Detailed Laboratory Report: Buffer Solutions and pH Experiment
VerifiedAdded on 2023/06/15
|11
|2083
|414
Report
AI Summary
This laboratory report investigates the behavior of buffer solutions, specifically focusing on how dilution affects their pH. The experiment aimed to compare the pH response of buffer solutions to that of non-buffered solutions upon dilution and to prepare standard buffers with accurate measurements. The report details the materials and methods used, including the preparation of Tris-HCl and Tri-Sodium Citrate (TSC) buffer solutions, and the procedures for determining the impact of concentration on pH. Results indicated that the pH of the buffer solutions decreased with reduced concentration; for instance, the pH of Tris-HCl solution dropped from 7.96 at 0.1M to 7.92 at 0.001M. The discussion interprets these findings, confirming the theoretical prediction that dilution lowers pH, and the conclusion emphasizes the importance of buffers in controlling pH in biochemical experiments. Desklib provides access to similar solved assignments and study tools for students.

LABORATORY REPORT 1
LABORATORY REPORT
By Name
Course
Instructor
Institution
Location
Date
LABORATORY REPORT
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

LABORATORY REPORT 2
Abstract
The control of pH is an essential aspect in carrying out biochemistry experiments; this is
because a certain pH is required for an experiment to be carried out. Buffers solutions refer to
the aqueous solutions which consist a mixture of conjugated base and a weak base and their
pH changes slightly when a small amount of strong base or acid is added to it, and they are
used to control the pH of an experiment. The central focus for carrying out this experiment
was to find out how the buffer solution reacts to the dilution in comparison to a non-buffered
solution. To produce some standard buffers while ensuring that all the components are
measured out accurately. The buffer solution with a pH less than 7 is referred to as acidic
buffer solution. Acid buffers are known to contain a weak acid and one of its salt which in
most cases is sodium salt. The Alkaline buffer solution I the buffer solution which has a pH
which is greater than 7 and in most cases made of a weak acid and one of its salts. The
following materials and apparatus were used during the experiment Solid buffers–Tri-Sodium
Citrate (TSC) and Tri-HCL, NaOH, 0.5M, HCL, 0.5M, PH meters, Beakers. The experiment
involved carrying out the following; Preparation of the buffer solutions, determining the
effects of concentration on the PH. It was concluded that the pH of the buffer solution was
lowered when the concentration was reduced. The pH value for the Tris-HCl solution when
the concentration was o.1M the pH was 7.96, and when the solution was diluted further by
add 90Ml of water to attain the concentration of 0.001M the pH was lowered to 7.92.
Abstract
The control of pH is an essential aspect in carrying out biochemistry experiments; this is
because a certain pH is required for an experiment to be carried out. Buffers solutions refer to
the aqueous solutions which consist a mixture of conjugated base and a weak base and their
pH changes slightly when a small amount of strong base or acid is added to it, and they are
used to control the pH of an experiment. The central focus for carrying out this experiment
was to find out how the buffer solution reacts to the dilution in comparison to a non-buffered
solution. To produce some standard buffers while ensuring that all the components are
measured out accurately. The buffer solution with a pH less than 7 is referred to as acidic
buffer solution. Acid buffers are known to contain a weak acid and one of its salt which in
most cases is sodium salt. The Alkaline buffer solution I the buffer solution which has a pH
which is greater than 7 and in most cases made of a weak acid and one of its salts. The
following materials and apparatus were used during the experiment Solid buffers–Tri-Sodium
Citrate (TSC) and Tri-HCL, NaOH, 0.5M, HCL, 0.5M, PH meters, Beakers. The experiment
involved carrying out the following; Preparation of the buffer solutions, determining the
effects of concentration on the PH. It was concluded that the pH of the buffer solution was
lowered when the concentration was reduced. The pH value for the Tris-HCl solution when
the concentration was o.1M the pH was 7.96, and when the solution was diluted further by
add 90Ml of water to attain the concentration of 0.001M the pH was lowered to 7.92.

LABORATORY REPORT 3
Introduction
Buffers solutions refer to the aqueous solutions which consist a mixture of conjugated base
and a weak base or vice versa. The pH of the buffer solution changes slightly when a small
amount of strong base or acid is added to it, and they are used to control the pH of an
experiment (Boyer, 2013, p. 45).
In most cases, the Buffer solutions acquire their resistance to the pH due to the presence of
the availability of an equilibrium between the acid and its conjugate base. By adding some
amount of a strong acid to the equilibrium of the mixture the weak acid and its conjugate base
usually shift to the left.
The objectives for carrying out this experiment was to find out how the buffer solution reacts
to the dilution in comparison to a non-buffered solution. To produce a number of standard
buffers while ensuring that all the components are measured out accurately (Cserhati, 2017,
p. 165).
The buffer solution with a pH less than 7 is referred to as acidic buffer solution. Acid buffers
are known to contain a weak acid and one of its salt which in most cases is sodium salt. The
Alkaline buffer solution I the buffer solution which has a pH which is greater than 7 and in
most cases made of a weak acid and one of its salts (Gueffroy, 2015, p. 201). It is possible to
change the pH of a buffer solution by altering the ratio of base or acid to the salt, or by
selecting a different base or acid and one of its salts (Garrett, 2016, p. 215).
The capacity of the buffer solutions depends upon two factors;
The ratio of the salt to that of the base or acid. The capacity of the buffer is maximum when
the ratio of the salt to the acid or the base is 1:1.
The total buffer concentration.
Introduction
Buffers solutions refer to the aqueous solutions which consist a mixture of conjugated base
and a weak base or vice versa. The pH of the buffer solution changes slightly when a small
amount of strong base or acid is added to it, and they are used to control the pH of an
experiment (Boyer, 2013, p. 45).
In most cases, the Buffer solutions acquire their resistance to the pH due to the presence of
the availability of an equilibrium between the acid and its conjugate base. By adding some
amount of a strong acid to the equilibrium of the mixture the weak acid and its conjugate base
usually shift to the left.
The objectives for carrying out this experiment was to find out how the buffer solution reacts
to the dilution in comparison to a non-buffered solution. To produce a number of standard
buffers while ensuring that all the components are measured out accurately (Cserhati, 2017,
p. 165).
The buffer solution with a pH less than 7 is referred to as acidic buffer solution. Acid buffers
are known to contain a weak acid and one of its salt which in most cases is sodium salt. The
Alkaline buffer solution I the buffer solution which has a pH which is greater than 7 and in
most cases made of a weak acid and one of its salts (Gueffroy, 2015, p. 201). It is possible to
change the pH of a buffer solution by altering the ratio of base or acid to the salt, or by
selecting a different base or acid and one of its salts (Garrett, 2016, p. 215).
The capacity of the buffer solutions depends upon two factors;
The ratio of the salt to that of the base or acid. The capacity of the buffer is maximum when
the ratio of the salt to the acid or the base is 1:1.
The total buffer concentration.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

LABORATORY REPORT 4
From the results which were obtained it was found out that the amount of Tris-HCL that was
required for the preparation of 100mL of O.1M of pH 8 was 1.576g while that was need for
the preparation of the 100mL TSC with 0.1M.
The pH for both the Tris-HCl and the TSC reduced when the concentration of the buffer
solution was reduced. The pH value for the Tris-HCl solution when the concentration was
o.1M the pH was 7.96, and when the solution was diluted further by add 90Ml of water to
attain the concentration of 0.001M the pH was lowered to 7.92.
This report contains the following sections; aims and objectives where the reasons for
carrying out the experiment are explained. The methodology which explains the procedures
followed during the experiment. The materials sections which outline and explain the
apparatus and materials used and how they were used. The results part which shows the data.
The discussion which discusses and explains the findings of the experiment.
Aims/objectives
The objectives of conducting this lab session were to:
To determine how the buffer solutions react to the dilution in comparison to a non-buffered
solution.
To prepare a variety of buffer solutions.
To find out the effects of concentration on the pH.
Materials and methods
During the lab session for the experiment, the following materials and apparatus were
required.
Solid buffers –Tri-Sodium Citrate (TSC) and Tri-HCL
From the results which were obtained it was found out that the amount of Tris-HCL that was
required for the preparation of 100mL of O.1M of pH 8 was 1.576g while that was need for
the preparation of the 100mL TSC with 0.1M.
The pH for both the Tris-HCl and the TSC reduced when the concentration of the buffer
solution was reduced. The pH value for the Tris-HCl solution when the concentration was
o.1M the pH was 7.96, and when the solution was diluted further by add 90Ml of water to
attain the concentration of 0.001M the pH was lowered to 7.92.
This report contains the following sections; aims and objectives where the reasons for
carrying out the experiment are explained. The methodology which explains the procedures
followed during the experiment. The materials sections which outline and explain the
apparatus and materials used and how they were used. The results part which shows the data.
The discussion which discusses and explains the findings of the experiment.
Aims/objectives
The objectives of conducting this lab session were to:
To determine how the buffer solutions react to the dilution in comparison to a non-buffered
solution.
To prepare a variety of buffer solutions.
To find out the effects of concentration on the pH.
Materials and methods
During the lab session for the experiment, the following materials and apparatus were
required.
Solid buffers –Tri-Sodium Citrate (TSC) and Tri-HCL
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

LABORATORY REPORT 5
NaOH, 0.5M
HCL, 0.5M
PH meters
Beakers
The following procedures were followed during the lab session to ensure that the experiment
was performed successfully.
Part 1: Preparation of the buffer solutions.
You will be making up a TSC buffer solution to pH 6.0 and a Tris-HCl buffer to pH 8.0.
The weight of the solid buffer that needed to prepare 100ml of 0.1M solution. Then the solid
buffer was weighed and dissolved in 50ml of water.
By using the pH meter the pH of the solution was measured and then changed to the desired
pH by using the HCL (0.5) or NaOH (0.5) a drop at the time, then the beaker was swirled
after the application of each drop.
More water was added until the 99mL of the solution was achieved and the pH was checked
once again. In the case where it had changed more NaOH or HCL as added to rectify it, it
was recommended to use a lower concentration of the acid or the base (McDougal, 2016, p.
284).
The volume of the solution was made to 10mL (Lundblad, 2015, p. 456).
Part 2: Determining the effects of concentration on the Ph.
10Ml of each of the buffer solution which was prepared in part was diluted by adding 90mL
of water to achieve a concentration of 0.01M.
10Ml of the diluted buffers was diluted further to achieve a concentration of 0.001m.
NaOH, 0.5M
HCL, 0.5M
PH meters
Beakers
The following procedures were followed during the lab session to ensure that the experiment
was performed successfully.
Part 1: Preparation of the buffer solutions.
You will be making up a TSC buffer solution to pH 6.0 and a Tris-HCl buffer to pH 8.0.
The weight of the solid buffer that needed to prepare 100ml of 0.1M solution. Then the solid
buffer was weighed and dissolved in 50ml of water.
By using the pH meter the pH of the solution was measured and then changed to the desired
pH by using the HCL (0.5) or NaOH (0.5) a drop at the time, then the beaker was swirled
after the application of each drop.
More water was added until the 99mL of the solution was achieved and the pH was checked
once again. In the case where it had changed more NaOH or HCL as added to rectify it, it
was recommended to use a lower concentration of the acid or the base (McDougal, 2016, p.
284).
The volume of the solution was made to 10mL (Lundblad, 2015, p. 456).
Part 2: Determining the effects of concentration on the Ph.
10Ml of each of the buffer solution which was prepared in part was diluted by adding 90mL
of water to achieve a concentration of 0.01M.
10Ml of the diluted buffers was diluted further to achieve a concentration of 0.001m.
LABORATORY REPORT 6
The pH of both the undiluted and the diluted buffers solution was measured (Metzler, 2014,
p. 89).
Preparation of standard solution
A weighed amount of substance was placed in a volumetric flask.
A small amount of water was added to the volumetric flask.
The solid is dissolved in water by gently swirling the volumetric flask.
More water is added to the solution until it reaches the etched mark on the neck of the
volumetric flask.
The solution was then mixed thoroughly by inverting the flask several times to enable the
solution to mix appropriately (Perrin, 2016, p. 45).
Results
The results which were obtained during the experiment were recorded and tabulated as shown
below.
Part 2
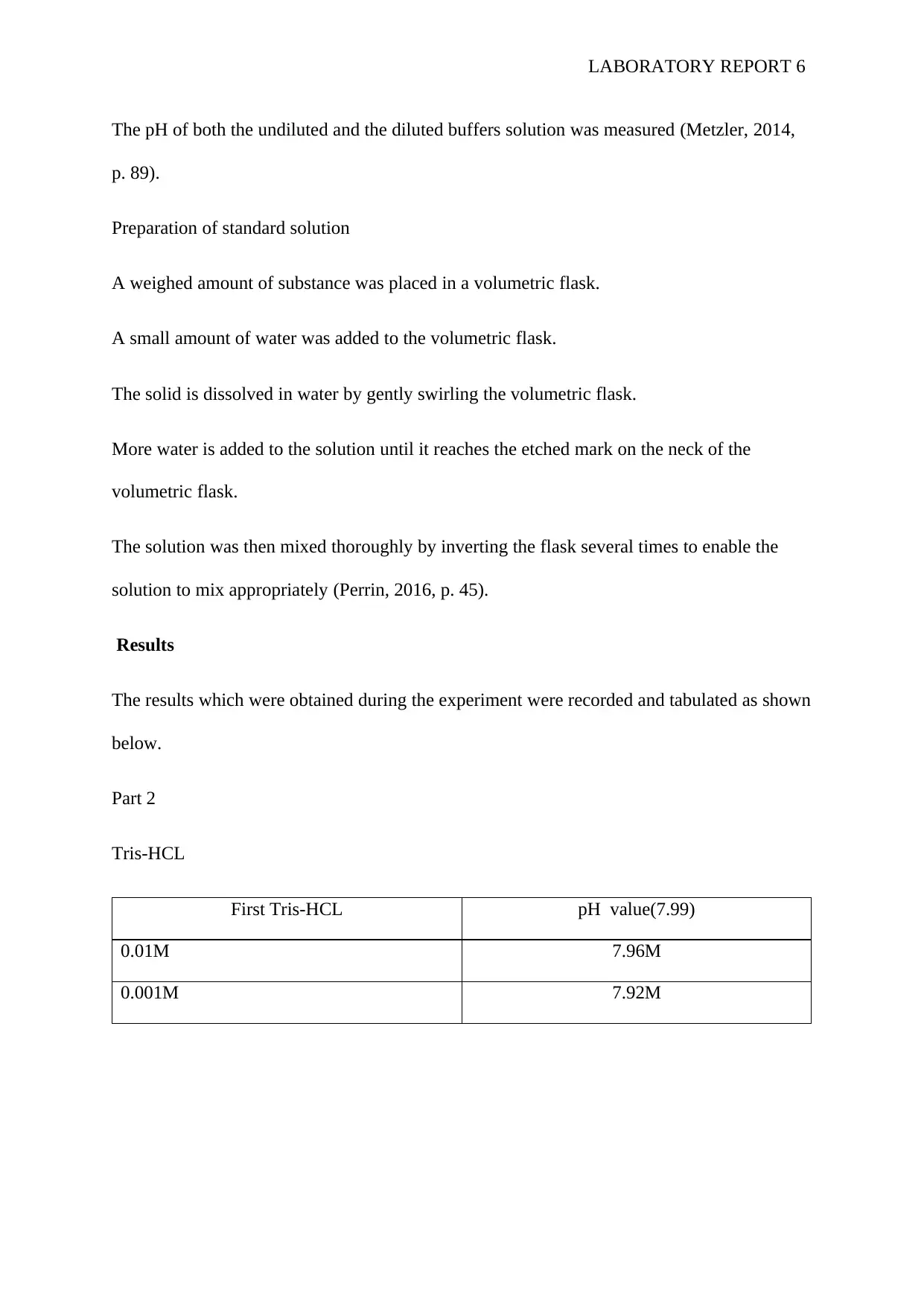
Tris-HCL
First Tris-HCL pH value(7.99)
0.01M 7.96M
0.001M 7.92M
The pH of both the undiluted and the diluted buffers solution was measured (Metzler, 2014,
p. 89).
Preparation of standard solution
A weighed amount of substance was placed in a volumetric flask.
A small amount of water was added to the volumetric flask.
The solid is dissolved in water by gently swirling the volumetric flask.
More water is added to the solution until it reaches the etched mark on the neck of the
volumetric flask.
The solution was then mixed thoroughly by inverting the flask several times to enable the
solution to mix appropriately (Perrin, 2016, p. 45).
Results
The results which were obtained during the experiment were recorded and tabulated as shown
below.
Part 2
Tris-HCL
First Tris-HCL pH value(7.99)
0.01M 7.96M
0.001M 7.92M
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
LABORATORY REPORT 7
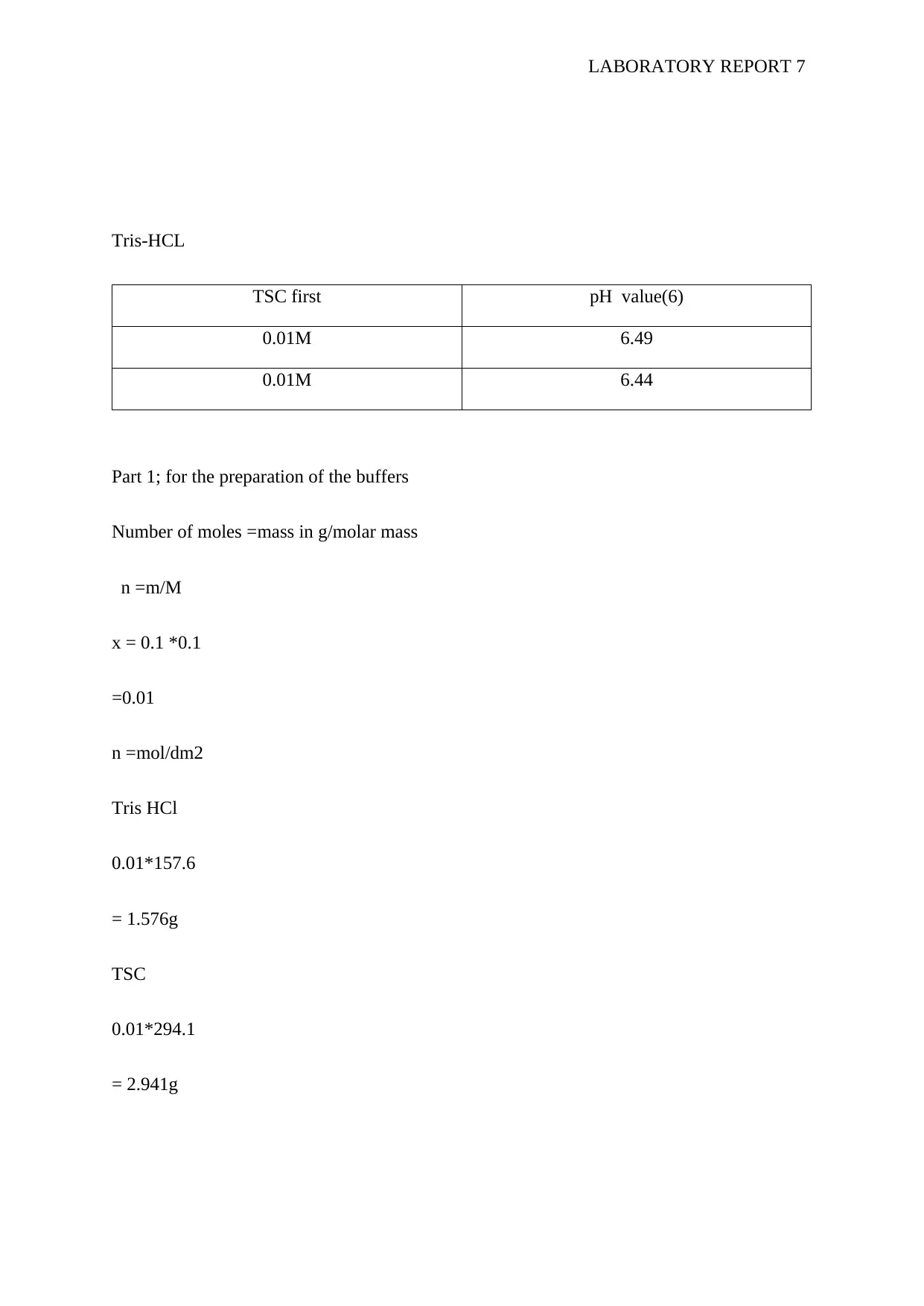
Tris-HCL
TSC first pH value(6)
0.01M 6.49
0.01M 6.44
Part 1; for the preparation of the buffers
Number of moles =mass in g/molar mass
n =m/M
x = 0.1 *0.1
=0.01
n =mol/dm2
Tris HCl
0.01*157.6
= 1.576g
TSC
0.01*294.1
= 2.941g
Tris-HCL
TSC first pH value(6)
0.01M 6.49
0.01M 6.44
Part 1; for the preparation of the buffers
Number of moles =mass in g/molar mass
n =m/M
x = 0.1 *0.1
=0.01
n =mol/dm2
Tris HCl
0.01*157.6
= 1.576g
TSC
0.01*294.1
= 2.941g
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

LABORATORY REPORT 8
Discussions
The data which was collected was very sufficient to achieve the goals of the experiment. The
experimental data which was collected confirms what was theoretically predicated; that the
pH value of the buffer solution was to be lowered when the concentration was reduced
(Stenesh, 2013, p. 147).
From the results which were obtained it was found out that the amount of Tris-HCL that was
required for the preparation of 100mL of O.1M of pH 8 was 1.576g while that was need for
the preparation of the 100mL TSC with 0.1M.
The pH for both the Tris-HCl and the TSC reduced when the concentration of the buffer
solution was reduced. The pH value for the Tris-HCl solution when the concentration was
o.1M the pH was 7.96, and when the solution was diluted further by add 90Ml of water to
attain the concentration of 0.001M the pH was lowered to 7.92.
For the case of TSC when the concentration was 0.01M the pH value was 6.49 and when the
solution was diluted further, and the concentration of 0.001M was attained the pH value of
the buffer reduced to 6.44.
Conclusion
In conclusion, Buffers solutions are the aqueous solutions which consist a mixture of
conjugated base and a weak base. The pH of the buffer solution changes slightly when a
small amount of strong base or acid is added to it, and they are used to control the pH of an
experiment (Katoch, 2013, p. 322).
Discussions
The data which was collected was very sufficient to achieve the goals of the experiment. The
experimental data which was collected confirms what was theoretically predicated; that the
pH value of the buffer solution was to be lowered when the concentration was reduced
(Stenesh, 2013, p. 147).
From the results which were obtained it was found out that the amount of Tris-HCL that was
required for the preparation of 100mL of O.1M of pH 8 was 1.576g while that was need for
the preparation of the 100mL TSC with 0.1M.
The pH for both the Tris-HCl and the TSC reduced when the concentration of the buffer
solution was reduced. The pH value for the Tris-HCl solution when the concentration was
o.1M the pH was 7.96, and when the solution was diluted further by add 90Ml of water to
attain the concentration of 0.001M the pH was lowered to 7.92.
For the case of TSC when the concentration was 0.01M the pH value was 6.49 and when the
solution was diluted further, and the concentration of 0.001M was attained the pH value of
the buffer reduced to 6.44.
Conclusion
In conclusion, Buffers solutions are the aqueous solutions which consist a mixture of
conjugated base and a weak base. The pH of the buffer solution changes slightly when a
small amount of strong base or acid is added to it, and they are used to control the pH of an
experiment (Katoch, 2013, p. 322).

LABORATORY REPORT 9
The central focus for carrying out this experiment was to find out how the buffer solution
reacts to the dilution in comparison to a non-buffered solution. To produce some standard
buffers while ensuring that all the components are measured out accurately (Wilson, 2012, p.
345).
Sufficient data was obtained during the lab session which was further analyzed. It was found
out from the results of the experiment that the pH of the buffer solution was lowered when
the concentration was reduced. The pH value for the Tris-HCl solution when the
concentration was o.1M the pH was 7.96, and when the solution was diluted further by add
90Ml of water to attain the concentration of 0.001M the pH was lowered to 7.92.
The central focus for carrying out this experiment was to find out how the buffer solution
reacts to the dilution in comparison to a non-buffered solution. To produce some standard
buffers while ensuring that all the components are measured out accurately (Wilson, 2012, p.
345).
Sufficient data was obtained during the lab session which was further analyzed. It was found
out from the results of the experiment that the pH of the buffer solution was lowered when
the concentration was reduced. The pH value for the Tris-HCl solution when the
concentration was o.1M the pH was 7.96, and when the solution was diluted further by add
90Ml of water to attain the concentration of 0.001M the pH was lowered to 7.92.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

LABORATORY REPORT 10
Bibliography
Boyer, R. F., 2013. Modern Experimental Biochemistry. 1st ed. Berlin: Benjamin/Cummings
Publishing Company.
Cserhati, T., 2017. Chromatographic Determination of Molecular Interactions Applications
in Biochemistry, Chemistry, and Biophysics. 3rd ed. London: CRC Press.
Garrett, R. H., 2016. Biochemistry. 2nd ed. Chicago: Cengage Learning.
Gueffroy, D. E., 2015. A Guide for the Preparation and Use of Buffers in Biological Systems.
1st ed. Berlin: Calbiochem-Behring Corporation.
Katoch, R., 2013. Analytical Techniques in Biochemistry and Molecular Biology. 2nd ed.
London: Springer Science & Business Media.
Lundblad, R. L., 2015. Handbook of Biochemistry and Molecular Biology, Fourth Edition.
1st ed. Texas: CRC Press.
McDougal, O. M., 2016. Biochemistry. 4th ed. Chicago: Cengage Learning.
Metzler, 2014. Biochemistry: The Chemical Reactions of Living Cells. 2nd ed. London:
Elsevier.
Perrin, D., 2016. Buffers for pH and Metal Ion Control. 4th ed. London: Springer Science &
Business Media.
Stenesh, J., 2013. Biochemistry. 2nd ed. London: Springer Science & Business Media.
Bibliography
Boyer, R. F., 2013. Modern Experimental Biochemistry. 1st ed. Berlin: Benjamin/Cummings
Publishing Company.
Cserhati, T., 2017. Chromatographic Determination of Molecular Interactions Applications
in Biochemistry, Chemistry, and Biophysics. 3rd ed. London: CRC Press.
Garrett, R. H., 2016. Biochemistry. 2nd ed. Chicago: Cengage Learning.
Gueffroy, D. E., 2015. A Guide for the Preparation and Use of Buffers in Biological Systems.
1st ed. Berlin: Calbiochem-Behring Corporation.
Katoch, R., 2013. Analytical Techniques in Biochemistry and Molecular Biology. 2nd ed.
London: Springer Science & Business Media.
Lundblad, R. L., 2015. Handbook of Biochemistry and Molecular Biology, Fourth Edition.
1st ed. Texas: CRC Press.
McDougal, O. M., 2016. Biochemistry. 4th ed. Chicago: Cengage Learning.
Metzler, 2014. Biochemistry: The Chemical Reactions of Living Cells. 2nd ed. London:
Elsevier.
Perrin, D., 2016. Buffers for pH and Metal Ion Control. 4th ed. London: Springer Science &
Business Media.
Stenesh, J., 2013. Biochemistry. 2nd ed. London: Springer Science & Business Media.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

LABORATORY REPORT 11
Stenesh, J., 2014. Biochemistry Biochemistry: Solutions Manual. 1st ed. Paris: Springer
Science & Business Media.
Wilson, K., 2012. Principles and Techniques of Biochemistry and Molecular Biology. 1st ed.
London: Cambridge University Press.
Stenesh, J., 2014. Biochemistry Biochemistry: Solutions Manual. 1st ed. Paris: Springer
Science & Business Media.
Wilson, K., 2012. Principles and Techniques of Biochemistry and Molecular Biology. 1st ed.
London: Cambridge University Press.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





