Critical Appraisal of Studies Report: PUBH6005 Epidemiology Assessment
VerifiedAdded on 2023/05/31
|3
|697
|72
Report
AI Summary
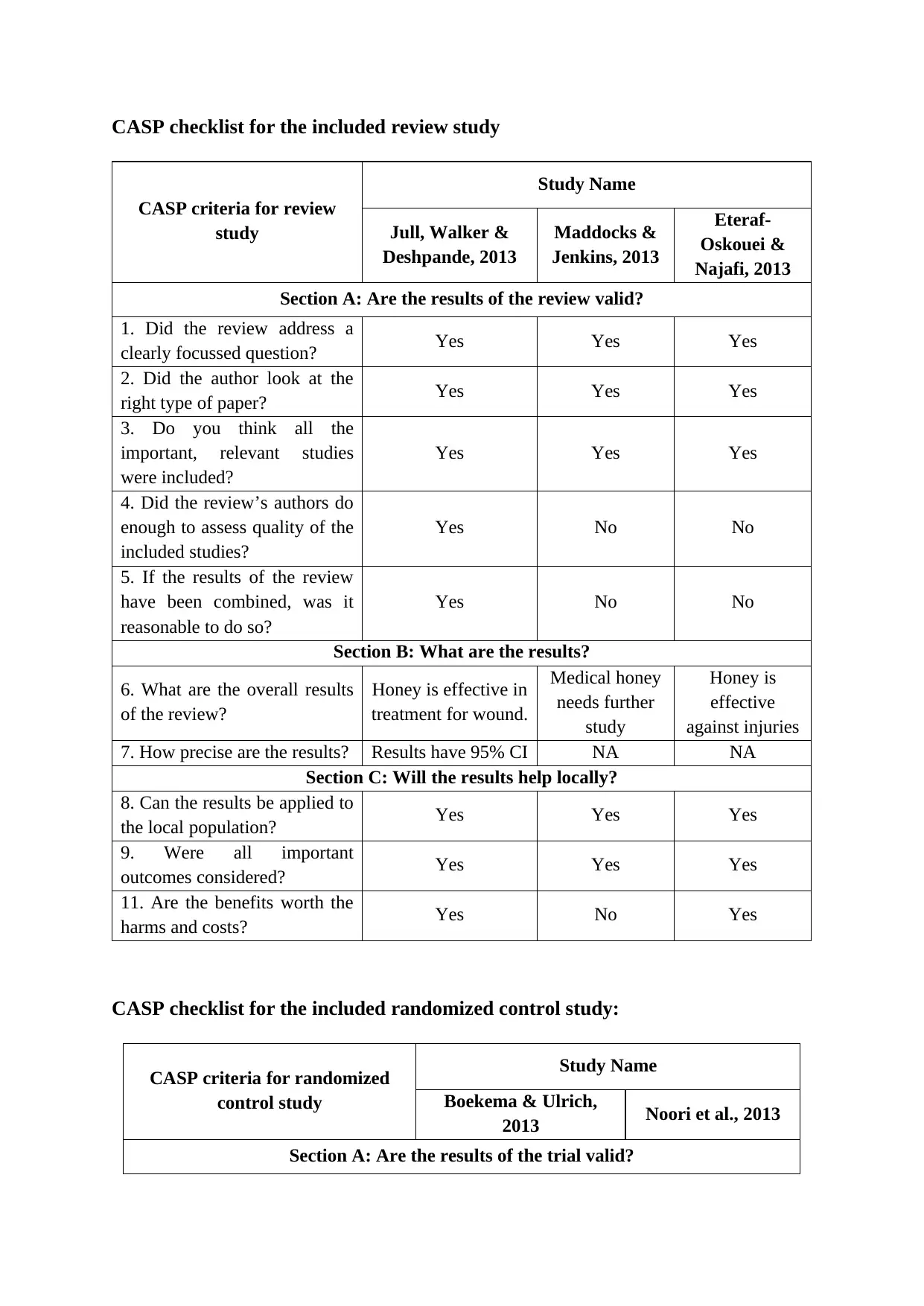
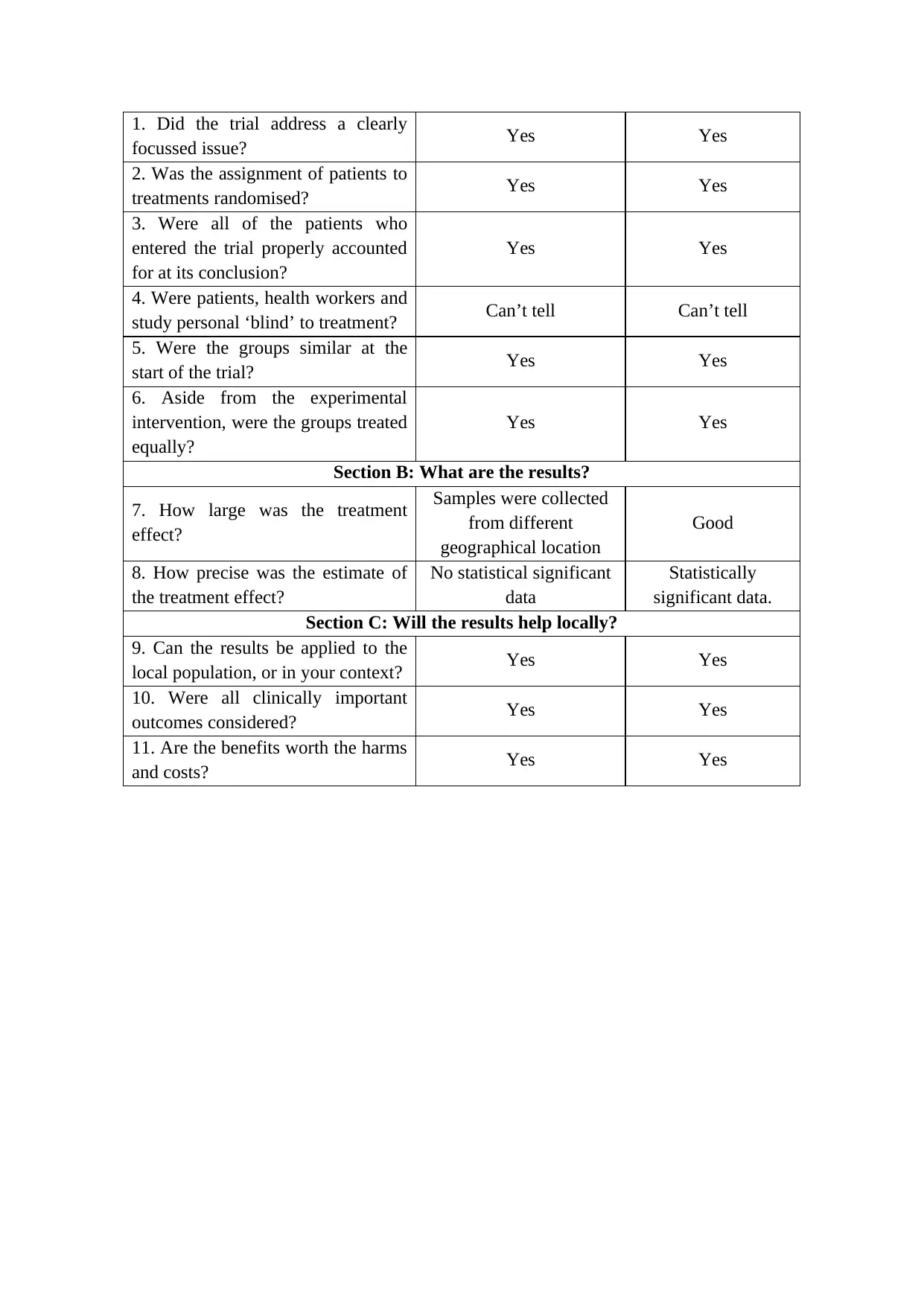
This report presents a critical appraisal of two types of studies: a review study and a randomized control study, using the CASP (Critical Appraisal Skills Programme) checklists. The review study, encompassing multiple papers, is evaluated based on its focus, methodology, inclusion of relevant studies, assessment of quality, and combining of results. The report examines the overall findings, precision of results, and their applicability to the local population. The randomized control study is assessed for its focus, randomization, accounting of patients, blinding, group similarity, and treatment equality. The report discusses the size and precision of the treatment effect and its local applicability. Both studies are analyzed in the context of the assessment brief, addressing antimicrobial resistance, a critical issue in global public health. The report concludes by synthesizing the strengths and limitations of the studies and their implications for epidemiological understanding and practice.
1 out of 3






![[object Object]](/_next/static/media/star-bottom.7253800d.svg)