Bleeding, Anticoagulation and the Cascade Pathway
VerifiedAdded on 2023/04/24
|20
|6127
|409
AI Summary
This paper provides a comprehensive overview of the physiological process of bleeding, the coagulation system, the cascade pathway, and a detailed discussion on the different types of anticoagulants, with a focus on the VKAs.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
Bleeding, anticoagulation and the cascade pathway
Name of the student:
Name of the university:
Author note:
Bleeding, anticoagulation and the cascade pathway
Name of the student:
Name of the university:
Author note:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
Table of Contents
Introduction:...............................................................................................................................2
The mechanism of bleeding:......................................................................................................2
Overview of the coagulation system:.........................................................................................3
The cascade pathway:................................................................................................................5
Anti-coagulative Drugs and types:.............................................................................................7
Vitamin K antagonists:...........................................................................................................7
Non-VKA Oral Anticoagulants (NOACs):..........................................................................11
Other anticoagulants:...........................................................................................................13
Conclusion:..............................................................................................................................15
References:...............................................................................................................................16
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
Table of Contents
Introduction:...............................................................................................................................2
The mechanism of bleeding:......................................................................................................2
Overview of the coagulation system:.........................................................................................3
The cascade pathway:................................................................................................................5
Anti-coagulative Drugs and types:.............................................................................................7
Vitamin K antagonists:...........................................................................................................7
Non-VKA Oral Anticoagulants (NOACs):..........................................................................11
Other anticoagulants:...........................................................................................................13
Conclusion:..............................................................................................................................15
References:...............................................................................................................................16

2
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
Introduction:
Bleeding and coagulation is one of the various different metabolic procedures of
human body, and alike the other human metabolic processes, this particular procedure is also
critically associated with an intricate genetic network. Elaborating more on the process of
bleeding, it is also known as haemorrhage, a process in which there is loss of blood from the
circulatory system. Bleeding or haemorrhage can occur facilitated by many causes, ranging
from small cuts, bruises and abrasions, to grave injuries and amputation (1). Bleeding is a
normal physiological procedure in human body which is immediately followed by the process
of coagulation. Coagulation on the other hand is the process in which the blood clot is formed
at the site of bleeding. This phase is also known as the secondary hemostasis, as is forms the
second stage of the process of arresting blood from any ruptured vessel. There are various
factors that can complicate of blood clot formation in the human body, which in turn leads to
a complicated and difficult to treat complications, further complicating the recovery
procedure (2). This paper will discuss in detail the physiological process of bleeding in the
human body, overview of the coagulation system, the cascade pathway, and a thorough and
detailed discussion on the Anticoagulative Drugs and types, such as Vitamin K antagonists
(warfarin, coumarin), Non-VKA Oral Anticoagulants (NOACs), aspirin, heparins etc.
The mechanism of bleeding:
Discussing the physiological mechanism associated with bleeding, there is an intricate
genetic network that guides and directs the process. Bleeding or haemorrhage, can be internal
or external and can be mediated or facilitated via a variety of reasons. The blood loss can
occur from any part of the human body; in case of internal bleeding, the blood is leaked or
released through a damaged blood vessel or organ. And on the other hand, the external
bleeding which is facilitated by a break or tear in the skin, through which the blood is
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
Introduction:
Bleeding and coagulation is one of the various different metabolic procedures of
human body, and alike the other human metabolic processes, this particular procedure is also
critically associated with an intricate genetic network. Elaborating more on the process of
bleeding, it is also known as haemorrhage, a process in which there is loss of blood from the
circulatory system. Bleeding or haemorrhage can occur facilitated by many causes, ranging
from small cuts, bruises and abrasions, to grave injuries and amputation (1). Bleeding is a
normal physiological procedure in human body which is immediately followed by the process
of coagulation. Coagulation on the other hand is the process in which the blood clot is formed
at the site of bleeding. This phase is also known as the secondary hemostasis, as is forms the
second stage of the process of arresting blood from any ruptured vessel. There are various
factors that can complicate of blood clot formation in the human body, which in turn leads to
a complicated and difficult to treat complications, further complicating the recovery
procedure (2). This paper will discuss in detail the physiological process of bleeding in the
human body, overview of the coagulation system, the cascade pathway, and a thorough and
detailed discussion on the Anticoagulative Drugs and types, such as Vitamin K antagonists
(warfarin, coumarin), Non-VKA Oral Anticoagulants (NOACs), aspirin, heparins etc.
The mechanism of bleeding:
Discussing the physiological mechanism associated with bleeding, there is an intricate
genetic network that guides and directs the process. Bleeding or haemorrhage, can be internal
or external and can be mediated or facilitated via a variety of reasons. The blood loss can
occur from any part of the human body; in case of internal bleeding, the blood is leaked or
released through a damaged blood vessel or organ. And on the other hand, the external
bleeding which is facilitated by a break or tear in the skin, through which the blood is

3
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
released or lost. An important contrast between the two mechanisms of bleeding is the fact
that in case of internal bleeding the blood is released inside the body in the internal cavities
and in case of external bleeding the blood is being lost outside the body (1). A very common
type of bleeding is known as the traumatic bleeding, which is directly caused by abrasions
that do not involve penetration below the skin, hematoma, lacerations or incisions, puncture
wounds that are generated from sharp objects such as needles or knives, crushing injuries,
and lastly, gunshot wounds. The medical conditions also are noted to cause bleeding in the
human body due to conditions such as haemophilia, leukemia, liver disorders, menorrhagia
which in turn causes uncontrolled or prolonged menstrual bleeding, thrombocytopenia which
in turn causes low blood platelet count, vitamin K deficiency, brain, trauma, bowel
obstruction, congestive heart failure (CHF), lung cancer, acute bronchitis, severe
hypothermia, and endometriosis (3).
Overview of the coagulation system:
Coagulation is the immediate immune response of the body to the process of bleeding,
and is mediated by the first and second line of immune defence of the body. The procedure is
maintained or controlled by the platelet mediated primary haemostasis. The process of the
coagulation is facilitated by the formation of the platelet plug, which in turn marks the initial
occlusion of the vascular lesion, the contributing factor to the bleeding (4). This series of
actions are closely riveted by the activation of the coagulation system, facilitated in response
to the rupture of the endothelium and the exposure of blood to the extravascular tissues of the
body. Elaborating more on the procedure of the primary haemostasis, the events are triggered
by the damaged vascular wall which is facilitated when the sub-endothelial tissues are
exposed to the blood after the injury. This phenomenon is followed by a multitude of
coordinated interactions occurring amongst the different tissue components, plasma proteins,
and receptors on platelets which in turn facilitates the process of initial sealing of the
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
released or lost. An important contrast between the two mechanisms of bleeding is the fact
that in case of internal bleeding the blood is released inside the body in the internal cavities
and in case of external bleeding the blood is being lost outside the body (1). A very common
type of bleeding is known as the traumatic bleeding, which is directly caused by abrasions
that do not involve penetration below the skin, hematoma, lacerations or incisions, puncture
wounds that are generated from sharp objects such as needles or knives, crushing injuries,
and lastly, gunshot wounds. The medical conditions also are noted to cause bleeding in the
human body due to conditions such as haemophilia, leukemia, liver disorders, menorrhagia
which in turn causes uncontrolled or prolonged menstrual bleeding, thrombocytopenia which
in turn causes low blood platelet count, vitamin K deficiency, brain, trauma, bowel
obstruction, congestive heart failure (CHF), lung cancer, acute bronchitis, severe
hypothermia, and endometriosis (3).
Overview of the coagulation system:
Coagulation is the immediate immune response of the body to the process of bleeding,
and is mediated by the first and second line of immune defence of the body. The procedure is
maintained or controlled by the platelet mediated primary haemostasis. The process of the
coagulation is facilitated by the formation of the platelet plug, which in turn marks the initial
occlusion of the vascular lesion, the contributing factor to the bleeding (4). This series of
actions are closely riveted by the activation of the coagulation system, facilitated in response
to the rupture of the endothelium and the exposure of blood to the extravascular tissues of the
body. Elaborating more on the procedure of the primary haemostasis, the events are triggered
by the damaged vascular wall which is facilitated when the sub-endothelial tissues are
exposed to the blood after the injury. This phenomenon is followed by a multitude of
coordinated interactions occurring amongst the different tissue components, plasma proteins,
and receptors on platelets which in turn facilitates the process of initial sealing of the
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

4
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
damaged area after the injury. In order to complete this procedure, the platelets undergo a
series of reactions, such as adhesion, aggregation, release of granule content, and
morphological changes. This results in the formation of the platelet plug (5).
The platelet plug formation is directly dependent on the interaction that occurs
between the platelets and von Willebrand factor (VWF). The von Willebrand factor (VWF) is
a complex plasma protein which is secreted into the blood post the body’s response to the
bleeding. This protein is composed of multiple disulphide-linked subunits and is less than 20
million Da in mass and 4 lm in length. This particular factor undergoes a proteolytic
processing while in the plasma, which is mediated by the ADAMTS 13, a metalloprotease.
This phenomenon results in the generation of VWF multimers of all sizes and with different
functional efficiency. These different sizes of VWF then perform platelet adhesion, by
serving as a bridge between the tissue and the platelets, binding both to collagen exposed at
sites of vascular injury and to the platelet membrane glycoprotein Ib-V-IX Journal of Internal
Medicine 2005; 257: 209–223 2005 Blackwell Publishing Ltd 209 (GPIb-V-IX). The larger
multimers of the VWF is more efficient in performing platelet adhesion than the smaller
ones, and the process of the adhesion of the platelet adhesion is reported to function better
under the shearing stress as well (6).
High shear unfolds the VWF thus exposing the binding sites for GPIb-V-IX.
Successful platelet adhesion is closely followed by major morphological changes of the
platelets with rearrangement of the membrane and exposure of negatively charged
phospholipids. Concomitant production and secretion of thromboxane A2 along with the
release of ADP, calcium, and serotonin from the platelet granules facilitates the process of
activation of additional platelets and as a result the smooth muscle cells of the vessel wall
contract (5). An inside-out signal facilitate the conformational change in the platelet integrin
glycoprotein IIb-IIIa (GPIIb-IIIa) and adhesive proteins such as the fibrinogen, VWF,
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
damaged area after the injury. In order to complete this procedure, the platelets undergo a
series of reactions, such as adhesion, aggregation, release of granule content, and
morphological changes. This results in the formation of the platelet plug (5).
The platelet plug formation is directly dependent on the interaction that occurs
between the platelets and von Willebrand factor (VWF). The von Willebrand factor (VWF) is
a complex plasma protein which is secreted into the blood post the body’s response to the
bleeding. This protein is composed of multiple disulphide-linked subunits and is less than 20
million Da in mass and 4 lm in length. This particular factor undergoes a proteolytic
processing while in the plasma, which is mediated by the ADAMTS 13, a metalloprotease.
This phenomenon results in the generation of VWF multimers of all sizes and with different
functional efficiency. These different sizes of VWF then perform platelet adhesion, by
serving as a bridge between the tissue and the platelets, binding both to collagen exposed at
sites of vascular injury and to the platelet membrane glycoprotein Ib-V-IX Journal of Internal
Medicine 2005; 257: 209–223 2005 Blackwell Publishing Ltd 209 (GPIb-V-IX). The larger
multimers of the VWF is more efficient in performing platelet adhesion than the smaller
ones, and the process of the adhesion of the platelet adhesion is reported to function better
under the shearing stress as well (6).
High shear unfolds the VWF thus exposing the binding sites for GPIb-V-IX.
Successful platelet adhesion is closely followed by major morphological changes of the
platelets with rearrangement of the membrane and exposure of negatively charged
phospholipids. Concomitant production and secretion of thromboxane A2 along with the
release of ADP, calcium, and serotonin from the platelet granules facilitates the process of
activation of additional platelets and as a result the smooth muscle cells of the vessel wall
contract (5). An inside-out signal facilitate the conformational change in the platelet integrin
glycoprotein IIb-IIIa (GPIIb-IIIa) and adhesive proteins such as the fibrinogen, VWF,

5
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
fibronectin, and thrombospondin binding sites are exposed. As a result the bridge between the
platelets are formed and the platelet aggregate is developed and this triggers the blood
coagulation cascade leading to the formation of thrombin and fibrin net (7).
(8)
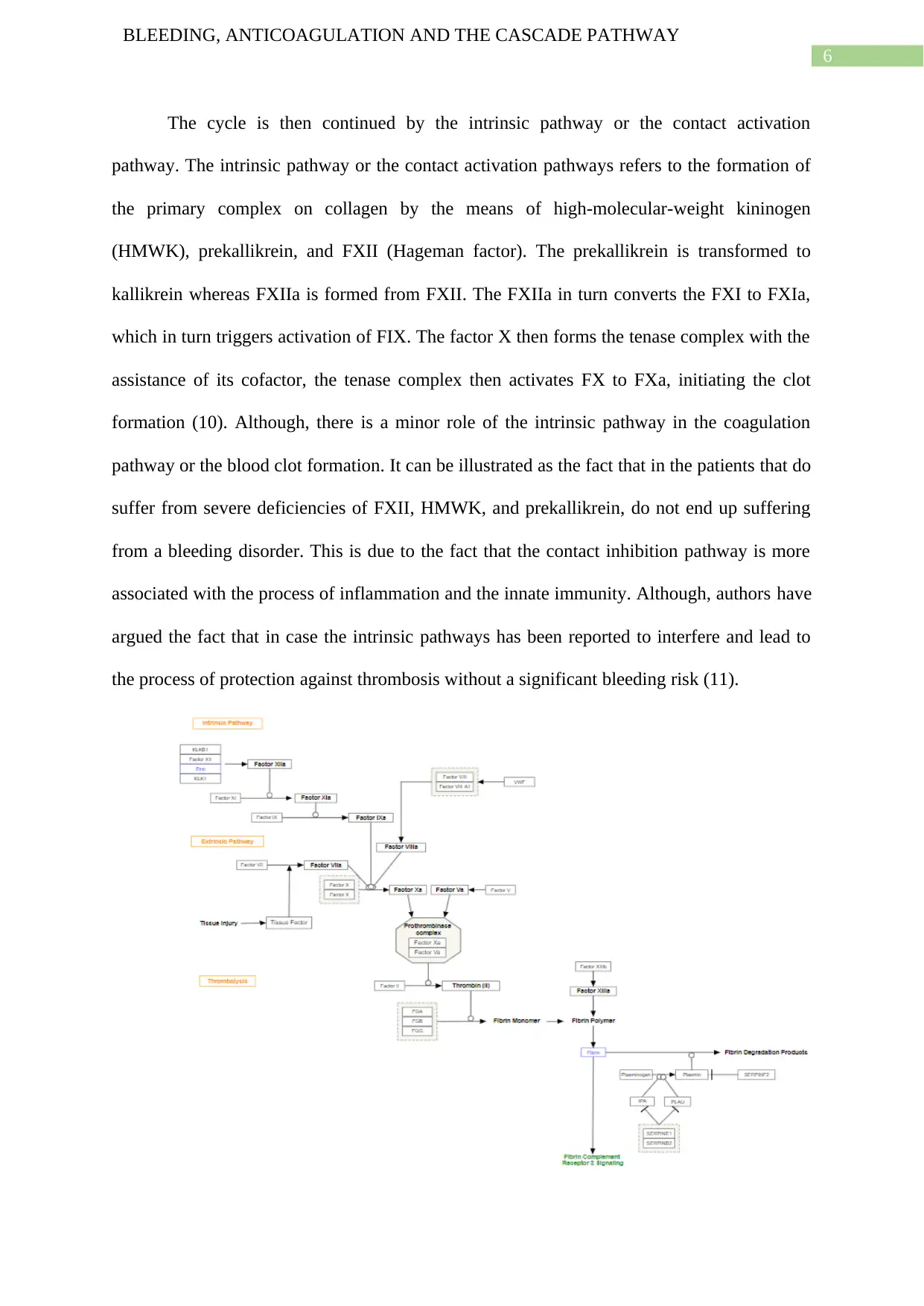
The cascade pathway:
Considering the coagulation cascade, there are two initial pathways which eventually
lead to the fibrin formation, these pathways are the contact activation pathway which is also
known as the intrinsic pathway, and the tissue factor pathway which in turn is also known as
the extrinsic pathway. The extrinsic or tissue factor pathway generates the thrombin burst,
which is the process in which the thrombin, the most important and pertinent component of
the coagulation cascade due to the feedback activation role (4). Due to the damage to the
blood vessel leading to the blood loss, FVII comes into contact with tissue factor which is
then expressed on the tissue-factor-bearing cells, which are the stromal fibroblasts and
leukocytes, forming the TF-FVIIa activated complex, which in turn then activates FIX and
FX. Thrombin, FXIa, FXII and FXa then together activate the FVII. The activation of FX by
TF-FVIIa is almost immediately inhibited by tissue factor pathway inhibitor (TFPI).
Thrombin then activates other components of the coagulation cascade, including FV and
FVIII (which forms a complex with FIX), and activates and releases FVIII from being bound
to vWF. FVIIIa is the co-factor of FIXa, and together they form the "tenase" complex, which
activates FX; and so the cycle continues (9).
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
fibronectin, and thrombospondin binding sites are exposed. As a result the bridge between the
platelets are formed and the platelet aggregate is developed and this triggers the blood
coagulation cascade leading to the formation of thrombin and fibrin net (7).
(8)
The cascade pathway:
Considering the coagulation cascade, there are two initial pathways which eventually
lead to the fibrin formation, these pathways are the contact activation pathway which is also
known as the intrinsic pathway, and the tissue factor pathway which in turn is also known as
the extrinsic pathway. The extrinsic or tissue factor pathway generates the thrombin burst,
which is the process in which the thrombin, the most important and pertinent component of
the coagulation cascade due to the feedback activation role (4). Due to the damage to the
blood vessel leading to the blood loss, FVII comes into contact with tissue factor which is
then expressed on the tissue-factor-bearing cells, which are the stromal fibroblasts and
leukocytes, forming the TF-FVIIa activated complex, which in turn then activates FIX and
FX. Thrombin, FXIa, FXII and FXa then together activate the FVII. The activation of FX by
TF-FVIIa is almost immediately inhibited by tissue factor pathway inhibitor (TFPI).
Thrombin then activates other components of the coagulation cascade, including FV and
FVIII (which forms a complex with FIX), and activates and releases FVIII from being bound
to vWF. FVIIIa is the co-factor of FIXa, and together they form the "tenase" complex, which
activates FX; and so the cycle continues (9).
6
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
The cycle is then continued by the intrinsic pathway or the contact activation
pathway. The intrinsic pathway or the contact activation pathways refers to the formation of
the primary complex on collagen by the means of high-molecular-weight kininogen
(HMWK), prekallikrein, and FXII (Hageman factor). The prekallikrein is transformed to
kallikrein whereas FXIIa is formed from FXII. The FXIIa in turn converts the FXI to FXIa,
which in turn triggers activation of FIX. The factor X then forms the tenase complex with the
assistance of its cofactor, the tenase complex then activates FX to FXa, initiating the clot
formation (10). Although, there is a minor role of the intrinsic pathway in the coagulation
pathway or the blood clot formation. It can be illustrated as the fact that in the patients that do
suffer from severe deficiencies of FXII, HMWK, and prekallikrein, do not end up suffering
from a bleeding disorder. This is due to the fact that the contact inhibition pathway is more
associated with the process of inflammation and the innate immunity. Although, authors have
argued the fact that in case the intrinsic pathways has been reported to interfere and lead to
the process of protection against thrombosis without a significant bleeding risk (11).
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
The cycle is then continued by the intrinsic pathway or the contact activation
pathway. The intrinsic pathway or the contact activation pathways refers to the formation of
the primary complex on collagen by the means of high-molecular-weight kininogen
(HMWK), prekallikrein, and FXII (Hageman factor). The prekallikrein is transformed to
kallikrein whereas FXIIa is formed from FXII. The FXIIa in turn converts the FXI to FXIa,
which in turn triggers activation of FIX. The factor X then forms the tenase complex with the
assistance of its cofactor, the tenase complex then activates FX to FXa, initiating the clot
formation (10). Although, there is a minor role of the intrinsic pathway in the coagulation
pathway or the blood clot formation. It can be illustrated as the fact that in the patients that do
suffer from severe deficiencies of FXII, HMWK, and prekallikrein, do not end up suffering
from a bleeding disorder. This is due to the fact that the contact inhibition pathway is more
associated with the process of inflammation and the innate immunity. Although, authors have
argued the fact that in case the intrinsic pathways has been reported to interfere and lead to
the process of protection against thrombosis without a significant bleeding risk (11).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
(11)
Anti-coagulative Drugs and types:
Although the blood coagulation is one of the most important aspect of the human
physiology, unwanted blood coagulation can be of extremely detrimental to the health and
welfare of the cardiac patients, renal disorder patients, and patients with bleeding disorders.
The anticoagulants are the drugs that are used on the patients that are at risk of developing
blood clots that might potentially block the blood vessels of a patient (12). In this case, the
blocked blood vessels of the patient leads to disrupting the blood flow across the body. Any
disruption in the blood flow might lead to many health adversities including strokes, transient
ischemic attacks, heart attacks, deep vein thrombosis, and pulmonary embolism. Hence, in
this case, the use of the anticoagulants helps in the eliminating or reducing the risk of blood
clot by prolonging the time required for the blood clot to be formed (13). Along with that, the
anticoagulants are also used abundantly to treat blood clots or thrombosis, such as deep vein
thrombosis and pulmonary embolism, the medication functions by stopping the clot from
getting any bigger in size, so that the innate immunity of the body can slowly reabsorb the
clot. Hence, the blood coagulants or the blood thinners are of extreme importance to medical
science, and there are different variants of blood thinners available, such as Vitamin K
antagonists or VKAs, Non-VKA Oral Anticoagulants, etc, and each type has different
pathways of functioning and differential usage (14).
Vitamin K antagonists:
Vitamin K antagonists had been first identified by K. P. Link, when he had been
investigating the haemorrhagic disease in cattle. Dicoumarol, a 3,3′-methyl-bis-4-
(hydroxycoumarin) had been the contributing factor that was discovered to have the
anticoagulant like activity. A chemical, clinically useful compound with functions equivalent
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
(11)
Anti-coagulative Drugs and types:
Although the blood coagulation is one of the most important aspect of the human
physiology, unwanted blood coagulation can be of extremely detrimental to the health and
welfare of the cardiac patients, renal disorder patients, and patients with bleeding disorders.
The anticoagulants are the drugs that are used on the patients that are at risk of developing
blood clots that might potentially block the blood vessels of a patient (12). In this case, the
blocked blood vessels of the patient leads to disrupting the blood flow across the body. Any
disruption in the blood flow might lead to many health adversities including strokes, transient
ischemic attacks, heart attacks, deep vein thrombosis, and pulmonary embolism. Hence, in
this case, the use of the anticoagulants helps in the eliminating or reducing the risk of blood
clot by prolonging the time required for the blood clot to be formed (13). Along with that, the
anticoagulants are also used abundantly to treat blood clots or thrombosis, such as deep vein
thrombosis and pulmonary embolism, the medication functions by stopping the clot from
getting any bigger in size, so that the innate immunity of the body can slowly reabsorb the
clot. Hence, the blood coagulants or the blood thinners are of extreme importance to medical
science, and there are different variants of blood thinners available, such as Vitamin K
antagonists or VKAs, Non-VKA Oral Anticoagulants, etc, and each type has different
pathways of functioning and differential usage (14).
Vitamin K antagonists:
Vitamin K antagonists had been first identified by K. P. Link, when he had been
investigating the haemorrhagic disease in cattle. Dicoumarol, a 3,3′-methyl-bis-4-
(hydroxycoumarin) had been the contributing factor that was discovered to have the
anticoagulant like activity. A chemical, clinically useful compound with functions equivalent

8
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
to that of dicoumarol, synthesized is the warfarin. The chemical composition of warfarin is 1-
(4′-hydroxy-3′-coumarinyl)-1-phenyl-3-butanone, and it is sold under the name of Coumadin
in US and is the first and most abundantly used Vitamin K antagonist (15).
Considering the functionality of the vitamin K antagonists or VKAs in the process of
anticoagulation is associated with inhibiting the action of vit K in the process of blood
coagulation. Vit K is an essential component which facilitates the synthesis of multiple blood
clotting factors, factor II (prothrombin), VII, IX and X, as well as protein C and protein S1;
and each of the factors have profound roles in the coagulation cascade. The vitamin K
antagonists inhibit the function of Vitamin K and restricts the formation of the above
mentioned factors, and inhibits the blood clot formation and reduces the process of the clot
increasing in size (16). The impact of Vit k inhibiting enzymes are associated with depleting
the active form of Vit k by the means of inhibiting the enzyme vitamin K epoxide reductase.
As a result, the inactive vitamin K epoxide is reduced back into the inactive form of Vit K,
which is incapable of activating the cascade that leads to the chain reaction that in turn
produces a variety of blood clotting factors, especially carboxylating the specific glutamic
acid residues on prothrombin, without which the appropriate thrombin conformation cannot
be reached and the platelet plug cannot be successfully formed. Therefore, Vitamin K
antagonists are successful anticoagulants and are used abundantly in the clinical setting to
avoid the fatality risks in the patients due to thrombosis (15).
Warfarin:
Warfarin is the most common oral anticoagulant drug used in the clinical practice,
with the chemical composition of 4-Hydroxy-3-(3-oxo-1-phenylbutyl) coumarin. It is a
coumarin anticoagulant, which is also considered as a racemic mixture encompassing two
different active isomers. The anticoagulant functions to treat and prevent thromboembolic
diseases such as thrombosis, thromboembolism, and pulmonary embolism. Although, a very
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
to that of dicoumarol, synthesized is the warfarin. The chemical composition of warfarin is 1-
(4′-hydroxy-3′-coumarinyl)-1-phenyl-3-butanone, and it is sold under the name of Coumadin
in US and is the first and most abundantly used Vitamin K antagonist (15).
Considering the functionality of the vitamin K antagonists or VKAs in the process of
anticoagulation is associated with inhibiting the action of vit K in the process of blood
coagulation. Vit K is an essential component which facilitates the synthesis of multiple blood
clotting factors, factor II (prothrombin), VII, IX and X, as well as protein C and protein S1;
and each of the factors have profound roles in the coagulation cascade. The vitamin K
antagonists inhibit the function of Vitamin K and restricts the formation of the above
mentioned factors, and inhibits the blood clot formation and reduces the process of the clot
increasing in size (16). The impact of Vit k inhibiting enzymes are associated with depleting
the active form of Vit k by the means of inhibiting the enzyme vitamin K epoxide reductase.
As a result, the inactive vitamin K epoxide is reduced back into the inactive form of Vit K,
which is incapable of activating the cascade that leads to the chain reaction that in turn
produces a variety of blood clotting factors, especially carboxylating the specific glutamic
acid residues on prothrombin, without which the appropriate thrombin conformation cannot
be reached and the platelet plug cannot be successfully formed. Therefore, Vitamin K
antagonists are successful anticoagulants and are used abundantly in the clinical setting to
avoid the fatality risks in the patients due to thrombosis (15).
Warfarin:
Warfarin is the most common oral anticoagulant drug used in the clinical practice,
with the chemical composition of 4-Hydroxy-3-(3-oxo-1-phenylbutyl) coumarin. It is a
coumarin anticoagulant, which is also considered as a racemic mixture encompassing two
different active isomers. The anticoagulant functions to treat and prevent thromboembolic
diseases such as thrombosis, thromboembolism, and pulmonary embolism. Although, a very

9
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
important aspect of using warfarin is to prevent ischemic stroke for the patients that have
atrial fibrillation. Discussing the mechanism of action, for Warfarin, alike any other vitamin
K antagonist, is by inhibiting the vitamin K reductase, depleting the reduced form of vitamin
K, which is the cofactor for the carboxylation of glutamate residues on the N-terminal regions
of vitamin K-dependent proteins, which in turn limits the gamma- carboxylation and their
subsequent activation of the vitamin K-dependent coagulant proteins (17). As a result the
chain of reactions leading to the successful production of the factors II, VII, IX, and X and
anticoagulant proteins C and S. As a direct result of the vitamin k dependent coagulation
factors, II, VII, and X, results directly in the decreased prothrombin levels which in turn
curbs the level of production of the thrombin and it’s binding to the fibrin proteins. This
series of events initiated by the vitamin K antagonists ultimately results in decreasing the
thrombogenicity of the clots, in turn not only inhibiting the production of blood clots but also
inhibits the process of increase in size for the clot (15).
Discussing the physiological properties of the drug, the drug is rapidly and
percutaneously absorbed following oral administration. The volume of distribution of the
drug is L/kg, and the protein binding capacity of the drug is 99%, primarily to albumin. With
respect to metabolism of the drug, the drug is metabolized stereo- and regio-selectively with
the assistance of hepatic microsomal enzymes. S-warfarin is mostly metabolized by
cytochrome P450 (CYP) 2C9 to produce the 6- and 7-hydroxylated metabolites. R-warfarin,
on the other hand, is metabolized by CYP1A1, 1A2, and 3A4 to produce 6-, 8-, and 10-
hydroxylated metabolites. Considering the route of elimination, warfarin is eliminated
majorly by the metabolism, and very little amount is excreted via the urine. Considering the
metabolites, they are principally excreted in the urine and slightly into the bile. The half-life
of the drug varies, for R-warfarin, it is 37-89 hours and for S-warfarin it is 21-43 hours (17).
Considering the drug-drug interaction, warfarin interacts with many compounds, such
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
important aspect of using warfarin is to prevent ischemic stroke for the patients that have
atrial fibrillation. Discussing the mechanism of action, for Warfarin, alike any other vitamin
K antagonist, is by inhibiting the vitamin K reductase, depleting the reduced form of vitamin
K, which is the cofactor for the carboxylation of glutamate residues on the N-terminal regions
of vitamin K-dependent proteins, which in turn limits the gamma- carboxylation and their
subsequent activation of the vitamin K-dependent coagulant proteins (17). As a result the
chain of reactions leading to the successful production of the factors II, VII, IX, and X and
anticoagulant proteins C and S. As a direct result of the vitamin k dependent coagulation
factors, II, VII, and X, results directly in the decreased prothrombin levels which in turn
curbs the level of production of the thrombin and it’s binding to the fibrin proteins. This
series of events initiated by the vitamin K antagonists ultimately results in decreasing the
thrombogenicity of the clots, in turn not only inhibiting the production of blood clots but also
inhibits the process of increase in size for the clot (15).
Discussing the physiological properties of the drug, the drug is rapidly and
percutaneously absorbed following oral administration. The volume of distribution of the
drug is L/kg, and the protein binding capacity of the drug is 99%, primarily to albumin. With
respect to metabolism of the drug, the drug is metabolized stereo- and regio-selectively with
the assistance of hepatic microsomal enzymes. S-warfarin is mostly metabolized by
cytochrome P450 (CYP) 2C9 to produce the 6- and 7-hydroxylated metabolites. R-warfarin,
on the other hand, is metabolized by CYP1A1, 1A2, and 3A4 to produce 6-, 8-, and 10-
hydroxylated metabolites. Considering the route of elimination, warfarin is eliminated
majorly by the metabolism, and very little amount is excreted via the urine. Considering the
metabolites, they are principally excreted in the urine and slightly into the bile. The half-life
of the drug varies, for R-warfarin, it is 37-89 hours and for S-warfarin it is 21-43 hours (17).
Considering the drug-drug interaction, warfarin interacts with many compounds, such
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

10
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
as 1,2,6,7-3H-Testosterone, 1-Testosterone, 18-methyl-19-nortestosterone, 2,5-Dimethoxy-
4-ethylthioamphetamine, 3-isobutyl-1-methyl-7H-xanthine, 3,4-
Methylenedioxyamphetamine, 3,5-diiodothyropropionic acid, and 4-Bromo-2,5-
dimethoxyamphetamine. Although, the drug has also been reported to interact with alcohol,
St. John's Wort, dietary Vitamin K, garlic, ginger, gingko, and horse chestnut. The adverse
effects of taking the drug includes unexplainable bruises that also expand in size, nosebleeds,
gum bleeding, intermittent or prolonged bleeding from the cuts, heavy or uncontrolled
menstrual bleeding, discoloration of urine and stool, hematuria, and lastly, coughing and
vomiting up blood. Similarly, in case of prolonged consumption of warfarin, it had been
observed that the drug can even lead to cellular necrosis or tissue necrosis, pain, change of
color or temperature of any of the area of the body, and purple toe syndrome (17).
Coumarin:
The second most commonly or abundantly used VKA drug is coumarin, is an
experimental group of small molecule, a Cytochrome P-450 CYP2C9 Substrates, with the
chemical formula being C9H6O2. The coumarin like compound is dicumarol, and this
anticoagulant acts by inhibiting the hepatic synthesis of vitamin K-dependent coagulation
factors such as prothrombin and factors VII, IX, and X. The compound is also abundantly
used in biochemical experiments as well. Considering the mechanism of action, this drug also
acts by inhibiting the action of vitamin K reductase, which in turn results in the depletion of
the reduced form of vitamin K (18). following a very similar format as that of warfarin it
ultimately results in reducing the thrombogenicity of the clots by the means of disrupting the
formation of the platelet plug. There is very little information available on the
pharmacokinetic properties of this drug type including the half-life, absorption, elimination,
toxicity, protein binding and metabolism. Although, this drug interacts largely with
testosterone and warfarin derivatives such as 1, 2, 6, 7-3H Testosterone, (R)-warfarin, (S)-
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
as 1,2,6,7-3H-Testosterone, 1-Testosterone, 18-methyl-19-nortestosterone, 2,5-Dimethoxy-
4-ethylthioamphetamine, 3-isobutyl-1-methyl-7H-xanthine, 3,4-
Methylenedioxyamphetamine, 3,5-diiodothyropropionic acid, and 4-Bromo-2,5-
dimethoxyamphetamine. Although, the drug has also been reported to interact with alcohol,
St. John's Wort, dietary Vitamin K, garlic, ginger, gingko, and horse chestnut. The adverse
effects of taking the drug includes unexplainable bruises that also expand in size, nosebleeds,
gum bleeding, intermittent or prolonged bleeding from the cuts, heavy or uncontrolled
menstrual bleeding, discoloration of urine and stool, hematuria, and lastly, coughing and
vomiting up blood. Similarly, in case of prolonged consumption of warfarin, it had been
observed that the drug can even lead to cellular necrosis or tissue necrosis, pain, change of
color or temperature of any of the area of the body, and purple toe syndrome (17).
Coumarin:
The second most commonly or abundantly used VKA drug is coumarin, is an
experimental group of small molecule, a Cytochrome P-450 CYP2C9 Substrates, with the
chemical formula being C9H6O2. The coumarin like compound is dicumarol, and this
anticoagulant acts by inhibiting the hepatic synthesis of vitamin K-dependent coagulation
factors such as prothrombin and factors VII, IX, and X. The compound is also abundantly
used in biochemical experiments as well. Considering the mechanism of action, this drug also
acts by inhibiting the action of vitamin K reductase, which in turn results in the depletion of
the reduced form of vitamin K (18). following a very similar format as that of warfarin it
ultimately results in reducing the thrombogenicity of the clots by the means of disrupting the
formation of the platelet plug. There is very little information available on the
pharmacokinetic properties of this drug type including the half-life, absorption, elimination,
toxicity, protein binding and metabolism. Although, this drug interacts largely with
testosterone and warfarin derivatives such as 1, 2, 6, 7-3H Testosterone, (R)-warfarin, (S)-

11
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
warfarin, 1-Testosterone, 18-methyl-19-nortestosterone, 2, 5-Dimethoxy-4-
ethylthioamphetamine, 3, 4-Methylenedioxyamphetamine, 3, 5-diiodothyropropionic acid, 4-
Bromo-2, 5-dimethoxyamphetamine, and 4-hydroxycoumarin. The adverse effects of
consuming uncontrolled amount of coumarin can lead to nosebleeds, bleeding from the gums,
bloody or discoloured urine, extensive bruising in the absence of injury or ecchymoses,
fatigue, shortness of breath or dyspnea, and fluid in lungs. In certain cases, uncontrolled
consumption of allergic skin reaction or hypersensitive allergies including redness and pain.
Prolonged exposure to the drug also leads to damage to the body organs as well (18).
Apart from these two major classes of VKA drugs, there are other formats or variants
of vitamin K antagonists, including Phenindione, phenprocoumon, acenocoumarol, and Ethyl
biscoumacetate. Whereas, phenprocoumon is used for venous thrombosis, thromboembolism,
and pulmonary embolism as well as for the prevention of ischemic stroke in patients with
atrial fibrillation, Phenindione is used for pulmonary embolism, cardiomyopathy, atrial
fibrillation and flutter, cerebral embolism, mural thrombosis, anticoagulant prophylaxis, and
thrombophilia. On the other hand, acenocoumarol is used for prevention of cerebral
embolism, deep vein thrombosis, pulmonary embolism, thromboembolism in infarction and
transient ischemic attacks (15).
Non-VKA Oral Anticoagulants (NOACs):
The second class of anti-coagulant drugs are the non VKA agents, the oral
anticoagulants which are also known as Non-VKA Oral Anticoagulants or NOACs. These are
the newest addition to the anticoagulant drug family, although their efficacy is associated
with similar bleeding risks, as observed in the VKA group of anticoagulants. Although,
prolonged usage of VKAs are associated with increased mortality risks, especially in the
target patient group with atrial fibrillation. In these cases, the addition of the non VKA
anticoagulant agents can help in reducing the mortality rates up to 2 year, which has been a
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
warfarin, 1-Testosterone, 18-methyl-19-nortestosterone, 2, 5-Dimethoxy-4-
ethylthioamphetamine, 3, 4-Methylenedioxyamphetamine, 3, 5-diiodothyropropionic acid, 4-
Bromo-2, 5-dimethoxyamphetamine, and 4-hydroxycoumarin. The adverse effects of
consuming uncontrolled amount of coumarin can lead to nosebleeds, bleeding from the gums,
bloody or discoloured urine, extensive bruising in the absence of injury or ecchymoses,
fatigue, shortness of breath or dyspnea, and fluid in lungs. In certain cases, uncontrolled
consumption of allergic skin reaction or hypersensitive allergies including redness and pain.
Prolonged exposure to the drug also leads to damage to the body organs as well (18).
Apart from these two major classes of VKA drugs, there are other formats or variants
of vitamin K antagonists, including Phenindione, phenprocoumon, acenocoumarol, and Ethyl
biscoumacetate. Whereas, phenprocoumon is used for venous thrombosis, thromboembolism,
and pulmonary embolism as well as for the prevention of ischemic stroke in patients with
atrial fibrillation, Phenindione is used for pulmonary embolism, cardiomyopathy, atrial
fibrillation and flutter, cerebral embolism, mural thrombosis, anticoagulant prophylaxis, and
thrombophilia. On the other hand, acenocoumarol is used for prevention of cerebral
embolism, deep vein thrombosis, pulmonary embolism, thromboembolism in infarction and
transient ischemic attacks (15).
Non-VKA Oral Anticoagulants (NOACs):
The second class of anti-coagulant drugs are the non VKA agents, the oral
anticoagulants which are also known as Non-VKA Oral Anticoagulants or NOACs. These are
the newest addition to the anticoagulant drug family, although their efficacy is associated
with similar bleeding risks, as observed in the VKA group of anticoagulants. Although,
prolonged usage of VKAs are associated with increased mortality risks, especially in the
target patient group with atrial fibrillation. In these cases, the addition of the non VKA
anticoagulant agents can help in reducing the mortality rates up to 2 year, which has been a

12
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
revolutionary change being brought forth in the health care industry. These drugs generally
function by inhibiting the FXa, an indispensable element of the blood clot formation or
coagulation process (19). The additional benefits associated with the use of non VKA oral
Anticoagulants is the fact that along with effectively inhibiting coagulation and reducing the
size of the clot already formed, these class of anti-coagulating medication also significantly
reduces the risk of stroke or systemic embolic events in non-valvular atrial fibrillation, a
challenge for patents on VKA drugs (12). There are a few options available for the NOAC
drugs, such as rivaroxaban, edoxaban, apixaban, dabigatran, etc.
Rivaroxaban:
This is the first identified and widely used direct factor Xa inhibitor, in an oral
consumption format. Unlike the VKA counterpart warfarin, there is no need for routine lab
monitoring necessary for rivaroxaban. The chemical formula of the medication is
C19H18ClN3O5S, and the medication is indicated generally for preventing enous
thromboembolic events (VTE), especially for patients with total hips replacements and total
knee replacement surgery. This is one of the only option in the oral anticoagulant drug variety
which can be used in conjunction with aspirin, making it ideal for peri-surgical scenarios.
Discussing the pharmacodynamics, the drug directly binds to factor Xa, and in turn
effectively blocks the amplification of coagulation cascade, and inhibits the formation of the
thrombus. The mechanism of action for the drug is by competitively inhibiting all free and
clot bound factor Xa, which is essential for activating the factor II and IIa, which in turn is
essential for activating fibrinogen to fibrin, the loose meshwork which completes the
coagulation cascade. The drug is absorbed rapidly, reaching peak plasma concentration in 2-4
hours, with volume distribution being 50 L and the plasma protein binding is within 92% to
95%. Although, only two-thirds of the medication dose is metabolized, by CYP3A4,
CYP3A5, CYP2J2 and CYP-independent mechanisms. The elimination of the drug is two-
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
revolutionary change being brought forth in the health care industry. These drugs generally
function by inhibiting the FXa, an indispensable element of the blood clot formation or
coagulation process (19). The additional benefits associated with the use of non VKA oral
Anticoagulants is the fact that along with effectively inhibiting coagulation and reducing the
size of the clot already formed, these class of anti-coagulating medication also significantly
reduces the risk of stroke or systemic embolic events in non-valvular atrial fibrillation, a
challenge for patents on VKA drugs (12). There are a few options available for the NOAC
drugs, such as rivaroxaban, edoxaban, apixaban, dabigatran, etc.
Rivaroxaban:
This is the first identified and widely used direct factor Xa inhibitor, in an oral
consumption format. Unlike the VKA counterpart warfarin, there is no need for routine lab
monitoring necessary for rivaroxaban. The chemical formula of the medication is
C19H18ClN3O5S, and the medication is indicated generally for preventing enous
thromboembolic events (VTE), especially for patients with total hips replacements and total
knee replacement surgery. This is one of the only option in the oral anticoagulant drug variety
which can be used in conjunction with aspirin, making it ideal for peri-surgical scenarios.
Discussing the pharmacodynamics, the drug directly binds to factor Xa, and in turn
effectively blocks the amplification of coagulation cascade, and inhibits the formation of the
thrombus. The mechanism of action for the drug is by competitively inhibiting all free and
clot bound factor Xa, which is essential for activating the factor II and IIa, which in turn is
essential for activating fibrinogen to fibrin, the loose meshwork which completes the
coagulation cascade. The drug is absorbed rapidly, reaching peak plasma concentration in 2-4
hours, with volume distribution being 50 L and the plasma protein binding is within 92% to
95%. Although, only two-thirds of the medication dose is metabolized, by CYP3A4,
CYP3A5, CYP2J2 and CYP-independent mechanisms. The elimination of the drug is two-
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

13
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
thirds into urine via tubular secretion, and rest one-third into feces. The toxicity of the drug is
via excessive bleeding due to over-dosage, which is treated with activated charcoal, coupled
with mechanical compression and hemodynamic support. This drug also interaction
negatively with 1-Testosterone, 18-methyl-19-nortestosterone, 3,5-diiodothyropropionic acid,
4-Hydroxytestosterone, 5-androstenedione, and 5beta-dihydrotestosterone. The side effects
include vomiting blood, nosebleeds, bleeding gums, and fatigue (19).
Apixaban:
Other more common NOAC drug consumed is Apixaban, an oral, direct, and highly
selective factor Xa (FXa) inhibitor, which is generally indicated for patients with nonvalvular
atrial fibrillation to prevent and treat thromboembolic diseases. The mechanism of action for
apixaban is by a very selective inhibition of the factor Xa, and the drug is absorbed in the
stomach and intestine, with 50% bio-availability. The volume of distribution is 21 L and the
protein binding is 87% plasma protein. The metabolism of the drug is via o-demethylation
and hydroxylation, and considering elimination, 25% of the administered dosage is excreted
via feces and urine. Considering interactions, the drug interacts with (1,2,6,7-
3H)Testosterone, warfarin, 1-Testosterone, 18-methyl-19-nortestosterone, 3,5-
diiodothyropropionic acid, 4-hydroxycoumarin, 4-Hydroxytestosterone, 5-androstenedione,
and 5beta-dihydrotestosterone. The adverse effects of the drug includes easy bruising,
unusual intermittent bleeding, heavy menstruation, discoloured urination, discoloration of
stool, severe headache, and coughing or vomiting blood (20).
Other anticoagulants:
Along with the common drugs for anticoagulation, other non-orthodox and
unconventional anticoagulation agents include aspirin and heparin.
Aspirin:
Aspirin is acetylsalicylic acid, an analgesic, antipyretic, antirheumatic, and anti-
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
thirds into urine via tubular secretion, and rest one-third into feces. The toxicity of the drug is
via excessive bleeding due to over-dosage, which is treated with activated charcoal, coupled
with mechanical compression and hemodynamic support. This drug also interaction
negatively with 1-Testosterone, 18-methyl-19-nortestosterone, 3,5-diiodothyropropionic acid,
4-Hydroxytestosterone, 5-androstenedione, and 5beta-dihydrotestosterone. The side effects
include vomiting blood, nosebleeds, bleeding gums, and fatigue (19).
Apixaban:
Other more common NOAC drug consumed is Apixaban, an oral, direct, and highly
selective factor Xa (FXa) inhibitor, which is generally indicated for patients with nonvalvular
atrial fibrillation to prevent and treat thromboembolic diseases. The mechanism of action for
apixaban is by a very selective inhibition of the factor Xa, and the drug is absorbed in the
stomach and intestine, with 50% bio-availability. The volume of distribution is 21 L and the
protein binding is 87% plasma protein. The metabolism of the drug is via o-demethylation
and hydroxylation, and considering elimination, 25% of the administered dosage is excreted
via feces and urine. Considering interactions, the drug interacts with (1,2,6,7-
3H)Testosterone, warfarin, 1-Testosterone, 18-methyl-19-nortestosterone, 3,5-
diiodothyropropionic acid, 4-hydroxycoumarin, 4-Hydroxytestosterone, 5-androstenedione,
and 5beta-dihydrotestosterone. The adverse effects of the drug includes easy bruising,
unusual intermittent bleeding, heavy menstruation, discoloured urination, discoloration of
stool, severe headache, and coughing or vomiting blood (20).
Other anticoagulants:
Along with the common drugs for anticoagulation, other non-orthodox and
unconventional anticoagulation agents include aspirin and heparin.
Aspirin:
Aspirin is acetylsalicylic acid, an analgesic, antipyretic, antirheumatic, and anti-

14
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
inflammatory agent, which also has anticoagulation properties. The mechanism of action for
aspirin to inhibit or interrupt coagulation procedure is by the fact that in low dosage (81 mg)
inhibits enzyme Cox-1, producer of thromboxane A-2, which is extremely essential for the
process of platelet aggregation (21). Aspirin has rapid absorption, protein binding is high
with 99.5% to albumin, metabolism is rapid hydrolyzation in the liver to salicylic acid, but is
fast conjugated with glycine and glucuronic acid. This drug also reacts largely to different
testosterone variants, 1,2,6,7-3H)Testosterone, 1-Testosterone, 8-methyl-19-nortestosterone,
along with warfarin, 1-(3-Mercapto-2-Methyl-Propionyl)-Pyrrolidine-2-Carboxylic Acid, 1-
benzylimidazole, 2,4-thiazolidinedione, 2,5-Dimethoxy-4-ethylamphetamine, and 2,5-
Dimethoxy-4-ethylthioamphetamine, along with alcohol. The adverse effects of aspirin
includes severe vomiting and nausea, vomiting, stomach ache, bloody or tarry stools, fever,
convulsions or seizures, hallucinations and confusion (22).
Heparin:
The last anticoagulant used in this paper is heparin, also known as unfractionated
heparin or UFH, used abundantly as an anticoagulant drug (23). Although the drug is
indicated for use in order to prevent or treat pulmonary embolism, arterial thromboembolism,
and deep vein thrombosis, in patients with atrial fibrillation or with unstable angina and/or
non-Q wave myocardial infarctions with acute coronary syndromes. It is a heterogenous
preparation of anionic sulfated glycosaminoglycan polymer, forming mucopolysaccharide
formed of equal parts of sulfated D-glucosamine and D-glucuronic acid with sulfaminic
bridges which is highly acidic (24). The mechanism of action of the drug is via inactivating
Factor Xa and thrombin, in turn inhibiting the conversion of fibrinogen to fibrin. Along with
that Heparin can also inhibits formation of the stable fibrin clot via preventing the activation
of fibrin stabilizing factor. The volume of distribution is 40-70 mL/mina and protein binding
is very high, mostly to low-density lipoproteins (25). The metabolism is via
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
inflammatory agent, which also has anticoagulation properties. The mechanism of action for
aspirin to inhibit or interrupt coagulation procedure is by the fact that in low dosage (81 mg)
inhibits enzyme Cox-1, producer of thromboxane A-2, which is extremely essential for the
process of platelet aggregation (21). Aspirin has rapid absorption, protein binding is high
with 99.5% to albumin, metabolism is rapid hydrolyzation in the liver to salicylic acid, but is
fast conjugated with glycine and glucuronic acid. This drug also reacts largely to different
testosterone variants, 1,2,6,7-3H)Testosterone, 1-Testosterone, 8-methyl-19-nortestosterone,
along with warfarin, 1-(3-Mercapto-2-Methyl-Propionyl)-Pyrrolidine-2-Carboxylic Acid, 1-
benzylimidazole, 2,4-thiazolidinedione, 2,5-Dimethoxy-4-ethylamphetamine, and 2,5-
Dimethoxy-4-ethylthioamphetamine, along with alcohol. The adverse effects of aspirin
includes severe vomiting and nausea, vomiting, stomach ache, bloody or tarry stools, fever,
convulsions or seizures, hallucinations and confusion (22).
Heparin:
The last anticoagulant used in this paper is heparin, also known as unfractionated
heparin or UFH, used abundantly as an anticoagulant drug (23). Although the drug is
indicated for use in order to prevent or treat pulmonary embolism, arterial thromboembolism,
and deep vein thrombosis, in patients with atrial fibrillation or with unstable angina and/or
non-Q wave myocardial infarctions with acute coronary syndromes. It is a heterogenous
preparation of anionic sulfated glycosaminoglycan polymer, forming mucopolysaccharide
formed of equal parts of sulfated D-glucosamine and D-glucuronic acid with sulfaminic
bridges which is highly acidic (24). The mechanism of action of the drug is via inactivating
Factor Xa and thrombin, in turn inhibiting the conversion of fibrinogen to fibrin. Along with
that Heparin can also inhibits formation of the stable fibrin clot via preventing the activation
of fibrin stabilizing factor. The volume of distribution is 40-70 mL/mina and protein binding
is very high, mostly to low-density lipoproteins (25). The metabolism is via

15
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
biotransformation in liver and reticulo-endothelial system; and the elimination of the drug is
via reticuloendothelial system, with a very small amount of unchanged heparin in the urine.
This anti-coagulating agent also reacts largely to different testosterone derivatives, such as,
(1,2,6,7-3H) Testosterone, 1-Testosterone, 18-methyl-19-nortestosterone, 4-
Hydroxytestosterone, 5beta-dihydrotestosterone, along with different formats of warfarin, 1-
(3-Mercapto-2-Methyl-Propionyl)-Pyrrolidine-2-Carboxylic Acid, 4-hydroxycoumarin, and
Abciximab. The adverse effects of the drug includes bleeding, bruising, pain, redness,
warmth, irritation, itching, and cyanosis in the skin (26).
Conclusion:
On a concluding note, this paper identified in detail the entire process, mechanism and
cascade of bleeding and coagulation, two physiological process that are intricately linked
with one another. The paper has successfully illustrated the entire cascade with clear genetic
interactions and molecular pathways of the entire coagulation cascade, and its relation as a
response mechanism to bleeding. Lastly, the paper has emphasized greatly on the different
anticoagulant medications, the different variants including Vitamin K antagonists, Non
vitamin K antagonist oral anticoagulants and drugs like aspirin and heparin, used abundantly
as anticoagulants. The thorough analysis of the drug, its composition, mechanism of action,
pharmacodynamics, interaction and adverse effects for the above mentioned classes of
anticoagulants has helped in developed a thorough understanding and expertise on the drugs
used, their indication for use, and necessary precautions, which will be extremely helpful in
my future practice.
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
biotransformation in liver and reticulo-endothelial system; and the elimination of the drug is
via reticuloendothelial system, with a very small amount of unchanged heparin in the urine.
This anti-coagulating agent also reacts largely to different testosterone derivatives, such as,
(1,2,6,7-3H) Testosterone, 1-Testosterone, 18-methyl-19-nortestosterone, 4-
Hydroxytestosterone, 5beta-dihydrotestosterone, along with different formats of warfarin, 1-
(3-Mercapto-2-Methyl-Propionyl)-Pyrrolidine-2-Carboxylic Acid, 4-hydroxycoumarin, and
Abciximab. The adverse effects of the drug includes bleeding, bruising, pain, redness,
warmth, irritation, itching, and cyanosis in the skin (26).
Conclusion:
On a concluding note, this paper identified in detail the entire process, mechanism and
cascade of bleeding and coagulation, two physiological process that are intricately linked
with one another. The paper has successfully illustrated the entire cascade with clear genetic
interactions and molecular pathways of the entire coagulation cascade, and its relation as a
response mechanism to bleeding. Lastly, the paper has emphasized greatly on the different
anticoagulant medications, the different variants including Vitamin K antagonists, Non
vitamin K antagonist oral anticoagulants and drugs like aspirin and heparin, used abundantly
as anticoagulants. The thorough analysis of the drug, its composition, mechanism of action,
pharmacodynamics, interaction and adverse effects for the above mentioned classes of
anticoagulants has helped in developed a thorough understanding and expertise on the drugs
used, their indication for use, and necessary precautions, which will be extremely helpful in
my future practice.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

16
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
References:
1. van Ryn J, Schurer J, Kink-Eiband M, Clemens A. Reversal of dabigatran-induced
bleeding by coagulation factor concentrates in a rat-tail bleeding model and lack of
effect on assays of coagulation. Anesthesiology: The Journal of the American Society
of Anesthesiologists. 2014 Jun 1;120(6):1429-40.
2. Haas T, Fries D, Tanaka KA, Asmis L, Curry NS, Schöchl H. Usefulness of standard
plasma coagulation tests in the management of perioperative coagulopathic bleeding:
is there any evidence?. British journal of anaesthesia. 2014 Sep 8;114(2):217-24.
3. Drolz A, Horvatits T, Roedl K, Rutter K, Staufer K, Kneidinger N, Holzinger U,
Zauner C, Schellongowski P, Heinz G, Perkmann T. Coagulation parameters and
major bleeding in critically ill patients with cirrhosis. Hepatology. 2016 Aug
1;64(2):556-68.
4. Bahin FF, Naidoo M, Williams SJ, Hourigan LF, Ormonde DG, Raftopoulos SC, Holt
BA, Sonson R, Bourke MJ. Prophylactic endoscopic coagulation to prevent bleeding
after wide-field endoscopic mucosal resection of large sessile colon polyps. Clinical
Gastroenterology and Hepatology. 2015 Apr 1;13(4):724-30.
5. Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian journal of
anaesthesia. 2014 Sep;58(5):515.
6. Kozek-Langenecker SA. Coagulation and transfusion in the postoperative bleeding
patient. Current opinion in critical care. 2014 Aug 1;20(4):460-6.
7. Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood
reviews. 2015 Jan 1;29(1):17-24.
8. Hall JE. Hemostasis and blood coagulation. Guyton and Hall Textbook of Medical
Physiology. 13th ed. Philadelphia, PA: Elsevier. 2016.
9. Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
References:
1. van Ryn J, Schurer J, Kink-Eiband M, Clemens A. Reversal of dabigatran-induced
bleeding by coagulation factor concentrates in a rat-tail bleeding model and lack of
effect on assays of coagulation. Anesthesiology: The Journal of the American Society
of Anesthesiologists. 2014 Jun 1;120(6):1429-40.
2. Haas T, Fries D, Tanaka KA, Asmis L, Curry NS, Schöchl H. Usefulness of standard
plasma coagulation tests in the management of perioperative coagulopathic bleeding:
is there any evidence?. British journal of anaesthesia. 2014 Sep 8;114(2):217-24.
3. Drolz A, Horvatits T, Roedl K, Rutter K, Staufer K, Kneidinger N, Holzinger U,
Zauner C, Schellongowski P, Heinz G, Perkmann T. Coagulation parameters and
major bleeding in critically ill patients with cirrhosis. Hepatology. 2016 Aug
1;64(2):556-68.
4. Bahin FF, Naidoo M, Williams SJ, Hourigan LF, Ormonde DG, Raftopoulos SC, Holt
BA, Sonson R, Bourke MJ. Prophylactic endoscopic coagulation to prevent bleeding
after wide-field endoscopic mucosal resection of large sessile colon polyps. Clinical
Gastroenterology and Hepatology. 2015 Apr 1;13(4):724-30.
5. Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian journal of
anaesthesia. 2014 Sep;58(5):515.
6. Kozek-Langenecker SA. Coagulation and transfusion in the postoperative bleeding
patient. Current opinion in critical care. 2014 Aug 1;20(4):460-6.
7. Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood
reviews. 2015 Jan 1;29(1):17-24.
8. Hall JE. Hemostasis and blood coagulation. Guyton and Hall Textbook of Medical
Physiology. 13th ed. Philadelphia, PA: Elsevier. 2016.
9. Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood

17
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
reviews. 2015 Jan 1;29(1):17-24.
10. Longstaff C, Kolev K. Basic mechanisms and regulation of fibrinolysis. Journal of
Thrombosis and Haemostasis. 2015 Jun;13:S98-105.
11. Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis.
Physiological reviews. 2013 Jan;93(1):327-58.
12. Amin A. Choosing non-Vitamin K antagonist oral anticoagulants: practical
considerations we need to know. The Ochsner Journal. 2016 Dec;16(4):531-41.
13. Salem JE, Sabouret P, Funck‐Brentano C, Hulot JS. Pharmacology and mechanisms
of action of new oral anticoagulants. Fundamental & clinical pharmacology. 2015
Feb;29(1):10-20.
14. Harter K, Levine M, Henderson SO. Anticoagulation drug therapy: a review. Western
Journal of Emergency Medicine. 2015 Jan;16(1):11.
15. Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma CS, Le Heuzey JY, Talajic M,
Scanavacca M, Vardas PE, Kirchhof P, Hemmrich M. Rivaroxaban vs. vitamin K
antagonists for cardioversion in atrial fibrillation. European heart journal. 2014 Sep
2;35(47):3346-55.
16. van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral
anticoagulants compared with vitamin K antagonists for acute symptomatic venous
thromboembolism: evidence from phase 3 trials. Blood. 2014 Jan 1:blood-2014.
17. Johnson JA, Cavallari LH. Warfarin pharmacogenetics. Trends in cardiovascular
medicine. 2015 Jan 1;25(1):33-41.
18. Sandhu S, Bansal Y, Silakari O, Bansal G. Coumarin hybrids as novel therapeutic
agents. Bioorganic & medicinal chemistry. 2014 Aug 1;22(15):3806-14.
19. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz
R, Alings M, Lonn EM, Anand SS, Widimsky P. Rivaroxaban with or without aspirin
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
reviews. 2015 Jan 1;29(1):17-24.
10. Longstaff C, Kolev K. Basic mechanisms and regulation of fibrinolysis. Journal of
Thrombosis and Haemostasis. 2015 Jun;13:S98-105.
11. Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis.
Physiological reviews. 2013 Jan;93(1):327-58.
12. Amin A. Choosing non-Vitamin K antagonist oral anticoagulants: practical
considerations we need to know. The Ochsner Journal. 2016 Dec;16(4):531-41.
13. Salem JE, Sabouret P, Funck‐Brentano C, Hulot JS. Pharmacology and mechanisms
of action of new oral anticoagulants. Fundamental & clinical pharmacology. 2015
Feb;29(1):10-20.
14. Harter K, Levine M, Henderson SO. Anticoagulation drug therapy: a review. Western
Journal of Emergency Medicine. 2015 Jan;16(1):11.
15. Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma CS, Le Heuzey JY, Talajic M,
Scanavacca M, Vardas PE, Kirchhof P, Hemmrich M. Rivaroxaban vs. vitamin K
antagonists for cardioversion in atrial fibrillation. European heart journal. 2014 Sep
2;35(47):3346-55.
16. van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral
anticoagulants compared with vitamin K antagonists for acute symptomatic venous
thromboembolism: evidence from phase 3 trials. Blood. 2014 Jan 1:blood-2014.
17. Johnson JA, Cavallari LH. Warfarin pharmacogenetics. Trends in cardiovascular
medicine. 2015 Jan 1;25(1):33-41.
18. Sandhu S, Bansal Y, Silakari O, Bansal G. Coumarin hybrids as novel therapeutic
agents. Bioorganic & medicinal chemistry. 2014 Aug 1;22(15):3806-14.
19. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz
R, Alings M, Lonn EM, Anand SS, Widimsky P. Rivaroxaban with or without aspirin

18
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
in stable cardiovascular disease. New England Journal of Medicine. 2017 Oct
5;377(14):1319-30.
20. Avezum A, Lopes RD, Schulte PJ, Lanas F, Gersh BJ, Hanna M, Pais P, Erol C, Diaz
R, Bahit MC, Bartunek J. Apixaban in comparison with warfarin in patients with
atrial fibrillation and valvular heart disease: findings from the Apixaban for Reduction
in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE)
trial. Circulation. 2015 Aug 25;132(8):624-32.
21. Hsu JC, Maddox TM, Kennedy K, Katz DF, Marzec LN, Lubitz SA, Gehi AK,
Turakhia MP, Marcus GM. Aspirin instead of oral anticoagulant prescription in atrial
fibrillation patients at risk for stroke. Journal of the American College of Cardiology.
2016 Jun 28;67(25):2913-23.
22. Dasenbrock HH, Yan SC, Gross BA, Guttieres D, Gormley WB, Frerichs KU, Aziz-
Sultan MA, Du R. The impact of aspirin and anticoagulant usage on outcomes after
aneurysmal subarachnoid hemorrhage: a Nationwide Inpatient Sample analysis.
Journal of neurosurgery. 2017 Feb 1;126(2):537-47.
23. de Jong PG, Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin and/or
heparin for women with unexplained recurrent miscarriage with or without inherited
thrombophilia. Cochrane Database of Systematic Reviews. 2014(7).
24. So CH, Eckman MH. Combined aspirin and anticoagulant therapy in patients with
atrial fibrillation. Journal of thrombosis and thrombolysis. 2017 Jan 1;43(1):7-17.
25. Chang CH, Lico LS, Huang TY, Lin SY, Chang CL, Arco SD, Hung SC. Synthesis of
the Heparin‐Based Anticoagulant Drug Fondaparinux. Angewandte Chemie
International Edition. 2014 Sep 8;53(37):9876-9.
26. Casu B, Naggi A, Torri G. Re-visiting the structure of heparin. Carbohydrate
research. 2015 Feb 11;403:60-8.
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
in stable cardiovascular disease. New England Journal of Medicine. 2017 Oct
5;377(14):1319-30.
20. Avezum A, Lopes RD, Schulte PJ, Lanas F, Gersh BJ, Hanna M, Pais P, Erol C, Diaz
R, Bahit MC, Bartunek J. Apixaban in comparison with warfarin in patients with
atrial fibrillation and valvular heart disease: findings from the Apixaban for Reduction
in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE)
trial. Circulation. 2015 Aug 25;132(8):624-32.
21. Hsu JC, Maddox TM, Kennedy K, Katz DF, Marzec LN, Lubitz SA, Gehi AK,
Turakhia MP, Marcus GM. Aspirin instead of oral anticoagulant prescription in atrial
fibrillation patients at risk for stroke. Journal of the American College of Cardiology.
2016 Jun 28;67(25):2913-23.
22. Dasenbrock HH, Yan SC, Gross BA, Guttieres D, Gormley WB, Frerichs KU, Aziz-
Sultan MA, Du R. The impact of aspirin and anticoagulant usage on outcomes after
aneurysmal subarachnoid hemorrhage: a Nationwide Inpatient Sample analysis.
Journal of neurosurgery. 2017 Feb 1;126(2):537-47.
23. de Jong PG, Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin and/or
heparin for women with unexplained recurrent miscarriage with or without inherited
thrombophilia. Cochrane Database of Systematic Reviews. 2014(7).
24. So CH, Eckman MH. Combined aspirin and anticoagulant therapy in patients with
atrial fibrillation. Journal of thrombosis and thrombolysis. 2017 Jan 1;43(1):7-17.
25. Chang CH, Lico LS, Huang TY, Lin SY, Chang CL, Arco SD, Hung SC. Synthesis of
the Heparin‐Based Anticoagulant Drug Fondaparinux. Angewandte Chemie
International Edition. 2014 Sep 8;53(37):9876-9.
26. Casu B, Naggi A, Torri G. Re-visiting the structure of heparin. Carbohydrate
research. 2015 Feb 11;403:60-8.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

19
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
BLEEDING, ANTICOAGULATION AND THE CASCADE PATHWAY
1 out of 20
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.



