Analysis of Boiling Points and Properties of Organic Compounds
VerifiedAdded on 2020/03/23
|7
|1307
|196
Report
AI Summary
This report investigates the boiling points of several organic compounds, including 1-pentanol, 1-hexanol, 2-methyl-1-butanol, and 3-methyl-2-butanone. The analysis focuses on how molecular weight, molecular structure, and the presence of hydrogen bonds influence the boiling points of these substances. The report explains that compounds with higher molecular weights generally exhibit higher boiling points due to the increased energy required to overcome intermolecular forces. The study also examines the role of molecular structure, with larger molecules having more bonds and thus requiring more heat to change state. The conclusion emphasizes that the size of the compound and its molecular structure are key factors in determining boiling point trends. The report uses structural diagrams to illustrate the molecular configurations of the compounds and supports the boiling point analysis with relevant literature from various authors, providing a comprehensive overview of the topic.

Boiling points of organic compounds 1
BOILING POINTS OF ORGANIC COMPOUND
By Name
Course
Instructor
Institution
Location
Date
BOILING POINTS OF ORGANIC COMPOUND
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Boiling points of organic compounds 2
Background information
The boiling point is the point at which a liquid will turn into a gas. This is realized when
the forces of attraction in the liquid break due to the application of heat (Acton, 2012). When the
forces of the attraction breaks and the losses, the forces formed will be very weak in which in
most cases they form weak van der Waal forces of the gases (forming gaseous substances). The
boiling points of a pure substance like for the compound given have a very sharp point and it is
affected by a number of factors of the molecular structures. Some of these common factors are
the weight, size, bonds and the molecular structure. A compound with a relatively higher
molecular weight will have a relatively higher boiling point while those with relatively lower
molecular weight will have a relatively lower boiling point (Hat, 2013).
This is because for a compound with a relatively higher molecular structure will require
relatively higher heat energy to break the molecular structure. Another important factor is the
forces of attraction and the molecular structure of a compound. A compound with a relatively
strong forces of attraction like hydrogen forces will require a relatively higher heat to help break
these forces thus become loose and forms gaseous substance(Anslyn, 2012). While the
compounds with relatively lower bonds and molecular structures like the weak van der Waal
forces will require relatively lower heat energy to break the forces of attraction to form a gaseous
compound. For the weak held bonds, they require a less heat to break the weakly held bonds to
form gaseous compound (Schlaf, 2012).
These four compounds have relatively higher boiling points because all of them have
hydrogen bonds. When their boiling points are reached then the hydrogen bonds are broken to
form weak van der Waal forces of the respective gases. From the list given of substances 1-
Background information
The boiling point is the point at which a liquid will turn into a gas. This is realized when
the forces of attraction in the liquid break due to the application of heat (Acton, 2012). When the
forces of the attraction breaks and the losses, the forces formed will be very weak in which in
most cases they form weak van der Waal forces of the gases (forming gaseous substances). The
boiling points of a pure substance like for the compound given have a very sharp point and it is
affected by a number of factors of the molecular structures. Some of these common factors are
the weight, size, bonds and the molecular structure. A compound with a relatively higher
molecular weight will have a relatively higher boiling point while those with relatively lower
molecular weight will have a relatively lower boiling point (Hat, 2013).
This is because for a compound with a relatively higher molecular structure will require
relatively higher heat energy to break the molecular structure. Another important factor is the
forces of attraction and the molecular structure of a compound. A compound with a relatively
strong forces of attraction like hydrogen forces will require a relatively higher heat to help break
these forces thus become loose and forms gaseous substance(Anslyn, 2012). While the
compounds with relatively lower bonds and molecular structures like the weak van der Waal
forces will require relatively lower heat energy to break the forces of attraction to form a gaseous
compound. For the weak held bonds, they require a less heat to break the weakly held bonds to
form gaseous compound (Schlaf, 2012).
These four compounds have relatively higher boiling points because all of them have
hydrogen bonds. When their boiling points are reached then the hydrogen bonds are broken to
form weak van der Waal forces of the respective gases. From the list given of substances 1-

Boiling points of organic compounds 3
pentanol, 1-hexanol, 2-methyl-1-butanol, 3-methyl-2-butanone, their boiling point actually does
not reduce as in the given list in the question but they reduce as below (Baev, 2013);
1. 1- Hexanol – 1570C
2. 1-pentanol -1380C
3. 2-Methyl-1-butanol-1290C
4. 3-methyl-2-butanone-940C
From the above boiling point it is clear that the boiling point reduces as follows;
1-hexanol, 1-pentanol, 2-methyl-1-butanol, 3-methyl-2-butanone. The decrease arrangement of
the boiling point of these four compounds can be substantiated by the molecular formula,
molecular mass and the structure of the above compound. The hydrogen bond cannot be applied
here to substantiate which compound is having a higher boiling point because all the four are
having the hydrogen bond. So for these compounds, the use of the molecular weight and the
structure are the best parameters to substantiate for the boiling point arrangement as given
above(Stoker, 2015).
1-Hexanol has a molecular weight of 102.17 g/ mol with a molecular formula of
CH3(CH2)4CH2OH ( condensed molecular formula), 1- pentanol has a molecular weight of
88.168g/ mol with a molecular formula of C5H12O, 2-Methyl-1-butanol has a molecular weight
of 88.148 g/mol with a molecular formula of CH3CH2CH(CH3)CH2OH (condensed molecular
structure) while the 3-methyl-2-butanone has a molecular weight of 86.13g /mol having a
molecular formula of C5H10O. The molecular weight of the compound fully substantiates the
trend of the above boiling point. And the variation in the boiling point is proportional to the
molecular weight (Brown, 2014). This can be seen where the variation between 1- Hexanol –
pentanol, 1-hexanol, 2-methyl-1-butanol, 3-methyl-2-butanone, their boiling point actually does
not reduce as in the given list in the question but they reduce as below (Baev, 2013);
1. 1- Hexanol – 1570C
2. 1-pentanol -1380C
3. 2-Methyl-1-butanol-1290C
4. 3-methyl-2-butanone-940C
From the above boiling point it is clear that the boiling point reduces as follows;
1-hexanol, 1-pentanol, 2-methyl-1-butanol, 3-methyl-2-butanone. The decrease arrangement of
the boiling point of these four compounds can be substantiated by the molecular formula,
molecular mass and the structure of the above compound. The hydrogen bond cannot be applied
here to substantiate which compound is having a higher boiling point because all the four are
having the hydrogen bond. So for these compounds, the use of the molecular weight and the
structure are the best parameters to substantiate for the boiling point arrangement as given
above(Stoker, 2015).
1-Hexanol has a molecular weight of 102.17 g/ mol with a molecular formula of
CH3(CH2)4CH2OH ( condensed molecular formula), 1- pentanol has a molecular weight of
88.168g/ mol with a molecular formula of C5H12O, 2-Methyl-1-butanol has a molecular weight
of 88.148 g/mol with a molecular formula of CH3CH2CH(CH3)CH2OH (condensed molecular
structure) while the 3-methyl-2-butanone has a molecular weight of 86.13g /mol having a
molecular formula of C5H10O. The molecular weight of the compound fully substantiates the
trend of the above boiling point. And the variation in the boiling point is proportional to the
molecular weight (Brown, 2014). This can be seen where the variation between 1- Hexanol –
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Boiling points of organic compounds 4
1570C and 1-pentanol -1380C is a relatively large range, this relatively large range is evidenced
in the molecular weight where 1- Hexanol is having 102.17g/mol and 1- pentanol having
88.168g /mol.
And in the cases where the discrepancy in the boiling point is very small, the discrepancy
in the molecular weight is also small. This is witnessed for the case of 1-pentanol -1380C and
Methyl-1-butanol-1290C, the discrepancy in their molecular weight is very small as the
compounds have the molecular weight of 88.168 g/ mol and 88.148g/mol. Relatively higher
amount of heat energy will be required to break the many bonds of the relatively heavy
compound as compared to the heat energy that will be required to break the bonds in the light
loaded compound (Dhingra, 2011). Therefore the molecular weight is a good parameter to
explain the variation of the boiling point of the four compounds. The diagram below illustrates
open molecular structure 1-pentanol;
Fig 1: open structure of1-pentanol
The below shows the open molecular structure of 1- Hexanol
1570C and 1-pentanol -1380C is a relatively large range, this relatively large range is evidenced
in the molecular weight where 1- Hexanol is having 102.17g/mol and 1- pentanol having
88.168g /mol.
And in the cases where the discrepancy in the boiling point is very small, the discrepancy
in the molecular weight is also small. This is witnessed for the case of 1-pentanol -1380C and
Methyl-1-butanol-1290C, the discrepancy in their molecular weight is very small as the
compounds have the molecular weight of 88.168 g/ mol and 88.148g/mol. Relatively higher
amount of heat energy will be required to break the many bonds of the relatively heavy
compound as compared to the heat energy that will be required to break the bonds in the light
loaded compound (Dhingra, 2011). Therefore the molecular weight is a good parameter to
explain the variation of the boiling point of the four compounds. The diagram below illustrates
open molecular structure 1-pentanol;
Fig 1: open structure of1-pentanol
The below shows the open molecular structure of 1- Hexanol
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Boiling points of organic compounds 5
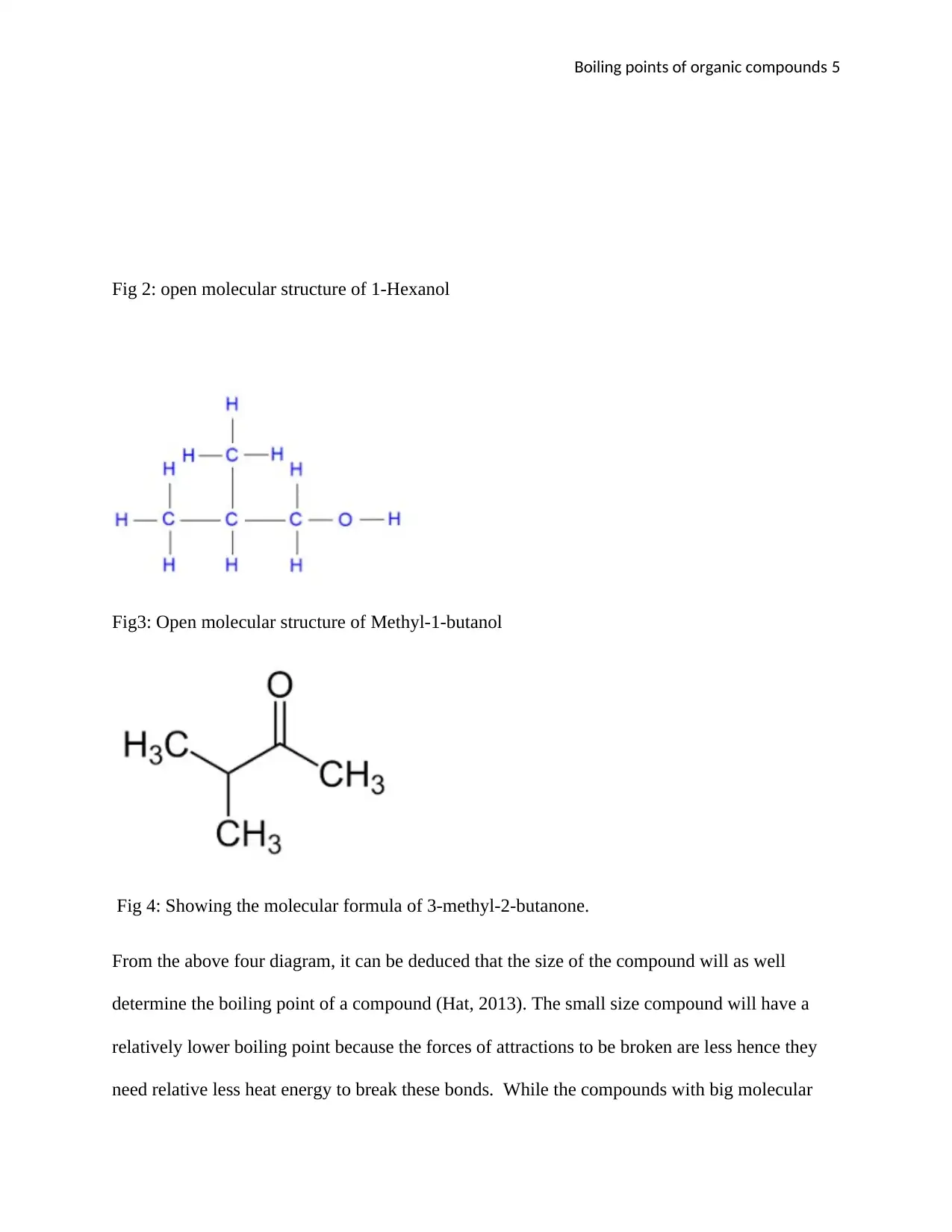
Fig 2: open molecular structure of 1-Hexanol
Fig3: Open molecular structure of Methyl-1-butanol
Fig 4: Showing the molecular formula of 3-methyl-2-butanone.
From the above four diagram, it can be deduced that the size of the compound will as well
determine the boiling point of a compound (Hat, 2013). The small size compound will have a
relatively lower boiling point because the forces of attractions to be broken are less hence they
need relative less heat energy to break these bonds. While the compounds with big molecular
Fig 2: open molecular structure of 1-Hexanol
Fig3: Open molecular structure of Methyl-1-butanol
Fig 4: Showing the molecular formula of 3-methyl-2-butanone.
From the above four diagram, it can be deduced that the size of the compound will as well
determine the boiling point of a compound (Hat, 2013). The small size compound will have a
relatively lower boiling point because the forces of attractions to be broken are less hence they
need relative less heat energy to break these bonds. While the compounds with big molecular

Boiling points of organic compounds 6
sizes have a relatively higher boiling point since there are relatively many bonds to be broken
hence relatively much heat energy is needed to break these bonds. From the above four
diagrams, 1-Hexanol is having six carbon atoms( the largest), 1-pentanol is having five carbon
atoms, Methyl-1-butanol is having four carbon atoms and 3-methyl-2-butanone is having three
carbon atoms indicating that it is the smallest of the four compounds(Stoker, 2015).
Conclusion
In conclusion, the boiling point of a compound can be substantiated by the type of the molecules
and bonds its forms. The molecular weight of a compound can also help to determine the trend in
the variation of the boiling point in the compound as seen in the above explanations. For the
organic compounds as in the above discussion the size of a compound (determined by a number
of carbon atoms) enables us to understand variation in the boiling point of a different compound
as seen above. For this comparison of the boiling point, the type bonds will not be employed
since the entire four compounds have the same type of bond (hydrogen bond).
sizes have a relatively higher boiling point since there are relatively many bonds to be broken
hence relatively much heat energy is needed to break these bonds. From the above four
diagrams, 1-Hexanol is having six carbon atoms( the largest), 1-pentanol is having five carbon
atoms, Methyl-1-butanol is having four carbon atoms and 3-methyl-2-butanone is having three
carbon atoms indicating that it is the smallest of the four compounds(Stoker, 2015).
Conclusion
In conclusion, the boiling point of a compound can be substantiated by the type of the molecules
and bonds its forms. The molecular weight of a compound can also help to determine the trend in
the variation of the boiling point in the compound as seen in the above explanations. For the
organic compounds as in the above discussion the size of a compound (determined by a number
of carbon atoms) enables us to understand variation in the boiling point of a different compound
as seen above. For this comparison of the boiling point, the type bonds will not be employed
since the entire four compounds have the same type of bond (hydrogen bond).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Boiling points of organic compounds 7
Bibliography
Acton, A., 2012. Cycloparaffins: Advances in Research and Application. 1st ed. Chicago: ScholarlyEditions.
Anslyn, E., 2012. Organic Chemistry. 6th ed. Hull: Cengage Learning.
Baev, A. K., 2013. The boiling point of the different organic compound. 4th ed. Hull: Springer Science &
Business Media.
Brown, W. H., 2014. Boiling points of Organic Chemistry. 4th ed. Leicester: Springer.
Dhingra, S., 2011. Comprehensive Practical Organic Chemistry: Qualitative Analysis. 5th ed. Birmingham:
Universities Press.
Hat, J., 2013. Liquid-Liquid InterfacesTheory and Methods. 2nd ed. Manchester: CRC Press.
Schlaf, M., 2012. Reaction Pathways and Mechanisms in Thermocatalytic Biomass Conversion. 3rd ed.
Stoke: Springer.
Stoker, H. S., 2015. General, Organic, and boiling points. 2nd ed. London: Cengage Learning.
Bibliography
Acton, A., 2012. Cycloparaffins: Advances in Research and Application. 1st ed. Chicago: ScholarlyEditions.
Anslyn, E., 2012. Organic Chemistry. 6th ed. Hull: Cengage Learning.
Baev, A. K., 2013. The boiling point of the different organic compound. 4th ed. Hull: Springer Science &
Business Media.
Brown, W. H., 2014. Boiling points of Organic Chemistry. 4th ed. Leicester: Springer.
Dhingra, S., 2011. Comprehensive Practical Organic Chemistry: Qualitative Analysis. 5th ed. Birmingham:
Universities Press.
Hat, J., 2013. Liquid-Liquid InterfacesTheory and Methods. 2nd ed. Manchester: CRC Press.
Schlaf, M., 2012. Reaction Pathways and Mechanisms in Thermocatalytic Biomass Conversion. 3rd ed.
Stoke: Springer.
Stoker, H. S., 2015. General, Organic, and boiling points. 2nd ed. London: Cengage Learning.
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





