University Research: Chemotherapy Treatments for Colorectal Cancer
VerifiedAdded on 2021/09/16
|25
|2287
|152
Report
AI Summary
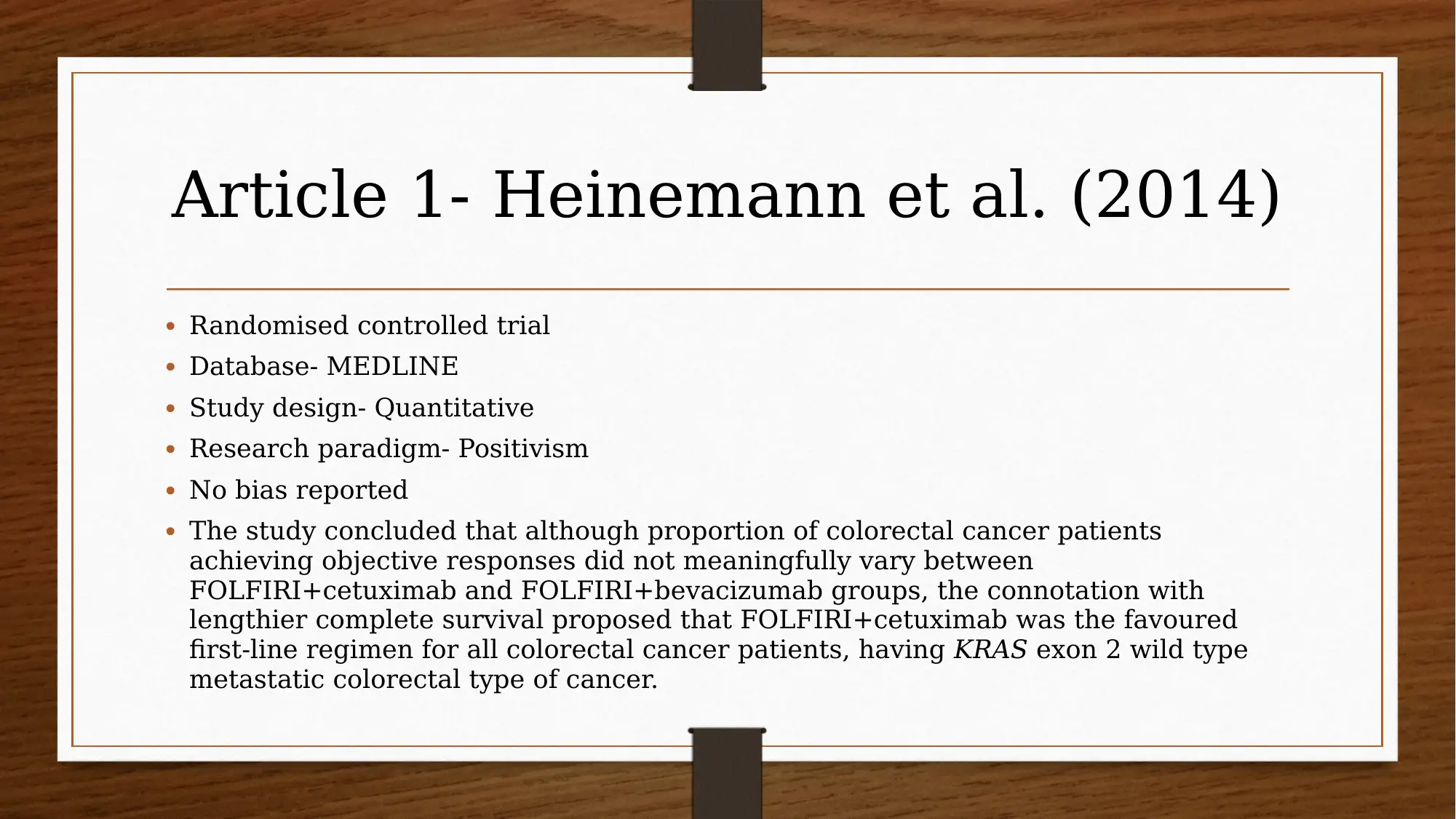
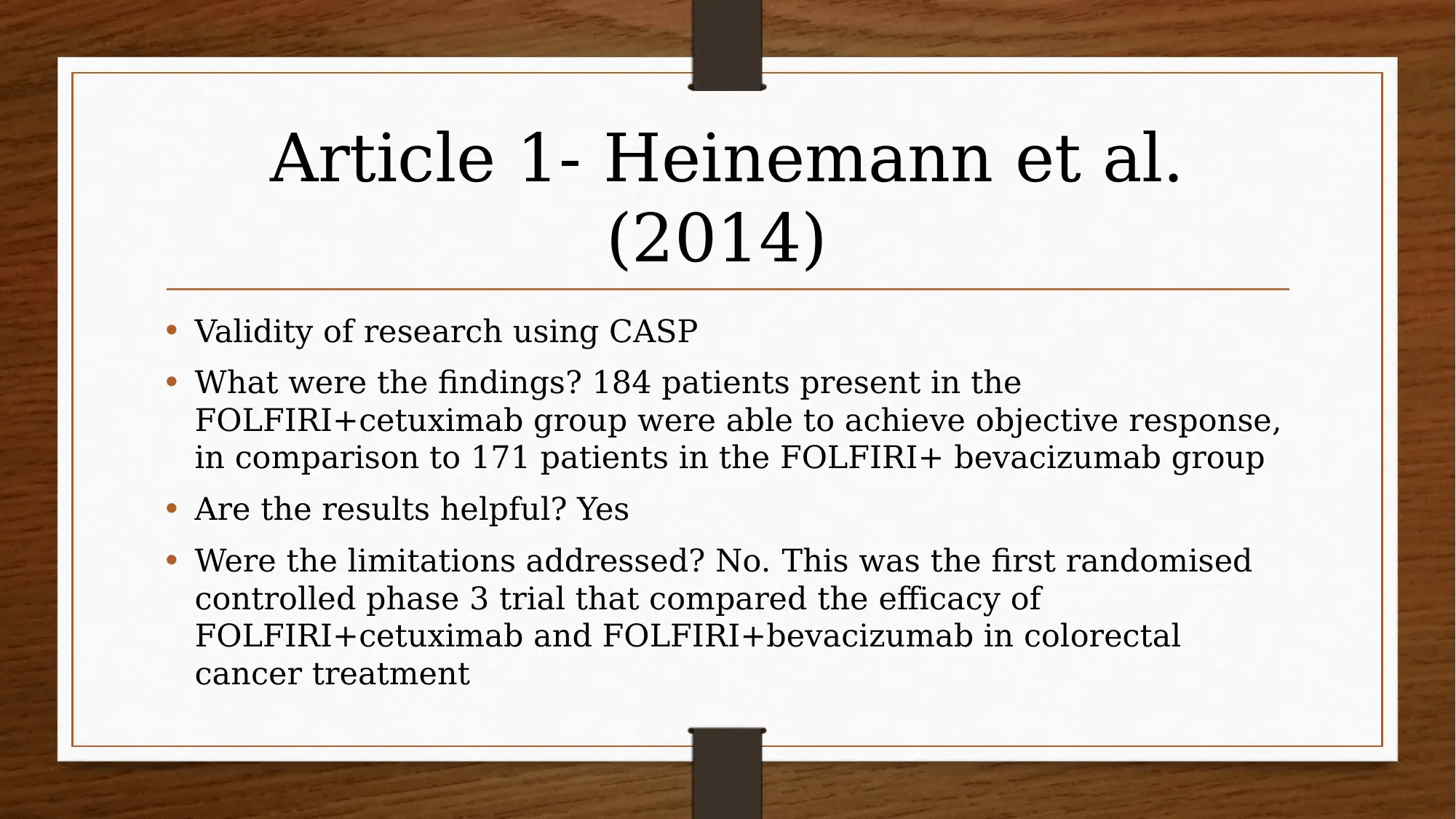
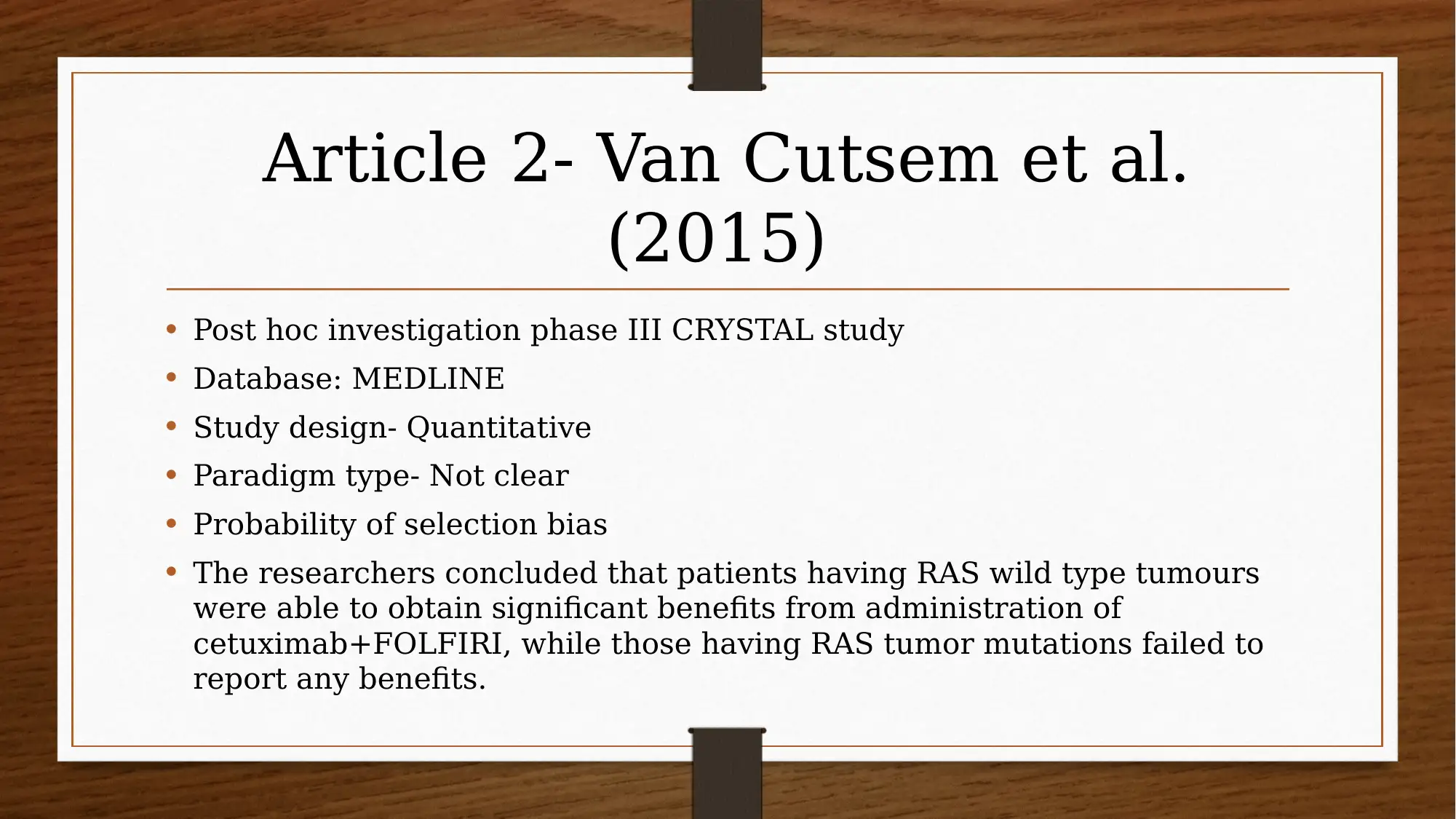
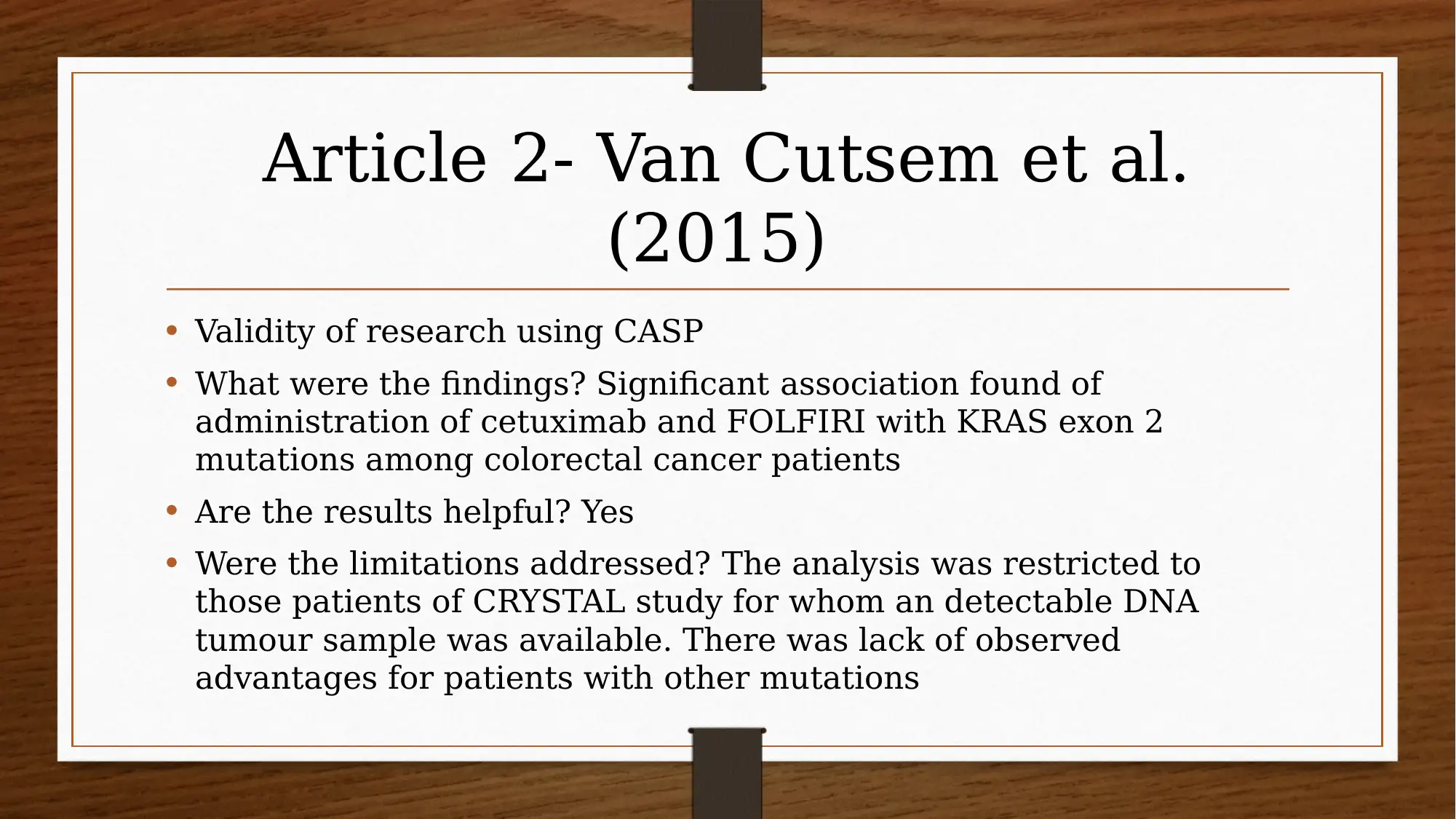
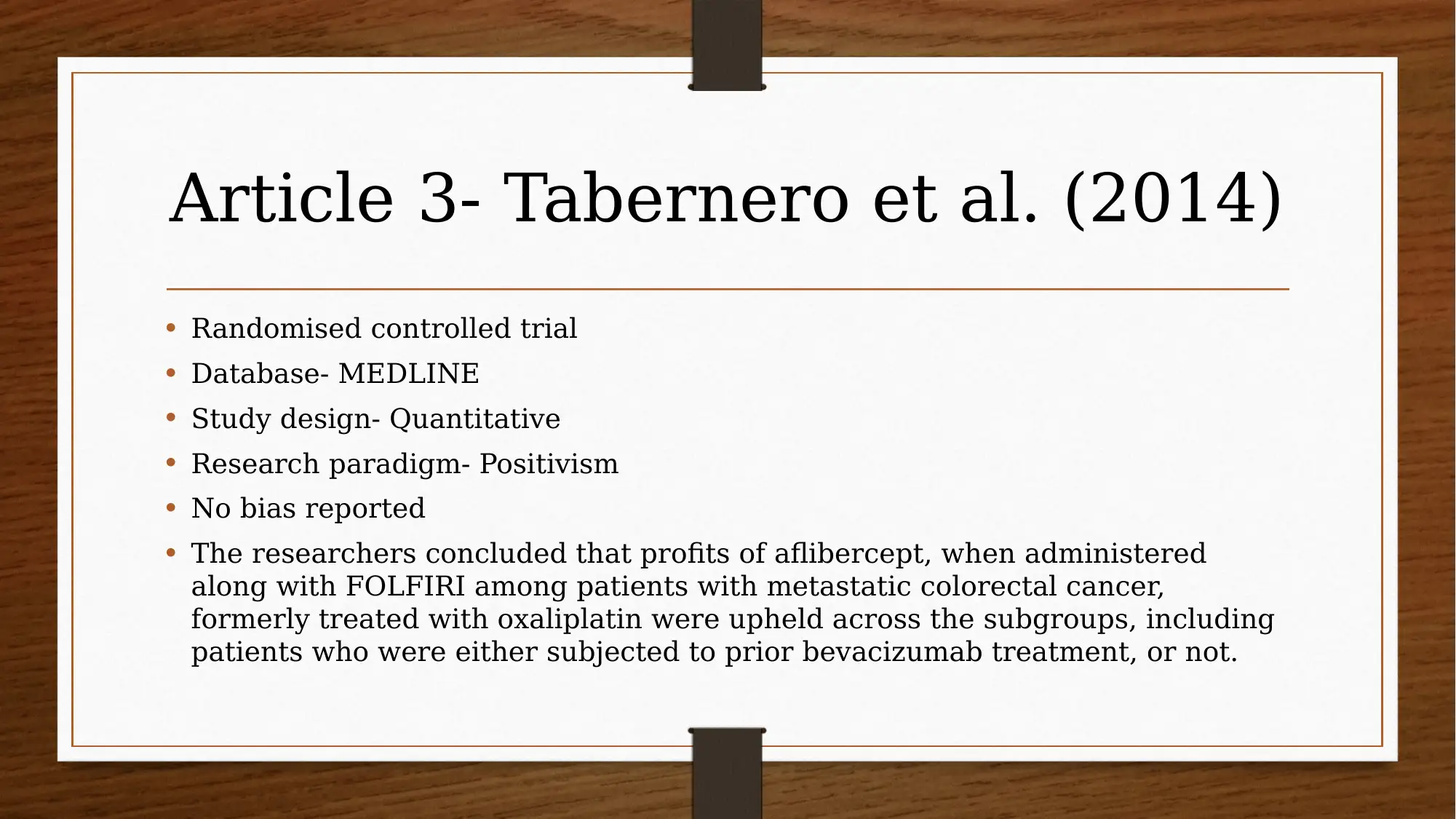
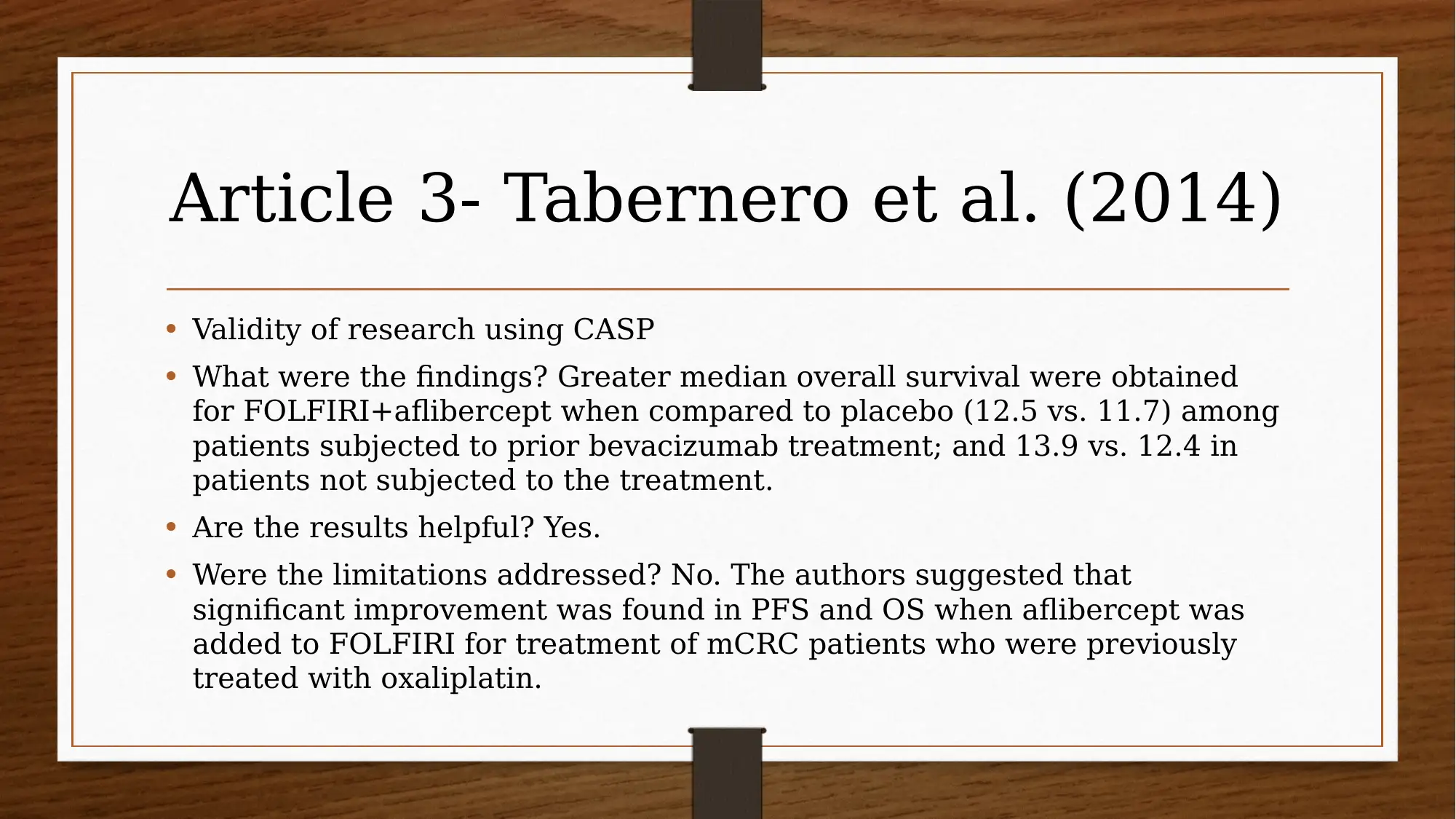
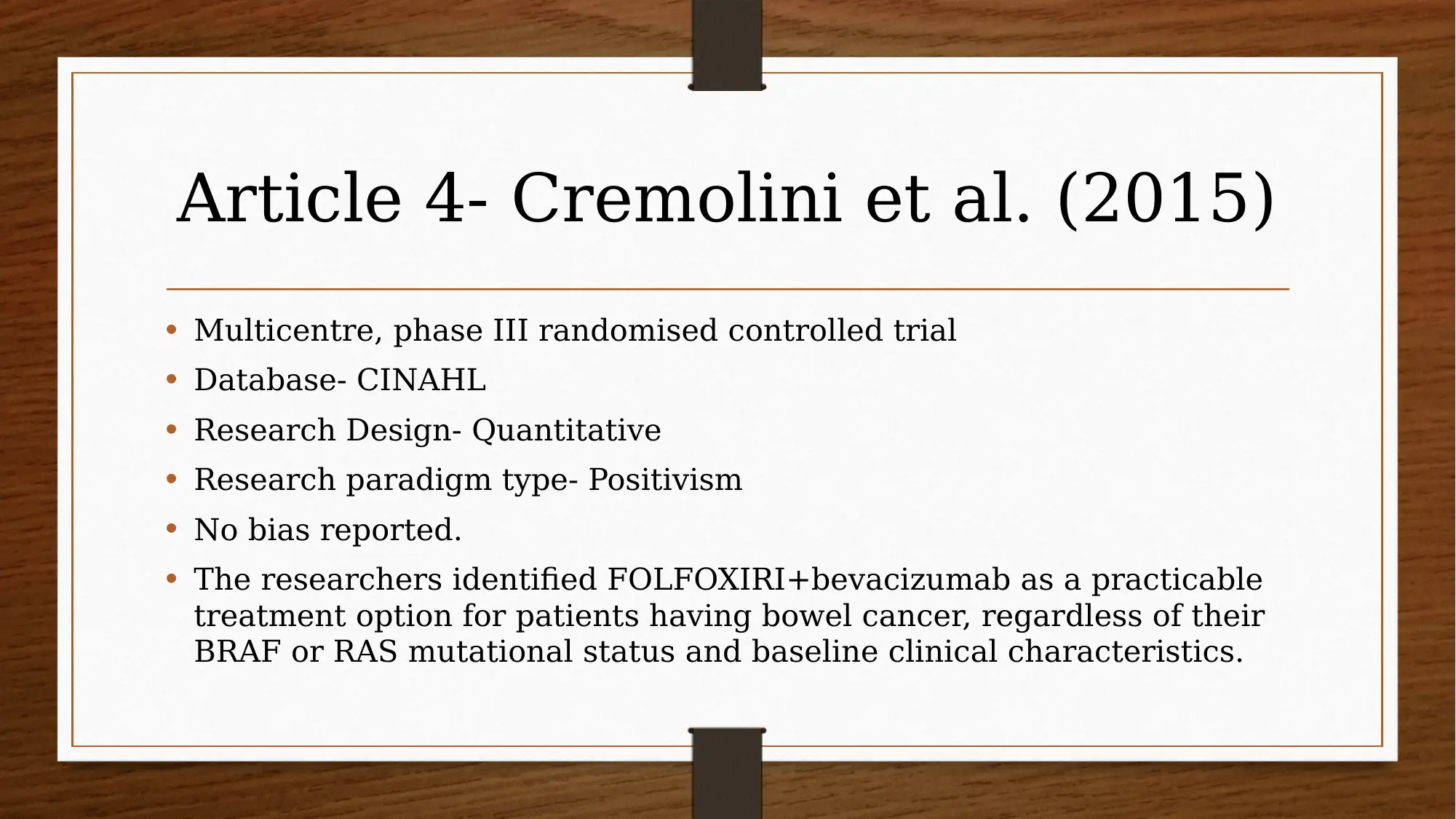
This report investigates the efficacy of chemotherapy drugs in treating colorectal cancer. The research utilizes the PICO question: "Are chemotherapy drugs effective in treating colorectal cancer?" The study employs MEDLINE and CINAHL databases, focusing on articles published after 2013, and uses CASP tools to assess the trustworthiness of the research. Key findings include the effectiveness of FOLFIRI combined with cetuximab or bevacizumab, and the benefits of FOLFIRI with aflibercept in certain patient groups. The report also explores patient perspectives using PROMs and clinician insights, acknowledging limitations like small sample sizes and potential drug interactions. The evidence suggests that oral chemotherapy can be a major part of the colorectal cancer patient’s prescription drug plan, providing them a greater sense of control. Several studies, including those by Heinemann et al. (2014), Van Cutsem et al. (2015), Tabernero et al. (2014), Cremolini et al. (2015), and Stintzing et al. (2016), support these findings.
1 out of 25






![[object Object]](/_next/static/media/star-bottom.7253800d.svg)