The Medicinal Use of Cannabis and Cannabinoids—An International Cross-Sectional Survey on Administration Forms
VerifiedAdded on 2023/06/10
|13
|10581
|87
AI Summary
The survey presented here was performed by the International Association for Cannabinoid Medicines (IACM), and is meant to contribute to the understanding of cannabinoid-based medicine by asking patients who used cannabis or cannabinoids detailed questions about their experiences with different methods of intake. The survey was completed by 953 participants from 31 countries, making this the largest international survey on a wide variety of users of cannabinoid-based medicine performed so far.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

This article was downloaded by: [University of Saskatchewan Library]
On: 20 September 2013, At: 05:31
Publisher: Routledge
Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House,
37-41 Mortimer Street, London W1T 3JH, UK
Journal of Psychoactive Drugs
Publication details, including instructions for authors and subscription information:
http://www.tandfonline.com/loi/ujpd20
The Medicinal Use of Cannabis and Cannabinoids
International Cross-Sectional Survey on Administ
Forms
Arno Hazekamp Ph.D.
a f , Mark A. Ware M.D.
b , Kirsten R. Muller-Vahl M.D.
c , Donald
Abrams M.D.
d & Franjo Grotenhermen M.D.
e
a Head of Research and Development at Bedrocan BV, The Netherlands; Department of
Metabolomics, Faculty of Science , Leiden University , Leiden , The Netherlands
b Departments of Family Medicine and Anesthesia , McGill University , Montreal , QC ,
Canada
c Clinic of Psychiatry, Social Psychiatry and Psychotherapy , Hannover Medical School ,
Hannover , Germany
d Division of Hematology-Oncology, San Francisco General Hospital , University of Califor
San Francisco , CA
e Nova-institut , Huerth , Germany
f Head of Research and Development at Bedrocan BV , The Netherlands
Published online: 02 Aug 2013.
To cite this article: Arno Hazekamp Ph.D. , Mark A. Ware M.D. , Kirsten R. Muller-Vahl M.D. , Donald Abrams M.D. & F
Grotenhermen M.D. (2013) The Medicinal Use of Cannabis and Cannabinoids—An International Cross-Sectional Survey
Administration Forms, Journal of Psychoactive Drugs, 45:3, 199-210, DOI: 10.1080/02791072.2013.805976
To link to this article: http://dx.doi.org/10.1080/02791072.2013.805976
PLEASE SCROLL DOWN FOR ARTICLE
Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained
in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no
representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the
Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and
are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and
should be independently verified with primary sources of information. Taylor and Francis shall not be liable for
any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever
or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of
the Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematic
reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any
form to anyone is expressly forbidden. Terms & Conditions of access and use can be found at http://
www.tandfonline.com/page/terms-and-conditions
On: 20 September 2013, At: 05:31
Publisher: Routledge
Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House,
37-41 Mortimer Street, London W1T 3JH, UK
Journal of Psychoactive Drugs
Publication details, including instructions for authors and subscription information:
http://www.tandfonline.com/loi/ujpd20
The Medicinal Use of Cannabis and Cannabinoids
International Cross-Sectional Survey on Administ
Forms
Arno Hazekamp Ph.D.
a f , Mark A. Ware M.D.
b , Kirsten R. Muller-Vahl M.D.
c , Donald
Abrams M.D.
d & Franjo Grotenhermen M.D.
e
a Head of Research and Development at Bedrocan BV, The Netherlands; Department of
Metabolomics, Faculty of Science , Leiden University , Leiden , The Netherlands
b Departments of Family Medicine and Anesthesia , McGill University , Montreal , QC ,
Canada
c Clinic of Psychiatry, Social Psychiatry and Psychotherapy , Hannover Medical School ,
Hannover , Germany
d Division of Hematology-Oncology, San Francisco General Hospital , University of Califor
San Francisco , CA
e Nova-institut , Huerth , Germany
f Head of Research and Development at Bedrocan BV , The Netherlands
Published online: 02 Aug 2013.
To cite this article: Arno Hazekamp Ph.D. , Mark A. Ware M.D. , Kirsten R. Muller-Vahl M.D. , Donald Abrams M.D. & F
Grotenhermen M.D. (2013) The Medicinal Use of Cannabis and Cannabinoids—An International Cross-Sectional Survey
Administration Forms, Journal of Psychoactive Drugs, 45:3, 199-210, DOI: 10.1080/02791072.2013.805976
To link to this article: http://dx.doi.org/10.1080/02791072.2013.805976
PLEASE SCROLL DOWN FOR ARTICLE
Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained
in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no
representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the
Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and
are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and
should be independently verified with primary sources of information. Taylor and Francis shall not be liable for
any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever
or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of
the Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematic
reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any
form to anyone is expressly forbidden. Terms & Conditions of access and use can be found at http://
www.tandfonline.com/page/terms-and-conditions
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Journal of Psychoactive Drugs, 45 (3), 199–210, 2013
Copyright © Taylor & Francis Group, LLC
ISSN: 0279-1072 print / 2159-9777 online
DOI: 10.1080/02791072.2013.805976
The Medicinal Use of Cannabis and
Cannabinoids—An International
Cross-Sectional Survey on
Administration Forms
Arno Hazekamp, Ph.D.a; Mark A. Ware, M.D.b; Kirsten R. Muller-Vahl, M.D.c; Donald Abrams, M.D.d
& Franjo Grotenhermen, M.D.e
Abstract — Cannabinoids, including tetrahydrocannabinol and cannabidiol, are the most important
active constituents of the cannabis plant. Over recent years, cannabinoid-based medicines (CBMs)
have become increasingly available to patients in many countries, both as pharmaceutical products and
as herbal cannabis (marijuana). While there seems to be a demand for multiple cannabinoid-based ther-
apeutic products, specifically for symptomatic amelioration in chronic diseases, therapeutic effects of
different CBMs have only been directly compared in a few clinical studies. The survey presented here
was performed by the International Association for Cannabinoid Medicines (IACM), and is meant to
contribute to the understanding of cannabinoid-based medicine by asking patients who used cannabis
or cannabinoids detailed questions about their experiences with different methods of intake. The survey
was completed by 953 participants from 31 countries, making this the largest international survey on
a wide variety of users of cannabinoid-based medicine performed so far. In general, herbal non-phar-
maceutical CBMs received higher appreciation scores by participants than pharmaceutical products
containing cannabinoids. However, the number of patients who reported experience with pharma-
ceutical products was low, limiting conclusions on preferences. Nevertheless, the reported data may
be useful for further development of safe and effective medications based on cannabis and single
cannabinoids.
Keywords — administration form, cannabinoids, cannabis, comparative study, survey
INTRODUCTION
In recent years, cannabinoid-based medicines (CBMs)
have become increasingly available to patients in many
countries. These include several pharmaceutical prepara-
tions containing pure cannabinoids or cannabis extracts,
aHead of Research and Development at Bedrocan BV, The
Netherlands; Department of Plant Metabolomics, Faculty of Science,
Leiden University, Leiden, The Netherlands.
bDepartments of Family Medicine and Anesthesia, McGill
University, Montreal, QC, Canada.
cClinic of Psychiatry, Social Psychiatry and Psychotherapy,
Hannover Medical School, Hannover, Germany.
dDivision of Hematology-Oncology, San Francisco General
Hospital, University of California, San Francisco, CA.
eNova-institut, Huerth, Germany.
Please address correspondence to Arno Hazekamp, Ph.D.,
Department of Plant Metabolomics, Faculty of Science, Leiden
University, Sylviusweg 72, 2333BE, Leiden, The Netherlands; email:
ahazekamp@bedrocan.nl
as well as herbal cannabis (marijuana) products. The
most commonly prescribed cannabinoid-based medicines
are dronabinol (marketed as Marinol ® since 1986, Abbott
Products Inc.) and nabilone (marketed as Cesamet ® since
1981, Valeant Pharmaceuticals International). Dronabinol
is the INN (international non-proprietary name) of the nat-
Journal of Psychoactive Drugs 199 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
Copyright © Taylor & Francis Group, LLC
ISSN: 0279-1072 print / 2159-9777 online
DOI: 10.1080/02791072.2013.805976
The Medicinal Use of Cannabis and
Cannabinoids—An International
Cross-Sectional Survey on
Administration Forms
Arno Hazekamp, Ph.D.a; Mark A. Ware, M.D.b; Kirsten R. Muller-Vahl, M.D.c; Donald Abrams, M.D.d
& Franjo Grotenhermen, M.D.e
Abstract — Cannabinoids, including tetrahydrocannabinol and cannabidiol, are the most important
active constituents of the cannabis plant. Over recent years, cannabinoid-based medicines (CBMs)
have become increasingly available to patients in many countries, both as pharmaceutical products and
as herbal cannabis (marijuana). While there seems to be a demand for multiple cannabinoid-based ther-
apeutic products, specifically for symptomatic amelioration in chronic diseases, therapeutic effects of
different CBMs have only been directly compared in a few clinical studies. The survey presented here
was performed by the International Association for Cannabinoid Medicines (IACM), and is meant to
contribute to the understanding of cannabinoid-based medicine by asking patients who used cannabis
or cannabinoids detailed questions about their experiences with different methods of intake. The survey
was completed by 953 participants from 31 countries, making this the largest international survey on
a wide variety of users of cannabinoid-based medicine performed so far. In general, herbal non-phar-
maceutical CBMs received higher appreciation scores by participants than pharmaceutical products
containing cannabinoids. However, the number of patients who reported experience with pharma-
ceutical products was low, limiting conclusions on preferences. Nevertheless, the reported data may
be useful for further development of safe and effective medications based on cannabis and single
cannabinoids.
Keywords — administration form, cannabinoids, cannabis, comparative study, survey
INTRODUCTION
In recent years, cannabinoid-based medicines (CBMs)
have become increasingly available to patients in many
countries. These include several pharmaceutical prepara-
tions containing pure cannabinoids or cannabis extracts,
aHead of Research and Development at Bedrocan BV, The
Netherlands; Department of Plant Metabolomics, Faculty of Science,
Leiden University, Leiden, The Netherlands.
bDepartments of Family Medicine and Anesthesia, McGill
University, Montreal, QC, Canada.
cClinic of Psychiatry, Social Psychiatry and Psychotherapy,
Hannover Medical School, Hannover, Germany.
dDivision of Hematology-Oncology, San Francisco General
Hospital, University of California, San Francisco, CA.
eNova-institut, Huerth, Germany.
Please address correspondence to Arno Hazekamp, Ph.D.,
Department of Plant Metabolomics, Faculty of Science, Leiden
University, Sylviusweg 72, 2333BE, Leiden, The Netherlands; email:
ahazekamp@bedrocan.nl
as well as herbal cannabis (marijuana) products. The
most commonly prescribed cannabinoid-based medicines
are dronabinol (marketed as Marinol ® since 1986, Abbott
Products Inc.) and nabilone (marketed as Cesamet ® since
1981, Valeant Pharmaceuticals International). Dronabinol
is the INN (international non-proprietary name) of the nat-
Journal of Psychoactive Drugs 199 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013

Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
ural cannabinoid (-)-trans-delta-9-tetrahydrocannabinol,
usually abbreviated THC (WHO 2006). It may be extracted
from the plant, gained by isomerization of cannabidiol
(CBD), or manufactured synthetically. Marinol® is a syn-
thetic form of THC dissolved in sesame oil and prepared
in gelatin capsules, while nabilone is a synthetic ago-
nist of the human cannabinoid receptor (CB1), structurally
derived from a major human metabolite of THC. Both
products are registered for the symptomatic treatment of
nausea and vomiting associated with cancer chemotherapy,
while dronabinol is also approved for treating anorexia and
cachexia related to HIV / AIDS. The patent on Marinol ®
expired in 2011, and an authorized generic version has
become available from Watson Pharmaceuticals. A generic
formulation of nabilone is now available in Canada from
Pharmascience Inc. In Germany, generic THC is supplied
by two companies (THC Pharm and Bionorica Ethics)
from which pharmacies can prepare capsules and solu-
tions (in oil or alcohol). The alcoholic solutions of THC
can either be used orally or inhaled by using a vapor-
izer (IACM 2009). About 7.5 kg of THC is delivered by
German pharmacies per year (WHO 2006).
Nabiximols (marketed as Sativex® since 2005 by
GW Pharmaceuticals, UK, and partners) is a sublingually
administered oromucosal spray based on a mixture of
two distinct standardized cannabis extracts. Its principal
active components are the plant-derived cannabinoids THC
and CBD. Sativex is currently registered in Canada, the
UK, Spain, Germany, Denmark, and New Zealand to treat
spasticity due to multiple sclerosis. In Canada, it is also
approved for the relief of neuropathic pain and advanced
cancer pain. Further approval is expected in other European
countries, based on the European Mutual Recognition
Procedure (GW Pharmaceuticals 2012).
Parallel to the development of pharmaceutical CBMs,
the number of countries providing a legal source of
medicinal-grade cannabis to chronically ill patients has
been growing as well. Canada (since 2001) and The
Netherlands (since 2003) have had government-run pro-
grams for the last decade, where quality-controlled herbal
cannabis is supplied by the specialized companies Prairie
Plant Systems Inc. and Bedrocan BV, respectively. Several
other countries are now setting up their own program
(Israel, Czech Republic) or importing products from the
Dutch program (Italy, Finland, Germany). In the US, the
number of states that introduced laws to permit the med-
ical use of cannabis has grown to 18 plus the District of
Columbia (DC), even though these state-level initiatives are
still prohibited by the federal government (IACM 2012).
In general, CBMs are used for symptomatic treat-
ment of chronic diseases refractory to standard treat-
ments. Because multiple biologically active cannabinoids
have been identified (including THC, CBD, tetrahy-
drocannabivarin (THCV) and THC-acid (THCA)), and
because CBMs can be administered in multiple ways (oral,
sublingual, inhaled), there seems to be a demand for multi-
ple cannabinoid-based therapeutic products. Nevertheless,
therapeutic effects of different CBMs have only been
directly compared in a few clinical studies. Most of these
studies compared an unregistered oral cannabis extract
(Cannador®) to Marinol ® (Zajicek 2003; 2005; Killestein
2002; Freeman 2006; Strasser 2006), while a few oth-
ers compared smoked cannabis to Marinol® (Haney 2005;
2007). The survey presented here was designed to con-
tribute to the understanding of cannabinoid-based medicine
by asking patients to compare the effects of different CBMs
and different administration forms. To the best of our
knowledge, this survey represents the largest systematic
study on actual patient experiences with cannabinoid-based
medicines currently available anywhere.
METHODOLOGY
We conducted an international, web-based, cross-
sectional survey to describe patients’ perceptions of dif-
ferent modes of administration for cannabinoid-based
medicines (complete survey available from first author).
The study was designed and conducted by the International
Association for Cannabinoid Medicines (IACM), which
has the goal to “advance knowledge on cannabis,
cannabinoids, the endocannabinoid system, and related
topics especially with regard to their therapeutic potential.”
The survey was posted on the official IACM website (http:/
/www.cannabis-med.org) from August 2009 until January
2010 in five different languages: English, French, Spanish,
German, and Dutch. To improve recruitment, the sur-
vey was brought to the attention of the approximately
5500 recipients of the IACM biweekly electronic newslet-
ter. The survey was approved by the Ethics Committee of
the Hannover Medical School, Germany.
Subjects were a self-selected “availability sample” of
visitors to the IACM website; eligible subjects needed
to have experience with at least two different CBMs or
administration forms to be included. Participants remained
anonymous and received no financial compensation.
The survey consisted of 21 structured (fixed) ques-
tions that were answered by yes / no responses, multiple
choice lists, and rating scales. In addition, two open-
ended questions allowed remarks, comments, and sugges-
tions. Information collected included demographics, details
on medical condition and symptoms, medical treatment,
cannabis-use patterns, and methods of former and cur-
rent intake of cannabis or cannabinoids. Participants were
asked to rate their personal experience with different modes
of delivery using Likert scale-style responses. Only com-
pleted surveys were included for final evaluation.
Participants were asked to evaluate different cannabis
administration forms, including: smoking of cannabis
(Smoking); inhalation of cannabis with a vaporizer
(Vaporizer); oral use of cannabis as a tea (Tea); oral use
Journal of Psychoactive Drugs 200 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
ural cannabinoid (-)-trans-delta-9-tetrahydrocannabinol,
usually abbreviated THC (WHO 2006). It may be extracted
from the plant, gained by isomerization of cannabidiol
(CBD), or manufactured synthetically. Marinol® is a syn-
thetic form of THC dissolved in sesame oil and prepared
in gelatin capsules, while nabilone is a synthetic ago-
nist of the human cannabinoid receptor (CB1), structurally
derived from a major human metabolite of THC. Both
products are registered for the symptomatic treatment of
nausea and vomiting associated with cancer chemotherapy,
while dronabinol is also approved for treating anorexia and
cachexia related to HIV / AIDS. The patent on Marinol ®
expired in 2011, and an authorized generic version has
become available from Watson Pharmaceuticals. A generic
formulation of nabilone is now available in Canada from
Pharmascience Inc. In Germany, generic THC is supplied
by two companies (THC Pharm and Bionorica Ethics)
from which pharmacies can prepare capsules and solu-
tions (in oil or alcohol). The alcoholic solutions of THC
can either be used orally or inhaled by using a vapor-
izer (IACM 2009). About 7.5 kg of THC is delivered by
German pharmacies per year (WHO 2006).
Nabiximols (marketed as Sativex® since 2005 by
GW Pharmaceuticals, UK, and partners) is a sublingually
administered oromucosal spray based on a mixture of
two distinct standardized cannabis extracts. Its principal
active components are the plant-derived cannabinoids THC
and CBD. Sativex is currently registered in Canada, the
UK, Spain, Germany, Denmark, and New Zealand to treat
spasticity due to multiple sclerosis. In Canada, it is also
approved for the relief of neuropathic pain and advanced
cancer pain. Further approval is expected in other European
countries, based on the European Mutual Recognition
Procedure (GW Pharmaceuticals 2012).
Parallel to the development of pharmaceutical CBMs,
the number of countries providing a legal source of
medicinal-grade cannabis to chronically ill patients has
been growing as well. Canada (since 2001) and The
Netherlands (since 2003) have had government-run pro-
grams for the last decade, where quality-controlled herbal
cannabis is supplied by the specialized companies Prairie
Plant Systems Inc. and Bedrocan BV, respectively. Several
other countries are now setting up their own program
(Israel, Czech Republic) or importing products from the
Dutch program (Italy, Finland, Germany). In the US, the
number of states that introduced laws to permit the med-
ical use of cannabis has grown to 18 plus the District of
Columbia (DC), even though these state-level initiatives are
still prohibited by the federal government (IACM 2012).
In general, CBMs are used for symptomatic treat-
ment of chronic diseases refractory to standard treat-
ments. Because multiple biologically active cannabinoids
have been identified (including THC, CBD, tetrahy-
drocannabivarin (THCV) and THC-acid (THCA)), and
because CBMs can be administered in multiple ways (oral,
sublingual, inhaled), there seems to be a demand for multi-
ple cannabinoid-based therapeutic products. Nevertheless,
therapeutic effects of different CBMs have only been
directly compared in a few clinical studies. Most of these
studies compared an unregistered oral cannabis extract
(Cannador®) to Marinol ® (Zajicek 2003; 2005; Killestein
2002; Freeman 2006; Strasser 2006), while a few oth-
ers compared smoked cannabis to Marinol® (Haney 2005;
2007). The survey presented here was designed to con-
tribute to the understanding of cannabinoid-based medicine
by asking patients to compare the effects of different CBMs
and different administration forms. To the best of our
knowledge, this survey represents the largest systematic
study on actual patient experiences with cannabinoid-based
medicines currently available anywhere.
METHODOLOGY
We conducted an international, web-based, cross-
sectional survey to describe patients’ perceptions of dif-
ferent modes of administration for cannabinoid-based
medicines (complete survey available from first author).
The study was designed and conducted by the International
Association for Cannabinoid Medicines (IACM), which
has the goal to “advance knowledge on cannabis,
cannabinoids, the endocannabinoid system, and related
topics especially with regard to their therapeutic potential.”
The survey was posted on the official IACM website (http:/
/www.cannabis-med.org) from August 2009 until January
2010 in five different languages: English, French, Spanish,
German, and Dutch. To improve recruitment, the sur-
vey was brought to the attention of the approximately
5500 recipients of the IACM biweekly electronic newslet-
ter. The survey was approved by the Ethics Committee of
the Hannover Medical School, Germany.
Subjects were a self-selected “availability sample” of
visitors to the IACM website; eligible subjects needed
to have experience with at least two different CBMs or
administration forms to be included. Participants remained
anonymous and received no financial compensation.
The survey consisted of 21 structured (fixed) ques-
tions that were answered by yes / no responses, multiple
choice lists, and rating scales. In addition, two open-
ended questions allowed remarks, comments, and sugges-
tions. Information collected included demographics, details
on medical condition and symptoms, medical treatment,
cannabis-use patterns, and methods of former and cur-
rent intake of cannabis or cannabinoids. Participants were
asked to rate their personal experience with different modes
of delivery using Likert scale-style responses. Only com-
pleted surveys were included for final evaluation.
Participants were asked to evaluate different cannabis
administration forms, including: smoking of cannabis
(Smoking); inhalation of cannabis with a vaporizer
(Vaporizer); oral use of cannabis as a tea (Tea); oral use
Journal of Psychoactive Drugs 200 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
TABLE 1
(a) Methods of Ingestion Ever Tried (Multiple Answers Possible), Most Preferred Method of Intake; (B)
Expressed as Total Number of Participants; and (C) Expressed as Percentage of All Users
Smoking
Vaporizer
Tea
Food/Tinc
Dronabinol
Nabilone
THC vap
Nabiximols
Other
GROUP 1 GROUP 2 OTHER
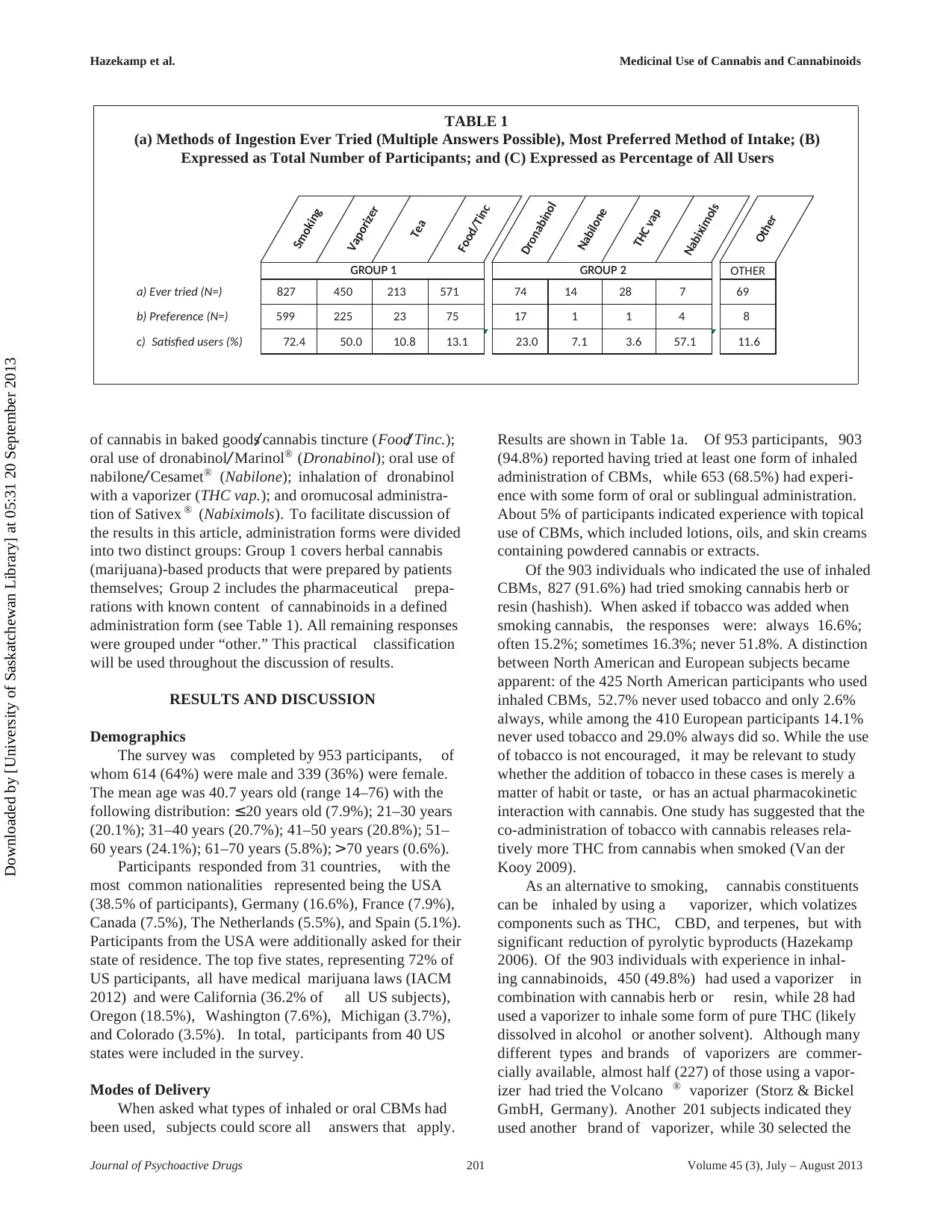
a) Ever tried (N=) 827 450 213 571 74 14 28 7 69
b) Preference (N=) 599 225 23 75 17 1 1 4 8
c) Satisfied users (%) 72.4 50.0 10.8 13.1 23.0 7.1 3.6 57.1 11.6
GROUP 1 GROUP 2
of cannabis in baked goods/ cannabis tincture (Food/ Tinc.);
oral use of dronabinol/ Marinol® (Dronabinol); oral use of
nabilone/ Cesamet® (Nabilone); inhalation of dronabinol
with a vaporizer (THC vap.); and oromucosal administra-
tion of Sativex ® (Nabiximols). To facilitate discussion of
the results in this article, administration forms were divided
into two distinct groups: Group 1 covers herbal cannabis
(marijuana)-based products that were prepared by patients
themselves; Group 2 includes the pharmaceutical prepa-
rations with known content of cannabinoids in a defined
administration form (see Table 1). All remaining responses
were grouped under “other.” This practical classification
will be used throughout the discussion of results.
RESULTS AND DISCUSSION
Demographics
The survey was completed by 953 participants, of
whom 614 (64%) were male and 339 (36%) were female.
The mean age was 40.7 years old (range 14–76) with the
following distribution: ≤20 years old (7.9%); 21–30 years
(20.1%); 31–40 years (20.7%); 41–50 years (20.8%); 51–
60 years (24.1%); 61–70 years (5.8%); >70 years (0.6%).
Participants responded from 31 countries, with the
most common nationalities represented being the USA
(38.5% of participants), Germany (16.6%), France (7.9%),
Canada (7.5%), The Netherlands (5.5%), and Spain (5.1%).
Participants from the USA were additionally asked for their
state of residence. The top five states, representing 72% of
US participants, all have medical marijuana laws (IACM
2012) and were California (36.2% of all US subjects),
Oregon (18.5%), Washington (7.6%), Michigan (3.7%),
and Colorado (3.5%). In total, participants from 40 US
states were included in the survey.
Modes of Delivery
When asked what types of inhaled or oral CBMs had
been used, subjects could score all answers that apply.
Results are shown in Table 1a. Of 953 participants, 903
(94.8%) reported having tried at least one form of inhaled
administration of CBMs, while 653 (68.5%) had experi-
ence with some form of oral or sublingual administration.
About 5% of participants indicated experience with topical
use of CBMs, which included lotions, oils, and skin creams
containing powdered cannabis or extracts.
Of the 903 individuals who indicated the use of inhaled
CBMs, 827 (91.6%) had tried smoking cannabis herb or
resin (hashish). When asked if tobacco was added when
smoking cannabis, the responses were: always 16.6%;
often 15.2%; sometimes 16.3%; never 51.8%. A distinction
between North American and European subjects became
apparent: of the 425 North American participants who used
inhaled CBMs, 52.7% never used tobacco and only 2.6%
always, while among the 410 European participants 14.1%
never used tobacco and 29.0% always did so. While the use
of tobacco is not encouraged, it may be relevant to study
whether the addition of tobacco in these cases is merely a
matter of habit or taste, or has an actual pharmacokinetic
interaction with cannabis. One study has suggested that the
co-administration of tobacco with cannabis releases rela-
tively more THC from cannabis when smoked (Van der
Kooy 2009).
As an alternative to smoking, cannabis constituents
can be inhaled by using a vaporizer, which volatizes
components such as THC, CBD, and terpenes, but with
significant reduction of pyrolytic byproducts (Hazekamp
2006). Of the 903 individuals with experience in inhal-
ing cannabinoids, 450 (49.8%) had used a vaporizer in
combination with cannabis herb or resin, while 28 had
used a vaporizer to inhale some form of pure THC (likely
dissolved in alcohol or another solvent). Although many
different types and brands of vaporizers are commer-
cially available, almost half (227) of those using a vapor-
izer had tried the Volcano ® vaporizer (Storz & Bickel
GmbH, Germany). Another 201 subjects indicated they
used another brand of vaporizer, while 30 selected the
Journal of Psychoactive Drugs 201 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
TABLE 1
(a) Methods of Ingestion Ever Tried (Multiple Answers Possible), Most Preferred Method of Intake; (B)
Expressed as Total Number of Participants; and (C) Expressed as Percentage of All Users
Smoking
Vaporizer
Tea
Food/Tinc
Dronabinol
Nabilone
THC vap
Nabiximols
Other
GROUP 1 GROUP 2 OTHER
a) Ever tried (N=) 827 450 213 571 74 14 28 7 69
b) Preference (N=) 599 225 23 75 17 1 1 4 8
c) Satisfied users (%) 72.4 50.0 10.8 13.1 23.0 7.1 3.6 57.1 11.6
GROUP 1 GROUP 2
of cannabis in baked goods/ cannabis tincture (Food/ Tinc.);
oral use of dronabinol/ Marinol® (Dronabinol); oral use of
nabilone/ Cesamet® (Nabilone); inhalation of dronabinol
with a vaporizer (THC vap.); and oromucosal administra-
tion of Sativex ® (Nabiximols). To facilitate discussion of
the results in this article, administration forms were divided
into two distinct groups: Group 1 covers herbal cannabis
(marijuana)-based products that were prepared by patients
themselves; Group 2 includes the pharmaceutical prepa-
rations with known content of cannabinoids in a defined
administration form (see Table 1). All remaining responses
were grouped under “other.” This practical classification
will be used throughout the discussion of results.
RESULTS AND DISCUSSION
Demographics
The survey was completed by 953 participants, of
whom 614 (64%) were male and 339 (36%) were female.
The mean age was 40.7 years old (range 14–76) with the
following distribution: ≤20 years old (7.9%); 21–30 years
(20.1%); 31–40 years (20.7%); 41–50 years (20.8%); 51–
60 years (24.1%); 61–70 years (5.8%); >70 years (0.6%).
Participants responded from 31 countries, with the
most common nationalities represented being the USA
(38.5% of participants), Germany (16.6%), France (7.9%),
Canada (7.5%), The Netherlands (5.5%), and Spain (5.1%).
Participants from the USA were additionally asked for their
state of residence. The top five states, representing 72% of
US participants, all have medical marijuana laws (IACM
2012) and were California (36.2% of all US subjects),
Oregon (18.5%), Washington (7.6%), Michigan (3.7%),
and Colorado (3.5%). In total, participants from 40 US
states were included in the survey.
Modes of Delivery
When asked what types of inhaled or oral CBMs had
been used, subjects could score all answers that apply.
Results are shown in Table 1a. Of 953 participants, 903
(94.8%) reported having tried at least one form of inhaled
administration of CBMs, while 653 (68.5%) had experi-
ence with some form of oral or sublingual administration.
About 5% of participants indicated experience with topical
use of CBMs, which included lotions, oils, and skin creams
containing powdered cannabis or extracts.
Of the 903 individuals who indicated the use of inhaled
CBMs, 827 (91.6%) had tried smoking cannabis herb or
resin (hashish). When asked if tobacco was added when
smoking cannabis, the responses were: always 16.6%;
often 15.2%; sometimes 16.3%; never 51.8%. A distinction
between North American and European subjects became
apparent: of the 425 North American participants who used
inhaled CBMs, 52.7% never used tobacco and only 2.6%
always, while among the 410 European participants 14.1%
never used tobacco and 29.0% always did so. While the use
of tobacco is not encouraged, it may be relevant to study
whether the addition of tobacco in these cases is merely a
matter of habit or taste, or has an actual pharmacokinetic
interaction with cannabis. One study has suggested that the
co-administration of tobacco with cannabis releases rela-
tively more THC from cannabis when smoked (Van der
Kooy 2009).
As an alternative to smoking, cannabis constituents
can be inhaled by using a vaporizer, which volatizes
components such as THC, CBD, and terpenes, but with
significant reduction of pyrolytic byproducts (Hazekamp
2006). Of the 903 individuals with experience in inhal-
ing cannabinoids, 450 (49.8%) had used a vaporizer in
combination with cannabis herb or resin, while 28 had
used a vaporizer to inhale some form of pure THC (likely
dissolved in alcohol or another solvent). Although many
different types and brands of vaporizers are commer-
cially available, almost half (227) of those using a vapor-
izer had tried the Volcano ® vaporizer (Storz & Bickel
GmbH, Germany). Another 201 subjects indicated they
used another brand of vaporizer, while 30 selected the
Journal of Psychoactive Drugs 201 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
FIGURE 1
Preferred Mode of Administration for Subjects in Each of the Top 5 Symptoms
0
10
20
30
40
50
60
70
80
% of participants
chronic pain (N = 278)
anxiety (N = 175)
loss of appetite and/or
weight (N = 102)
depression (N = 50)
insomnia or sleeping
disorder (N = 49)
answer “does not apply,” which could indicate they assem-
bled their own device for vaporizing.
Of the 653 participants who indicated experience with
oral or sublingual forms of CBM, 571 subjects (87.4%)
had used herbal cannabis in foods, baked goods, or tinc-
tures. Another 231 subjects (35.4%) had used cannabis
prepared as a tea. Fewer participants had experience with
dronabinol (74; 11.3%), nabilone (14; 2.1%), or nabiximols
(7; 1.1%).
Subjects were asked which method of intake they
would prefer, if given a single choice. Preferences are
shown in Table 1b. While 97% chose an herbal CBM
(Group 1) to treat their medical condition, this may reflect
the fact that many patients have not had the opportunity
to try any of the pharmaceutical preparations covered by
the survey. We therefore compared the total number of
users of each CBM to the number of subjects who would
choose that particular CBM as their preference. The result-
ing percentage (Table 1c) may be loosely regarded as a
“satisfaction-score,” independent of the number of patients
who have actually tried each product.
Medical Condition
Participants were asked to select from a list of 47 med-
ical conditions the main condition or disease for which
they seek symptomatic relief by using CBMs. The results,
as shown in Appendix 1, indicated that participants used
CBMs for a wide variety of medical conditions. The top
five conditions were back pain (11.9%), sleeping disorder
(6.9%), depression (6.7%), pain resulting from injury or
accident (6.2%), and multiple sclerosis (4.1%). Eighty par-
ticipants (8.4%) marked “other.” Most participants (80.4%)
indicated they were, or had been, under medical treatment
by a doctor for their particular condition.
Participants were also asked to select the main symp-
tom for which they sought relief by using CBMs from a
list of 22 options. The top five consisted of chronic pain
(29.2%), anxiety (18.3%), loss of appetite and / or weight
(10.7%), depression (5.2%), and insomnia or sleeping dis-
order (5.1%) (see Appendix 2). While chronic pain and
loss of appetite and / or weight are specifically targeted
indications of pharmaceutical products such as Marinol
and Sativex, anxiety, depression, and insomnia have not
yet been targeted as indications for pharmaceutical drug
development.
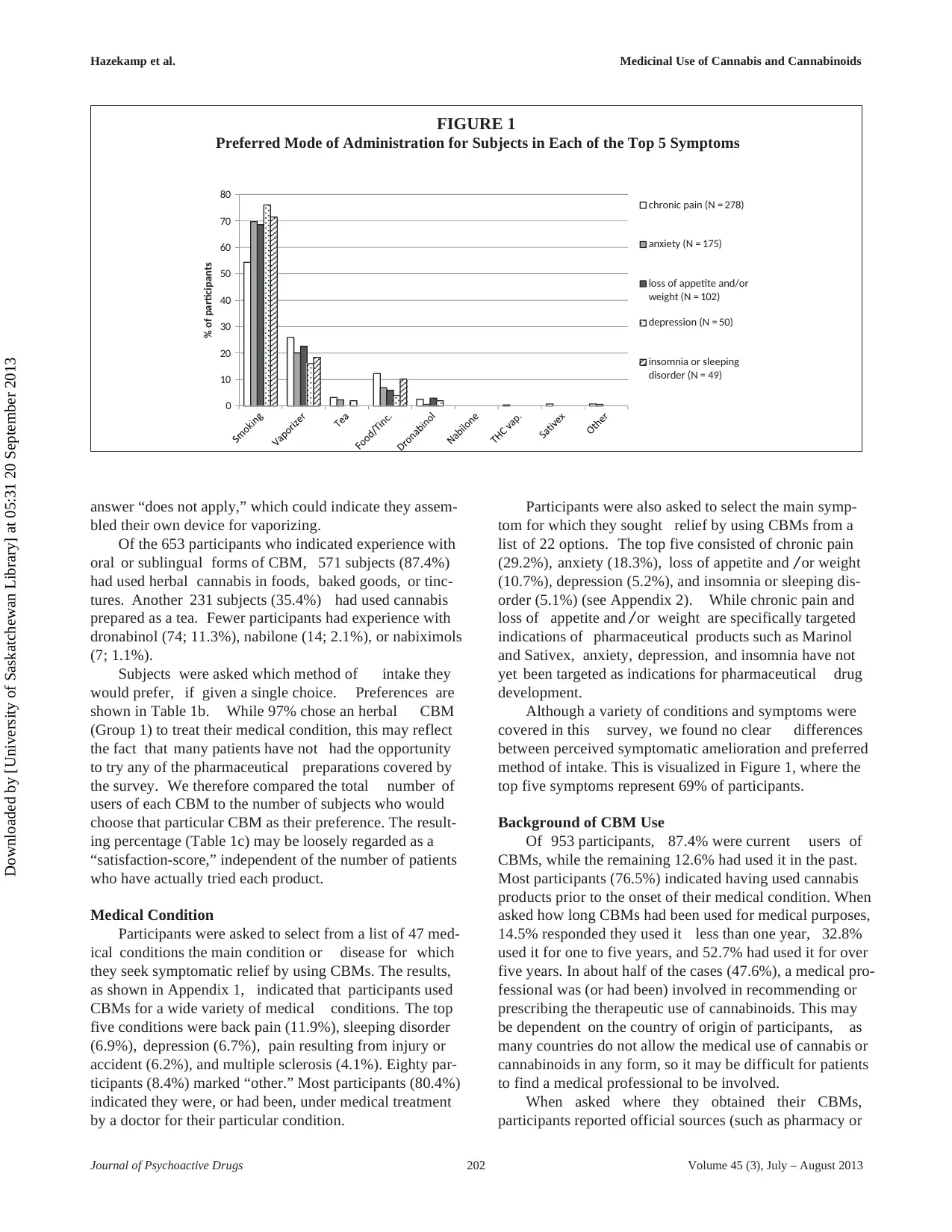
Although a variety of conditions and symptoms were
covered in this survey, we found no clear differences
between perceived symptomatic amelioration and preferred
method of intake. This is visualized in Figure 1, where the
top five symptoms represent 69% of participants.
Background of CBM Use
Of 953 participants, 87.4% were current users of
CBMs, while the remaining 12.6% had used it in the past.
Most participants (76.5%) indicated having used cannabis
products prior to the onset of their medical condition. When
asked how long CBMs had been used for medical purposes,
14.5% responded they used it less than one year, 32.8%
used it for one to five years, and 52.7% had used it for over
five years. In about half of the cases (47.6%), a medical pro-
fessional was (or had been) involved in recommending or
prescribing the therapeutic use of cannabinoids. This may
be dependent on the country of origin of participants, as
many countries do not allow the medical use of cannabis or
cannabinoids in any form, so it may be difficult for patients
to find a medical professional to be involved.
When asked where they obtained their CBMs,
participants reported official sources (such as pharmacy or
Journal of Psychoactive Drugs 202 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
FIGURE 1
Preferred Mode of Administration for Subjects in Each of the Top 5 Symptoms
0
10
20
30
40
50
60
70
80
% of participants
chronic pain (N = 278)
anxiety (N = 175)
loss of appetite and/or
weight (N = 102)
depression (N = 50)
insomnia or sleeping
disorder (N = 49)
answer “does not apply,” which could indicate they assem-
bled their own device for vaporizing.
Of the 653 participants who indicated experience with
oral or sublingual forms of CBM, 571 subjects (87.4%)
had used herbal cannabis in foods, baked goods, or tinc-
tures. Another 231 subjects (35.4%) had used cannabis
prepared as a tea. Fewer participants had experience with
dronabinol (74; 11.3%), nabilone (14; 2.1%), or nabiximols
(7; 1.1%).
Subjects were asked which method of intake they
would prefer, if given a single choice. Preferences are
shown in Table 1b. While 97% chose an herbal CBM
(Group 1) to treat their medical condition, this may reflect
the fact that many patients have not had the opportunity
to try any of the pharmaceutical preparations covered by
the survey. We therefore compared the total number of
users of each CBM to the number of subjects who would
choose that particular CBM as their preference. The result-
ing percentage (Table 1c) may be loosely regarded as a
“satisfaction-score,” independent of the number of patients
who have actually tried each product.
Medical Condition
Participants were asked to select from a list of 47 med-
ical conditions the main condition or disease for which
they seek symptomatic relief by using CBMs. The results,
as shown in Appendix 1, indicated that participants used
CBMs for a wide variety of medical conditions. The top
five conditions were back pain (11.9%), sleeping disorder
(6.9%), depression (6.7%), pain resulting from injury or
accident (6.2%), and multiple sclerosis (4.1%). Eighty par-
ticipants (8.4%) marked “other.” Most participants (80.4%)
indicated they were, or had been, under medical treatment
by a doctor for their particular condition.
Participants were also asked to select the main symp-
tom for which they sought relief by using CBMs from a
list of 22 options. The top five consisted of chronic pain
(29.2%), anxiety (18.3%), loss of appetite and / or weight
(10.7%), depression (5.2%), and insomnia or sleeping dis-
order (5.1%) (see Appendix 2). While chronic pain and
loss of appetite and / or weight are specifically targeted
indications of pharmaceutical products such as Marinol
and Sativex, anxiety, depression, and insomnia have not
yet been targeted as indications for pharmaceutical drug
development.
Although a variety of conditions and symptoms were
covered in this survey, we found no clear differences
between perceived symptomatic amelioration and preferred
method of intake. This is visualized in Figure 1, where the
top five symptoms represent 69% of participants.
Background of CBM Use
Of 953 participants, 87.4% were current users of
CBMs, while the remaining 12.6% had used it in the past.
Most participants (76.5%) indicated having used cannabis
products prior to the onset of their medical condition. When
asked how long CBMs had been used for medical purposes,
14.5% responded they used it less than one year, 32.8%
used it for one to five years, and 52.7% had used it for over
five years. In about half of the cases (47.6%), a medical pro-
fessional was (or had been) involved in recommending or
prescribing the therapeutic use of cannabinoids. This may
be dependent on the country of origin of participants, as
many countries do not allow the medical use of cannabis or
cannabinoids in any form, so it may be difficult for patients
to find a medical professional to be involved.
When asked where they obtained their CBMs,
participants reported official sources (such as pharmacy or
Journal of Psychoactive Drugs 202 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
FIGURE 2
Source of CBMS, Comparing Participants Using CBMS with Prescription Vs. No Prescription; Totals Exceed
100% Because Participants Could Check Multiple Sources
0
10
20
30
40
50
60
70
% of participants
prescription (N = 454)
no prescription (N = 499)
TABLE 2
Daily Dose, Daily Frequency, and Onset of Effects; Mean Values are Shown
Smoking
Vaporizer
Tea
Food/Tinc
Dronabinol
Nabilone
THC vap
Nabiximols
Other
a) Daily use
(units are indicated) 3.0 3.0 2.4 3.4 30.1 4.4 35.1 17.3 3.2
gram gram gram gram mg mg mg sprays gram
b) Daily frequency
(times per day) 6.0 5.2 1.9 1.8 2.5 3.2 8.8 10.9 3.3
c) First onset of effects
(minutes) 7.0 6.5 28.9 45.5 52.9 39.4 2.5 13.1 15.3
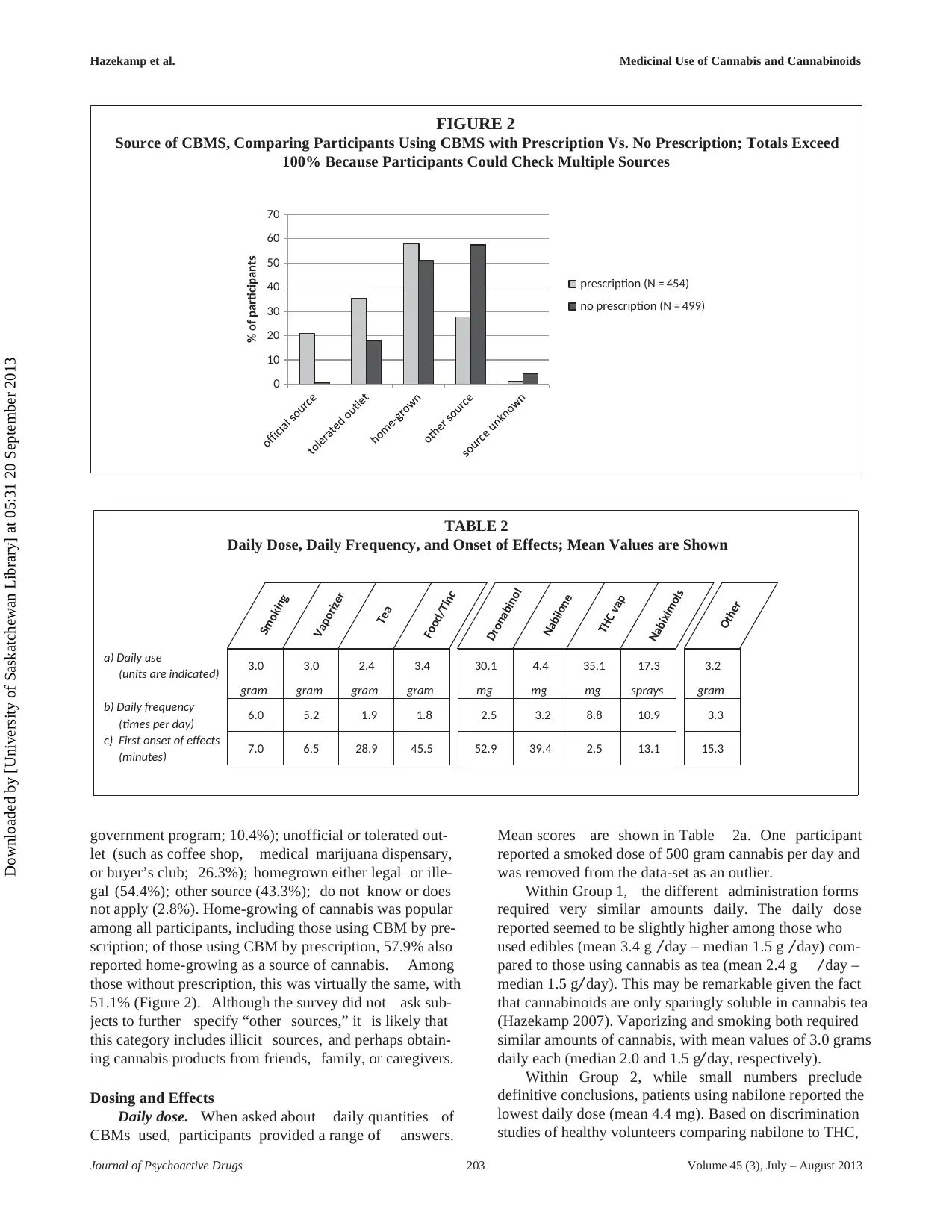
government program; 10.4%); unofficial or tolerated out-
let (such as coffee shop, medical marijuana dispensary,
or buyer’s club; 26.3%); homegrown either legal or ille-
gal (54.4%); other source (43.3%); do not know or does
not apply (2.8%). Home-growing of cannabis was popular
among all participants, including those using CBM by pre-
scription; of those using CBM by prescription, 57.9% also
reported home-growing as a source of cannabis. Among
those without prescription, this was virtually the same, with
51.1% (Figure 2). Although the survey did not ask sub-
jects to further specify “other sources,” it is likely that
this category includes illicit sources, and perhaps obtain-
ing cannabis products from friends, family, or caregivers.
Dosing and Effects
Daily dose. When asked about daily quantities of
CBMs used, participants provided a range of answers.
Mean scores are shown in Table 2a. One participant
reported a smoked dose of 500 gram cannabis per day and
was removed from the data-set as an outlier.
Within Group 1, the different administration forms
required very similar amounts daily. The daily dose
reported seemed to be slightly higher among those who
used edibles (mean 3.4 g / day – median 1.5 g / day) com-
pared to those using cannabis as tea (mean 2.4 g / day –
median 1.5 g/ day). This may be remarkable given the fact
that cannabinoids are only sparingly soluble in cannabis tea
(Hazekamp 2007). Vaporizing and smoking both required
similar amounts of cannabis, with mean values of 3.0 grams
daily each (median 2.0 and 1.5 g/ day, respectively).
Within Group 2, while small numbers preclude
definitive conclusions, patients using nabilone reported the
lowest daily dose (mean 4.4 mg). Based on discrimination
studies of healthy volunteers comparing nabilone to THC,
Journal of Psychoactive Drugs 203 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
FIGURE 2
Source of CBMS, Comparing Participants Using CBMS with Prescription Vs. No Prescription; Totals Exceed
100% Because Participants Could Check Multiple Sources
0
10
20
30
40
50
60
70
% of participants
prescription (N = 454)
no prescription (N = 499)
TABLE 2
Daily Dose, Daily Frequency, and Onset of Effects; Mean Values are Shown
Smoking
Vaporizer
Tea
Food/Tinc
Dronabinol
Nabilone
THC vap
Nabiximols
Other
a) Daily use
(units are indicated) 3.0 3.0 2.4 3.4 30.1 4.4 35.1 17.3 3.2
gram gram gram gram mg mg mg sprays gram
b) Daily frequency
(times per day) 6.0 5.2 1.9 1.8 2.5 3.2 8.8 10.9 3.3
c) First onset of effects
(minutes) 7.0 6.5 28.9 45.5 52.9 39.4 2.5 13.1 15.3
government program; 10.4%); unofficial or tolerated out-
let (such as coffee shop, medical marijuana dispensary,
or buyer’s club; 26.3%); homegrown either legal or ille-
gal (54.4%); other source (43.3%); do not know or does
not apply (2.8%). Home-growing of cannabis was popular
among all participants, including those using CBM by pre-
scription; of those using CBM by prescription, 57.9% also
reported home-growing as a source of cannabis. Among
those without prescription, this was virtually the same, with
51.1% (Figure 2). Although the survey did not ask sub-
jects to further specify “other sources,” it is likely that
this category includes illicit sources, and perhaps obtain-
ing cannabis products from friends, family, or caregivers.
Dosing and Effects
Daily dose. When asked about daily quantities of
CBMs used, participants provided a range of answers.
Mean scores are shown in Table 2a. One participant
reported a smoked dose of 500 gram cannabis per day and
was removed from the data-set as an outlier.
Within Group 1, the different administration forms
required very similar amounts daily. The daily dose
reported seemed to be slightly higher among those who
used edibles (mean 3.4 g / day – median 1.5 g / day) com-
pared to those using cannabis as tea (mean 2.4 g / day –
median 1.5 g/ day). This may be remarkable given the fact
that cannabinoids are only sparingly soluble in cannabis tea
(Hazekamp 2007). Vaporizing and smoking both required
similar amounts of cannabis, with mean values of 3.0 grams
daily each (median 2.0 and 1.5 g/ day, respectively).
Within Group 2, while small numbers preclude
definitive conclusions, patients using nabilone reported the
lowest daily dose (mean 4.4 mg). Based on discrimination
studies of healthy volunteers comparing nabilone to THC,
Journal of Psychoactive Drugs 203 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013

Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
it may be estimated that 1 mg of nabilone equals about
7-8 mg of THC (Lile 2011; Bedi 2012). For nabiximols,
when converting the number of sprays to total dose of THC
and CBD (2.7/ 2.5 mg per spray, respectively), the reported
daily dose of cannabinoids was a mean of 46 mg THC and
43 mg CBD per day.
Number of intakes. CBM preparations were further
evaluated according to the number of daily doses used
to treat the symptoms or condition of the participants.
Mean values are shown in Table 2b. Oral use of cannabis
in the form of tea, together with baked products or tinc-
ture, required the fewest intakes, with little less than two
administrations daily. Smoking and vaporizing cannabis
required a higher number of intakes, with an average of
five to six administrations daily. Oral cannabinoids are
known to have a longer, although more erratic, duration of
effect (Grotenhermen 2003; McGilveray 2005). As a result,
cannabis smokers may use more doses per day because
they are able to titrate to desired effect with multiple
smaller doses that have rapid onset. The sublingual product
nabiximols required the highest number of administrations
(mean 10.9) per day.
First onset of effects. The time needed to first onset
of effects is an important pharmacodynamic consideration
of a medicine. Together with total duration of effect, it has
a major impact on how quickly patients attain drug effi-
cacy, and therefore may influence adherence to the drug
regimen. The survey therefore asked how long it took on
average before first therapeutic effects became apparent
using the different preparations. Mean scores are shown
in Table 2c. Comparing CBMs in Group 1, subjects using
inhalation (smoking and vaporizing) reported a first effect
after about seven minutes. This is in agreement with a study
(Abrams 2007) which showed that smoking and vaporiz-
ing the same quantity of cannabis, respectively, resulted in
similar blood serum levels of THC over time. Although
tea and baked goods / tincture are both taken orally, sub-
jects using cannabis as tea reported more rapid onset of
effect (mean 29 min.) than other oral preparations (mean
46 min.). Uptake of cannabinoids can be significantly
delayed depending on the nature of the food present in
the gastrointestinal (GI) tract. For example, fatty foods can
significantly delay absorption (Grotenhermen 2002).
Subjects using vaporizers reported the onset of effects
more rapidly with pure THC (mean 2.5 min) than
herbal cannabis (mean 6.5 min). It is possible that
non-cannabinoid constituents present in the plant, such
as terpenes, delay or modulate the onset of effects of
cannabinoids (Russo 2011). Patients using nabilone or
dronabinol reported the longest time before first onset of
effect, likely due to the delay in GI absorption, resulting
in similar scores compared to cannabis taken in food or
as a tincture. Participants using nabiximols experienced
first effects after an average of 13 minutes, suggesting that
nabiximols may be absorbed (at least in part) sublingually,
speeding up the process of absorption of cannabinoids.
Advantages of Different Modes of Delivery
Subjects were asked to compare their satisfaction with
nine parameters regarding their experience using differ-
ent modes of delivery. These parameters included: dose
needed, onset of effect, duration of effect, ease of dose find-
ing, ease of exact dosing, ease of preparation and intake,
irritation of lungs (if applicable), side-effects, and cost
involved. The satisfaction rating scale ranged from 0 to 10,
with 0 representing absolutely no satisfaction and 10 rep-
resenting perfect satisfaction by the participant. A total
number of 4414 ratings were obtained. The results are
shown in Table 3.
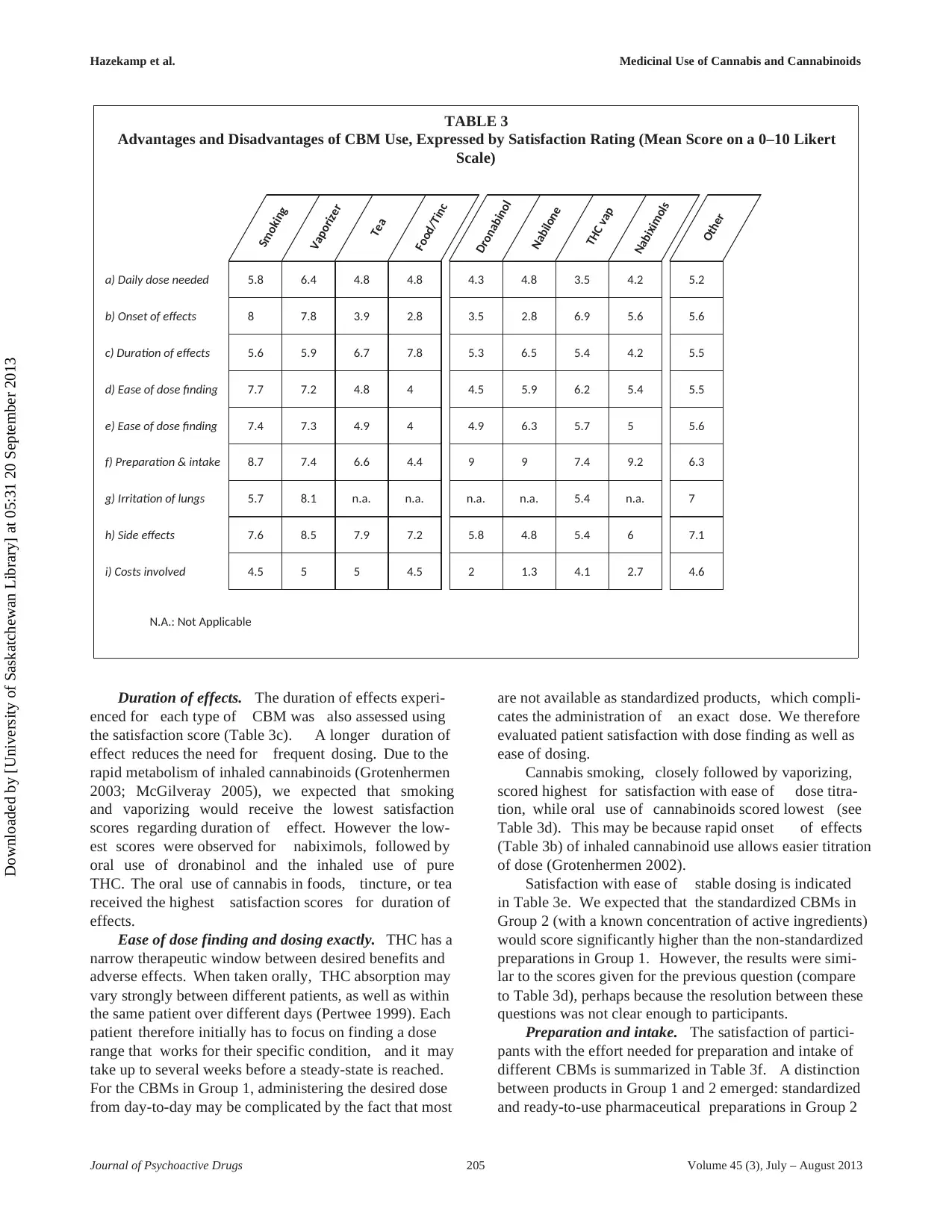
Daily dose needed. The highest rating of dose sat-
isfaction (representing “only low dose needed”) was
obtained for inhalation of herbal cannabis with a vapor-
izer (6.4), closely followed by smoking (5.8). The inhala-
tion of pure THC, using a vaporizer, received the
lowest score. This is notable, since laboratory stud-
ies have shown pure THC to evaporate more effi-
ciently (at the same temperature) than an equivalent
amount of THC present in herbal material (Hazekamp
2006). The use of products in Group 2 (range 3.5
– 4.8) scored consistently lower in dose satisfaction
than plant-based preparations in Group 1 (range 4.8 –
6.4).
The lower scores for Group 2 may be related to the fact
that these pharmaceutical preparations are generally per-
ceived as more costly than the preparations in Group 1 (see
section on “cost” below), which may subjectively add to the
sense of needing “too much” for a single dose. It should be
noted that the dose units are different between Groups 1 and
2 (gram versus milligram), and that the herbal cannabis
mentioned in the majority of cases has no standardized or
known chemical composition.
Onset of effects. Participants were asked to rate their
satisfaction with the time needed before first (therapeutic)
effects became apparent. The faster the first onset of effects,
the higher CBMs are scored on this scale (Table 3b). Note
that this scoring system assumed that rapid onset of effect
is always more satisfactory, which may not always be the
case for chronic stable conditions.
The highest satisfaction scores for onset of action
was obtained for smoking (8.0), vaporizing of herbal
cannabis (7.8), and inhaled administration of pure THC
(6.9). Indeed, the inhaled administration of cannabinoids
is known to be the most rapid way to induce measurable
serum levels of cannabinoids (Grotenhermen 2003). The
obtained results are compatible with Table 2c, where these
three inhaled administration forms all showed first effects
in less than 10 minutes. The administration forms with low-
est scores were all oral preparations with slow GI absorp-
tion and potential first-pass effects by liver metabolism.
Nabiximols received an intermediate score, suggesting that
patients experience pharmacodynamic effects of oromu-
cosal administration of cannabinoids somewhere between
inhaled and oral administration forms.
Journal of Psychoactive Drugs 204 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
it may be estimated that 1 mg of nabilone equals about
7-8 mg of THC (Lile 2011; Bedi 2012). For nabiximols,
when converting the number of sprays to total dose of THC
and CBD (2.7/ 2.5 mg per spray, respectively), the reported
daily dose of cannabinoids was a mean of 46 mg THC and
43 mg CBD per day.
Number of intakes. CBM preparations were further
evaluated according to the number of daily doses used
to treat the symptoms or condition of the participants.
Mean values are shown in Table 2b. Oral use of cannabis
in the form of tea, together with baked products or tinc-
ture, required the fewest intakes, with little less than two
administrations daily. Smoking and vaporizing cannabis
required a higher number of intakes, with an average of
five to six administrations daily. Oral cannabinoids are
known to have a longer, although more erratic, duration of
effect (Grotenhermen 2003; McGilveray 2005). As a result,
cannabis smokers may use more doses per day because
they are able to titrate to desired effect with multiple
smaller doses that have rapid onset. The sublingual product
nabiximols required the highest number of administrations
(mean 10.9) per day.
First onset of effects. The time needed to first onset
of effects is an important pharmacodynamic consideration
of a medicine. Together with total duration of effect, it has
a major impact on how quickly patients attain drug effi-
cacy, and therefore may influence adherence to the drug
regimen. The survey therefore asked how long it took on
average before first therapeutic effects became apparent
using the different preparations. Mean scores are shown
in Table 2c. Comparing CBMs in Group 1, subjects using
inhalation (smoking and vaporizing) reported a first effect
after about seven minutes. This is in agreement with a study
(Abrams 2007) which showed that smoking and vaporiz-
ing the same quantity of cannabis, respectively, resulted in
similar blood serum levels of THC over time. Although
tea and baked goods / tincture are both taken orally, sub-
jects using cannabis as tea reported more rapid onset of
effect (mean 29 min.) than other oral preparations (mean
46 min.). Uptake of cannabinoids can be significantly
delayed depending on the nature of the food present in
the gastrointestinal (GI) tract. For example, fatty foods can
significantly delay absorption (Grotenhermen 2002).
Subjects using vaporizers reported the onset of effects
more rapidly with pure THC (mean 2.5 min) than
herbal cannabis (mean 6.5 min). It is possible that
non-cannabinoid constituents present in the plant, such
as terpenes, delay or modulate the onset of effects of
cannabinoids (Russo 2011). Patients using nabilone or
dronabinol reported the longest time before first onset of
effect, likely due to the delay in GI absorption, resulting
in similar scores compared to cannabis taken in food or
as a tincture. Participants using nabiximols experienced
first effects after an average of 13 minutes, suggesting that
nabiximols may be absorbed (at least in part) sublingually,
speeding up the process of absorption of cannabinoids.
Advantages of Different Modes of Delivery
Subjects were asked to compare their satisfaction with
nine parameters regarding their experience using differ-
ent modes of delivery. These parameters included: dose
needed, onset of effect, duration of effect, ease of dose find-
ing, ease of exact dosing, ease of preparation and intake,
irritation of lungs (if applicable), side-effects, and cost
involved. The satisfaction rating scale ranged from 0 to 10,
with 0 representing absolutely no satisfaction and 10 rep-
resenting perfect satisfaction by the participant. A total
number of 4414 ratings were obtained. The results are
shown in Table 3.
Daily dose needed. The highest rating of dose sat-
isfaction (representing “only low dose needed”) was
obtained for inhalation of herbal cannabis with a vapor-
izer (6.4), closely followed by smoking (5.8). The inhala-
tion of pure THC, using a vaporizer, received the
lowest score. This is notable, since laboratory stud-
ies have shown pure THC to evaporate more effi-
ciently (at the same temperature) than an equivalent
amount of THC present in herbal material (Hazekamp
2006). The use of products in Group 2 (range 3.5
– 4.8) scored consistently lower in dose satisfaction
than plant-based preparations in Group 1 (range 4.8 –
6.4).
The lower scores for Group 2 may be related to the fact
that these pharmaceutical preparations are generally per-
ceived as more costly than the preparations in Group 1 (see
section on “cost” below), which may subjectively add to the
sense of needing “too much” for a single dose. It should be
noted that the dose units are different between Groups 1 and
2 (gram versus milligram), and that the herbal cannabis
mentioned in the majority of cases has no standardized or
known chemical composition.
Onset of effects. Participants were asked to rate their
satisfaction with the time needed before first (therapeutic)
effects became apparent. The faster the first onset of effects,
the higher CBMs are scored on this scale (Table 3b). Note
that this scoring system assumed that rapid onset of effect
is always more satisfactory, which may not always be the
case for chronic stable conditions.
The highest satisfaction scores for onset of action
was obtained for smoking (8.0), vaporizing of herbal
cannabis (7.8), and inhaled administration of pure THC
(6.9). Indeed, the inhaled administration of cannabinoids
is known to be the most rapid way to induce measurable
serum levels of cannabinoids (Grotenhermen 2003). The
obtained results are compatible with Table 2c, where these
three inhaled administration forms all showed first effects
in less than 10 minutes. The administration forms with low-
est scores were all oral preparations with slow GI absorp-
tion and potential first-pass effects by liver metabolism.
Nabiximols received an intermediate score, suggesting that
patients experience pharmacodynamic effects of oromu-
cosal administration of cannabinoids somewhere between
inhaled and oral administration forms.
Journal of Psychoactive Drugs 204 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
TABLE 3
Advantages and Disadvantages of CBM Use, Expressed by Satisfaction Rating (Mean Score on a 0–10 Likert
Scale)
Smoking
Vaporizer
Tea
Food/Tinc
Dronabinol
Nabilone
THC vap
Nabiximols
Other
a) Daily dose needed 5.8 6.4 4.8 4.8 4.3 4.8 3.5 4.2 5.2
b) Onset of effects 8 7.8 3.9 2.8 3.5 2.8 6.9 5.6 5.6
c) Duration of effects 5.6 5.9 6.7 7.8 5.3 6.5 5.4 4.2 5.5
d) Ease of dose finding 7.7 7.2 4.8 4 4.5 5.9 6.2 5.4 5.5
e) Ease of dose finding 7.4 7.3 4.9 4 4.9 6.3 5.7 5 5.6
f) Preparation & intake 8.7 7.4 6.6 4.4 9 9 7.4 9.2 6.3
g) Irritation of lungs 5.7 8.1 n.a. n.a. n.a. n.a. 5.4 n.a. 7
h) Side effects 7.6 8.5 7.9 7.2 5.8 4.8 5.4 6 7.1
i) Costs involved
N.A.: Not Applicable
4.5 5 5 4.5 2 1.3 4.1 2.7 4.6
Duration of effects. The duration of effects experi-
enced for each type of CBM was also assessed using
the satisfaction score (Table 3c). A longer duration of
effect reduces the need for frequent dosing. Due to the
rapid metabolism of inhaled cannabinoids (Grotenhermen
2003; McGilveray 2005), we expected that smoking
and vaporizing would receive the lowest satisfaction
scores regarding duration of effect. However the low-
est scores were observed for nabiximols, followed by
oral use of dronabinol and the inhaled use of pure
THC. The oral use of cannabis in foods, tincture, or tea
received the highest satisfaction scores for duration of
effects.
Ease of dose finding and dosing exactly. THC has a
narrow therapeutic window between desired benefits and
adverse effects. When taken orally, THC absorption may
vary strongly between different patients, as well as within
the same patient over different days (Pertwee 1999). Each
patient therefore initially has to focus on finding a dose
range that works for their specific condition, and it may
take up to several weeks before a steady-state is reached.
For the CBMs in Group 1, administering the desired dose
from day-to-day may be complicated by the fact that most
are not available as standardized products, which compli-
cates the administration of an exact dose. We therefore
evaluated patient satisfaction with dose finding as well as
ease of dosing.
Cannabis smoking, closely followed by vaporizing,
scored highest for satisfaction with ease of dose titra-
tion, while oral use of cannabinoids scored lowest (see
Table 3d). This may be because rapid onset of effects
(Table 3b) of inhaled cannabinoid use allows easier titration
of dose (Grotenhermen 2002).
Satisfaction with ease of stable dosing is indicated
in Table 3e. We expected that the standardized CBMs in
Group 2 (with a known concentration of active ingredients)
would score significantly higher than the non-standardized
preparations in Group 1. However, the results were simi-
lar to the scores given for the previous question (compare
to Table 3d), perhaps because the resolution between these
questions was not clear enough to participants.
Preparation and intake. The satisfaction of partici-
pants with the effort needed for preparation and intake of
different CBMs is summarized in Table 3f. A distinction
between products in Group 1 and 2 emerged: standardized
and ready-to-use pharmaceutical preparations in Group 2
Journal of Psychoactive Drugs 205 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
TABLE 3
Advantages and Disadvantages of CBM Use, Expressed by Satisfaction Rating (Mean Score on a 0–10 Likert
Scale)
Smoking
Vaporizer
Tea
Food/Tinc
Dronabinol
Nabilone
THC vap
Nabiximols
Other
a) Daily dose needed 5.8 6.4 4.8 4.8 4.3 4.8 3.5 4.2 5.2
b) Onset of effects 8 7.8 3.9 2.8 3.5 2.8 6.9 5.6 5.6
c) Duration of effects 5.6 5.9 6.7 7.8 5.3 6.5 5.4 4.2 5.5
d) Ease of dose finding 7.7 7.2 4.8 4 4.5 5.9 6.2 5.4 5.5
e) Ease of dose finding 7.4 7.3 4.9 4 4.9 6.3 5.7 5 5.6
f) Preparation & intake 8.7 7.4 6.6 4.4 9 9 7.4 9.2 6.3
g) Irritation of lungs 5.7 8.1 n.a. n.a. n.a. n.a. 5.4 n.a. 7
h) Side effects 7.6 8.5 7.9 7.2 5.8 4.8 5.4 6 7.1
i) Costs involved
N.A.: Not Applicable
4.5 5 5 4.5 2 1.3 4.1 2.7 4.6
Duration of effects. The duration of effects experi-
enced for each type of CBM was also assessed using
the satisfaction score (Table 3c). A longer duration of
effect reduces the need for frequent dosing. Due to the
rapid metabolism of inhaled cannabinoids (Grotenhermen
2003; McGilveray 2005), we expected that smoking
and vaporizing would receive the lowest satisfaction
scores regarding duration of effect. However the low-
est scores were observed for nabiximols, followed by
oral use of dronabinol and the inhaled use of pure
THC. The oral use of cannabis in foods, tincture, or tea
received the highest satisfaction scores for duration of
effects.
Ease of dose finding and dosing exactly. THC has a
narrow therapeutic window between desired benefits and
adverse effects. When taken orally, THC absorption may
vary strongly between different patients, as well as within
the same patient over different days (Pertwee 1999). Each
patient therefore initially has to focus on finding a dose
range that works for their specific condition, and it may
take up to several weeks before a steady-state is reached.
For the CBMs in Group 1, administering the desired dose
from day-to-day may be complicated by the fact that most
are not available as standardized products, which compli-
cates the administration of an exact dose. We therefore
evaluated patient satisfaction with dose finding as well as
ease of dosing.
Cannabis smoking, closely followed by vaporizing,
scored highest for satisfaction with ease of dose titra-
tion, while oral use of cannabinoids scored lowest (see
Table 3d). This may be because rapid onset of effects
(Table 3b) of inhaled cannabinoid use allows easier titration
of dose (Grotenhermen 2002).
Satisfaction with ease of stable dosing is indicated
in Table 3e. We expected that the standardized CBMs in
Group 2 (with a known concentration of active ingredients)
would score significantly higher than the non-standardized
preparations in Group 1. However, the results were simi-
lar to the scores given for the previous question (compare
to Table 3d), perhaps because the resolution between these
questions was not clear enough to participants.
Preparation and intake. The satisfaction of partici-
pants with the effort needed for preparation and intake of
different CBMs is summarized in Table 3f. A distinction
between products in Group 1 and 2 emerged: standardized
and ready-to-use pharmaceutical preparations in Group 2
Journal of Psychoactive Drugs 205 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013

Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
scored generally higher than the herbal cannabis-based
preparations in Group 1. One notable exception is the
smoked use of cannabis, which receives a high score.
A possible reason may be that most participants had pre-
vious experience with smoking cannabis, before as well as
during onset of their illness. It is likely that those partic-
ipants are familiar with preparing cannabis cigarettes and
therefore do not find it bothersome to do so when they get
ill. It is also possible that participants conveniently used a
pipe or bong to inhale herbal cannabis (not evaluated in this
study). The low score for baked products or tincture may be
due to the extended time needed to prepare such products.
With baked products specifically, limited shelf-life stability
may also play a role.
Irritation of the lungs. Satisfaction with lung irri-
tation (Table 3g; low scores suggest more irritation) is
only relevant for inhaled preparations. While smoking and
vaporizing herbal cannabis received similar scores in previ-
ous questions, the advantage of vaporizing (score 8.1) over
smoking (score 5.7) now becomes more obvious. This
advantage was not recognized for using the vaporizer with
pure THC, which scored similar to smoking. Although
pyrolytic by-products of combustion may be responsible
for pulmonary irritation, it seems that THC alone is also
capable of this (Tashkin 1977; Naef 2004). Higher satisfac-
tion with vaporized cannabis compared to THC alone may
be due to the presence of non-THC constituents, including
anti-inflammatory terpenes that protect the lungs from irri-
tation (Russo 2011). Another potential cause of irritation
from pure THC preparations may be the presence of resid-
ual solvents (e.g., ethanol) that are needed to solubilize the
sticky pure THC (Žuškin 1981).
Side-effects. Participants were asked to rate their
satisfaction with side-effects experienced with the different
CBMs (Table 3h). Unfortunately, the survey did not ask
more explicitly what side-effects were experienced, as
this could have added significantly to our understanding
of CBMs in a large population. Here, more than for any
other parameter that we assessed, the differences between
preparations in Group 1 and 2 were very distinct. The
herbal cannabis-based products received mean scores in
the range of 7.2 – 8.5 (meaning high overall satisfaction
with side-effect profiles), while the pharmaceutical prepa-
rations scored notably lower with a range of 4.8 – 6.0.
The major reason for this may be that the majority of
herbal cannabis users (76%) admitted to using cannabis
products before onset of their condition, potentially giving
them more experience with these CBMs and, hence, with
their potential side-effects. The lowest satisfaction with
side-effects was reported with the use of nabilone, even
though the average daily dose of nabilone reported by
subjects (4.4 mg; Table 2) was well below the maximum
of 3 mg twice daily suggested by the product monograph.
Vaporizing cannabis had the highest side-effect satisfaction
score (score 8.5), which was higher than that reported for
smoking (score 7.6).
Costs involved. For chronically ill patients, disabil-
ity and unemployment render “cost” to be an important
factor in CBM use. Overall, low satisfaction ratings were
observed for all products (highest mean score was 5.0; see
Table 3i), suggesting that the cost of using CBMs is a major
issue for patients everywhere.
Open Questions
In order to assess what the perfect CBM would look
like, if participants could develop their own product, the
survey concluded with two open-ended questions. A total
of 375 suggestions were obtained (39.3% response).
A tincture based on whole cannabis was found to be
the most popular choice for a new product to be developed,
mainly because it can be used in a multitude of ways: as
oral drops, in baking and tea, and for vaporizing as well
as smoking. According to participants, this allows for max-
imal flexibility of using cannabinoids throughout the day.
Furthermore, a tincture can be used discretely in public
(i.e., at work, visiting friends) and does not have a strong,
distinct smell. Other common suggestions were legally
growing your own cannabis, developing standardized edi-
bles, and capsules containing whole powdered cannabis
plant.
Issues that participants wanted to see addressed
included bad taste, adverse events such as drowsiness,
uncontrollable appetite (munchies), and mental effects
(getting high). The latter observation is of particular inter-
est, as getting high is generally the effect that recreational
users seek when using cannabis. Multiple participants fur-
ther suggested that different administration forms may be
preferred in the privacy of one’s home and in public.
CONCLUSION
To our knowledge, this survey represents the largest
systematic study of patient experiences with CBMs con-
ducted to date. Although other, and sometimes larger,
surveys have been reported, they did not compare patients’
experiences with multiple CBM products or administra-
tions forms, or they were restricted to a single country
and/ or focused on a single medical condition (Hazekamp
2013; Corless 2009; O’Connell 2007; Chong 2006; Ware
2005; Prentiss 2004; Page 2003; Ware 2003; Braitstein
2001; Ogborne 2000). The majority of subjects in our
study were current users who had a health professional
involved in the management of their illness, and were using
CBMs for at least several years. The inclusion criterion
that participants should have experience with at least two
administration forms was easily met in the study popula-
tion; on average, those who (ever) tried smoking cannabis
had experience with 2.4 different administration forms
(lowest value), while those who had (ever) used nabiximols
(Sativex®) had tried 4.4 different administration forms
(highest value) in total.
Journal of Psychoactive Drugs 206 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
scored generally higher than the herbal cannabis-based
preparations in Group 1. One notable exception is the
smoked use of cannabis, which receives a high score.
A possible reason may be that most participants had pre-
vious experience with smoking cannabis, before as well as
during onset of their illness. It is likely that those partic-
ipants are familiar with preparing cannabis cigarettes and
therefore do not find it bothersome to do so when they get
ill. It is also possible that participants conveniently used a
pipe or bong to inhale herbal cannabis (not evaluated in this
study). The low score for baked products or tincture may be
due to the extended time needed to prepare such products.
With baked products specifically, limited shelf-life stability
may also play a role.
Irritation of the lungs. Satisfaction with lung irri-
tation (Table 3g; low scores suggest more irritation) is
only relevant for inhaled preparations. While smoking and
vaporizing herbal cannabis received similar scores in previ-
ous questions, the advantage of vaporizing (score 8.1) over
smoking (score 5.7) now becomes more obvious. This
advantage was not recognized for using the vaporizer with
pure THC, which scored similar to smoking. Although
pyrolytic by-products of combustion may be responsible
for pulmonary irritation, it seems that THC alone is also
capable of this (Tashkin 1977; Naef 2004). Higher satisfac-
tion with vaporized cannabis compared to THC alone may
be due to the presence of non-THC constituents, including
anti-inflammatory terpenes that protect the lungs from irri-
tation (Russo 2011). Another potential cause of irritation
from pure THC preparations may be the presence of resid-
ual solvents (e.g., ethanol) that are needed to solubilize the
sticky pure THC (Žuškin 1981).
Side-effects. Participants were asked to rate their
satisfaction with side-effects experienced with the different
CBMs (Table 3h). Unfortunately, the survey did not ask
more explicitly what side-effects were experienced, as
this could have added significantly to our understanding
of CBMs in a large population. Here, more than for any
other parameter that we assessed, the differences between
preparations in Group 1 and 2 were very distinct. The
herbal cannabis-based products received mean scores in
the range of 7.2 – 8.5 (meaning high overall satisfaction
with side-effect profiles), while the pharmaceutical prepa-
rations scored notably lower with a range of 4.8 – 6.0.
The major reason for this may be that the majority of
herbal cannabis users (76%) admitted to using cannabis
products before onset of their condition, potentially giving
them more experience with these CBMs and, hence, with
their potential side-effects. The lowest satisfaction with
side-effects was reported with the use of nabilone, even
though the average daily dose of nabilone reported by
subjects (4.4 mg; Table 2) was well below the maximum
of 3 mg twice daily suggested by the product monograph.
Vaporizing cannabis had the highest side-effect satisfaction
score (score 8.5), which was higher than that reported for
smoking (score 7.6).
Costs involved. For chronically ill patients, disabil-
ity and unemployment render “cost” to be an important
factor in CBM use. Overall, low satisfaction ratings were
observed for all products (highest mean score was 5.0; see
Table 3i), suggesting that the cost of using CBMs is a major
issue for patients everywhere.
Open Questions
In order to assess what the perfect CBM would look
like, if participants could develop their own product, the
survey concluded with two open-ended questions. A total
of 375 suggestions were obtained (39.3% response).
A tincture based on whole cannabis was found to be
the most popular choice for a new product to be developed,
mainly because it can be used in a multitude of ways: as
oral drops, in baking and tea, and for vaporizing as well
as smoking. According to participants, this allows for max-
imal flexibility of using cannabinoids throughout the day.
Furthermore, a tincture can be used discretely in public
(i.e., at work, visiting friends) and does not have a strong,
distinct smell. Other common suggestions were legally
growing your own cannabis, developing standardized edi-
bles, and capsules containing whole powdered cannabis
plant.
Issues that participants wanted to see addressed
included bad taste, adverse events such as drowsiness,
uncontrollable appetite (munchies), and mental effects
(getting high). The latter observation is of particular inter-
est, as getting high is generally the effect that recreational
users seek when using cannabis. Multiple participants fur-
ther suggested that different administration forms may be
preferred in the privacy of one’s home and in public.
CONCLUSION
To our knowledge, this survey represents the largest
systematic study of patient experiences with CBMs con-
ducted to date. Although other, and sometimes larger,
surveys have been reported, they did not compare patients’
experiences with multiple CBM products or administra-
tions forms, or they were restricted to a single country
and/ or focused on a single medical condition (Hazekamp
2013; Corless 2009; O’Connell 2007; Chong 2006; Ware
2005; Prentiss 2004; Page 2003; Ware 2003; Braitstein
2001; Ogborne 2000). The majority of subjects in our
study were current users who had a health professional
involved in the management of their illness, and were using
CBMs for at least several years. The inclusion criterion
that participants should have experience with at least two
administration forms was easily met in the study popula-
tion; on average, those who (ever) tried smoking cannabis
had experience with 2.4 different administration forms
(lowest value), while those who had (ever) used nabiximols
(Sativex®) had tried 4.4 different administration forms
(highest value) in total.
Journal of Psychoactive Drugs 206 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013

Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
The main goal of this survey was to compare different
administration forms of cannabinoids and identify their rel-
ative advantages and disadvantages as described by actual
users. Before any conclusions may be drawn, however, the
potential limitations of the study must be clearly addressed.
Most participants of the survey had experience with herbal
cannabis before onset of their medical condition, and smok-
ing of cannabis was the most common method of intake
participants had tried. So although many different methods
of intake were represented in the survey, the results may
be biased towards the use of herbal cannabis. Also, people
who were satisfied with their first cannabinoid medication
(e.g., smoked cannabis, dronabinol, nabiximols) and used
it without problems were not included, because they did
not meet the inclusion criterion of having experience with
multiple methods of intake. As homemade cannabis prod-
ucts allow more experimentation with administration forms
and dosing than standardized oral products, the survey con-
sequently may have favored participants who use herbal
cannabis. Because of this potential for bias, we caution
against drawing any conclusions with respect to the effi-
cacy of any CBM from this study. In addition, circulating
the survey through the IACM may have attracted responses
from subjects who are already familiar with cannabis
effects and may have produced a bias towards more posi-
tive responses overall. In addition, one should keep in mind
that some CBMs (such as nabiximols) have been available
to patients only for a relatively short time. But despite these
limitations, we believe that these results contribute to our
understanding of patient preferences for specific methods
of intake (administration forms) for cannabinoids.
Patient-reported advantages and disadvantages for
each administration form varied widely. Many parame-
ters measured (e.g., time before first onset, duration of
effects) showed a distinction between oral and inhaled
forms of cannabinoids, reflecting known differences in
pharmacokinetics and/ or pharmacodynamics. Nabiximols
was often rated between oral and inhaled administration,
reflecting the different nature of the oromucosal admin-
istration form. Many participants also provided scores in
the category “other use,” suggesting there may have been
administration forms that were overlooked in this study.
Future surveys may want to further identify such products.
In general, products in Group 1 (i.e., herbal non-
pharmaceutical CBMs) received the higher scores in most
categories. Products of Group 2 (i.e., pharmaceutical, stan-
dardized products) scored consistently higher than the
herbal preparations only for “ease of preparation and
intake.” Indeed, herbal products are generally lacking con-
venient, reliable, and standardized administration forms, in
contrast to conventional approved medicines.
There was low patient satisfaction with costs for
all CBMs studied. This may be because most health-
care systems do not provide for reimbursement or health
insurance coverage of CBMs, with the exception of the
Dutch program, where cannabis is covered by an increasing
number of health insurers (NCSM 2012), and Canada
where nabilone is covered on most provincial formula-
ries. Indeed, cost factors may have influenced our data:
when medication costs are covered by health insurance,
patients may be able to use higher doses. Conversely, when
patients have to cover the costs by themselves (probably
most often when cannabis is used without prescription)
the doses used may be lower. Perhaps those patients pre-
ferring herbal cannabis are those who need a very high
dose of cannabinoids, which cannot be covered by the cur-
rently available pharmaceutical cannabinoid preparations,
both practically and economically. As a result, costs may
also be a reason for patients to grow their own cannabis.
The source where CBMs are obtained is worth fur-
ther consideration, as we found that homegrowing is very
popular despite clear legal risks in most countries. Patients
who use CBMs on prescription, and simultaneously grow
their own cannabis, may raise questions about the legiti-
macy of their medical use of cannabinoids. For researchers
as well as policy makers, it would be of interest to under-
stand the motivations for patients to choose a particular
source of herbal CBMs. Besides cost, another potential rea-
son for continuing home-growing despite having access to
a legal source may be a lack of cannabis varieties currently
available to patients. Indeed, differences in chemical com-
position between varieties can be significant (Fischedick
2010; Hazekamp 2012). In Canada, a recent review of the
national medical marijuana program indicated that access
to multiple cannabis varieties is an important issue for
patients (Health Canada 2011).
In conclusion, we believe that this survey presents a
broad picture of the current state of CBM use. The reported
data may be useful to guide the development of safe
and effective cannabinoid-based medications that meet the
needs of patients. Besides the need for such products to be
standardized and quality controlled, our data suggest that
overall there is good satisfaction with whole plant prepa-
rations that are affordable and administered in an inhaled
manner, or in the form of a tincture. Relatively new admin-
istration forms of herbal cannabis, such as juicing of raw
leaves and buds (Courtney 2012), or the preparation of con-
centrated extracts such as Simpson oil (Simpson 2012), are
not covered by this survey. Future studies should therefore
be aware of these newer cannabis preparations, and ask
more detailed questions in order to properly explore such
upcoming cannabinoid based treatments.
CONFLICT OF INTEREST STATEMENTS
The authors, with the exception of DA, are members
of the Board of Directors of the IACM. FG is also the
Executive Director of the IACM. AH receives income in
his role as head of Research and Development at Bedrocan
BV. MW has received grant support from GW, Bedrocan,
Journal of Psychoactive Drugs 207 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
The main goal of this survey was to compare different
administration forms of cannabinoids and identify their rel-
ative advantages and disadvantages as described by actual
users. Before any conclusions may be drawn, however, the
potential limitations of the study must be clearly addressed.
Most participants of the survey had experience with herbal
cannabis before onset of their medical condition, and smok-
ing of cannabis was the most common method of intake
participants had tried. So although many different methods
of intake were represented in the survey, the results may
be biased towards the use of herbal cannabis. Also, people
who were satisfied with their first cannabinoid medication
(e.g., smoked cannabis, dronabinol, nabiximols) and used
it without problems were not included, because they did
not meet the inclusion criterion of having experience with
multiple methods of intake. As homemade cannabis prod-
ucts allow more experimentation with administration forms
and dosing than standardized oral products, the survey con-
sequently may have favored participants who use herbal
cannabis. Because of this potential for bias, we caution
against drawing any conclusions with respect to the effi-
cacy of any CBM from this study. In addition, circulating
the survey through the IACM may have attracted responses
from subjects who are already familiar with cannabis
effects and may have produced a bias towards more posi-
tive responses overall. In addition, one should keep in mind
that some CBMs (such as nabiximols) have been available
to patients only for a relatively short time. But despite these
limitations, we believe that these results contribute to our
understanding of patient preferences for specific methods
of intake (administration forms) for cannabinoids.
Patient-reported advantages and disadvantages for
each administration form varied widely. Many parame-
ters measured (e.g., time before first onset, duration of
effects) showed a distinction between oral and inhaled
forms of cannabinoids, reflecting known differences in
pharmacokinetics and/ or pharmacodynamics. Nabiximols
was often rated between oral and inhaled administration,
reflecting the different nature of the oromucosal admin-
istration form. Many participants also provided scores in
the category “other use,” suggesting there may have been
administration forms that were overlooked in this study.
Future surveys may want to further identify such products.
In general, products in Group 1 (i.e., herbal non-
pharmaceutical CBMs) received the higher scores in most
categories. Products of Group 2 (i.e., pharmaceutical, stan-
dardized products) scored consistently higher than the
herbal preparations only for “ease of preparation and
intake.” Indeed, herbal products are generally lacking con-
venient, reliable, and standardized administration forms, in
contrast to conventional approved medicines.
There was low patient satisfaction with costs for
all CBMs studied. This may be because most health-
care systems do not provide for reimbursement or health
insurance coverage of CBMs, with the exception of the
Dutch program, where cannabis is covered by an increasing
number of health insurers (NCSM 2012), and Canada
where nabilone is covered on most provincial formula-
ries. Indeed, cost factors may have influenced our data:
when medication costs are covered by health insurance,
patients may be able to use higher doses. Conversely, when
patients have to cover the costs by themselves (probably
most often when cannabis is used without prescription)
the doses used may be lower. Perhaps those patients pre-
ferring herbal cannabis are those who need a very high
dose of cannabinoids, which cannot be covered by the cur-
rently available pharmaceutical cannabinoid preparations,
both practically and economically. As a result, costs may
also be a reason for patients to grow their own cannabis.
The source where CBMs are obtained is worth fur-
ther consideration, as we found that homegrowing is very
popular despite clear legal risks in most countries. Patients
who use CBMs on prescription, and simultaneously grow
their own cannabis, may raise questions about the legiti-
macy of their medical use of cannabinoids. For researchers
as well as policy makers, it would be of interest to under-
stand the motivations for patients to choose a particular
source of herbal CBMs. Besides cost, another potential rea-
son for continuing home-growing despite having access to
a legal source may be a lack of cannabis varieties currently
available to patients. Indeed, differences in chemical com-
position between varieties can be significant (Fischedick
2010; Hazekamp 2012). In Canada, a recent review of the
national medical marijuana program indicated that access
to multiple cannabis varieties is an important issue for
patients (Health Canada 2011).
In conclusion, we believe that this survey presents a
broad picture of the current state of CBM use. The reported
data may be useful to guide the development of safe
and effective cannabinoid-based medications that meet the
needs of patients. Besides the need for such products to be
standardized and quality controlled, our data suggest that
overall there is good satisfaction with whole plant prepa-
rations that are affordable and administered in an inhaled
manner, or in the form of a tincture. Relatively new admin-
istration forms of herbal cannabis, such as juicing of raw
leaves and buds (Courtney 2012), or the preparation of con-
centrated extracts such as Simpson oil (Simpson 2012), are
not covered by this survey. Future studies should therefore
be aware of these newer cannabis preparations, and ask
more detailed questions in order to properly explore such
upcoming cannabinoid based treatments.
CONFLICT OF INTEREST STATEMENTS
The authors, with the exception of DA, are members
of the Board of Directors of the IACM. FG is also the
Executive Director of the IACM. AH receives income in
his role as head of Research and Development at Bedrocan
BV. MW has received grant support from GW, Bedrocan,
Journal of Psychoactive Drugs 207 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
and Valeant and has consulted for companies developing
cannabinoid medications (AstraZeneca, Boehringer,
Ironwood). Apart from this support, no other funding was
received for conducting the survey or publishing the data.
The survey results have been presented, in part, at an
IACM conference in Bonn, Germany, in September, 2011.
An abstract was posted on the website of the IACM (http://
www.cannabis-med.org).
REFERENCES
Bedi, G.; Cooper, Z.D. & Haney, M. 2012. Subjective, cognitive and
cardiovascular dose-effect profile of nabilone and dronabinol in
marijuana smokers. Addiction Biology (in press).
Braitstein, P.; Kendall. T.; Chan, K.; Wood, E.; Montaner, J.S.;
O’Shaughnessy, M.V. & Hogg, R.S. 2001. Mary-Jane and
her patients: Sociodemographic and clinical characteristics of
HIVpositive individuals using medical marijuana and antiretroviral
agents. AIDS 15 (4): 532–3.
Chong, M.S.; Wolff, K.; Wise, K.; Tanton, C.; Winstock, A. & Silber,
E. 2006. Cannabis use in patients with multiple sclerosis. Multiple
Sclerosis 12 (5): 646–51.
Corless, I.B.; Lindgren, T.; Holzemer, W.; Robinson, L.; Moezzi, S.;
Kirksey, K.; Coleman, C.; Tsai, Y.F.; Sanzero Eller, L.; Hamilton,
M.J.; Sefcik, E.F.; Canaval, G.E.; Rivero Mendez, M.; Kemppainen,
J.K.; Bunch, E.H.; Nicholas, P.K.; Nokes, K.M.; Dole, P. &
Reynolds, N. 2009. Marijuana effectiveness as an HIV self-care
strategy. Clinical Nursing Research 18(2): 172–93.
Courtney, W. 2012. Cannabis International, official website. Available
at:http://www.cannabisinternational.org.
Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H. & Verpoorte, R.
2010. Metabolic fingerprinting of Cannabis sativa L. cannabinoids
and terpenoids for chemotaxonomic and drug standardization pur-
poses. Phytochemistry 71: 2058–73.
Freeman, R.M.; Adekanmi, O.; Waterfield, M.R.; Waterfield, A.E.;
Wright, D. & Zajicek, J. 2006. The effect of cannabis on urge
incontinence in patients with multiple sclerosis: A multicentre,
randomised placebo-controlled trial (CAMS-LUTS). International
Urogynecology Journal and Pelvic Floor Dysfunction 17 (6):
636–41.
Grotenhermen, F. & Russo, E. 2002. Cannabis and Cannabinoids:
Pharmacology, Toxicology and Therapeutic Potential. New York:
Haworth Press.
Grotenhermen, F. 2003. Pharmacokinetics and pharmacodynamics of
cannabinoids. Clinical Pharmacokinetics 42 (4): 327–60.
GW Pharmaceuticals. 2012. Sativex® Mutual Recognition Procedure
Closes with Recommendation for Approval in Ten European
Countries. Available at:http://www.gwpharm.com/Sativex%
20Mutual%20Recognition%20Procedure%20Closes%20with%
20Recommendation%20for%20Approval%20in%20Ten%
20European%20Countries.aspx.
Haney, M.; Rabkin, J.; Gunderson, E. & Foltin, R.W. 2005. Dronabinol
and marijuana in HIV( +) marijuana smokers: Acute effects on
caloric intake and mood. Psychopharmacology (Berlin) 181 (1):
170–78.
Haney, M.; Gunderson, E.W.; Rabkin, J.; Hart, C.L.; Vosburg, S.K.;
Comer, S.D. & Foltin, R.W. 2007. Dronabinol and marijuana in
HIV-positive marijuana smokers: Caloric intake, mood, and sleep.
Journal of Acquired Immune Deficiency Syndromes 45 (5): 545–54.
Hazekamp, A.; Ruhaak, R.; Zuurman, L.; van Gerven, J. & Verpoorte,
R. 2006. Evaluation of a vaporizing device (Volcano®) for
the pulmonary delivery of tetrahydrocannabinol. Journal of
Pharmaceutical Sciences 95 (6): 1308–17.
Hazekamp, A.; Bastola, K.; Rashidi, H.; Bender, J. & Verpoorte, R. 2007.
Cannabis tea revisited: A systematic evaluation of the cannabinoid
composition of cannabis tea. Journal of Ethnopharmacology 113:
85–90.
Hazekamp, A. & Fischedick, J.T. 2012. Cannabis - from cultivar to
chemovar: Towards a better definition of cannabis potency. Drug
Testing and Analysis 4: 660–7.
Hazekamp, A. & Heerdink E.R. 2013. The prevalence and incidence of
medicinal cannabis on prescription in The Netherlands. European
Journal of Clinical Pharmacology (in press).
Health Canada. 2011. Canada Federal Department of Health. Medical
Marihuana Regulatory Reform 2011 Consultations Results.
Available at:http://www.hc-sc.gc.ca/dhp-mps/consultation/
marihuana/_2011/program/consult_reform-eng.php.
IACM (International Association for Cannabinoid Medicines). 2009.
Laws and Politics of Germany, November 27, 2009. Available
at:http://www.cannabis-med.org/index.php?tpl=page&id=44&
lng=en.
IACM (International Association for Cannabinoid Medicines). 2012.
USA: Massachusetts becomes the 18th state to legalize the medical
use of cannabis, November 18, 2012. Available at:http://www.
cannabis-med.org/english/bulletin/ww_en_db_cannabis_artikel.
php?id=386#1.
Killestein, J.; Hoogervorst, E.L.; Reif, M.; Kalkers, N.F.; Van Loenen,
A.C.; Staats, P.G.; Gorter, R.W.; Uitdehaag, B.M. & Polman,
C.H. 2002. Safety, tolerability, and efficacy of orally administered
cannabinoids in MS. Neurology 58 (9): 1404–7.
Lile, J.A.; Kelly, T.H. & Hays, L.R. 2011. Separate and combined effects
of the cannabinoid agonists nabilone and 9-THC in humans dis-
criminating 9-THC. Drug and Alcohol Dependence 116 (1–3):
86–92.
McGilveray, I.J. 2005. Pharmacokinetics of cannabinoids. Pain Research
& Management 10 (Suppl A): 15A–22A.
Naef, M.; Russmann, S.; Petersen-Felix, S. & Brenneisen, R. 2004.
Development and pharmacokinetic characterization of pulmonal and
intravenous delta-9-tetrahydrocannabinol (THC) in humans. Journal
of Pharmaceutical Sciences 93: 1176–84.
NCSM (Dutch Foundation for Legal Cannabis and its Constituents
as Medicine). 2012. Reimbursement of medicinal cannabis
by Dutch health insurance companies, March 28, 2012 (in
Dutch). Available at:http://www.ncsm.nl/cfsystem/userData/pdf/
1342695429__document__ncsm-vergoeding-medicinale-cannabis-
2012.pdf.
O’Connell, T.J. & Bou-Matar, C.B. 2007. Long term marijuana
users seeking medical cannabis in California (2001–2007):
Demographics, social characteristics, patterns of cannabis and other
drug use of 4117 applicants. Harm Reduction Journal 4: 16.
Ogborne, A.C.; Smart, R.G. & Adlaf, E.M. 2000. Self-reported medical
use of marijuana: A survey of the general population. Canadian
Medical Association Journal 162 (12): 1685–6.
Page, S.A.; Verhoef, M.J.; Stebbins, R.A.; Metz, L.M. & Levy, J.C.
2003. Cannabis use as described by people with multiple sclerosis.
Canadian Journal of Neurological Sciences 30 (3): 201–5.
Pertwee, R.G. 1999. Cannabis and cannabinoids: Pharmacology and ratio-
nale for clinical use. Forschende Komplementärmedizin 6 (Suppl 3):
12–5.
Prentiss, D.; Power, R.; Balmas, G.; Tzuang, G. & Israelski, D.M. 2004.
Patterns of marijuana use among patients with HIV / AIDS fol-
lowed in a public health care setting. Journal of Acquired Immune
Deficiency Syndromes 35 (1): 38–45.
Journal of Psychoactive Drugs 208 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
and Valeant and has consulted for companies developing
cannabinoid medications (AstraZeneca, Boehringer,
Ironwood). Apart from this support, no other funding was
received for conducting the survey or publishing the data.
The survey results have been presented, in part, at an
IACM conference in Bonn, Germany, in September, 2011.
An abstract was posted on the website of the IACM (http://
www.cannabis-med.org).
REFERENCES
Bedi, G.; Cooper, Z.D. & Haney, M. 2012. Subjective, cognitive and
cardiovascular dose-effect profile of nabilone and dronabinol in
marijuana smokers. Addiction Biology (in press).
Braitstein, P.; Kendall. T.; Chan, K.; Wood, E.; Montaner, J.S.;
O’Shaughnessy, M.V. & Hogg, R.S. 2001. Mary-Jane and
her patients: Sociodemographic and clinical characteristics of
HIVpositive individuals using medical marijuana and antiretroviral
agents. AIDS 15 (4): 532–3.
Chong, M.S.; Wolff, K.; Wise, K.; Tanton, C.; Winstock, A. & Silber,
E. 2006. Cannabis use in patients with multiple sclerosis. Multiple
Sclerosis 12 (5): 646–51.
Corless, I.B.; Lindgren, T.; Holzemer, W.; Robinson, L.; Moezzi, S.;
Kirksey, K.; Coleman, C.; Tsai, Y.F.; Sanzero Eller, L.; Hamilton,
M.J.; Sefcik, E.F.; Canaval, G.E.; Rivero Mendez, M.; Kemppainen,
J.K.; Bunch, E.H.; Nicholas, P.K.; Nokes, K.M.; Dole, P. &
Reynolds, N. 2009. Marijuana effectiveness as an HIV self-care
strategy. Clinical Nursing Research 18(2): 172–93.
Courtney, W. 2012. Cannabis International, official website. Available
at:http://www.cannabisinternational.org.
Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H. & Verpoorte, R.
2010. Metabolic fingerprinting of Cannabis sativa L. cannabinoids
and terpenoids for chemotaxonomic and drug standardization pur-
poses. Phytochemistry 71: 2058–73.
Freeman, R.M.; Adekanmi, O.; Waterfield, M.R.; Waterfield, A.E.;
Wright, D. & Zajicek, J. 2006. The effect of cannabis on urge
incontinence in patients with multiple sclerosis: A multicentre,
randomised placebo-controlled trial (CAMS-LUTS). International
Urogynecology Journal and Pelvic Floor Dysfunction 17 (6):
636–41.
Grotenhermen, F. & Russo, E. 2002. Cannabis and Cannabinoids:
Pharmacology, Toxicology and Therapeutic Potential. New York:
Haworth Press.
Grotenhermen, F. 2003. Pharmacokinetics and pharmacodynamics of
cannabinoids. Clinical Pharmacokinetics 42 (4): 327–60.
GW Pharmaceuticals. 2012. Sativex® Mutual Recognition Procedure
Closes with Recommendation for Approval in Ten European
Countries. Available at:http://www.gwpharm.com/Sativex%
20Mutual%20Recognition%20Procedure%20Closes%20with%
20Recommendation%20for%20Approval%20in%20Ten%
20European%20Countries.aspx.
Haney, M.; Rabkin, J.; Gunderson, E. & Foltin, R.W. 2005. Dronabinol
and marijuana in HIV( +) marijuana smokers: Acute effects on
caloric intake and mood. Psychopharmacology (Berlin) 181 (1):
170–78.
Haney, M.; Gunderson, E.W.; Rabkin, J.; Hart, C.L.; Vosburg, S.K.;
Comer, S.D. & Foltin, R.W. 2007. Dronabinol and marijuana in
HIV-positive marijuana smokers: Caloric intake, mood, and sleep.
Journal of Acquired Immune Deficiency Syndromes 45 (5): 545–54.
Hazekamp, A.; Ruhaak, R.; Zuurman, L.; van Gerven, J. & Verpoorte,
R. 2006. Evaluation of a vaporizing device (Volcano®) for
the pulmonary delivery of tetrahydrocannabinol. Journal of
Pharmaceutical Sciences 95 (6): 1308–17.
Hazekamp, A.; Bastola, K.; Rashidi, H.; Bender, J. & Verpoorte, R. 2007.
Cannabis tea revisited: A systematic evaluation of the cannabinoid
composition of cannabis tea. Journal of Ethnopharmacology 113:
85–90.
Hazekamp, A. & Fischedick, J.T. 2012. Cannabis - from cultivar to
chemovar: Towards a better definition of cannabis potency. Drug
Testing and Analysis 4: 660–7.
Hazekamp, A. & Heerdink E.R. 2013. The prevalence and incidence of
medicinal cannabis on prescription in The Netherlands. European
Journal of Clinical Pharmacology (in press).
Health Canada. 2011. Canada Federal Department of Health. Medical
Marihuana Regulatory Reform 2011 Consultations Results.
Available at:http://www.hc-sc.gc.ca/dhp-mps/consultation/
marihuana/_2011/program/consult_reform-eng.php.
IACM (International Association for Cannabinoid Medicines). 2009.
Laws and Politics of Germany, November 27, 2009. Available
at:http://www.cannabis-med.org/index.php?tpl=page&id=44&
lng=en.
IACM (International Association for Cannabinoid Medicines). 2012.
USA: Massachusetts becomes the 18th state to legalize the medical
use of cannabis, November 18, 2012. Available at:http://www.
cannabis-med.org/english/bulletin/ww_en_db_cannabis_artikel.
php?id=386#1.
Killestein, J.; Hoogervorst, E.L.; Reif, M.; Kalkers, N.F.; Van Loenen,
A.C.; Staats, P.G.; Gorter, R.W.; Uitdehaag, B.M. & Polman,
C.H. 2002. Safety, tolerability, and efficacy of orally administered
cannabinoids in MS. Neurology 58 (9): 1404–7.
Lile, J.A.; Kelly, T.H. & Hays, L.R. 2011. Separate and combined effects
of the cannabinoid agonists nabilone and 9-THC in humans dis-
criminating 9-THC. Drug and Alcohol Dependence 116 (1–3):
86–92.
McGilveray, I.J. 2005. Pharmacokinetics of cannabinoids. Pain Research
& Management 10 (Suppl A): 15A–22A.
Naef, M.; Russmann, S.; Petersen-Felix, S. & Brenneisen, R. 2004.
Development and pharmacokinetic characterization of pulmonal and
intravenous delta-9-tetrahydrocannabinol (THC) in humans. Journal
of Pharmaceutical Sciences 93: 1176–84.
NCSM (Dutch Foundation for Legal Cannabis and its Constituents
as Medicine). 2012. Reimbursement of medicinal cannabis
by Dutch health insurance companies, March 28, 2012 (in
Dutch). Available at:http://www.ncsm.nl/cfsystem/userData/pdf/
1342695429__document__ncsm-vergoeding-medicinale-cannabis-
2012.pdf.
O’Connell, T.J. & Bou-Matar, C.B. 2007. Long term marijuana
users seeking medical cannabis in California (2001–2007):
Demographics, social characteristics, patterns of cannabis and other
drug use of 4117 applicants. Harm Reduction Journal 4: 16.
Ogborne, A.C.; Smart, R.G. & Adlaf, E.M. 2000. Self-reported medical
use of marijuana: A survey of the general population. Canadian
Medical Association Journal 162 (12): 1685–6.
Page, S.A.; Verhoef, M.J.; Stebbins, R.A.; Metz, L.M. & Levy, J.C.
2003. Cannabis use as described by people with multiple sclerosis.
Canadian Journal of Neurological Sciences 30 (3): 201–5.
Pertwee, R.G. 1999. Cannabis and cannabinoids: Pharmacology and ratio-
nale for clinical use. Forschende Komplementärmedizin 6 (Suppl 3):
12–5.
Prentiss, D.; Power, R.; Balmas, G.; Tzuang, G. & Israelski, D.M. 2004.
Patterns of marijuana use among patients with HIV / AIDS fol-
lowed in a public health care setting. Journal of Acquired Immune
Deficiency Syndromes 35 (1): 38–45.
Journal of Psychoactive Drugs 208 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013

Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
Russo, E.B. 2011. Taming THC: Potential cannabis synergy and
phytocannabinoid-terpenoid entourage effects. British Journal of
Pharmacology 163: 1344–64.
Simpson, R. 2012. February 1, 2013. Available at:http://phoenixtears.ca/.
Strasser, F.; Luftnerl, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.;
Meissner, W.; Ko, Y.D.; Schnelle, M.; Reif, M. & Cerny, T.
2006. Comparison of orally administered cannabis extract and
delta-9-tetrahydrocannabinol in treating patients with cancer-related
anorexia-cachexia syndrome: A multicenter, phase III, randomized,
double-blind, placebo-controlled clinical trial from the Cannabis-
In-Cachexia-Study-Group. Journal of Clinical Oncology 24 (21):
3394–400.
Tashkin, D.P.; Reiss, S.; Shapiro, B.J.; Calvarese, B.; Olsen, J.L.
& Lodge, J.W. 1977. Bronchial effects of aerosolized delta 9-
tetrahydrocannabinol in healthy and asthmatic subjects. American
Review of Respiratory Disease 115 (1): 57–65.
Van der Kooy, F.; Pomahacova, B. & Verpoorte, R. 2009. Cannabis smoke
condensate II: Influence of tobacco on tetrahydrocannabinol levels.
Inhalation Toxicology 21 (2): 87–90.
Ware, M.A.; Doyle, C.R.; Woods, R.; Lynch, M.E. & Clark, A.J. 2003.
Cannabis use for chronic non-cancer pain: Results of a prospective
survey. Pain 102 (1–2): 211–6.
Ware, M.A.; Adams, H. & Guy, G.W. 2005. The medicinal use of cannabis
in the UK: Results of a nationwide survey. International Journal of
Clinical Practice 59 (3): 291–95.
WHO (World Health Organization). 2006. Norms and Standards:
Quality, Safety and Efficacy of Medicines. Available at:http://
www.who.int/medicines/areas/quality_safety/4.2DronabinolCrit
Review.pdf.
Zajicek, J.; Fox, P.; Sanders, H.; Wright, D.; Vickery, J.; Nunn, A.
& Thompson, A. 2003. Cannabinoids for treatment of spasticity
and other symptoms related to multiple sclerosis (CAMS study):
Multicentre randomised placebo-controlled trial. Lancet 362 (9395):
1517–26.
Zajicek, J.P.; Sanders, H.P.; Wright, D.E.; Vickery, P.J.; Ingram, W.M.;
Reilly, S.M.; Nunn, A.J.; Teare, L.J.; Fox, P.J. & Thompson, A.J.
2005. Cannabinoids in multiple sclerosis (CAMS) study: Safety
and efficacy data for 12 months follow-up. Journal of Neurology,
Neurosurgery and Psychiatry 76 (12): 1664–9.
Žuškin, E.; Bouhuys, A. & Šari, M. 1981. Lung function changes
by ethanol inhalation. Clinical & Experimental Allergy 11 (3):
243–8.
Journal of Psychoactive Drugs 209 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
Russo, E.B. 2011. Taming THC: Potential cannabis synergy and
phytocannabinoid-terpenoid entourage effects. British Journal of
Pharmacology 163: 1344–64.
Simpson, R. 2012. February 1, 2013. Available at:http://phoenixtears.ca/.
Strasser, F.; Luftnerl, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.;
Meissner, W.; Ko, Y.D.; Schnelle, M.; Reif, M. & Cerny, T.
2006. Comparison of orally administered cannabis extract and
delta-9-tetrahydrocannabinol in treating patients with cancer-related
anorexia-cachexia syndrome: A multicenter, phase III, randomized,
double-blind, placebo-controlled clinical trial from the Cannabis-
In-Cachexia-Study-Group. Journal of Clinical Oncology 24 (21):
3394–400.
Tashkin, D.P.; Reiss, S.; Shapiro, B.J.; Calvarese, B.; Olsen, J.L.
& Lodge, J.W. 1977. Bronchial effects of aerosolized delta 9-
tetrahydrocannabinol in healthy and asthmatic subjects. American
Review of Respiratory Disease 115 (1): 57–65.
Van der Kooy, F.; Pomahacova, B. & Verpoorte, R. 2009. Cannabis smoke
condensate II: Influence of tobacco on tetrahydrocannabinol levels.
Inhalation Toxicology 21 (2): 87–90.
Ware, M.A.; Doyle, C.R.; Woods, R.; Lynch, M.E. & Clark, A.J. 2003.
Cannabis use for chronic non-cancer pain: Results of a prospective
survey. Pain 102 (1–2): 211–6.
Ware, M.A.; Adams, H. & Guy, G.W. 2005. The medicinal use of cannabis
in the UK: Results of a nationwide survey. International Journal of
Clinical Practice 59 (3): 291–95.
WHO (World Health Organization). 2006. Norms and Standards:
Quality, Safety and Efficacy of Medicines. Available at:http://
www.who.int/medicines/areas/quality_safety/4.2DronabinolCrit
Review.pdf.
Zajicek, J.; Fox, P.; Sanders, H.; Wright, D.; Vickery, J.; Nunn, A.
& Thompson, A. 2003. Cannabinoids for treatment of spasticity
and other symptoms related to multiple sclerosis (CAMS study):
Multicentre randomised placebo-controlled trial. Lancet 362 (9395):
1517–26.
Zajicek, J.P.; Sanders, H.P.; Wright, D.E.; Vickery, P.J.; Ingram, W.M.;
Reilly, S.M.; Nunn, A.J.; Teare, L.J.; Fox, P.J. & Thompson, A.J.
2005. Cannabinoids in multiple sclerosis (CAMS) study: Safety
and efficacy data for 12 months follow-up. Journal of Neurology,
Neurosurgery and Psychiatry 76 (12): 1664–9.
Žuškin, E.; Bouhuys, A. & Šari, M. 1981. Lung function changes
by ethanol inhalation. Clinical & Experimental Allergy 11 (3):
243–8.
Journal of Psychoactive Drugs 209 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
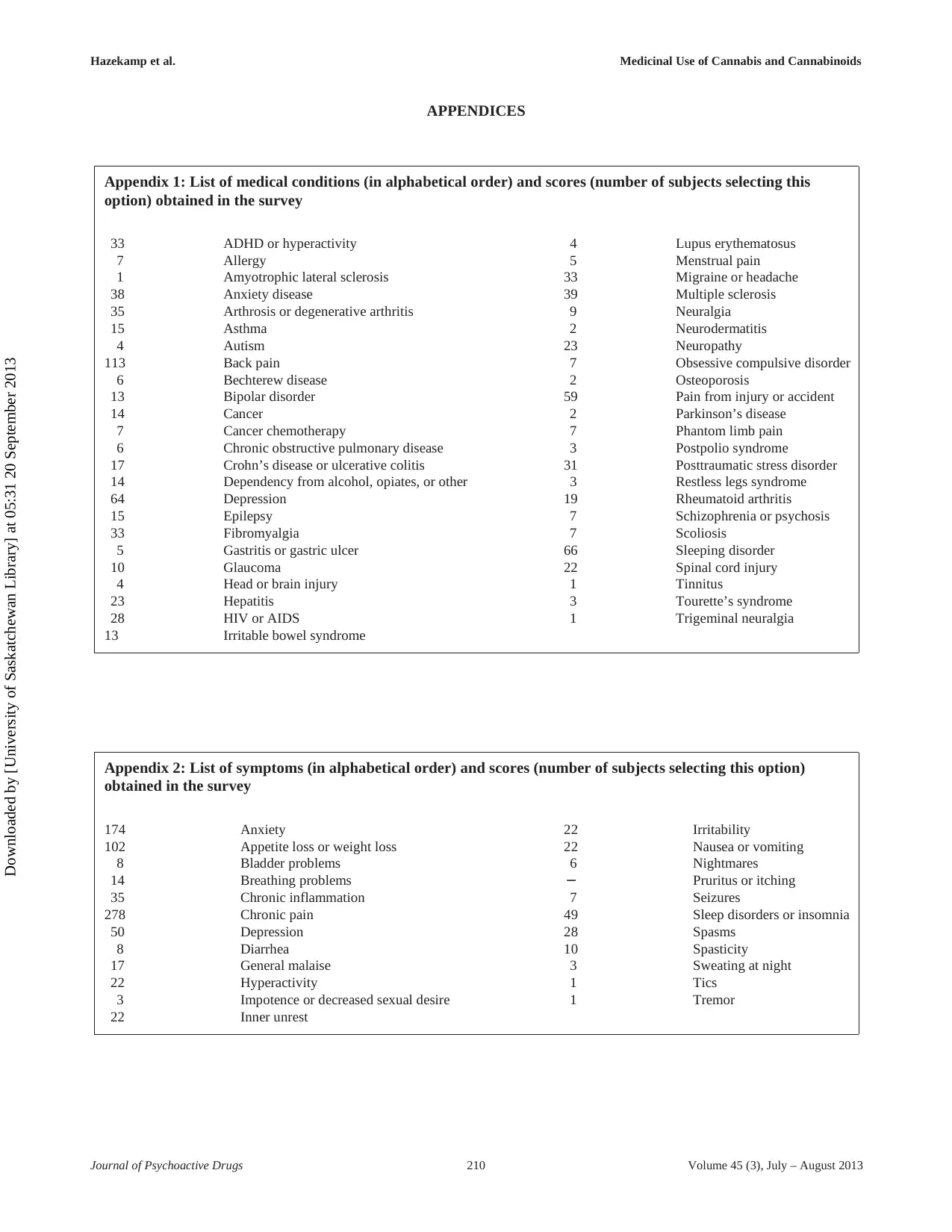
Hazekamp et al. Medicinal Use of Cannabis and Cannabinoids
APPENDICES
Appendix 1: List of medical conditions (in alphabetical order) and scores (number of subjects selecting this
option) obtained in the survey
33 ADHD or hyperactivity 4 Lupus erythematosus
7 Allergy 5 Menstrual pain
1 Amyotrophic lateral sclerosis 33 Migraine or headache
38 Anxiety disease 39 Multiple sclerosis
35 Arthrosis or degenerative arthritis 9 Neuralgia
15 Asthma 2 Neurodermatitis
4 Autism 23 Neuropathy
113 Back pain 7 Obsessive compulsive disorder
6 Bechterew disease 2 Osteoporosis
13 Bipolar disorder 59 Pain from injury or accident
14 Cancer 2 Parkinson’s disease
7 Cancer chemotherapy 7 Phantom limb pain
6 Chronic obstructive pulmonary disease 3 Postpolio syndrome
17 Crohn’s disease or ulcerative colitis 31 Posttraumatic stress disorder
14 Dependency from alcohol, opiates, or other 3 Restless legs syndrome
64 Depression 19 Rheumatoid arthritis
15 Epilepsy 7 Schizophrenia or psychosis
33 Fibromyalgia 7 Scoliosis
5 Gastritis or gastric ulcer 66 Sleeping disorder
10 Glaucoma 22 Spinal cord injury
4 Head or brain injury 1 Tinnitus
23 Hepatitis 3 Tourette’s syndrome
28 HIV or AIDS 1 Trigeminal neuralgia
13 Irritable bowel syndrome
Appendix 2: List of symptoms (in alphabetical order) and scores (number of subjects selecting this option)
obtained in the survey
174 Anxiety 22 Irritability
102 Appetite loss or weight loss 22 Nausea or vomiting
8 Bladder problems 6 Nightmares
14 Breathing problems − Pruritus or itching
35 Chronic inflammation 7 Seizures
278 Chronic pain 49 Sleep disorders or insomnia
50 Depression 28 Spasms
8 Diarrhea 10 Spasticity
17 General malaise 3 Sweating at night
22 Hyperactivity 1 Tics
3 Impotence or decreased sexual desire 1 Tremor
22 Inner unrest
Journal of Psychoactive Drugs 210 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
APPENDICES
Appendix 1: List of medical conditions (in alphabetical order) and scores (number of subjects selecting this
option) obtained in the survey
33 ADHD or hyperactivity 4 Lupus erythematosus
7 Allergy 5 Menstrual pain
1 Amyotrophic lateral sclerosis 33 Migraine or headache
38 Anxiety disease 39 Multiple sclerosis
35 Arthrosis or degenerative arthritis 9 Neuralgia
15 Asthma 2 Neurodermatitis
4 Autism 23 Neuropathy
113 Back pain 7 Obsessive compulsive disorder
6 Bechterew disease 2 Osteoporosis
13 Bipolar disorder 59 Pain from injury or accident
14 Cancer 2 Parkinson’s disease
7 Cancer chemotherapy 7 Phantom limb pain
6 Chronic obstructive pulmonary disease 3 Postpolio syndrome
17 Crohn’s disease or ulcerative colitis 31 Posttraumatic stress disorder
14 Dependency from alcohol, opiates, or other 3 Restless legs syndrome
64 Depression 19 Rheumatoid arthritis
15 Epilepsy 7 Schizophrenia or psychosis
33 Fibromyalgia 7 Scoliosis
5 Gastritis or gastric ulcer 66 Sleeping disorder
10 Glaucoma 22 Spinal cord injury
4 Head or brain injury 1 Tinnitus
23 Hepatitis 3 Tourette’s syndrome
28 HIV or AIDS 1 Trigeminal neuralgia
13 Irritable bowel syndrome
Appendix 2: List of symptoms (in alphabetical order) and scores (number of subjects selecting this option)
obtained in the survey
174 Anxiety 22 Irritability
102 Appetite loss or weight loss 22 Nausea or vomiting
8 Bladder problems 6 Nightmares
14 Breathing problems − Pruritus or itching
35 Chronic inflammation 7 Seizures
278 Chronic pain 49 Sleep disorders or insomnia
50 Depression 28 Spasms
8 Diarrhea 10 Spasticity
17 General malaise 3 Sweating at night
22 Hyperactivity 1 Tics
3 Impotence or decreased sexual desire 1 Tremor
22 Inner unrest
Journal of Psychoactive Drugs 210 Volume 45 (3), July – August 2013
Downloaded by [University of Saskatchewan Library] at 05:31 20 September 2013
1 out of 13
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
