223BMS: Temperature Effects on Buccal Cell Morphology & DNA Analysis
VerifiedAdded on 2023/06/13
|11
|3216
|444
Report
AI Summary
This report investigates the effects of long-term storage of buccal swabs at various temperatures on cell morphology and DNA permeability. Buccal cells were collected, stained with Haematoxylin and Eosin (H&E), and examined under a microscope. The results indicate that temperature significantly impacts cell and cytoplasm diameters, with higher temperatures leading to smaller cell sizes. Additionally, alcohol treatment increased cell permeability. The study highlights the importance of storage conditions in maintaining the quality of buccal cells for genetic analysis, referencing findings from 223BMS Biological & Forensic Sciences mini-project, which is critical for forensic DNA analysis and other applications. The report concludes that careful control of storage temperature and the use of alcohol can enhance DNA permeability and cell observation.

UNIVERSITY
ASSIGNMENT
Biological & Forensic Sciences
Lab report
NAME
ASSIGNMENT
Biological & Forensic Sciences
Lab report
NAME
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biological & Forensic Sciences 2
Abstract
Effects of long term storage of buccal swabs at various temperature conditions have shown to
have direct impact on the PCR amplification and other cell and cytoplasm parameters.
Various reports have evaluated the different sampling procedures’ and the storage conditions
effects on the size and quantity of isolated DNA. This experimental study undertakes
assessment of buccal cells in various conditions of temperature and solvent applications. The
buccal cells swabs were obtained from participants and treated under Haematoxylin and
Eosin (H&E) stains to be assessed on microcopy examination. Results showed that
temperature has an effect on the storage and quantity size of cell and cytoplasm diameters of
the buccal cells. Higher cytoplasm and nucleus diameter was observed under room temperate,
lower size being observed at higher temperature of 40 degrees Celsius, on subjecting to
alcohol solvent, there was increased permeability ability on the cells. Thus qualities of buccal
cells depend on the storage conditions which have effects on the genetic permeability of the
cells.
Abstract
Effects of long term storage of buccal swabs at various temperature conditions have shown to
have direct impact on the PCR amplification and other cell and cytoplasm parameters.
Various reports have evaluated the different sampling procedures’ and the storage conditions
effects on the size and quantity of isolated DNA. This experimental study undertakes
assessment of buccal cells in various conditions of temperature and solvent applications. The
buccal cells swabs were obtained from participants and treated under Haematoxylin and
Eosin (H&E) stains to be assessed on microcopy examination. Results showed that
temperature has an effect on the storage and quantity size of cell and cytoplasm diameters of
the buccal cells. Higher cytoplasm and nucleus diameter was observed under room temperate,
lower size being observed at higher temperature of 40 degrees Celsius, on subjecting to
alcohol solvent, there was increased permeability ability on the cells. Thus qualities of buccal
cells depend on the storage conditions which have effects on the genetic permeability of the
cells.

Biological & Forensic Sciences 3
Introduction
Buccal cells (squamous epithelial cells from the oral cavity) are used extensively as a
source of DNA for the forensic identification of individuals using the DNA profiling
technique known as ‘DNA17’. The interest of getting clearer concept of genetics and various
diseases and various body processes have increased, requiring the isolation of DNA. Over the
years blood samples have been an excellent source of obtaining DNA, however extraction of
DNA from the buccal cells has brought new perspective in DNA analysis, (Benschop et al.,
2010 pp. 118).
There are currently two types of procedures which are currently available for the buccal
cell collection, dry and wet collection methods are currently available. Wet method entails
swishing liquids in mouth and spitting them into a collecting cup. This process has been
shown to yield higher amounts of longer fragments of DNA which requires more
cumbersome and higher costs. Dry procedures use buccal swabs and cytobrushes. These
methods are often simpler and cheap and less sensitive to effect of long term storage methods
at room temperature. According to recent studies , the utilization of cytobruches have
appeared to be more appropriate in facilitating collection of human DNA genome exhibiting
good quality and high security, (Thomasma & Foran, 2013, pp 466).
However, the microscopic comparison of these buccal cells remains an under-evaluated
area of study. A study by Donald et al. in 2013, compared the buccal mucosal cells from 400
females to determine changes related to the hormonal status of the donors and this indicated
that it may be possible to distinguish cells from different age ranges and menstrual cycle
stages of females. This was achieved through measurement of the average Cell Diameter
(CD) and Nucleus Diameter (ND) and the ratio of these two measured values (ND:CD).
Various factors have been advanced on DNA degradation as it contains in buccal cells.
DNA degradation occurs when the samples are exposed to varied environmental insults and
chemical, (Dowlman et al, 2010 pp 168). These include factors such as light, humidity which
is attributed to more effect on quality rather the quantity, elevated temperature and moisture
content which degrades DNA and make it difficult for profiling of fungal contamination and
interval post mortem. The survival of these DNA however is dependent on various factors
and varied environmental conditions, (Verdon, Mitchell & Van, 2013 pp 168).
Obtaining DNA genome from buccal epithelial cells has overcome many problems with
venipuncture which require extraction of blood through phlebotomist to draw blood. Self
collection of buccal cells can be undertaken. This collection is less invasive technique which
often requires less invasive technique to obtain the DNA samples, (Nandita et al, 2014 pp
Introduction
Buccal cells (squamous epithelial cells from the oral cavity) are used extensively as a
source of DNA for the forensic identification of individuals using the DNA profiling
technique known as ‘DNA17’. The interest of getting clearer concept of genetics and various
diseases and various body processes have increased, requiring the isolation of DNA. Over the
years blood samples have been an excellent source of obtaining DNA, however extraction of
DNA from the buccal cells has brought new perspective in DNA analysis, (Benschop et al.,
2010 pp. 118).
There are currently two types of procedures which are currently available for the buccal
cell collection, dry and wet collection methods are currently available. Wet method entails
swishing liquids in mouth and spitting them into a collecting cup. This process has been
shown to yield higher amounts of longer fragments of DNA which requires more
cumbersome and higher costs. Dry procedures use buccal swabs and cytobrushes. These
methods are often simpler and cheap and less sensitive to effect of long term storage methods
at room temperature. According to recent studies , the utilization of cytobruches have
appeared to be more appropriate in facilitating collection of human DNA genome exhibiting
good quality and high security, (Thomasma & Foran, 2013, pp 466).
However, the microscopic comparison of these buccal cells remains an under-evaluated
area of study. A study by Donald et al. in 2013, compared the buccal mucosal cells from 400
females to determine changes related to the hormonal status of the donors and this indicated
that it may be possible to distinguish cells from different age ranges and menstrual cycle
stages of females. This was achieved through measurement of the average Cell Diameter
(CD) and Nucleus Diameter (ND) and the ratio of these two measured values (ND:CD).
Various factors have been advanced on DNA degradation as it contains in buccal cells.
DNA degradation occurs when the samples are exposed to varied environmental insults and
chemical, (Dowlman et al, 2010 pp 168). These include factors such as light, humidity which
is attributed to more effect on quality rather the quantity, elevated temperature and moisture
content which degrades DNA and make it difficult for profiling of fungal contamination and
interval post mortem. The survival of these DNA however is dependent on various factors
and varied environmental conditions, (Verdon, Mitchell & Van, 2013 pp 168).
Obtaining DNA genome from buccal epithelial cells has overcome many problems with
venipuncture which require extraction of blood through phlebotomist to draw blood. Self
collection of buccal cells can be undertaken. This collection is less invasive technique which
often requires less invasive technique to obtain the DNA samples, (Nandita et al, 2014 pp
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Biological & Forensic Sciences 4
59).
Various staining process have been undertaken with regard to buccal cells. Use of
hematoxylin and eosin has been used over a century and it is still essential in identifying
various tissue types and different morphological changes in cells. The stain works well with
many varied fixatives, (Verdon, Mitchell & Van, 2014 pp 1080). Hematoxylin has a deep
purple color which offers staining on the nucleus through complex reaction. On the other end
Eosin is pink in color and offer staining to proteins non specifically. In typical tissues, nuclei
are stained to be blue while the cytoplasm and the extracellular matrix have varied degrees of
staining. Hence staining are very essential in disclosing the abundant structure of structural
information which have specific functionalities.
Focus on exfoliative cytology has been undertaken with key focus on microscopy
examination being undertaken, (Kumar et al, 2011 pp 293). Mucous membrane has been
focused with this approach. Haematoxylin and Eosin (H&E) stains used in the buccal swabs
have been effective in using different color on the cellular differentiation. With
advancements used in quantitative exfoliative cytology, oral cytology process has been
enhanced and used as an emerging diagnostic tool in diagnosing oral lesions, (Marshall et al,
2014 pp 192).
Assistance of computer morph metric analysis have been shown to improve on the
overall parameters such as the nuclei diameter, cell diameter and nuclear area, cytoplasm area
and nuclear to cytoplasm ratio, (Goregen, Ankul & Gundogdu, 2011 pp 10). The yield of the
buccal cells have shown to vary in terms of quantity from those obtained from blood cells and
vary in term s of how the cells have been acquired, stored and transported. Thus this
experimental study seeks to investigate the effect of Haematoxylin and Eosin (H&E) stains
on heat treatment conditions through microscopic observation.
Methods
The present study has been conducted on four participants in the academic institution.
The subjects were of age group 25-30 years and it entails 2 males and 2 females. This study
utilized experimental design research to investigate the effects of temperature on storage of
buccal cells under Haematoxylin and Eosin (H&E) stains and with buccal swabs.
Ethical clearance was sought from the department of Biological sciences at the
university. Patient data sheet was obtained to explain to the participant on the required
information of the study, there after a count was sought from the participants.
The participants were asked to rinse their mouth to remove debris in the oral cavity.
Buccal cells were obtained and transferred using buccal swap on a dry glass slide. There after
59).
Various staining process have been undertaken with regard to buccal cells. Use of
hematoxylin and eosin has been used over a century and it is still essential in identifying
various tissue types and different morphological changes in cells. The stain works well with
many varied fixatives, (Verdon, Mitchell & Van, 2014 pp 1080). Hematoxylin has a deep
purple color which offers staining on the nucleus through complex reaction. On the other end
Eosin is pink in color and offer staining to proteins non specifically. In typical tissues, nuclei
are stained to be blue while the cytoplasm and the extracellular matrix have varied degrees of
staining. Hence staining are very essential in disclosing the abundant structure of structural
information which have specific functionalities.
Focus on exfoliative cytology has been undertaken with key focus on microscopy
examination being undertaken, (Kumar et al, 2011 pp 293). Mucous membrane has been
focused with this approach. Haematoxylin and Eosin (H&E) stains used in the buccal swabs
have been effective in using different color on the cellular differentiation. With
advancements used in quantitative exfoliative cytology, oral cytology process has been
enhanced and used as an emerging diagnostic tool in diagnosing oral lesions, (Marshall et al,
2014 pp 192).
Assistance of computer morph metric analysis have been shown to improve on the
overall parameters such as the nuclei diameter, cell diameter and nuclear area, cytoplasm area
and nuclear to cytoplasm ratio, (Goregen, Ankul & Gundogdu, 2011 pp 10). The yield of the
buccal cells have shown to vary in terms of quantity from those obtained from blood cells and
vary in term s of how the cells have been acquired, stored and transported. Thus this
experimental study seeks to investigate the effect of Haematoxylin and Eosin (H&E) stains
on heat treatment conditions through microscopic observation.
Methods
The present study has been conducted on four participants in the academic institution.
The subjects were of age group 25-30 years and it entails 2 males and 2 females. This study
utilized experimental design research to investigate the effects of temperature on storage of
buccal cells under Haematoxylin and Eosin (H&E) stains and with buccal swabs.
Ethical clearance was sought from the department of Biological sciences at the
university. Patient data sheet was obtained to explain to the participant on the required
information of the study, there after a count was sought from the participants.
The participants were asked to rinse their mouth to remove debris in the oral cavity.
Buccal cells were obtained and transferred using buccal swap on a dry glass slide. There after
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Biological & Forensic Sciences 5
staining was done. Rapid PAP kit of biolab was used for staining. The smears were fixed in
tap water followed by rapid PAP in 45 s. The stained smears were observed under 50X
microscope, with measurements of cell. Different temperature ranges were subjected to the
buccal cells; photo micrographic images were under taken. The captured images captured for
further analysis for calibration of image. Assessment of morph metric parameters was done.
Nuclear diameter and cell diameter was undertaken using digitalized interactive measurement
tool used for tracing the perpendicular lines.
Statistical methods applied on this experimental study included mann Whitney U test
comparison for group analysis for nuclear diameter and cytplasmic diameter.
Results
The results of the experiment revealed the following results when the buccal cells were
subjected to varying temperatures
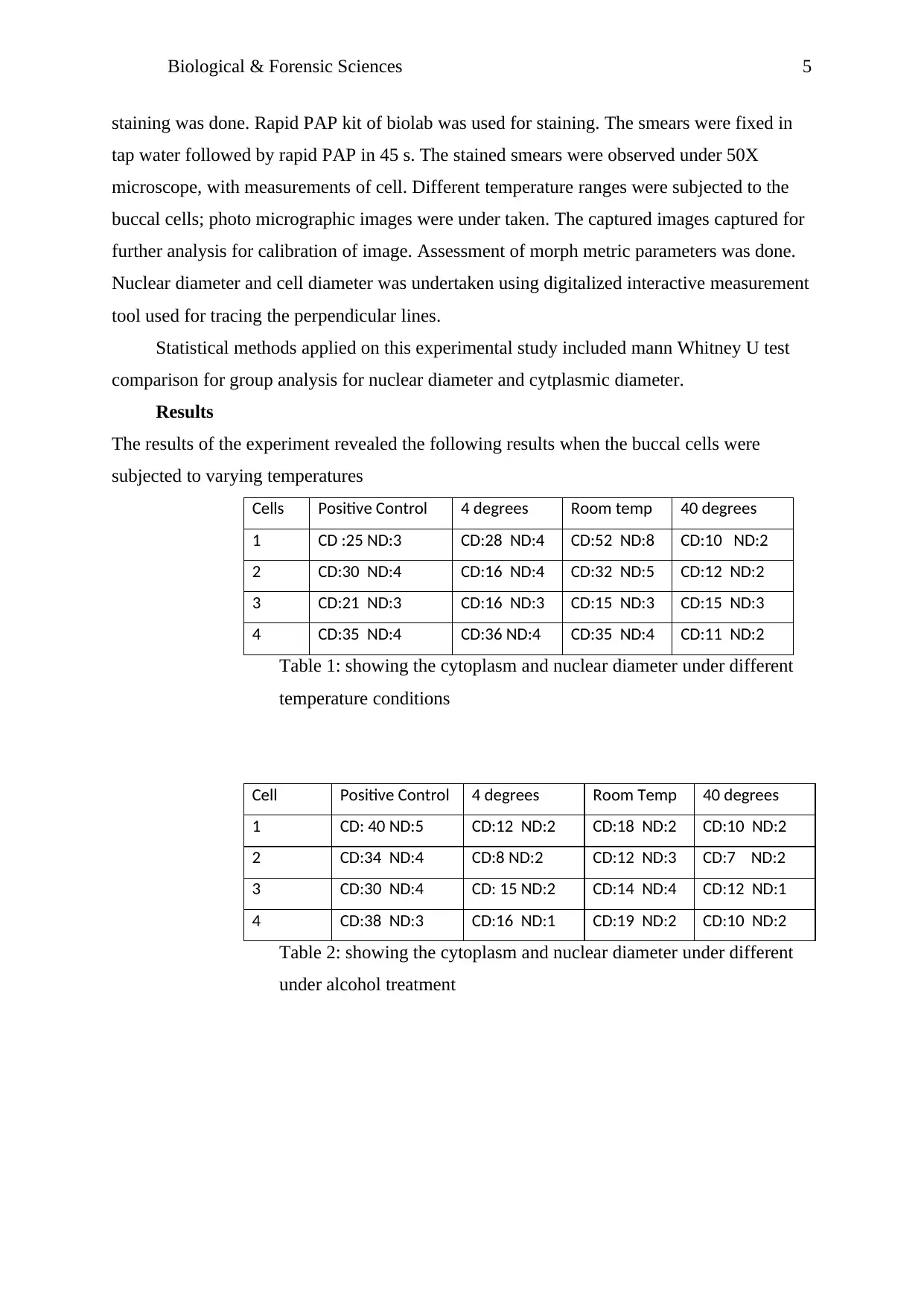
Cells Positive Control 4 degrees Room temp 40 degrees
1 CD :25 ND:3 CD:28 ND:4 CD:52 ND:8 CD:10 ND:2
2 CD:30 ND:4 CD:16 ND:4 CD:32 ND:5 CD:12 ND:2
3 CD:21 ND:3 CD:16 ND:3 CD:15 ND:3 CD:15 ND:3
4 CD:35 ND:4 CD:36 ND:4 CD:35 ND:4 CD:11 ND:2
Table 1: showing the cytoplasm and nuclear diameter under different
temperature conditions
Cell Positive Control 4 degrees Room Temp 40 degrees
1 CD: 40 ND:5 CD:12 ND:2 CD:18 ND:2 CD:10 ND:2
2 CD:34 ND:4 CD:8 ND:2 CD:12 ND:3 CD:7 ND:2
3 CD:30 ND:4 CD: 15 ND:2 CD:14 ND:4 CD:12 ND:1
4 CD:38 ND:3 CD:16 ND:1 CD:19 ND:2 CD:10 ND:2
Table 2: showing the cytoplasm and nuclear diameter under different
under alcohol treatment
staining was done. Rapid PAP kit of biolab was used for staining. The smears were fixed in
tap water followed by rapid PAP in 45 s. The stained smears were observed under 50X
microscope, with measurements of cell. Different temperature ranges were subjected to the
buccal cells; photo micrographic images were under taken. The captured images captured for
further analysis for calibration of image. Assessment of morph metric parameters was done.
Nuclear diameter and cell diameter was undertaken using digitalized interactive measurement
tool used for tracing the perpendicular lines.
Statistical methods applied on this experimental study included mann Whitney U test
comparison for group analysis for nuclear diameter and cytplasmic diameter.
Results
The results of the experiment revealed the following results when the buccal cells were
subjected to varying temperatures
Cells Positive Control 4 degrees Room temp 40 degrees
1 CD :25 ND:3 CD:28 ND:4 CD:52 ND:8 CD:10 ND:2
2 CD:30 ND:4 CD:16 ND:4 CD:32 ND:5 CD:12 ND:2
3 CD:21 ND:3 CD:16 ND:3 CD:15 ND:3 CD:15 ND:3
4 CD:35 ND:4 CD:36 ND:4 CD:35 ND:4 CD:11 ND:2
Table 1: showing the cytoplasm and nuclear diameter under different
temperature conditions
Cell Positive Control 4 degrees Room Temp 40 degrees
1 CD: 40 ND:5 CD:12 ND:2 CD:18 ND:2 CD:10 ND:2
2 CD:34 ND:4 CD:8 ND:2 CD:12 ND:3 CD:7 ND:2
3 CD:30 ND:4 CD: 15 ND:2 CD:14 ND:4 CD:12 ND:1
4 CD:38 ND:3 CD:16 ND:1 CD:19 ND:2 CD:10 ND:2
Table 2: showing the cytoplasm and nuclear diameter under different
under alcohol treatment
Biological & Forensic Sciences 6
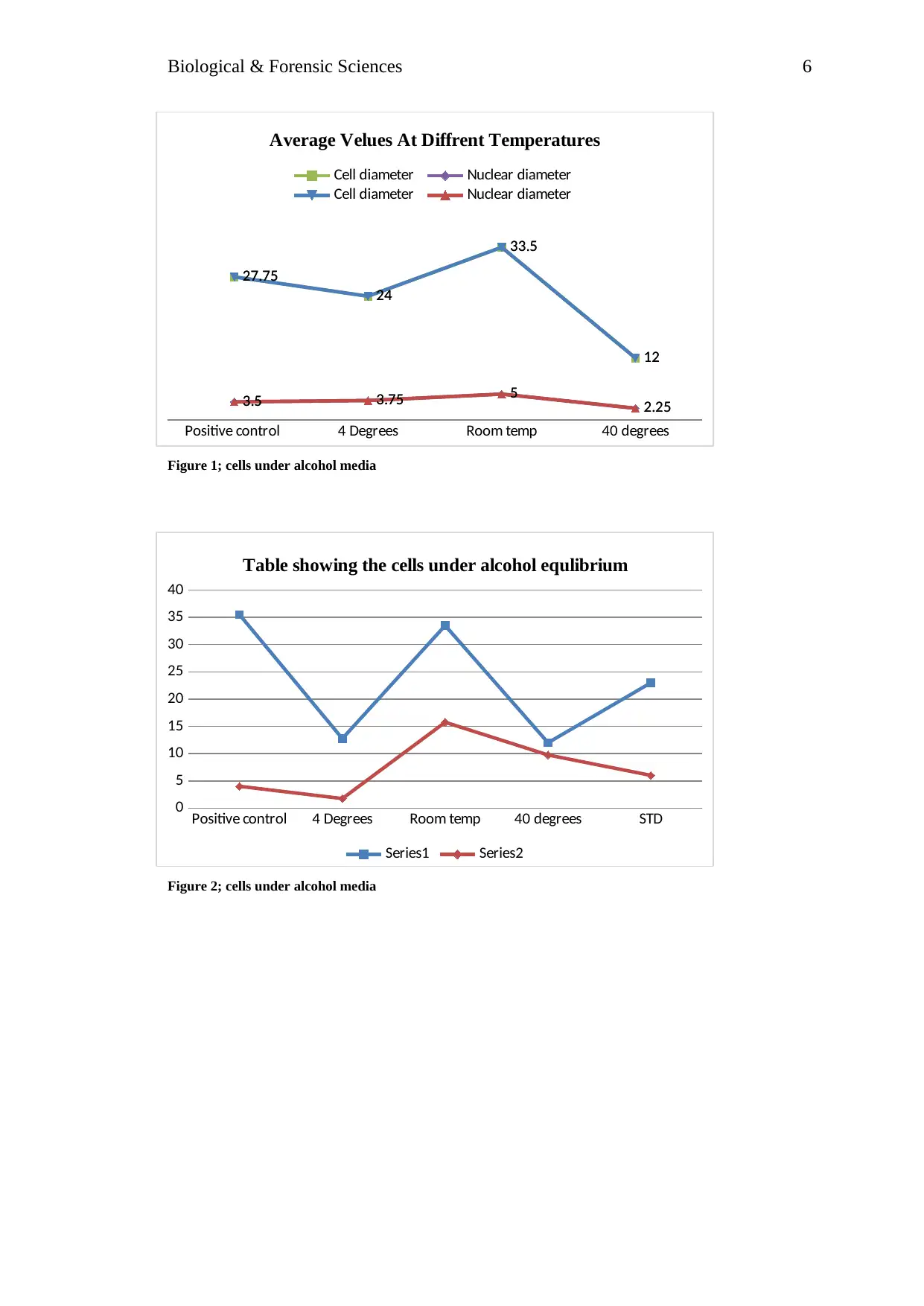
Positive control 4 Degrees Room temp 40 degrees
27.75
24
33.5
12
3.5 3.75 5 2.25
27.75
24
33.5
12
3.5 3.75 5 2.25
Average Velues At Diffrent Temperatures
Cell diameter Nuclear diameter
Cell diameter Nuclear diameter
Figure 1; cells under alcohol media
Positive control 4 Degrees Room temp 40 degrees STD
0
5
10
15
20
25
30
35
40
Table showing the cells under alcohol equlibrium
Series1 Series2
Figure 2; cells under alcohol media
Positive control 4 Degrees Room temp 40 degrees
27.75
24
33.5
12
3.5 3.75 5 2.25
27.75
24
33.5
12
3.5 3.75 5 2.25
Average Velues At Diffrent Temperatures
Cell diameter Nuclear diameter
Cell diameter Nuclear diameter
Figure 1; cells under alcohol media
Positive control 4 Degrees Room temp 40 degrees STD
0
5
10
15
20
25
30
35
40
Table showing the cells under alcohol equlibrium
Series1 Series2
Figure 2; cells under alcohol media
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Biological & Forensic Sciences 7
Positive control 4 Degrees Room temp 40 degrees
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
Table showing the cells under alcohol equlibrium
Axis Title
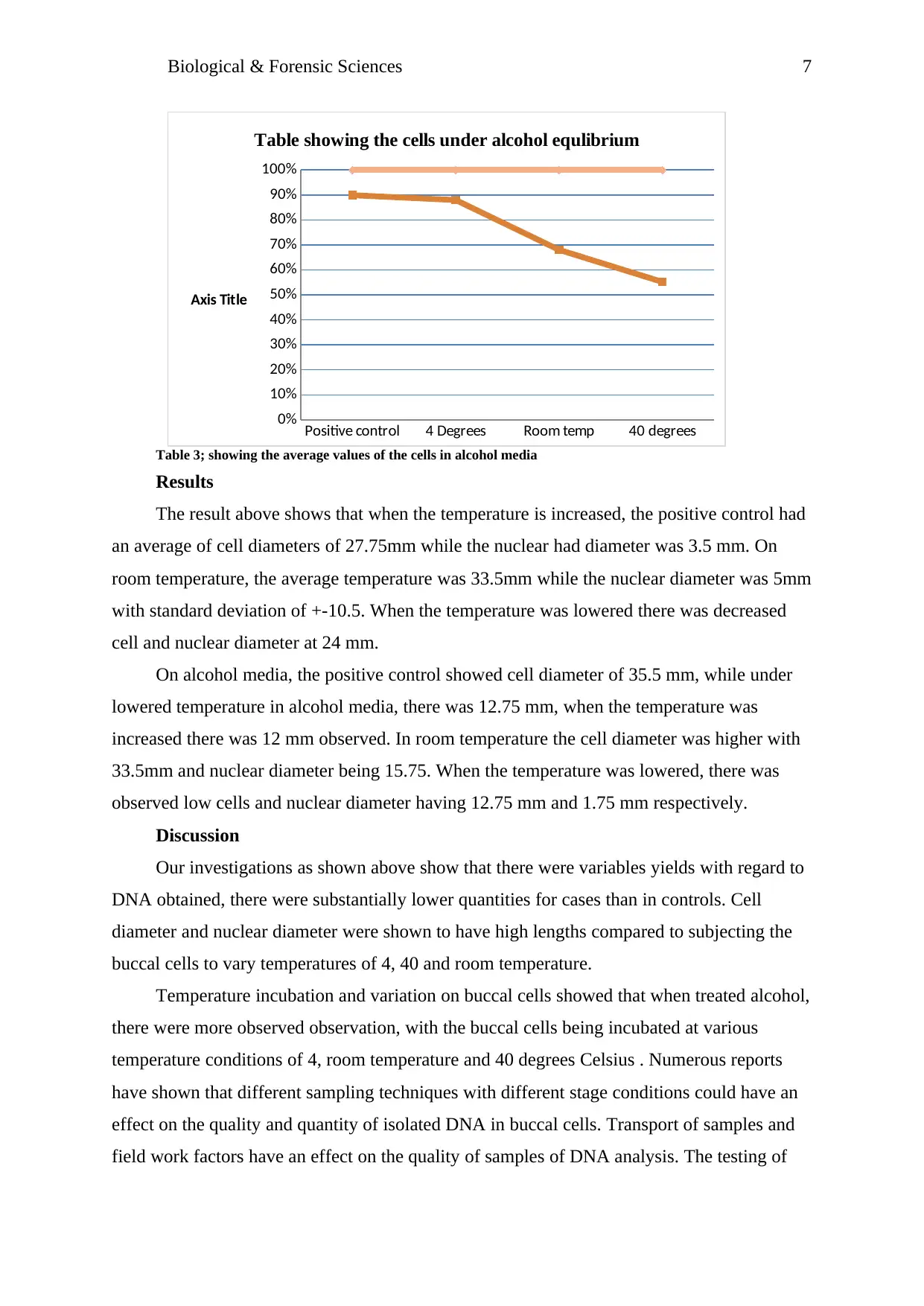
Table 3; showing the average values of the cells in alcohol media
Results
The result above shows that when the temperature is increased, the positive control had
an average of cell diameters of 27.75mm while the nuclear had diameter was 3.5 mm. On
room temperature, the average temperature was 33.5mm while the nuclear diameter was 5mm
with standard deviation of +-10.5. When the temperature was lowered there was decreased
cell and nuclear diameter at 24 mm.
On alcohol media, the positive control showed cell diameter of 35.5 mm, while under
lowered temperature in alcohol media, there was 12.75 mm, when the temperature was
increased there was 12 mm observed. In room temperature the cell diameter was higher with
33.5mm and nuclear diameter being 15.75. When the temperature was lowered, there was
observed low cells and nuclear diameter having 12.75 mm and 1.75 mm respectively.
Discussion
Our investigations as shown above show that there were variables yields with regard to
DNA obtained, there were substantially lower quantities for cases than in controls. Cell
diameter and nuclear diameter were shown to have high lengths compared to subjecting the
buccal cells to vary temperatures of 4, 40 and room temperature.
Temperature incubation and variation on buccal cells showed that when treated alcohol,
there were more observed observation, with the buccal cells being incubated at various
temperature conditions of 4, room temperature and 40 degrees Celsius . Numerous reports
have shown that different sampling techniques with different stage conditions could have an
effect on the quality and quantity of isolated DNA in buccal cells. Transport of samples and
field work factors have an effect on the quality of samples of DNA analysis. The testing of
Positive control 4 Degrees Room temp 40 degrees
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
Table showing the cells under alcohol equlibrium
Axis Title
Table 3; showing the average values of the cells in alcohol media
Results
The result above shows that when the temperature is increased, the positive control had
an average of cell diameters of 27.75mm while the nuclear had diameter was 3.5 mm. On
room temperature, the average temperature was 33.5mm while the nuclear diameter was 5mm
with standard deviation of +-10.5. When the temperature was lowered there was decreased
cell and nuclear diameter at 24 mm.
On alcohol media, the positive control showed cell diameter of 35.5 mm, while under
lowered temperature in alcohol media, there was 12.75 mm, when the temperature was
increased there was 12 mm observed. In room temperature the cell diameter was higher with
33.5mm and nuclear diameter being 15.75. When the temperature was lowered, there was
observed low cells and nuclear diameter having 12.75 mm and 1.75 mm respectively.
Discussion
Our investigations as shown above show that there were variables yields with regard to
DNA obtained, there were substantially lower quantities for cases than in controls. Cell
diameter and nuclear diameter were shown to have high lengths compared to subjecting the
buccal cells to vary temperatures of 4, 40 and room temperature.
Temperature incubation and variation on buccal cells showed that when treated alcohol,
there were more observed observation, with the buccal cells being incubated at various
temperature conditions of 4, room temperature and 40 degrees Celsius . Numerous reports
have shown that different sampling techniques with different stage conditions could have an
effect on the quality and quantity of isolated DNA in buccal cells. Transport of samples and
field work factors have an effect on the quality of samples of DNA analysis. The testing of
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biological & Forensic Sciences 8
the prolongation of storage time in buccal swabs at the three tier temperature ranges shows
that are that DNA quantity can have an effect, (Thomasma & Foran, 2013 pp 468).
Permeability of DNA was further observed with the treatment of alcohol on the buccal
cells. This was exhibited by the large proportion of DNA in the bacterial growth. Compared
to positive control treatment on alcohol, there was significant difference on the different
temporary treatment son the samples.
Forensic DNA analysis has often utilized buccal swabs for extraction of DNA cells.
Testing done have shown that significant quantities of the DNA are often retained in the swab
and later get lost. Testing assessment has indicated that variation of temperature plays key
role. The incubation time protocol and temperature with added stains are key in enhancing
permeability of the cell and cytoplasm nucleus. Incubations process in this study under
various temperature states and subjecting it to alcohol staining. Samples incubated under
alcohol showed increase permeability and observation of DN in the buccal cells, (Zech,
Malik & Thali, 2012 pp 1038).
Other studies linking buccal cells incubation with temperature have shown varied
results. Increasing the temperature at the incubation with buccal cells that there were lower
quantity of buccal cells at 65°C. When incubation was done at shaking of 900 rpm at 65°C,
the average quantification time had lowered than the 55°C. The buccal cell quantification and
difference conditions were shown to indicate significant difference in this study. There was
an average increase in cell diameter and cytoplasm diameter for the positives controls
compared to variation of the different temperature states.
DNA available in buccal cells can be rapidly degraded by post mortem which initial
enzymes of the cells dies thus losing its structure and overall integrity which affect later the
environmental conditions. Temperatures play crucial role and degradation of DNA and its
overall survival. This is relying on the integrity conditions of the biological conditions of the
location, (Suvarna et al, 2012). When temperature increases often damage occurs this is rapid
over time.
Microbes often don’t have any way of regulating internal temperature so they evolve
temperature and environmental adaptations. Changes in temperature have an effect on the
enzymes and their activity. Temperatures below the optimal often leads to faster metabolism
which yields decrease enzyme activity and lowered metabolism rate, while higher
temperature have been shown to have denaturing effect on the proteins such as the enzymes.
Microbes have growth curve which have an optimal temperature which the growth peaks and
minimum and maximum temperature which exhibit growth, (Sumanthi et al, 2012).
the prolongation of storage time in buccal swabs at the three tier temperature ranges shows
that are that DNA quantity can have an effect, (Thomasma & Foran, 2013 pp 468).
Permeability of DNA was further observed with the treatment of alcohol on the buccal
cells. This was exhibited by the large proportion of DNA in the bacterial growth. Compared
to positive control treatment on alcohol, there was significant difference on the different
temporary treatment son the samples.
Forensic DNA analysis has often utilized buccal swabs for extraction of DNA cells.
Testing done have shown that significant quantities of the DNA are often retained in the swab
and later get lost. Testing assessment has indicated that variation of temperature plays key
role. The incubation time protocol and temperature with added stains are key in enhancing
permeability of the cell and cytoplasm nucleus. Incubations process in this study under
various temperature states and subjecting it to alcohol staining. Samples incubated under
alcohol showed increase permeability and observation of DN in the buccal cells, (Zech,
Malik & Thali, 2012 pp 1038).
Other studies linking buccal cells incubation with temperature have shown varied
results. Increasing the temperature at the incubation with buccal cells that there were lower
quantity of buccal cells at 65°C. When incubation was done at shaking of 900 rpm at 65°C,
the average quantification time had lowered than the 55°C. The buccal cell quantification and
difference conditions were shown to indicate significant difference in this study. There was
an average increase in cell diameter and cytoplasm diameter for the positives controls
compared to variation of the different temperature states.
DNA available in buccal cells can be rapidly degraded by post mortem which initial
enzymes of the cells dies thus losing its structure and overall integrity which affect later the
environmental conditions. Temperatures play crucial role and degradation of DNA and its
overall survival. This is relying on the integrity conditions of the biological conditions of the
location, (Suvarna et al, 2012). When temperature increases often damage occurs this is rapid
over time.
Microbes often don’t have any way of regulating internal temperature so they evolve
temperature and environmental adaptations. Changes in temperature have an effect on the
enzymes and their activity. Temperatures below the optimal often leads to faster metabolism
which yields decrease enzyme activity and lowered metabolism rate, while higher
temperature have been shown to have denaturing effect on the proteins such as the enzymes.
Microbes have growth curve which have an optimal temperature which the growth peaks and
minimum and maximum temperature which exhibit growth, (Sumanthi et al, 2012).

Biological & Forensic Sciences 9
Humans exhibit mesophile microbes , which have growth optima of 37 degrees Celsius
and ranges for 20-45 degrees Celsius. Nearly all human micro flora fall in this category with
other human pathogens. This mesophiles have same occupation of environment which
humans have. On the other end , thermophiles have same spectrum as they prefer higher
temperature. Thermophils have temperature ranges ranging from 45degrees to 80 degrees
with growth optimum of 60 degrees Celsius, (Warnakulasuriya et al, 2014 pp 268).
Further other factors have been assessed with regard to humidity and the survival of the
buccal cells DNA. Exposure of biological material to the external environment with high
quantity of water and elevated temperature often leads to growth of micro organisms such as
bacteria and fungi.
Thus from the results it is evident that, temperature has effect on permeability for
buccal cells. At room temperature the buccal cells exhibited increased permeability as it was
observable with the cell and cytoplasm diameters compared to lowered temperature and
higher temperature environments. Thus storage temperature of buccal cells plays critical
factor in its cell mediation and permeability. When the cells are subjected to alcohol
conditions, the positive control showed increased permeability of the cells compared to
normal room temperature, at lower temperature and at an increased environmental condition.
Humans exhibit mesophile microbes , which have growth optima of 37 degrees Celsius
and ranges for 20-45 degrees Celsius. Nearly all human micro flora fall in this category with
other human pathogens. This mesophiles have same occupation of environment which
humans have. On the other end , thermophiles have same spectrum as they prefer higher
temperature. Thermophils have temperature ranges ranging from 45degrees to 80 degrees
with growth optimum of 60 degrees Celsius, (Warnakulasuriya et al, 2014 pp 268).
Further other factors have been assessed with regard to humidity and the survival of the
buccal cells DNA. Exposure of biological material to the external environment with high
quantity of water and elevated temperature often leads to growth of micro organisms such as
bacteria and fungi.
Thus from the results it is evident that, temperature has effect on permeability for
buccal cells. At room temperature the buccal cells exhibited increased permeability as it was
observable with the cell and cytoplasm diameters compared to lowered temperature and
higher temperature environments. Thus storage temperature of buccal cells plays critical
factor in its cell mediation and permeability. When the cells are subjected to alcohol
conditions, the positive control showed increased permeability of the cells compared to
normal room temperature, at lower temperature and at an increased environmental condition.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Biological & Forensic Sciences 10
References
Benschop, C.C., Wiebosch, D.C., Kloosterman, A.D. and Sijen, T., 2010. Post-coital vaginal
sampling with nylon flocked swabs improves DNA typing. Forensic science international:
genetics, 4(2), pp.115-121.
Donald, P.M., George, R., Sriram, G., Kavitha, B. and Sivapathasundharam, B., 2013.
Hormonal changes in exfoliated normal buccal mucosal cells. Journal of Cytology/Indian
Academy of Cytologists, 30(4), p.252.
Dowlman, E.A., Martin, N.C., Foy, M.J., Lochner, T. and Neocleous, T., 2010. The
prevalence of mixed DNA profiles on fingernail swabs. Science and Justice, 50(2), pp.64-71.
GÖREGEN, M., AKGÜL, H.M. and GÜNDOĞDU, C., 2011. The cytomorphological
analysis of buccal mucosa cells in smokers. Turkish Journal of Medical Sciences, 41(2),
pp.205-210.
Kumar, S., Vezhavendhan, N. and Priya, S., 2011. Role of oral exfoliative cytology in oral
leukoplakia and squamous cell carcinoma. International Journal of Clinical Dental Science,
2(1).
Marshall, P.L., Stoljarova, M., Larue, B.L., King, J.L. and Budowle, B., 2014. Evaluation of
a novel material, Diomics X-Swab™, for collection of DNA. Forensic Science International:
Genetics, 12, pp.192-198.
Nandita, K.P., Boaz, K., Srikant, N., Lewis, A.J. and Manaktala, N., 2014. Oral epithelium in
diabetics: A cytomorphometric correlation. Dental Hypotheses, 5(2), p.59.
Sumanthi, J., Reddy, G.S., Anuradha, C.H., Sekhar, P.C., Prasad, L.K. and Reddy, B.R.,
2012. A study on cytomorphometric analysis of exfoliative buccal cells in iron deficiency
anemic patients. Contemporary clinical dentistry, 3(Suppl 2), p.S156.
Suvarna, M., Anuradha, C., Kumar, K.K., Sekhar, P.C., Chandra, K.L.P. and Reddy, B.R.,
2012. Cytomorphometric analysis of exfoliative buccal cells in type II diabetic patients.
Journal of Dr. NTR University of Health Sciences, 1(1), p.33.
Thomasma, S.M. and Foran, D.R., 2013. The influence of swabbing solutions on DNA
recovery from touch samples. Journal of Forensic sciences, 58(2), pp.465-469.
References
Benschop, C.C., Wiebosch, D.C., Kloosterman, A.D. and Sijen, T., 2010. Post-coital vaginal
sampling with nylon flocked swabs improves DNA typing. Forensic science international:
genetics, 4(2), pp.115-121.
Donald, P.M., George, R., Sriram, G., Kavitha, B. and Sivapathasundharam, B., 2013.
Hormonal changes in exfoliated normal buccal mucosal cells. Journal of Cytology/Indian
Academy of Cytologists, 30(4), p.252.
Dowlman, E.A., Martin, N.C., Foy, M.J., Lochner, T. and Neocleous, T., 2010. The
prevalence of mixed DNA profiles on fingernail swabs. Science and Justice, 50(2), pp.64-71.
GÖREGEN, M., AKGÜL, H.M. and GÜNDOĞDU, C., 2011. The cytomorphological
analysis of buccal mucosa cells in smokers. Turkish Journal of Medical Sciences, 41(2),
pp.205-210.
Kumar, S., Vezhavendhan, N. and Priya, S., 2011. Role of oral exfoliative cytology in oral
leukoplakia and squamous cell carcinoma. International Journal of Clinical Dental Science,
2(1).
Marshall, P.L., Stoljarova, M., Larue, B.L., King, J.L. and Budowle, B., 2014. Evaluation of
a novel material, Diomics X-Swab™, for collection of DNA. Forensic Science International:
Genetics, 12, pp.192-198.
Nandita, K.P., Boaz, K., Srikant, N., Lewis, A.J. and Manaktala, N., 2014. Oral epithelium in
diabetics: A cytomorphometric correlation. Dental Hypotheses, 5(2), p.59.
Sumanthi, J., Reddy, G.S., Anuradha, C.H., Sekhar, P.C., Prasad, L.K. and Reddy, B.R.,
2012. A study on cytomorphometric analysis of exfoliative buccal cells in iron deficiency
anemic patients. Contemporary clinical dentistry, 3(Suppl 2), p.S156.
Suvarna, M., Anuradha, C., Kumar, K.K., Sekhar, P.C., Chandra, K.L.P. and Reddy, B.R.,
2012. Cytomorphometric analysis of exfoliative buccal cells in type II diabetic patients.
Journal of Dr. NTR University of Health Sciences, 1(1), p.33.
Thomasma, S.M. and Foran, D.R., 2013. The influence of swabbing solutions on DNA
recovery from touch samples. Journal of Forensic sciences, 58(2), pp.465-469.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biological & Forensic Sciences 11
Verdon, T.J., Mitchell, R.J. and Oorschot, R.A., 2014. Swabs as DNA collection devices for
sampling different biological materials from different substrates. Journal of forensic sciences,
59(4), pp.1080-1089.
Verdon, T.J., Mitchell, R.J. and van Oorschot, R.A., 2013. The influence of substrate on
DNA transfer and extraction efficiency. Forensic Science International: Genetics, 7(1),
pp.167-175.
Warnakulasuriya S, Tilakaretne WM. Potentially malignant disorders. In: Silverman S Jr,
Thongprasom K, Warnakulasuriya S, editors. Oral Medicine and Pathology: A Guide to
Diagnosis and Management. New Delhi: Jaypee Brothers Medical Publishers; 2014. pp. 268–
70.
Zech, W.D., Malik, N. and Thali, M., 2012. Applicability of DNA analysis on adhesive tape
in forensic casework. Journal of forensic sciences, 57(4), pp.1036-1041.
Verdon, T.J., Mitchell, R.J. and Oorschot, R.A., 2014. Swabs as DNA collection devices for
sampling different biological materials from different substrates. Journal of forensic sciences,
59(4), pp.1080-1089.
Verdon, T.J., Mitchell, R.J. and van Oorschot, R.A., 2013. The influence of substrate on
DNA transfer and extraction efficiency. Forensic Science International: Genetics, 7(1),
pp.167-175.
Warnakulasuriya S, Tilakaretne WM. Potentially malignant disorders. In: Silverman S Jr,
Thongprasom K, Warnakulasuriya S, editors. Oral Medicine and Pathology: A Guide to
Diagnosis and Management. New Delhi: Jaypee Brothers Medical Publishers; 2014. pp. 268–
70.
Zech, W.D., Malik, N. and Thali, M., 2012. Applicability of DNA analysis on adhesive tape
in forensic casework. Journal of forensic sciences, 57(4), pp.1036-1041.
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
