Boiling Point and Intermolecular Forces in Aldehydes and Hydroxyl Group Compounds
VerifiedAdded on 2023/06/18
|8
|1622
|405
AI Summary
This report discusses the boiling point and intermolecular forces in aldehydes and hydroxyl group compounds. It compares the boiling points of different compounds and explains the role of intermolecular forces in determining boiling points. The report focuses on four compounds, 2-hexanol, hexanal, 1-hexanol, and pentanal, and their physical properties. The report also includes a table comparing the boiling points and intermolecular forces of these compounds.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

CHEMISTRY
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Table of Contents
INTRDOUCTION...........................................................................................................................1
MAIN BODY...................................................................................................................................1
CONCLUSION................................................................................................................................4
REFERENCES................................................................................................................................5
INTRDOUCTION...........................................................................................................................1
MAIN BODY...................................................................................................................................1
CONCLUSION................................................................................................................................4
REFERENCES................................................................................................................................5

INTRDOUCTION
The boiling point is defined as the temperature at which the pressure is exerted by the core of
surrounding that is based upon in form of liquid which is majorly equalled by the core pressure
that is exerted by the form of vapour which is associated with liquid. In this, the major of
compound have their own boiling and forces which may intermolecular and intramolecular
forces. Moreover, the intramolecular forces are referring a forces which have mediate interaction
between molecule which is properly include force of attraction or the course of repulsion which
act between the atom and other type of neighbouring particle. In this report, the major of
discussion is based on the compound with their boiling point and intramolecular forces. The
compound is from aldehyde and hydroxyl group.
MAIN BODY
The boiling point is the temperature at which the vapour of gas is generated by the phase
change from a liquid to a gas and is equivalent to 1 atmospheric pressure (Dearden 2003).
Boiling points depend on three essential factors, this includes the molecular weight, the shape of
the molecule and the intensity of the intermolecular forces present (Struyf 2017). Boiling point
property is affected by intermolecular forces (Dearden 2003). Intermolecular forces between
molecules in the liquid overcome by thermal energy. The type and strength of intermolecular
forces that are discovered in the molecular compound predetermine the boiling point (Burrows
2017).
The stronger the intermolecular forces, the lesser the vapour pressure of the compound
and the greater is the boiling point (Lewis 2015). The strength of the three intermolecular forces
from strongest to weakest are hydrogen bonding, dipole-dipole interaction, and dispersion
(Burrows 2017).
While comparing the boiling points of Hexanal and 1-hexanol, the theory of hydrogen
bonding has the strongest attraction (Struyf 2011). These forces have electrostatic attractions,
which develop unshielded hydrogen on the molecule, and a pair of electronegative atoms on an
adjacent molecule (Struyf 2011).
1
The boiling point is defined as the temperature at which the pressure is exerted by the core of
surrounding that is based upon in form of liquid which is majorly equalled by the core pressure
that is exerted by the form of vapour which is associated with liquid. In this, the major of
compound have their own boiling and forces which may intermolecular and intramolecular
forces. Moreover, the intramolecular forces are referring a forces which have mediate interaction
between molecule which is properly include force of attraction or the course of repulsion which
act between the atom and other type of neighbouring particle. In this report, the major of
discussion is based on the compound with their boiling point and intramolecular forces. The
compound is from aldehyde and hydroxyl group.
MAIN BODY
The boiling point is the temperature at which the vapour of gas is generated by the phase
change from a liquid to a gas and is equivalent to 1 atmospheric pressure (Dearden 2003).
Boiling points depend on three essential factors, this includes the molecular weight, the shape of
the molecule and the intensity of the intermolecular forces present (Struyf 2017). Boiling point
property is affected by intermolecular forces (Dearden 2003). Intermolecular forces between
molecules in the liquid overcome by thermal energy. The type and strength of intermolecular
forces that are discovered in the molecular compound predetermine the boiling point (Burrows
2017).
The stronger the intermolecular forces, the lesser the vapour pressure of the compound
and the greater is the boiling point (Lewis 2015). The strength of the three intermolecular forces
from strongest to weakest are hydrogen bonding, dipole-dipole interaction, and dispersion
(Burrows 2017).
While comparing the boiling points of Hexanal and 1-hexanol, the theory of hydrogen
bonding has the strongest attraction (Struyf 2011). These forces have electrostatic attractions,
which develop unshielded hydrogen on the molecule, and a pair of electronegative atoms on an
adjacent molecule (Struyf 2011).
1
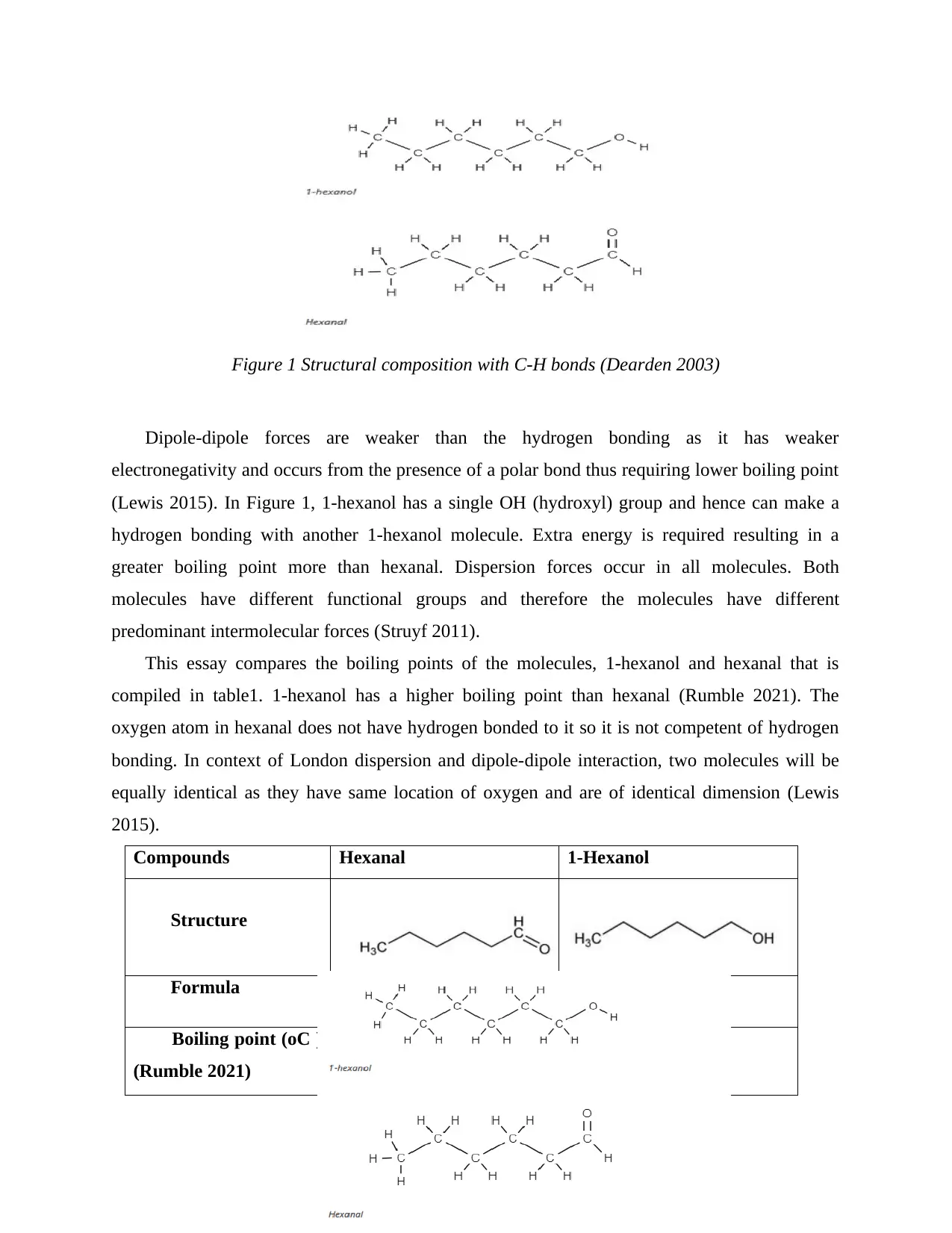
Figure 1 Structural composition with C-H bonds (Dearden 2003)
Dipole-dipole forces are weaker than the hydrogen bonding as it has weaker
electronegativity and occurs from the presence of a polar bond thus requiring lower boiling point
(Lewis 2015). In Figure 1, 1-hexanol has a single OH (hydroxyl) group and hence can make a
hydrogen bonding with another 1-hexanol molecule. Extra energy is required resulting in a
greater boiling point more than hexanal. Dispersion forces occur in all molecules. Both
molecules have different functional groups and therefore the molecules have different
predominant intermolecular forces (Struyf 2011).
This essay compares the boiling points of the molecules, 1-hexanol and hexanal that is
compiled in table1. 1-hexanol has a higher boiling point than hexanal (Rumble 2021). The
oxygen atom in hexanal does not have hydrogen bonded to it so it is not competent of hydrogen
bonding. In context of London dispersion and dipole-dipole interaction, two molecules will be
equally identical as they have same location of oxygen and are of identical dimension (Lewis
2015).
Compounds Hexanal 1-Hexanol
Structure
Formula C6H12O C6H14O
Boiling point (oC )
(Rumble 2021) 129.6 oC 156.9 oC
2
Dipole-dipole forces are weaker than the hydrogen bonding as it has weaker
electronegativity and occurs from the presence of a polar bond thus requiring lower boiling point
(Lewis 2015). In Figure 1, 1-hexanol has a single OH (hydroxyl) group and hence can make a
hydrogen bonding with another 1-hexanol molecule. Extra energy is required resulting in a
greater boiling point more than hexanal. Dispersion forces occur in all molecules. Both
molecules have different functional groups and therefore the molecules have different
predominant intermolecular forces (Struyf 2011).
This essay compares the boiling points of the molecules, 1-hexanol and hexanal that is
compiled in table1. 1-hexanol has a higher boiling point than hexanal (Rumble 2021). The
oxygen atom in hexanal does not have hydrogen bonded to it so it is not competent of hydrogen
bonding. In context of London dispersion and dipole-dipole interaction, two molecules will be
equally identical as they have same location of oxygen and are of identical dimension (Lewis
2015).
Compounds Hexanal 1-Hexanol
Structure
Formula C6H12O C6H14O
Boiling point (oC )
(Rumble 2021) 129.6 oC 156.9 oC
2
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Intermolecular Force dipole-dipole interaction,
Van der Waal forces
Hydrogen Bonding, dipole-
dipole interaction, dispersion
force
In conclusion, the boiling points are a measure of intermolecular forces. Molecules with
high chain branching and a smaller molecular mass will have lower boiling point while
molecules with hydrogen bonding, more carbons will have higher boiling points. By observing
the structural composition and the functional group present in the molecule, the structure can
play an essential role in determining the boiling point of the molecule. (Lewis 2015).
In this, the new compound which is selected to create an overview aspect on the boiling and
intramolecular force is with 2-hexanol, hexanal, 1-hexanol and pentanal. Moreover, the
compound is majorly from the aldehyde and hydroxyl group. With this aspect the physical
properties of aldehyde and ketone which is show their boiling point which is -19 degree Celsius
and lower as per the change in compound. However, there are four compound which is focus in
the study with the base of boiling and intramolecular forces (Hu and et. al., 2021). 2-hexanol
have molecular formula is C16H14O also called as hexa-2-ol. The molecular weight which is
considered with this compound is 102.17. whereas, the hexan-2-ol is a secondary alcohol and a
hexanol. The boiling point of the compound is identified up to 136 degrees Celsius. In this, the
intramolecular forces are strong due the number of carbon and make the bond with hydroxyl
compound. That develop and create a high range of intramolecular forces (Van Den Einde and et.
al., 2017). Hexanal have molecular formula is C6H12O. they are also called hexa-2-ol. In this,
the boiling point which is raise up to the 129 degree Celsius. The density of the compound which
is based 814 kg/m3. In this, there is six carbons which is linked with twelve hydrogens. The
physical property of compound is strong. Perhaps, the intramolecular forces are high in which
they are encounter with the aspect of physical property. Moreover, there are number of
compound who is essentially bound with such level of boiling and they are completely showing a
high range of intramolecular aspects (Fuller and et. al., 2017). 1-hexanol have also called as
hexa-1-ol. As per this, the molecular formula of the compound is C6H14O. Usually, this is a
colourless liquid which is slightly soluble in water but they are miscible with diethyl ether and
ethanol. Moreover, the boiling point which is 157 degrees Celsius. Moreover, due the strong
3
Van der Waal forces
Hydrogen Bonding, dipole-
dipole interaction, dispersion
force
In conclusion, the boiling points are a measure of intermolecular forces. Molecules with
high chain branching and a smaller molecular mass will have lower boiling point while
molecules with hydrogen bonding, more carbons will have higher boiling points. By observing
the structural composition and the functional group present in the molecule, the structure can
play an essential role in determining the boiling point of the molecule. (Lewis 2015).
In this, the new compound which is selected to create an overview aspect on the boiling and
intramolecular force is with 2-hexanol, hexanal, 1-hexanol and pentanal. Moreover, the
compound is majorly from the aldehyde and hydroxyl group. With this aspect the physical
properties of aldehyde and ketone which is show their boiling point which is -19 degree Celsius
and lower as per the change in compound. However, there are four compound which is focus in
the study with the base of boiling and intramolecular forces (Hu and et. al., 2021). 2-hexanol
have molecular formula is C16H14O also called as hexa-2-ol. The molecular weight which is
considered with this compound is 102.17. whereas, the hexan-2-ol is a secondary alcohol and a
hexanol. The boiling point of the compound is identified up to 136 degrees Celsius. In this, the
intramolecular forces are strong due the number of carbon and make the bond with hydroxyl
compound. That develop and create a high range of intramolecular forces (Van Den Einde and et.
al., 2017). Hexanal have molecular formula is C6H12O. they are also called hexa-2-ol. In this,
the boiling point which is raise up to the 129 degree Celsius. The density of the compound which
is based 814 kg/m3. In this, there is six carbons which is linked with twelve hydrogens. The
physical property of compound is strong. Perhaps, the intramolecular forces are high in which
they are encounter with the aspect of physical property. Moreover, there are number of
compound who is essentially bound with such level of boiling and they are completely showing a
high range of intramolecular aspects (Fuller and et. al., 2017). 1-hexanol have also called as
hexa-1-ol. As per this, the molecular formula of the compound is C6H14O. Usually, this is a
colourless liquid which is slightly soluble in water but they are miscible with diethyl ether and
ethanol. Moreover, the boiling point which is 157 degrees Celsius. Moreover, due the strong
3
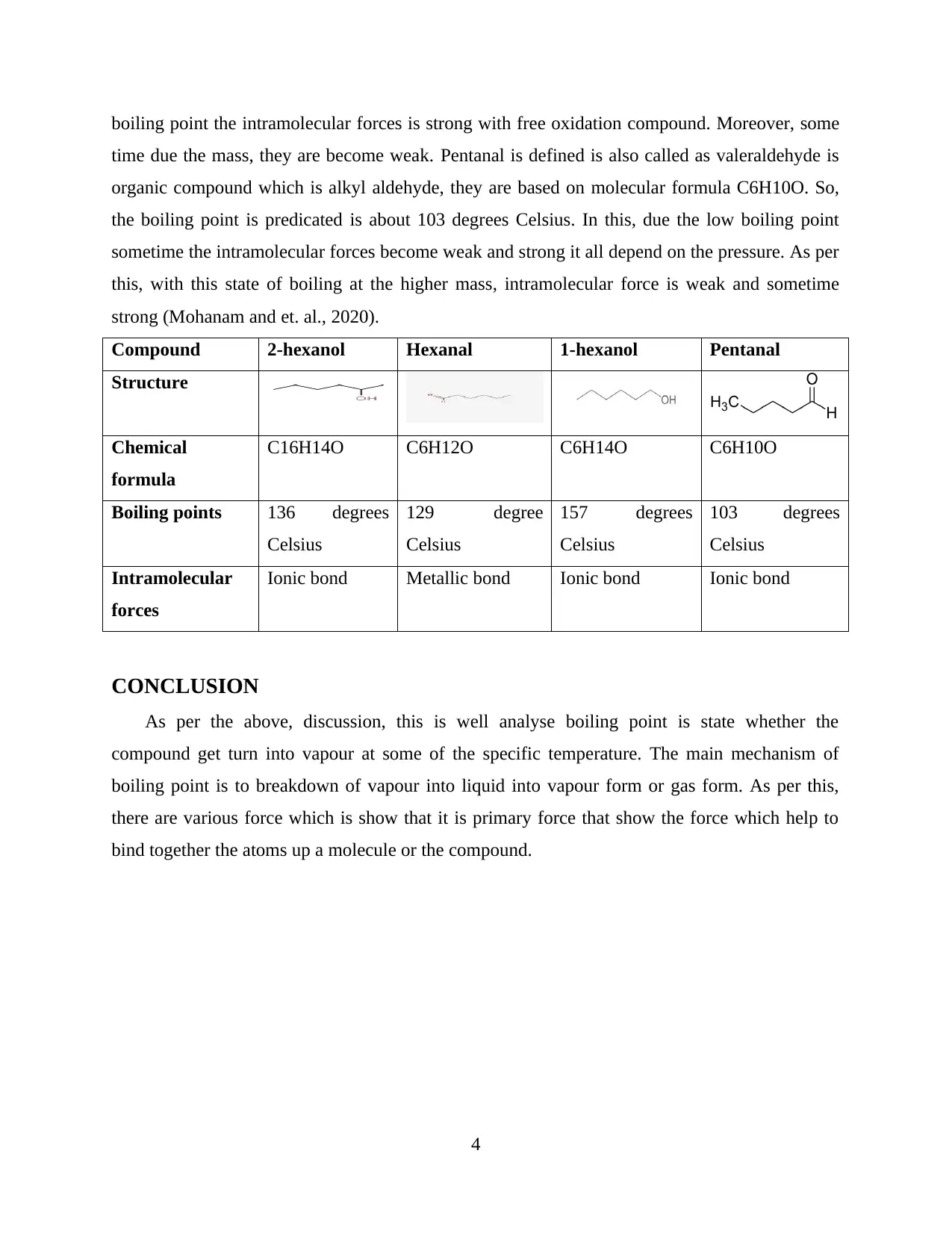
boiling point the intramolecular forces is strong with free oxidation compound. Moreover, some
time due the mass, they are become weak. Pentanal is defined is also called as valeraldehyde is
organic compound which is alkyl aldehyde, they are based on molecular formula C6H10O. So,
the boiling point is predicated is about 103 degrees Celsius. In this, due the low boiling point
sometime the intramolecular forces become weak and strong it all depend on the pressure. As per
this, with this state of boiling at the higher mass, intramolecular force is weak and sometime
strong (Mohanam and et. al., 2020).
Compound 2-hexanol Hexanal 1-hexanol Pentanal
Structure
Chemical
formula
C16H14O C6H12O C6H14O C6H10O
Boiling points 136 degrees
Celsius
129 degree
Celsius
157 degrees
Celsius
103 degrees
Celsius
Intramolecular
forces
Ionic bond Metallic bond Ionic bond Ionic bond
CONCLUSION
As per the above, discussion, this is well analyse boiling point is state whether the
compound get turn into vapour at some of the specific temperature. The main mechanism of
boiling point is to breakdown of vapour into liquid into vapour form or gas form. As per this,
there are various force which is show that it is primary force that show the force which help to
bind together the atoms up a molecule or the compound.
4
time due the mass, they are become weak. Pentanal is defined is also called as valeraldehyde is
organic compound which is alkyl aldehyde, they are based on molecular formula C6H10O. So,
the boiling point is predicated is about 103 degrees Celsius. In this, due the low boiling point
sometime the intramolecular forces become weak and strong it all depend on the pressure. As per
this, with this state of boiling at the higher mass, intramolecular force is weak and sometime
strong (Mohanam and et. al., 2020).
Compound 2-hexanol Hexanal 1-hexanol Pentanal
Structure
Chemical
formula
C16H14O C6H12O C6H14O C6H10O
Boiling points 136 degrees
Celsius
129 degree
Celsius
157 degrees
Celsius
103 degrees
Celsius
Intramolecular
forces
Ionic bond Metallic bond Ionic bond Ionic bond
CONCLUSION
As per the above, discussion, this is well analyse boiling point is state whether the
compound get turn into vapour at some of the specific temperature. The main mechanism of
boiling point is to breakdown of vapour into liquid into vapour form or gas form. As per this,
there are various force which is show that it is primary force that show the force which help to
bind together the atoms up a molecule or the compound.
4

REFERENCES
Books and Journals
Burrows, A Holman, J & Parsons, A, 2017, ‘Chemistry³: Introducing inorganic, organic and
physical chemistry’, 3rd edn, pp.542-581.
Dearden, J 2003, ‘Quantitative structure-property relationships for prediction of boiling point,
vapor pressure, and melting point’, Environmental Toxicology and Chemistry, viewed 9
August 2021
Lewis, B 2015, ‘Intermolecular forces learning objectives: learn to identify and explain the three
types of intermolecular forces: Van der Waals permanent, viewed 11 August
2021,<https://slideplayer.com/slide/6612984/>
Rumble, John (ed.), 2021, CRC Handbook of Chemistry and Physics, 102nd edition (Internet
Version). 102nd edn. CRC Press/Taylor and Francis, Boca Raton USA. Physical
Constants of Organic Compound viewed 6 August 2021.
Struyf, J (ed.), 2011, An analytical approach for relating boiling points of mono functional
organic compounds to intermolecular forces, Journal of Chemical Education, vol. 87. pp.
923-935.
Mohanam and et. al., 2020. Intermolecular Forces Game: Using a Card Game to Engage
Students in Reviewing Intermolecular Forces and Their Relationship to Boiling
Points. Journal of Chemical Education, 97(11), pp.4044-4048.
Fuller and et. al., 2017. Solvatochromism in perylene diimides; experiment and theory. Physical
Chemistry Chemical Physics, 19(47), pp.31781-31787.
Van Den Einde and et. al., 2017. The effects of intermolecular energy transfers on power cycle
efficiency. Physics Essays, 30(2), pp.224-225.
Hu and et. al., 2021. Preparation and characterization of organic soluble polyimides with low
dielectric constant containing trifluoromethyl for optoelectronic application. European
Polymer Journal, 157, p.110566.
5
Books and Journals
Burrows, A Holman, J & Parsons, A, 2017, ‘Chemistry³: Introducing inorganic, organic and
physical chemistry’, 3rd edn, pp.542-581.
Dearden, J 2003, ‘Quantitative structure-property relationships for prediction of boiling point,
vapor pressure, and melting point’, Environmental Toxicology and Chemistry, viewed 9
August 2021
Lewis, B 2015, ‘Intermolecular forces learning objectives: learn to identify and explain the three
types of intermolecular forces: Van der Waals permanent, viewed 11 August
2021,<https://slideplayer.com/slide/6612984/>
Rumble, John (ed.), 2021, CRC Handbook of Chemistry and Physics, 102nd edition (Internet
Version). 102nd edn. CRC Press/Taylor and Francis, Boca Raton USA. Physical
Constants of Organic Compound viewed 6 August 2021.
Struyf, J (ed.), 2011, An analytical approach for relating boiling points of mono functional
organic compounds to intermolecular forces, Journal of Chemical Education, vol. 87. pp.
923-935.
Mohanam and et. al., 2020. Intermolecular Forces Game: Using a Card Game to Engage
Students in Reviewing Intermolecular Forces and Their Relationship to Boiling
Points. Journal of Chemical Education, 97(11), pp.4044-4048.
Fuller and et. al., 2017. Solvatochromism in perylene diimides; experiment and theory. Physical
Chemistry Chemical Physics, 19(47), pp.31781-31787.
Van Den Einde and et. al., 2017. The effects of intermolecular energy transfers on power cycle
efficiency. Physics Essays, 30(2), pp.224-225.
Hu and et. al., 2021. Preparation and characterization of organic soluble polyimides with low
dielectric constant containing trifluoromethyl for optoelectronic application. European
Polymer Journal, 157, p.110566.
5
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

6
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.





