An Overview of Biological Therapy: Immunotherapy for Cancer Treatment
VerifiedAdded on 2023/04/23
|15
|3936
|463
Essay
AI Summary
This essay provides an overview of biological therapy, focusing on immunotherapy as a cancer treatment. It delves into the mechanisms of action, specifically highlighting immune checkpoint inhibitors like CTLA-4 and PD-1. The essay explains how these therapies work by targeting and modulating the immune system to combat cancer cells. It covers the role of T-cells, the tumor microenvironment, and the impact of these therapies on tumor rejection. The discussion includes specific examples of cancer types where immunotherapy is utilized, such as melanoma and non-small cell lung cancer. Furthermore, the essay details the molecular mechanisms of CTLA-4 and PD-1 blockade, including their effects on T-cell activation and the overall immune response. The essay also discusses the clinical applications and effectiveness of these therapies, referencing relevant studies and research findings. In conclusion, the essay emphasizes the significance of biological therapy in advancing cancer treatment and its potential to improve patient outcomes.

Running head: BIOLOGICAL THERAPY
Biological Therapy
Name of the Student
Name of the University
Author Note
Biological Therapy
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1BIOLOGICAL THERAPY
Biological therapy is a special type of treatment process in which a living organism is used to
treat a specific disease. Not only a living organism, but any substances derived from any
organism and any agent produced in a laboratory can also be used as a substance to treat a
disease (Priyadarshani, Li and Yao 2016). In recent time, the use of biological therapy has
become very common in the treatment of Cancer therapy. The biological therapies have
positively contributed to the advancement of the cancer therapies and often it is reported that, in
treatment ofadvanced-stage nonsmall cell lung cancer (NSCLC) the use of the biological
therapies has reduced the mortality rate in the world. Such biological therapies that are used for
the cancer treatment, are generally attacks the cancer cells by stimulating the immune system of
the body (Hersh 2016). In this biological therapy genotype is targetedwith druggable targets
with predefined clinical actionability and those genes, that are targeted, are HER2, RET, BRAF,
MET, NTRK1. These types of biological therapy used for the cancer, are referred to as
immunotherapy. However, this technique does not attack the cancer cells directly (McGranahan
and Swanton 2015). Therefore, there are several biological therapies, such as antibody therapies,
targeted therapies are associated with the direct interaction with the cancer cells. Immunotherapy
is one of the most common biological therapy that can be used for the treatment of cancer in a
wide manner. Therefore most of the biological therapy technique generally use the mechanism
of immune checkpoint inhibitor in the biological therapy of various cancer treatment. (Li et al.
2017). In this essay, the biological therapy, specifically the immunotherapy for cancer and use of
this therapy are highlighted in a brief manner.
Immunotherapy for the cancer treatment has various types such as immune checkpoint inhibitors,
adoptive cell transfer and non-specific immune stimulation. The use of the check point inhibitors
are used in a wide manner in recent times in the treatment of the cancer. The use of immune
Biological therapy is a special type of treatment process in which a living organism is used to
treat a specific disease. Not only a living organism, but any substances derived from any
organism and any agent produced in a laboratory can also be used as a substance to treat a
disease (Priyadarshani, Li and Yao 2016). In recent time, the use of biological therapy has
become very common in the treatment of Cancer therapy. The biological therapies have
positively contributed to the advancement of the cancer therapies and often it is reported that, in
treatment ofadvanced-stage nonsmall cell lung cancer (NSCLC) the use of the biological
therapies has reduced the mortality rate in the world. Such biological therapies that are used for
the cancer treatment, are generally attacks the cancer cells by stimulating the immune system of
the body (Hersh 2016). In this biological therapy genotype is targetedwith druggable targets
with predefined clinical actionability and those genes, that are targeted, are HER2, RET, BRAF,
MET, NTRK1. These types of biological therapy used for the cancer, are referred to as
immunotherapy. However, this technique does not attack the cancer cells directly (McGranahan
and Swanton 2015). Therefore, there are several biological therapies, such as antibody therapies,
targeted therapies are associated with the direct interaction with the cancer cells. Immunotherapy
is one of the most common biological therapy that can be used for the treatment of cancer in a
wide manner. Therefore most of the biological therapy technique generally use the mechanism
of immune checkpoint inhibitor in the biological therapy of various cancer treatment. (Li et al.
2017). In this essay, the biological therapy, specifically the immunotherapy for cancer and use of
this therapy are highlighted in a brief manner.
Immunotherapy for the cancer treatment has various types such as immune checkpoint inhibitors,
adoptive cell transfer and non-specific immune stimulation. The use of the check point inhibitors
are used in a wide manner in recent times in the treatment of the cancer. The use of immune

2BIOLOGICAL THERAPY
system against the cancer cells in the body, influences the fate of development of the cancer cells
in the body and as a part of this, the agents also act as an extrinsic tumor suppressing agent that
are associated with the destruction of the tumors and restrict the development of the tumor cells.
It is observed that immune suppression can be mediated through the use of cytotoxic T-
lymphocyte associated antigen-4 (CTLA-4) and through Programmed death-1 (PD-1) (Sharma et
al. 2017). These two agents are used as an immunosuppressing agents as they are expressed on
the T cells. The immune checkpoint techniques can be used in various types of cancers such as
melanoma, renal cell carcinoma, non-small cell lung cancer. The immune therapy for the cancer
therapy mainly tries to give a safeguard to the immune system of the body. The T-cell therapy
and immune blockade checkpoints are mostly used immunotherapy for the treatment of cancer.
The blocking of negative immune regulators will help the human body to combat against the
cancer. As a part of the immune check point therapy, BCG is widely used as a treatment process
of cancer treatment (Nagarsheth, Wicha and Zou 2017). BCG was first approved by FDA as an
immunotherapy reagent and it is observed that in US the use of this therapy had lowered the risks
of Bladder cancer (BC) in the US by 70% approximately. There are various inhibitory immune
checkpoints such as APC cells, cancer cells and T cells. The T cell is activated when it makes
association with the Major histocompatibility complex (MHC) on the APCs or on the surface of
the tumor cells. Whenever, a T cell interacts with an antigen that is presented on the surface of
the cancer cell by the MHC class I molecules, the cancer cells shows cognate ligands to be
associated with the inhibitory checkpoints expressed on the surface of the T cell. Generally it can
be said that, the immune checkpoint proteins are located on the cell surfaces of the lymphocytes
which are specific for the tumor cells (Farkona, Diamandis and Blasutig 2016). In order to
inhibit the functions of cancer cells, the interaction property between the checkpoint ligands and
system against the cancer cells in the body, influences the fate of development of the cancer cells
in the body and as a part of this, the agents also act as an extrinsic tumor suppressing agent that
are associated with the destruction of the tumors and restrict the development of the tumor cells.
It is observed that immune suppression can be mediated through the use of cytotoxic T-
lymphocyte associated antigen-4 (CTLA-4) and through Programmed death-1 (PD-1) (Sharma et
al. 2017). These two agents are used as an immunosuppressing agents as they are expressed on
the T cells. The immune checkpoint techniques can be used in various types of cancers such as
melanoma, renal cell carcinoma, non-small cell lung cancer. The immune therapy for the cancer
therapy mainly tries to give a safeguard to the immune system of the body. The T-cell therapy
and immune blockade checkpoints are mostly used immunotherapy for the treatment of cancer.
The blocking of negative immune regulators will help the human body to combat against the
cancer. As a part of the immune check point therapy, BCG is widely used as a treatment process
of cancer treatment (Nagarsheth, Wicha and Zou 2017). BCG was first approved by FDA as an
immunotherapy reagent and it is observed that in US the use of this therapy had lowered the risks
of Bladder cancer (BC) in the US by 70% approximately. There are various inhibitory immune
checkpoints such as APC cells, cancer cells and T cells. The T cell is activated when it makes
association with the Major histocompatibility complex (MHC) on the APCs or on the surface of
the tumor cells. Whenever, a T cell interacts with an antigen that is presented on the surface of
the cancer cell by the MHC class I molecules, the cancer cells shows cognate ligands to be
associated with the inhibitory checkpoints expressed on the surface of the T cell. Generally it can
be said that, the immune checkpoint proteins are located on the cell surfaces of the lymphocytes
which are specific for the tumor cells (Farkona, Diamandis and Blasutig 2016). In order to
inhibit the functions of cancer cells, the interaction property between the checkpoint ligands and
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3BIOLOGICAL THERAPY
their ligands can be used and as result, the immune system will be engaged against the tumor
cells of the body. CLAT-4 can be used as am immune checkpoint blockers and it exerts its
inhibitory effects when it binds with the ligands named CD86 , CD 80 and they are quite similar
with the function of CD28. However, both the two agents have much higher affinity than that to
the CD28. This competitive bindings inhibits CD-28 induced activation of the T-cell. As a result,
the production rate of cytokine is also hampered. Not only the CTLA-4 but , the PD-1 can also
be used as a blocking agent of immune check points and it is also widely used in the treatment of
cancer therapy. The PD-1 is one of the activated protein that is expressed on the surface of the T
cell. The PD-1 generally can bind with the two types of receptor that are PDL1 and PDL2. The
PDL1 is highly expressed in the APCs, epithelial cells, T-lymphocytes and in the tumor cells
(Baumeister et al. 2016). The secretion of PDL1 from the overexpressing tumor cells protect the
tumor cells from the CD8+ mediated cell lysis. So it can be said that blocking of PDL1 will
inhibit the binding of the PD1 with its receptor and this will allow the T cells to destroy the
tumor cells. In a study it was noted that, use ofPidilizumab, a humanized monoclonal IgG4
antibody is capable of targeting the receptor of PD1, has become quite successful in the
treatment of advanced melanoma patients. In the phase I of this study, 37-38% of patients had
reported about positive outcome (Gotwals et al. 2017). In another study by Ohaegbulam et al.
(2015) , also showed the effectiveness of Pidilizumab in the blocking of PD1. In this, study, the
blocker is used in the treatment of various melanoma patients and it was observed that the
objective response rate was almost 66% among the study group of 32 patients and the patients
who were given in a rate of 10mg/kg ,showed the highest response.
Mechanism of CTLA-4 Mediated therapy
their ligands can be used and as result, the immune system will be engaged against the tumor
cells of the body. CLAT-4 can be used as am immune checkpoint blockers and it exerts its
inhibitory effects when it binds with the ligands named CD86 , CD 80 and they are quite similar
with the function of CD28. However, both the two agents have much higher affinity than that to
the CD28. This competitive bindings inhibits CD-28 induced activation of the T-cell. As a result,
the production rate of cytokine is also hampered. Not only the CTLA-4 but , the PD-1 can also
be used as a blocking agent of immune check points and it is also widely used in the treatment of
cancer therapy. The PD-1 is one of the activated protein that is expressed on the surface of the T
cell. The PD-1 generally can bind with the two types of receptor that are PDL1 and PDL2. The
PDL1 is highly expressed in the APCs, epithelial cells, T-lymphocytes and in the tumor cells
(Baumeister et al. 2016). The secretion of PDL1 from the overexpressing tumor cells protect the
tumor cells from the CD8+ mediated cell lysis. So it can be said that blocking of PDL1 will
inhibit the binding of the PD1 with its receptor and this will allow the T cells to destroy the
tumor cells. In a study it was noted that, use ofPidilizumab, a humanized monoclonal IgG4
antibody is capable of targeting the receptor of PD1, has become quite successful in the
treatment of advanced melanoma patients. In the phase I of this study, 37-38% of patients had
reported about positive outcome (Gotwals et al. 2017). In another study by Ohaegbulam et al.
(2015) , also showed the effectiveness of Pidilizumab in the blocking of PD1. In this, study, the
blocker is used in the treatment of various melanoma patients and it was observed that the
objective response rate was almost 66% among the study group of 32 patients and the patients
who were given in a rate of 10mg/kg ,showed the highest response.
Mechanism of CTLA-4 Mediated therapy
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4BIOLOGICAL THERAPY
The function and expression pof CTLA-4 is directly linked with the activation of the T-cell.
After the activation of the T-cell receptor, the CTLA-4 is upregulated. However, CTLA-4 lowers
TCR signaling mechanism through the competitive stimulation pathway of CD28. In the CD28
the ligand binding part that is B7, in CD80 the ligand binding part is B7-1 and the B7-2 in the
CD86 and for these ligand binding sites, CTLA-4 has higher affinity and avidity ( Fig-1). The
main reason behind this enhanced affinity towards these two ligand binding sites are that both of
these ligand binding sites show positive costimulatory responses through the CD28 and the
competitive inhibition of these two molecules is very crucial in order to attenuate T-cell
activation in an effective manner (Marthey et al. 2016). The rapid binding mechanism of CTLA-
4 and CD28 with the B7-1 causes the rapid competitive inhibition by the CTLA-4. Moreover, the
upregulation of CTLA-4 in comparison with the T-cell activation causes the more activation of
CTLA-4 and that contains intracellular vesicles which are rapidly trafficked to the immunologic
synapses. Therefore, the number of CTLA-4 trafficked to immunogenic synapses is correlated
with the strength of the TCR signaling. After the trafficking of CTLA-4 to the immunogenic
synapses, the CTLA-4 is stabilized by the binding of B7 ligand. By busing this mechanism,
CTLA4 causes positive correlation with the help of CD28 and a result the downstream of CD28
signaling process is limited and this process is mediated by the AKT and PI3K. This in turn
causes robust regulation of the T cell activity through the reduction of TCR signaling amplitude
(Carosella et al. 2015). It is observed that negative costimulation of the CTLA-4 is also
associated with the down regulation of the expression of B7 ligands and CD28 co-signaling
pathway. However, the CTLA-4 primarily controls the activity of the T-cells at the region of T-
cell priming site. Due to the primary role in T cell activation, negative costimulation of the
CTLA-4 can be very critical to tolerate. Moreover the cell-intrinsic functions are also involvedin
The function and expression pof CTLA-4 is directly linked with the activation of the T-cell.
After the activation of the T-cell receptor, the CTLA-4 is upregulated. However, CTLA-4 lowers
TCR signaling mechanism through the competitive stimulation pathway of CD28. In the CD28
the ligand binding part that is B7, in CD80 the ligand binding part is B7-1 and the B7-2 in the
CD86 and for these ligand binding sites, CTLA-4 has higher affinity and avidity ( Fig-1). The
main reason behind this enhanced affinity towards these two ligand binding sites are that both of
these ligand binding sites show positive costimulatory responses through the CD28 and the
competitive inhibition of these two molecules is very crucial in order to attenuate T-cell
activation in an effective manner (Marthey et al. 2016). The rapid binding mechanism of CTLA-
4 and CD28 with the B7-1 causes the rapid competitive inhibition by the CTLA-4. Moreover, the
upregulation of CTLA-4 in comparison with the T-cell activation causes the more activation of
CTLA-4 and that contains intracellular vesicles which are rapidly trafficked to the immunologic
synapses. Therefore, the number of CTLA-4 trafficked to immunogenic synapses is correlated
with the strength of the TCR signaling. After the trafficking of CTLA-4 to the immunogenic
synapses, the CTLA-4 is stabilized by the binding of B7 ligand. By busing this mechanism,
CTLA4 causes positive correlation with the help of CD28 and a result the downstream of CD28
signaling process is limited and this process is mediated by the AKT and PI3K. This in turn
causes robust regulation of the T cell activity through the reduction of TCR signaling amplitude
(Carosella et al. 2015). It is observed that negative costimulation of the CTLA-4 is also
associated with the down regulation of the expression of B7 ligands and CD28 co-signaling
pathway. However, the CTLA-4 primarily controls the activity of the T-cells at the region of T-
cell priming site. Due to the primary role in T cell activation, negative costimulation of the
CTLA-4 can be very critical to tolerate. Moreover the cell-intrinsic functions are also involvedin

5BIOLOGICAL THERAPY
the attenuation of the T-cell activation by CTLA-4. Often it is observed that, the CTLA-4
competent T-cells are capable of preventing the lymphoproliferation of the and most of the
suppressive function of the CTLA-4 is mediated by the Tregs. Moreover, the CTLA-4 depletion
in the Tregs can induce the autoimmunity and aberrant T-cell activation. So it can be sad that in
order to maintain the immunogenic tolerance in the body, it is very necessary to maintain the
activity of CTLA-4. Hence, the CTLA-4 expressed by the Tregs can cause T-cell activation by
the cell-extrinsic manner and simultaneously, the availability of the B7-1 and B7-2 ligands are
reduced and as result the CD28 mediated costimulation of the T –cells are reduced (Wei, Duffy
and Allison 2018). However, it is also reported that, the CTLA-4 can also reduce the overall
availability of the B7 ligands by the transendocytosis mechanism. In recent studies it was found
that the conditional deletion of CTLA4 in the Tregs is enough for conferring resistance to the
EAE and it is indicating towards the fact that enhanced expression of Treg CTLA-4 can prevent
the autoimmunity in cancer. The Treg deletion may cause reduction of CTLA-4 induced Treg
cells and it will increase the efficacy of anti-CTLA-4 therapy. Tregs are selected as it has higher
affinity for the TCR on the MHC complexes and it is also associated with the signaling strengths
of the TCR activation (De Velasco et al. 2017). The strong TCR signaling will cause the more
activation of T-cells. The CLTA-4 blocking leads to enhancement of tumor neoantigen specific
CD8 cells in the tumor micro environment and it will cause the deletion of tumor in the
microenvironment.The CTLA-4 blocking will cause adecrease the autoimmunity. The blocking
of the CTLA-4 will cause more activation of the T-Cells and the blockade of this CTLA-4 will
induce the rejection of tumor in the body. The anti-tumor immunity is induced in the body by the
increased blockade of the CTLA4 and it is mediated through the enhancement of the particular
T-cell populations (Wei, Duffy and Allison 2018).
the attenuation of the T-cell activation by CTLA-4. Often it is observed that, the CTLA-4
competent T-cells are capable of preventing the lymphoproliferation of the and most of the
suppressive function of the CTLA-4 is mediated by the Tregs. Moreover, the CTLA-4 depletion
in the Tregs can induce the autoimmunity and aberrant T-cell activation. So it can be sad that in
order to maintain the immunogenic tolerance in the body, it is very necessary to maintain the
activity of CTLA-4. Hence, the CTLA-4 expressed by the Tregs can cause T-cell activation by
the cell-extrinsic manner and simultaneously, the availability of the B7-1 and B7-2 ligands are
reduced and as result the CD28 mediated costimulation of the T –cells are reduced (Wei, Duffy
and Allison 2018). However, it is also reported that, the CTLA-4 can also reduce the overall
availability of the B7 ligands by the transendocytosis mechanism. In recent studies it was found
that the conditional deletion of CTLA4 in the Tregs is enough for conferring resistance to the
EAE and it is indicating towards the fact that enhanced expression of Treg CTLA-4 can prevent
the autoimmunity in cancer. The Treg deletion may cause reduction of CTLA-4 induced Treg
cells and it will increase the efficacy of anti-CTLA-4 therapy. Tregs are selected as it has higher
affinity for the TCR on the MHC complexes and it is also associated with the signaling strengths
of the TCR activation (De Velasco et al. 2017). The strong TCR signaling will cause the more
activation of T-cells. The CLTA-4 blocking leads to enhancement of tumor neoantigen specific
CD8 cells in the tumor micro environment and it will cause the deletion of tumor in the
microenvironment.The CTLA-4 blocking will cause adecrease the autoimmunity. The blocking
of the CTLA-4 will cause more activation of the T-Cells and the blockade of this CTLA-4 will
induce the rejection of tumor in the body. The anti-tumor immunity is induced in the body by the
increased blockade of the CTLA4 and it is mediated through the enhancement of the particular
T-cell populations (Wei, Duffy and Allison 2018).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
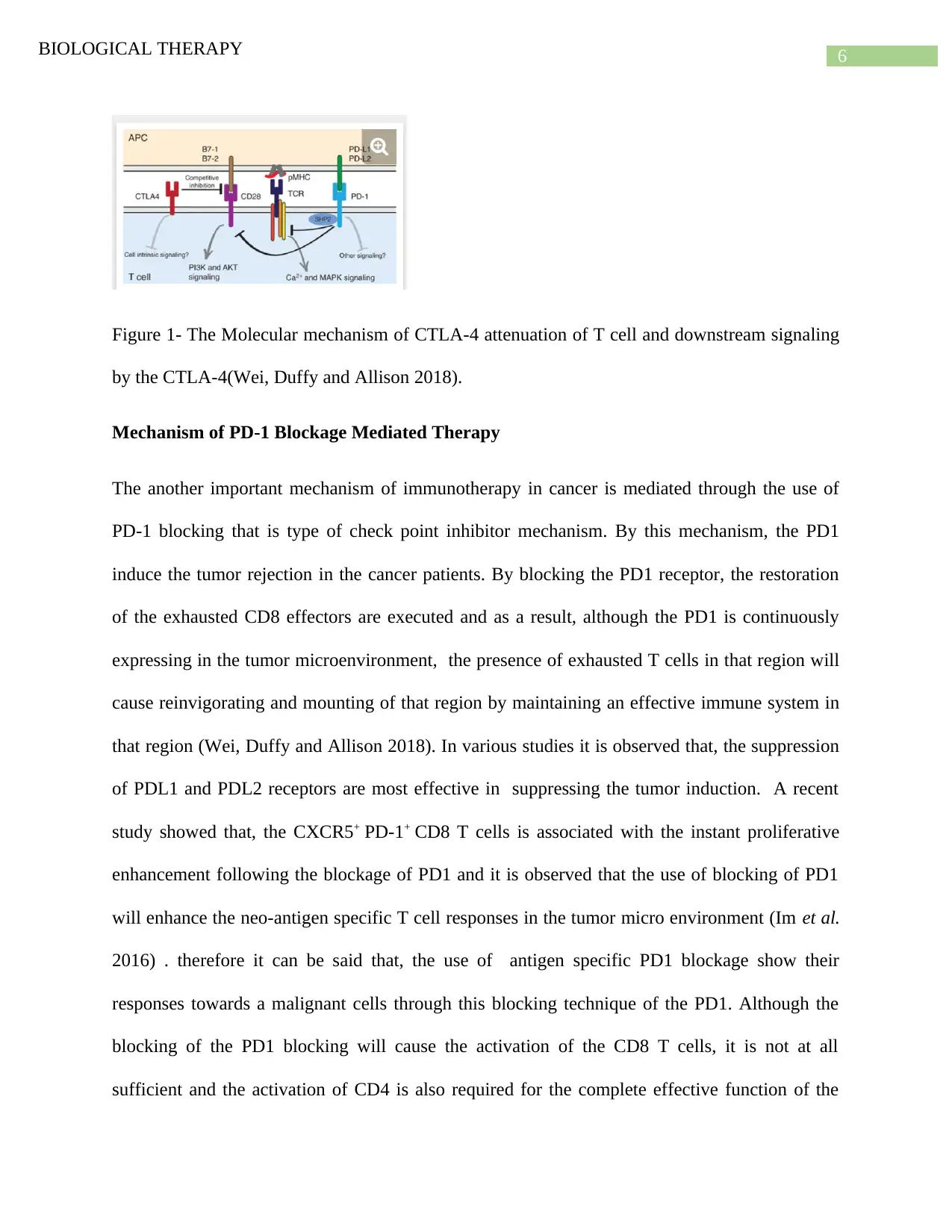
6BIOLOGICAL THERAPY
Figure 1- The Molecular mechanism of CTLA-4 attenuation of T cell and downstream signaling
by the CTLA-4(Wei, Duffy and Allison 2018).
Mechanism of PD-1 Blockage Mediated Therapy
The another important mechanism of immunotherapy in cancer is mediated through the use of
PD-1 blocking that is type of check point inhibitor mechanism. By this mechanism, the PD1
induce the tumor rejection in the cancer patients. By blocking the PD1 receptor, the restoration
of the exhausted CD8 effectors are executed and as a result, although the PD1 is continuously
expressing in the tumor microenvironment, the presence of exhausted T cells in that region will
cause reinvigorating and mounting of that region by maintaining an effective immune system in
that region (Wei, Duffy and Allison 2018). In various studies it is observed that, the suppression
of PDL1 and PDL2 receptors are most effective in suppressing the tumor induction. A recent
study showed that, the CXCR5+ PD-1+ CD8 T cells is associated with the instant proliferative
enhancement following the blockage of PD1 and it is observed that the use of blocking of PD1
will enhance the neo-antigen specific T cell responses in the tumor micro environment (Im et al.
2016) . therefore it can be said that, the use of antigen specific PD1 blockage show their
responses towards a malignant cells through this blocking technique of the PD1. Although the
blocking of the PD1 blocking will cause the activation of the CD8 T cells, it is not at all
sufficient and the activation of CD4 is also required for the complete effective function of the
Figure 1- The Molecular mechanism of CTLA-4 attenuation of T cell and downstream signaling
by the CTLA-4(Wei, Duffy and Allison 2018).
Mechanism of PD-1 Blockage Mediated Therapy
The another important mechanism of immunotherapy in cancer is mediated through the use of
PD-1 blocking that is type of check point inhibitor mechanism. By this mechanism, the PD1
induce the tumor rejection in the cancer patients. By blocking the PD1 receptor, the restoration
of the exhausted CD8 effectors are executed and as a result, although the PD1 is continuously
expressing in the tumor microenvironment, the presence of exhausted T cells in that region will
cause reinvigorating and mounting of that region by maintaining an effective immune system in
that region (Wei, Duffy and Allison 2018). In various studies it is observed that, the suppression
of PDL1 and PDL2 receptors are most effective in suppressing the tumor induction. A recent
study showed that, the CXCR5+ PD-1+ CD8 T cells is associated with the instant proliferative
enhancement following the blockage of PD1 and it is observed that the use of blocking of PD1
will enhance the neo-antigen specific T cell responses in the tumor micro environment (Im et al.
2016) . therefore it can be said that, the use of antigen specific PD1 blockage show their
responses towards a malignant cells through this blocking technique of the PD1. Although the
blocking of the PD1 blocking will cause the activation of the CD8 T cells, it is not at all
sufficient and the activation of CD4 is also required for the complete effective function of the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7BIOLOGICAL THERAPY
PD1 blocking. However, it is not completely understood about the fact how the activation of
CD4 is related with this mechanism (Iwai et al. 2017). Hence it is assumed that, the CD helper T
cells are associated with the formation of immune memory and this may also enhance the
antitumor activity by enhancing the entry of CD8 T cells into the peripheral tissues of the tumor
micro environment sites (Granier et al. 2017). As supportive fact of PD1 blockage effectiveness
it can be highlighted that, the anti- PD1 therapy has become successful in suppressing the tumor
and it is observed that, the use of PD1 blocking as a part of the immunotherapy will cause
activation of additional mechanisms that has immense role in inducing the therapeutic activity in
the treatment of cancer (Ohaegbulam et al. 2015). Along with this, the blocking of PD1 will
cause activation of antibodies that are directly targeting the PDL1 and as a result the induction of
immune tumor rejection occur. Th1 cytokines are associated with the induction of PDL1,
whereas the PDL2 is induced by the Th2 cytokines. It is observed that PDL1 blockade will cause
skewed response of Th1 and that condition is required for the antitumor immune responses. So, it
can be said that, the PD-1 blocking will promote the T-cell mediated tumor cell killing through
the cell non autonomous and autonomous mechanism(Wei, Duffy and Allison 2018). The
mechanism is shown in the figure.2.
PD1 blocking. However, it is not completely understood about the fact how the activation of
CD4 is related with this mechanism (Iwai et al. 2017). Hence it is assumed that, the CD helper T
cells are associated with the formation of immune memory and this may also enhance the
antitumor activity by enhancing the entry of CD8 T cells into the peripheral tissues of the tumor
micro environment sites (Granier et al. 2017). As supportive fact of PD1 blockage effectiveness
it can be highlighted that, the anti- PD1 therapy has become successful in suppressing the tumor
and it is observed that, the use of PD1 blocking as a part of the immunotherapy will cause
activation of additional mechanisms that has immense role in inducing the therapeutic activity in
the treatment of cancer (Ohaegbulam et al. 2015). Along with this, the blocking of PD1 will
cause activation of antibodies that are directly targeting the PDL1 and as a result the induction of
immune tumor rejection occur. Th1 cytokines are associated with the induction of PDL1,
whereas the PDL2 is induced by the Th2 cytokines. It is observed that PDL1 blockade will cause
skewed response of Th1 and that condition is required for the antitumor immune responses. So, it
can be said that, the PD-1 blocking will promote the T-cell mediated tumor cell killing through
the cell non autonomous and autonomous mechanism(Wei, Duffy and Allison 2018). The
mechanism is shown in the figure.2.
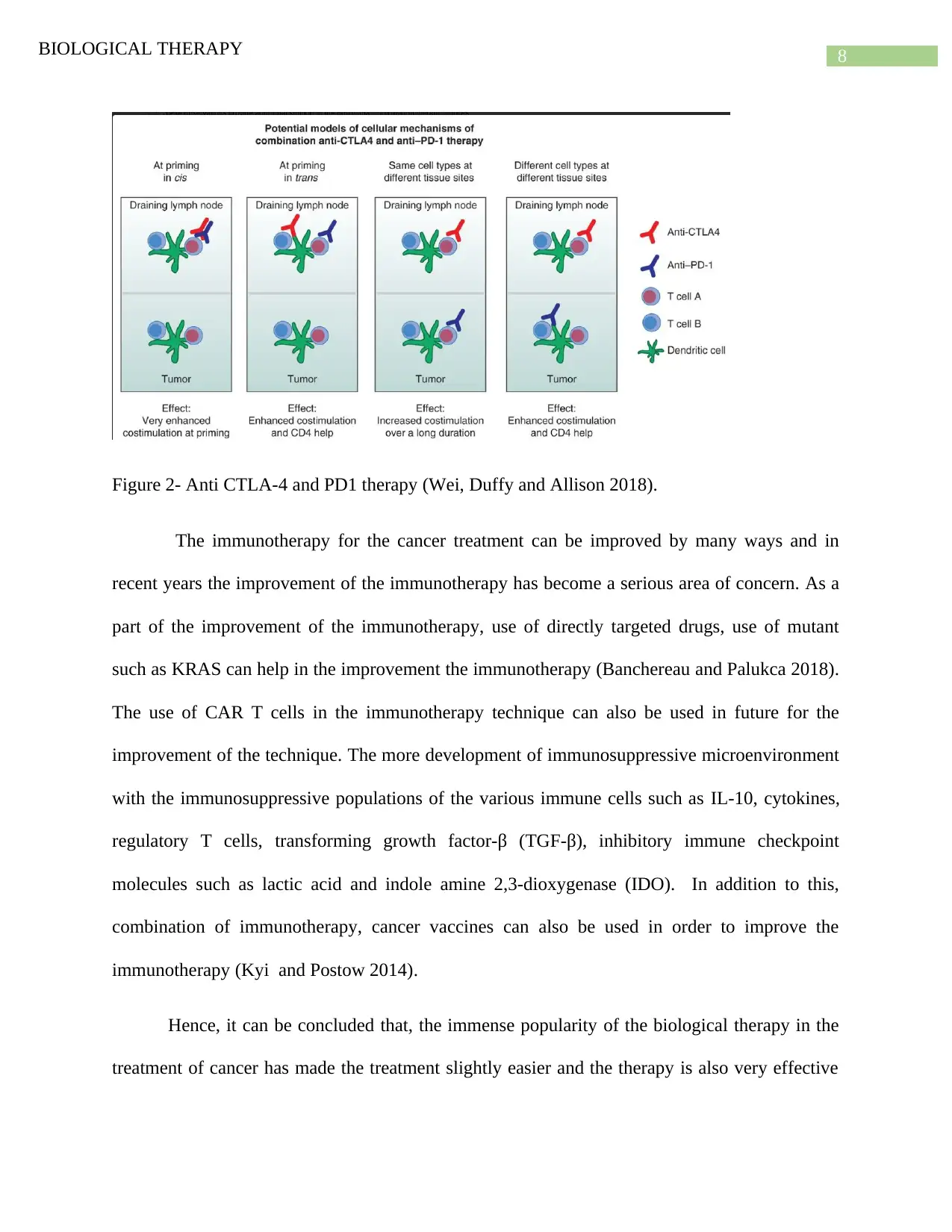
8BIOLOGICAL THERAPY
Figure 2- Anti CTLA-4 and PD1 therapy (Wei, Duffy and Allison 2018).
The immunotherapy for the cancer treatment can be improved by many ways and in
recent years the improvement of the immunotherapy has become a serious area of concern. As a
part of the improvement of the immunotherapy, use of directly targeted drugs, use of mutant
such as KRAS can help in the improvement the immunotherapy (Banchereau and Palukca 2018).
The use of CAR T cells in the immunotherapy technique can also be used in future for the
improvement of the technique. The more development of immunosuppressive microenvironment
with the immunosuppressive populations of the various immune cells such as IL-10, cytokines,
regulatory T cells, transforming growth factor-β (TGF-β), inhibitory immune checkpoint
molecules such as lactic acid and indole amine 2,3-dioxygenase (IDO). In addition to this,
combination of immunotherapy, cancer vaccines can also be used in order to improve the
immunotherapy (Kyi and Postow 2014).
Hence, it can be concluded that, the immense popularity of the biological therapy in the
treatment of cancer has made the treatment slightly easier and the therapy is also very effective
Figure 2- Anti CTLA-4 and PD1 therapy (Wei, Duffy and Allison 2018).
The immunotherapy for the cancer treatment can be improved by many ways and in
recent years the improvement of the immunotherapy has become a serious area of concern. As a
part of the improvement of the immunotherapy, use of directly targeted drugs, use of mutant
such as KRAS can help in the improvement the immunotherapy (Banchereau and Palukca 2018).
The use of CAR T cells in the immunotherapy technique can also be used in future for the
improvement of the technique. The more development of immunosuppressive microenvironment
with the immunosuppressive populations of the various immune cells such as IL-10, cytokines,
regulatory T cells, transforming growth factor-β (TGF-β), inhibitory immune checkpoint
molecules such as lactic acid and indole amine 2,3-dioxygenase (IDO). In addition to this,
combination of immunotherapy, cancer vaccines can also be used in order to improve the
immunotherapy (Kyi and Postow 2014).
Hence, it can be concluded that, the immense popularity of the biological therapy in the
treatment of cancer has made the treatment slightly easier and the therapy is also very effective
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9BIOLOGICAL THERAPY
in nature. Immunotherapy is one of the most popular biological therapy technique.
Immunotherapy in recent time has introduced a new era of cancer treatment. However, the use of
two techniques that is CTLA-4 and PD1 results in an immense success in reducing the death rate
of the disease. In various studies, it was observed that, the use of the immune blocking therapy
in the treatment of cancer has faced immense success rate among the patients. However, there
are various other therapies, but the immune blocking therapy is much more effective in nature.
Although the check point blocking strategy in the immune blocking system is very effective,
there are various areas in this techniques that can be improved and a few strategies has also been
identified in order to make the therapy more effective in nature.
in nature. Immunotherapy is one of the most popular biological therapy technique.
Immunotherapy in recent time has introduced a new era of cancer treatment. However, the use of
two techniques that is CTLA-4 and PD1 results in an immense success in reducing the death rate
of the disease. In various studies, it was observed that, the use of the immune blocking therapy
in the treatment of cancer has faced immense success rate among the patients. However, there
are various other therapies, but the immune blocking therapy is much more effective in nature.
Although the check point blocking strategy in the immune blocking system is very effective,
there are various areas in this techniques that can be improved and a few strategies has also been
identified in order to make the therapy more effective in nature.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10BIOLOGICAL THERAPY
References
Banchereau, J. and Palucka, K., 2018. Immunotherapy: Cancer vaccines on the move. Nature
reviews Clinical oncology, 15(1), p.9.
Baumeister, S.H., Freeman, G.J., Dranoff, G. and Sharpe, A.H., 2016. Coinhibitory pathways in
immunotherapy for cancer. Annual review of immunology, 34, pp.539-573.
Carosella, E.D., Ploussard, G., LeMaoult, J. and Desgrandchamps, F., 2015. A systematic review
of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and
HLA-G. European urology, 68(2), pp.267-279.
Chen, L. and Han, X., 2015. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and
future. The Journal of clinical investigation, 125(9), pp.3384-3391.
De Velasco, G., Je, Y., Bossé, D., Awad, M.M., Ott, P.A., Moreira, R.B., Schutz, F., Bellmunt,
J., Sonpavde, G.P., Hodi, F.S. and Choueiri, T.K., 2017. Comprehensive meta-analysis of key
immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer
patients. Cancer immunology research.
Farkona, S., Diamandis, E.P. and Blasutig, I.M., 2016. Cancer immunotherapy: the beginning of
the end of cancer?. BMC medicine, 14(1), p.73.
Gotwals, P., Cameron, S., Cipolletta, D., Cremasco, V., Crystal, A., Hewes, B., Mueller, B.,
Quaratino, S., Sabatos-Peyton, C., Petruzzelli, L. and Engelman, J.A., 2017. Prospects for
combining targeted and conventional cancer therapy with immunotherapy. Nature Reviews
Cancer, 17(5), p.286.
References
Banchereau, J. and Palucka, K., 2018. Immunotherapy: Cancer vaccines on the move. Nature
reviews Clinical oncology, 15(1), p.9.
Baumeister, S.H., Freeman, G.J., Dranoff, G. and Sharpe, A.H., 2016. Coinhibitory pathways in
immunotherapy for cancer. Annual review of immunology, 34, pp.539-573.
Carosella, E.D., Ploussard, G., LeMaoult, J. and Desgrandchamps, F., 2015. A systematic review
of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and
HLA-G. European urology, 68(2), pp.267-279.
Chen, L. and Han, X., 2015. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and
future. The Journal of clinical investigation, 125(9), pp.3384-3391.
De Velasco, G., Je, Y., Bossé, D., Awad, M.M., Ott, P.A., Moreira, R.B., Schutz, F., Bellmunt,
J., Sonpavde, G.P., Hodi, F.S. and Choueiri, T.K., 2017. Comprehensive meta-analysis of key
immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer
patients. Cancer immunology research.
Farkona, S., Diamandis, E.P. and Blasutig, I.M., 2016. Cancer immunotherapy: the beginning of
the end of cancer?. BMC medicine, 14(1), p.73.
Gotwals, P., Cameron, S., Cipolletta, D., Cremasco, V., Crystal, A., Hewes, B., Mueller, B.,
Quaratino, S., Sabatos-Peyton, C., Petruzzelli, L. and Engelman, J.A., 2017. Prospects for
combining targeted and conventional cancer therapy with immunotherapy. Nature Reviews
Cancer, 17(5), p.286.

11BIOLOGICAL THERAPY
Granier, C., De Guillebon, E., Blanc, C., Roussel, H., Badoual, C., Colin, E., Saldmann, A., Gey,
A., Oudard, S. and Tartour, E., 2017. Mechanisms of action and rationale for the use of
checkpoint inhibitors in cancer. ESMO open, 2(2), p.e000213.
Hersh, E.M., 2016. Current status of immunotherapy and biological therapy of cancer. Advances
in Immunopharmacology, 3, p.47.
Im, S.J., Hashimoto, M., Gerner, M.Y., Lee, J., Kissick, H.T., Burger, M.C., Shan, Q., Hale, J.S.,
Lee, J., Nasti, T.H. and Sharpe, A.H., 2016. Defining CD8+ T cells that provide the proliferative
burst after PD-1 therapy. Nature, 537(7620), p.417.
Iwai, Y., Hamanishi, J., Chamoto, K. and Honjo, T., 2017. Cancer immunotherapies targeting the
PD-1 signaling pathway. Journal of biomedical science, 24(1), p.26.
Kim, J., 2016. Immune checkpoint blockade therapy for bladder cancer treatment. Investigative
and clinical urology, 57(Suppl 1), pp.S98-S105.
Kyi, C. and Postow, M.A., 2014. Checkpoint blocking antibodies in cancer
immunotherapy. FEBS letters, 588(2), pp.368-376.
Li, D., Ma, Y., Du, J., Tao, W., Du, X., Yang, X. and Wang, J., 2017. Tumor acidity/NIR
controlled interaction of transformable nanoparticle with biological systems for cancer
therapy. Nano letters, 17(5), pp.2871-2878.
Marthey, L., Mateus, C., Mussini, C., Nachury, M., Nancey, S., Grange, F., Zallot, C., Peyrin-
Biroulet, L., Rahier, J.F., Bourdier de Beauregard, M. and Mortier, L., 2016. Cancer
immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel
disease. Journal of Crohn's and Colitis, 10(4), pp.395-401.
Granier, C., De Guillebon, E., Blanc, C., Roussel, H., Badoual, C., Colin, E., Saldmann, A., Gey,
A., Oudard, S. and Tartour, E., 2017. Mechanisms of action and rationale for the use of
checkpoint inhibitors in cancer. ESMO open, 2(2), p.e000213.
Hersh, E.M., 2016. Current status of immunotherapy and biological therapy of cancer. Advances
in Immunopharmacology, 3, p.47.
Im, S.J., Hashimoto, M., Gerner, M.Y., Lee, J., Kissick, H.T., Burger, M.C., Shan, Q., Hale, J.S.,
Lee, J., Nasti, T.H. and Sharpe, A.H., 2016. Defining CD8+ T cells that provide the proliferative
burst after PD-1 therapy. Nature, 537(7620), p.417.
Iwai, Y., Hamanishi, J., Chamoto, K. and Honjo, T., 2017. Cancer immunotherapies targeting the
PD-1 signaling pathway. Journal of biomedical science, 24(1), p.26.
Kim, J., 2016. Immune checkpoint blockade therapy for bladder cancer treatment. Investigative
and clinical urology, 57(Suppl 1), pp.S98-S105.
Kyi, C. and Postow, M.A., 2014. Checkpoint blocking antibodies in cancer
immunotherapy. FEBS letters, 588(2), pp.368-376.
Li, D., Ma, Y., Du, J., Tao, W., Du, X., Yang, X. and Wang, J., 2017. Tumor acidity/NIR
controlled interaction of transformable nanoparticle with biological systems for cancer
therapy. Nano letters, 17(5), pp.2871-2878.
Marthey, L., Mateus, C., Mussini, C., Nachury, M., Nancey, S., Grange, F., Zallot, C., Peyrin-
Biroulet, L., Rahier, J.F., Bourdier de Beauregard, M. and Mortier, L., 2016. Cancer
immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel
disease. Journal of Crohn's and Colitis, 10(4), pp.395-401.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 15
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





