent and the
peptide bonds. Using this, the protein concentrations can be identified for unknown samples. The
more the concentration of BSA, the more the number of proteins present in the sample and from
this relationship, protein concentrations in unknown samples is calculated. As observed from the
curve, the less the concentration, the less the absorbance obtained and the less the number of
proteins obtained (Legendre et al. 2017).
CONCLUSION
Biuret assay of proteins enables the estimation of the number of proteins in unknown samples in
hospitals and health facilities. The number of proteins in urine can be estimated using this
process to help in the urine analysis for kidney examination where a high concentration of
proteins in urine may signify a disorder in the kidneys such as kidney failure.

Biochemistry Laboratory Report 9
References
Astrof, N.S. and Horowitz, G., 2018. Protein Colorimetry Experiments That Incorporate
Intentional Discrepancies and Historical Narratives. Journal of Chemical Education, 95(7),
pp.1198-1204.
Ferreira, L.G., Dos Santos, R.N., Oliva, G. and Andricopulo, A.D., 2015. Molecular docking and
structure-based drug design strategies. Molecules, 20(7), pp.13384-13421.
Legendre, K.P., Leissinger, M., Le Donne, V., Grasperge, B.J. and Gaunt, S.D., 2017. The effect
of urea on refractometric total protein measurement in dogs and cats with azotemia. Veterinary
clinical pathology, 46(1), pp.138-142.
Liu, Z. and Pan, J., 2017. A practical method for extending the biuret assay to protein
determination of corn-based products. Food chemistry, 224, pp.289-293.
O'sullivan, J., Murray, B., Flynn, C. and Norton, I., 2016. The effect of ultrasound treatment on
the structural, physical and emulsifying properties of animal and vegetable proteins. Food
hydrocolloids, 53, pp.141-154.
Piana, S., Donchev, A.G., Robustelli, P. and Shaw, D.E., 2015. Water dispersion interactions
strongly influence simulated structural properties of disordered protein states. The journal of
physical chemistry B, 119(16), pp.5113-5123.
Rose, A., Funk, D. and Neiger, R., 2016. Comparison of refractometry and biuret assay for
measurement of total protein concentration in canine abdominal and pleural fluid specimens.
Journal of the American Veterinary Medical Association, 248(7), pp.789-794.
Zheng, K., Wu, L., He, Z., Yang, B. and Yang, Y., 2017. Measurement of the total protein in
serum by biuret method with uncertainty evaluation. Measurement, 112, pp.16-21.
References
Astrof, N.S. and Horowitz, G., 2018. Protein Colorimetry Experiments That Incorporate
Intentional Discrepancies and Historical Narratives. Journal of Chemical Education, 95(7),
pp.1198-1204.
Ferreira, L.G., Dos Santos, R.N., Oliva, G. and Andricopulo, A.D., 2015. Molecular docking and
structure-based drug design strategies. Molecules, 20(7), pp.13384-13421.
Legendre, K.P., Leissinger, M., Le Donne, V., Grasperge, B.J. and Gaunt, S.D., 2017. The effect
of urea on refractometric total protein measurement in dogs and cats with azotemia. Veterinary
clinical pathology, 46(1), pp.138-142.
Liu, Z. and Pan, J., 2017. A practical method for extending the biuret assay to protein
determination of corn-based products. Food chemistry, 224, pp.289-293.
O'sullivan, J., Murray, B., Flynn, C. and Norton, I., 2016. The effect of ultrasound treatment on
the structural, physical and emulsifying properties of animal and vegetable proteins. Food
hydrocolloids, 53, pp.141-154.
Piana, S., Donchev, A.G., Robustelli, P. and Shaw, D.E., 2015. Water dispersion interactions
strongly influence simulated structural properties of disordered protein states. The journal of
physical chemistry B, 119(16), pp.5113-5123.
Rose, A., Funk, D. and Neiger, R., 2016. Comparison of refractometry and biuret assay for
measurement of total protein concentration in canine abdominal and pleural fluid specimens.
Journal of the American Veterinary Medical Association, 248(7), pp.789-794.
Zheng, K., Wu, L., He, Z., Yang, B. and Yang, Y., 2017. Measurement of the total protein in
serum by biuret method with uncertainty evaluation. Measurement, 112, pp.16-21.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





