CHANGES IN ENTHALPY DOWN THE ALCOHOL GROUP WITH INCREASING CARBON ATOMS CHAINS Introduction Alcohols
VerifiedAdded on 2023/04/24
|13
|3734
|80
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

CHANGES IN ENTHALPY DOWN THE ALCOHOL GROUP WITH INCREASING CARBON
ATOMS CHAINS
Introduction
Alcohols refer to a group of organic compounds known as hydrocarbons that contain
the –OH group. The group comprises of three major elements, namely Carbon, Oxygen, and
Hydrogen while the -OH group contributes by determining the manner in which the
hydrocarbon compound reacts. This group has a general chemical formula of CnH2n+1OH
where the value of n represents the number of carbon atoms in the compound. Since
alcohols are organic compounds, their combustion is known to produce high amounts of
energy because their combustion reactions are exothermic (Bruno and Smith, 2016, p.
2109). Regardless of this, their exothermic trait of the fuel does not guarantee that the
alcohols will be fuels that will release the highest amount of energy which makes the best
fuels. The amount of energy released in an exothermic reaction is determined by the
enthalpy change that occurs when the compound is combusted. Enthalpy is a term used to
define the amount of heat energy that will be yielded when one mole of a substance is
combusted in the presence of oxygen (Wiberg, Crocker, and Morgan, 2016, p. 2147).
Since enthalpy change is affected by the mole ratios of the reactants in this combustion
reaction, the molecular mass of the compound has an impact on the values of enthalpy that
are attained when a compound is combusted (Bruno and Smith, 2016, p. 2111). This is
because the alcohol chain to be combusted continues to become longer as a unit CH2
(methylene) molecule continues to be added to the chain. This implies that much more
energy will be required to break these long chains and more covalent bonds and Van der
Waal’s forces in the compound (Thornton, 2017, p. 197). As the number of carbon atoms in
the alcohols continues to increase, the enthalpy of the oxidation reaction continues to
decrease thus making the enthalpy of combustion to become more negative increasing
carbon atoms in the chain. The impact of an even higher enthalpy change with every
increase in the carbon atom in the long hydrocarbon chains is a longer time taken for the
water to reach the boiling point (Thornton, 2017, p. 199). This will indicate that the fuel is
taking even longer to break the compound bonds during the exothermic combustion
reaction which releases the heat required to boil the water in the calorimeter.
ATOMS CHAINS
Introduction
Alcohols refer to a group of organic compounds known as hydrocarbons that contain
the –OH group. The group comprises of three major elements, namely Carbon, Oxygen, and
Hydrogen while the -OH group contributes by determining the manner in which the
hydrocarbon compound reacts. This group has a general chemical formula of CnH2n+1OH
where the value of n represents the number of carbon atoms in the compound. Since
alcohols are organic compounds, their combustion is known to produce high amounts of
energy because their combustion reactions are exothermic (Bruno and Smith, 2016, p.
2109). Regardless of this, their exothermic trait of the fuel does not guarantee that the
alcohols will be fuels that will release the highest amount of energy which makes the best
fuels. The amount of energy released in an exothermic reaction is determined by the
enthalpy change that occurs when the compound is combusted. Enthalpy is a term used to
define the amount of heat energy that will be yielded when one mole of a substance is
combusted in the presence of oxygen (Wiberg, Crocker, and Morgan, 2016, p. 2147).
Since enthalpy change is affected by the mole ratios of the reactants in this combustion
reaction, the molecular mass of the compound has an impact on the values of enthalpy that
are attained when a compound is combusted (Bruno and Smith, 2016, p. 2111). This is
because the alcohol chain to be combusted continues to become longer as a unit CH2
(methylene) molecule continues to be added to the chain. This implies that much more
energy will be required to break these long chains and more covalent bonds and Van der
Waal’s forces in the compound (Thornton, 2017, p. 197). As the number of carbon atoms in
the alcohols continues to increase, the enthalpy of the oxidation reaction continues to
decrease thus making the enthalpy of combustion to become more negative increasing
carbon atoms in the chain. The impact of an even higher enthalpy change with every
increase in the carbon atom in the long hydrocarbon chains is a longer time taken for the
water to reach the boiling point (Thornton, 2017, p. 199). This will indicate that the fuel is
taking even longer to break the compound bonds during the exothermic combustion
reaction which releases the heat required to boil the water in the calorimeter.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Hypothesis
As the number of carbon atoms in the alcohol chains continues to increase, the enthalpy of
combustion increases
Theory
The molecular formulae for the alcohols that will be considered are as follows. These
formulae are important for the computation of the molecular masses of the alcohols.
Alcohol Chemical Formula Molecular Mass
Methanol CH3OH 32
Ethanol CH3 CH2OH 44
Propan-1-ol CH3 CH2 CH2OH 60
Propan-2-ol (CH3)2CHOH 60
Butan-1-ol CH3(CH2)3OH 74
Pentan-1-ol CH3(CH2)4OH 88
Hexan-1-ol CH3(CH2)5OH 102
The above table is able to demonstrates that as the carbon atoms increase in this
homologous group, the chains become complex and longer, and this in effect changes the
physical properties of the alcohols as you go down the homologous group.
Combustion can be defined as the chemical reaction that occurs when carbon compounds
are oxidized in the presence of oxygen to form CO2 and H2O.
The combustion reaction for alcohols follows the following chemical formula.
CnH2n+1OH n CO2 + 2n H2O
Since the reaction is exothermic, heat energy is also yielded in this process. Enthalpy, on the
other hand, refers to the amount of heat energy that will be yielded when one mole of a
substance is combusted in the presence of oxygen (Wiberg, Crocker, and Morgan, 2016, p.
2147). Since as the carbon atoms increase, the amount covalent and hydrogen bonds as
well as the Van der Waals forces to be broken down during the combustion reaction also
increase. This implies that less energy will be released per mole of alcohol being burnt as
Oxygen
As the number of carbon atoms in the alcohol chains continues to increase, the enthalpy of
combustion increases
Theory
The molecular formulae for the alcohols that will be considered are as follows. These
formulae are important for the computation of the molecular masses of the alcohols.
Alcohol Chemical Formula Molecular Mass
Methanol CH3OH 32
Ethanol CH3 CH2OH 44
Propan-1-ol CH3 CH2 CH2OH 60
Propan-2-ol (CH3)2CHOH 60
Butan-1-ol CH3(CH2)3OH 74
Pentan-1-ol CH3(CH2)4OH 88
Hexan-1-ol CH3(CH2)5OH 102
The above table is able to demonstrates that as the carbon atoms increase in this
homologous group, the chains become complex and longer, and this in effect changes the
physical properties of the alcohols as you go down the homologous group.
Combustion can be defined as the chemical reaction that occurs when carbon compounds
are oxidized in the presence of oxygen to form CO2 and H2O.
The combustion reaction for alcohols follows the following chemical formula.
CnH2n+1OH n CO2 + 2n H2O
Since the reaction is exothermic, heat energy is also yielded in this process. Enthalpy, on the
other hand, refers to the amount of heat energy that will be yielded when one mole of a
substance is combusted in the presence of oxygen (Wiberg, Crocker, and Morgan, 2016, p.
2147). Since as the carbon atoms increase, the amount covalent and hydrogen bonds as
well as the Van der Waals forces to be broken down during the combustion reaction also
increase. This implies that less energy will be released per mole of alcohol being burnt as
Oxygen

you go down the homologous group and thus the enthalpy change will thus become more
and more negative with the increasing number of carbon atoms in the chain.
Research Question
Is there a possible relationship between the number of carbon atoms in an alcohol and the
energy released as a result of enthalpy change during combustion?
Variables
Independent Variable --The independent variables are the different types of alcohols whose
enthalpy will be tested. They are: Methanol, Ethanol, Propan-1-ol, propan-2-ol-butan-1-ol,
Pentan-1-ol and Hexan-1-ol
Dependent Variable -- The dependent variable will be the amount of the alcohol that was
burnt during the combustion reaction
Controlled Variables --These include the experiment parameters that will not be altered
during the experiment. They include the temperature increase in the water, the distance
between the bottom of the copper calorimeter and the burner, the mass of the water, the
length of time it took for the water in the calorimeter to come to a boil, the mass of the
alcohol to be burnt, the apparatus that will be utilized for the experiment, the
environmental conditions of temperature and pressure during the experiment.
Safety
The alcohol which serves as the independent variable in this experiment is changed through
replacing the alcohol being used with the next specimen with a different amount of carbon
atom. The safety of the experiment will also further be reinforced by ensuring that the
temperature increase is recorded with a thermometer and then the change in mass is
recorded on a balance away from the source of heat. To ensure that there are minimal
changes in the recorded data, the balance, burner, thermometer, and even the calorimeter
used ought to be the same one for each trial. Changes in the temperatures recorded will
also be maintained through encouraging air conditioning in the environment and the use of
a ruler to ensure the distance between the burner and the calorimeter (Anderson, 2016, p.
4807). This distance ought to be kept as constant as possible since when it varies, the
and more negative with the increasing number of carbon atoms in the chain.
Research Question
Is there a possible relationship between the number of carbon atoms in an alcohol and the
energy released as a result of enthalpy change during combustion?
Variables
Independent Variable --The independent variables are the different types of alcohols whose
enthalpy will be tested. They are: Methanol, Ethanol, Propan-1-ol, propan-2-ol-butan-1-ol,
Pentan-1-ol and Hexan-1-ol
Dependent Variable -- The dependent variable will be the amount of the alcohol that was
burnt during the combustion reaction
Controlled Variables --These include the experiment parameters that will not be altered
during the experiment. They include the temperature increase in the water, the distance
between the bottom of the copper calorimeter and the burner, the mass of the water, the
length of time it took for the water in the calorimeter to come to a boil, the mass of the
alcohol to be burnt, the apparatus that will be utilized for the experiment, the
environmental conditions of temperature and pressure during the experiment.
Safety
The alcohol which serves as the independent variable in this experiment is changed through
replacing the alcohol being used with the next specimen with a different amount of carbon
atom. The safety of the experiment will also further be reinforced by ensuring that the
temperature increase is recorded with a thermometer and then the change in mass is
recorded on a balance away from the source of heat. To ensure that there are minimal
changes in the recorded data, the balance, burner, thermometer, and even the calorimeter
used ought to be the same one for each trial. Changes in the temperatures recorded will
also be maintained through encouraging air conditioning in the environment and the use of
a ruler to ensure the distance between the burner and the calorimeter (Anderson, 2016, p.
4807). This distance ought to be kept as constant as possible since when it varies, the

amount of heat lost to the environment and the amount of heat energy heating the water in
the calorimeter also vary. This variation may impact the results of the experiment by causing
the temperature rise in the experiment to be inaccurately observed and thus cause the final
computations of the enthalpy to be inaccurate.
For the sake of personal safety, I wore protective equipment’s like lab coats, and goggles in
the lab as well as an emphasis placed on the use tongs to handle the hot apparatus were
emphasized to avoid any risk of injury. This is because some alcohols have the ability to
corrode the skin of its users to ensure that safety in the lab is guaranteed and optimized.
Materials
Weighing Balance (±0.001g)
Thermometer (±0.05 °C)
Copper calorimeter
Spirit Lamp
Tongs
Clamp and stand
Methanol
Ethanol
Propan-1-ol
Propan-2-ol
Butan-1-ol
pentan-1-ol
Hexan-1-ol
Match sticks
Distilled water
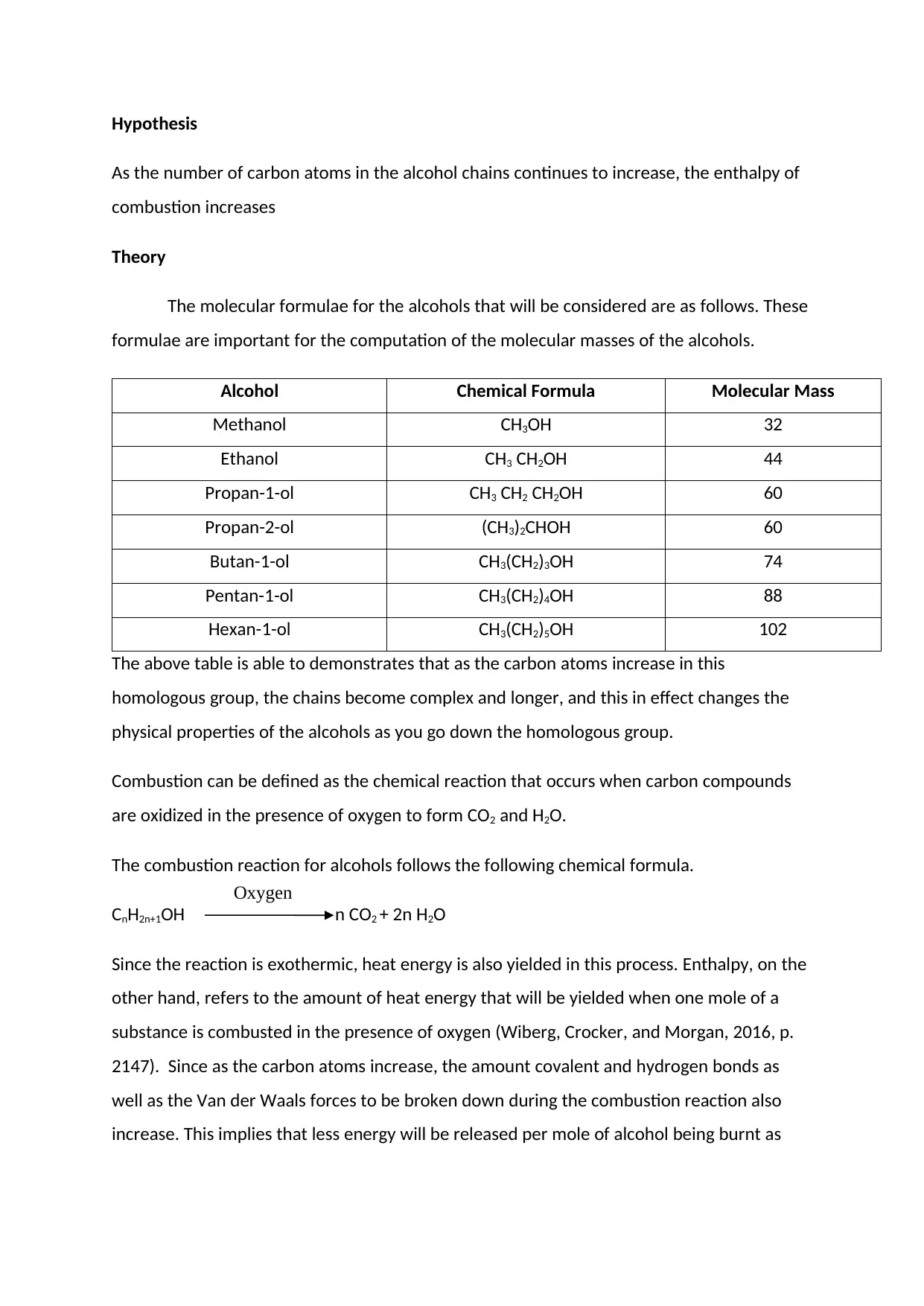
Methodology
50 cm3 was measured and poured into the burner. The burner was then weighed on the
weighing balance and I recorded the new weight. 100g of water was measured and poured
into the copper calorimeter that had earlier been clamped to a waiting stand. It was
necessary that I covered the alcohols in this stage as they might have evaporated due to
their volatile nature. The initial temperature of the water in the calorimeter was taken and
recorded. The calorimeter was then insulated and covered then the thermometer and
stirrer were placed inside it. The burner was then lit and the calorimeter containing the
water placed over it. I began immediately timing with a stopwatch when the alcohol lit up
and the temperature rise observed while the amount of time taken to bring the water to a
boil recorded. As the water began to heat up, the thermometer I used was to continuously
stir the water to ensure that the heat distribution in the water is even throughout the
the calorimeter also vary. This variation may impact the results of the experiment by causing
the temperature rise in the experiment to be inaccurately observed and thus cause the final
computations of the enthalpy to be inaccurate.
For the sake of personal safety, I wore protective equipment’s like lab coats, and goggles in
the lab as well as an emphasis placed on the use tongs to handle the hot apparatus were
emphasized to avoid any risk of injury. This is because some alcohols have the ability to
corrode the skin of its users to ensure that safety in the lab is guaranteed and optimized.
Materials
Weighing Balance (±0.001g)
Thermometer (±0.05 °C)
Copper calorimeter
Spirit Lamp
Tongs
Clamp and stand
Methanol
Ethanol
Propan-1-ol
Propan-2-ol
Butan-1-ol
pentan-1-ol
Hexan-1-ol
Match sticks
Distilled water
Methodology
50 cm3 was measured and poured into the burner. The burner was then weighed on the
weighing balance and I recorded the new weight. 100g of water was measured and poured
into the copper calorimeter that had earlier been clamped to a waiting stand. It was
necessary that I covered the alcohols in this stage as they might have evaporated due to
their volatile nature. The initial temperature of the water in the calorimeter was taken and
recorded. The calorimeter was then insulated and covered then the thermometer and
stirrer were placed inside it. The burner was then lit and the calorimeter containing the
water placed over it. I began immediately timing with a stopwatch when the alcohol lit up
and the temperature rise observed while the amount of time taken to bring the water to a
boil recorded. As the water began to heat up, the thermometer I used was to continuously
stir the water to ensure that the heat distribution in the water is even throughout the
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
container. The colour of the flame was observed while the water was continuously stirred to
ensure minimum heat was lost and uniform distribution. After 10 minutes I recorded the
temperature of the water, and the weight of the burner. The mass of the water after the
time lapsed was noted down as swift as possible to prevent the vaporization. I repeated
these steps for ethanol, propan-1-ol, butan-1-ol, pentan-1-ol, hexan-1-ol.
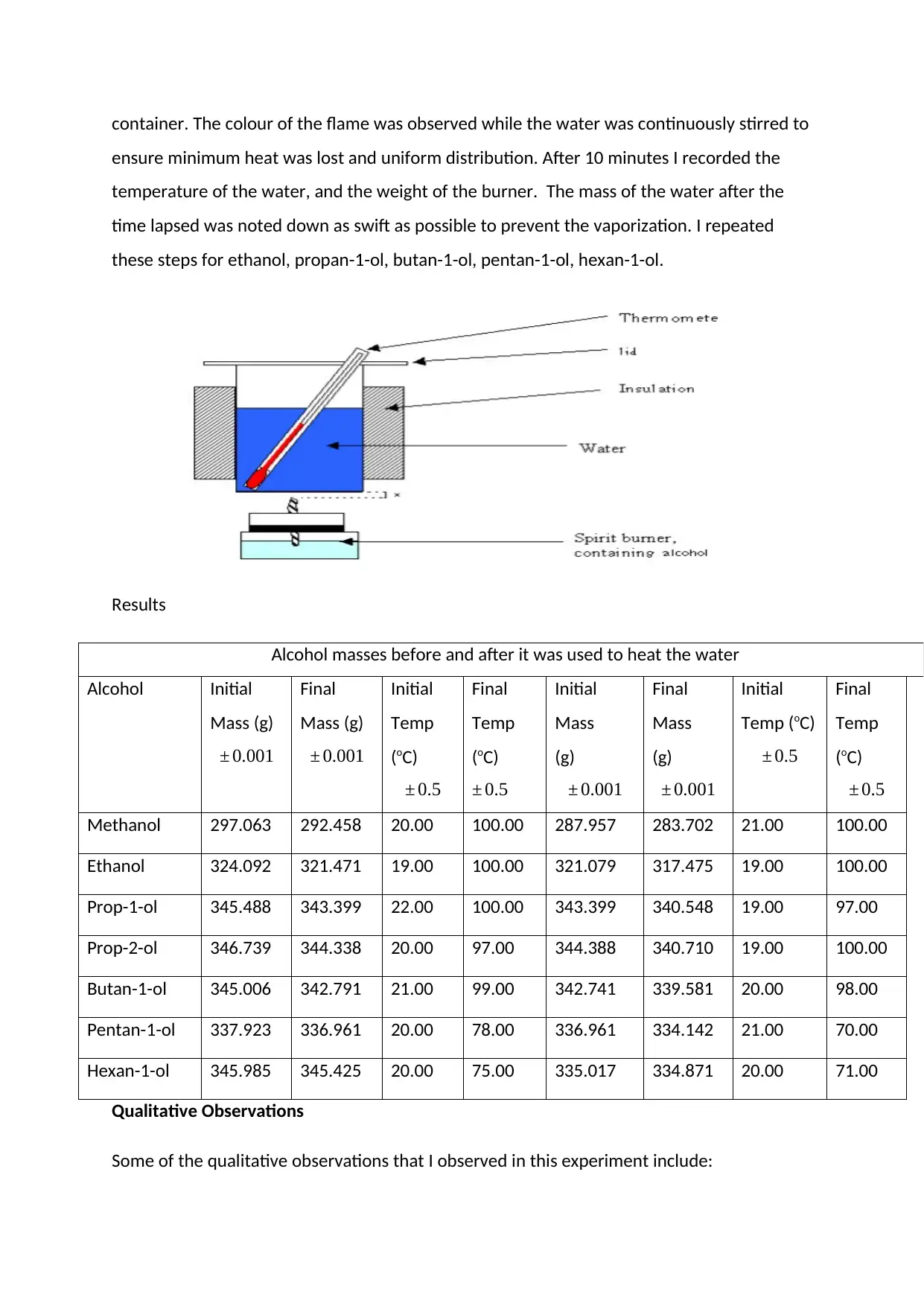
Results
Alcohol masses before and after it was used to heat the water
Alcohol Initial
Mass (g)
± 0.001
Final
Mass (g)
± 0.001
Initial
Temp
(oC)
± 0.5
Final
Temp
(oC)
± 0.5
Initial
Mass
(g)
± 0.001
Final
Mass
(g)
± 0.001
Initial
Temp (oC)
± 0.5
Final
Temp
(oC)
± 0.5
Methanol 297.063 292.458 20.00 100.00 287.957 283.702 21.00 100.00
Ethanol 324.092 321.471 19.00 100.00 321.079 317.475 19.00 100.00
Prop-1-ol 345.488 343.399 22.00 100.00 343.399 340.548 19.00 97.00
Prop-2-ol 346.739 344.338 20.00 97.00 344.388 340.710 19.00 100.00
Butan-1-ol 345.006 342.791 21.00 99.00 342.741 339.581 20.00 98.00
Pentan-1-ol 337.923 336.961 20.00 78.00 336.961 334.142 21.00 70.00
Hexan-1-ol 345.985 345.425 20.00 75.00 335.017 334.871 20.00 71.00
Qualitative Observations
Some of the qualitative observations that I observed in this experiment include:
ensure minimum heat was lost and uniform distribution. After 10 minutes I recorded the
temperature of the water, and the weight of the burner. The mass of the water after the
time lapsed was noted down as swift as possible to prevent the vaporization. I repeated
these steps for ethanol, propan-1-ol, butan-1-ol, pentan-1-ol, hexan-1-ol.
Results
Alcohol masses before and after it was used to heat the water
Alcohol Initial
Mass (g)
± 0.001
Final
Mass (g)
± 0.001
Initial
Temp
(oC)
± 0.5
Final
Temp
(oC)
± 0.5
Initial
Mass
(g)
± 0.001
Final
Mass
(g)
± 0.001
Initial
Temp (oC)
± 0.5
Final
Temp
(oC)
± 0.5
Methanol 297.063 292.458 20.00 100.00 287.957 283.702 21.00 100.00
Ethanol 324.092 321.471 19.00 100.00 321.079 317.475 19.00 100.00
Prop-1-ol 345.488 343.399 22.00 100.00 343.399 340.548 19.00 97.00
Prop-2-ol 346.739 344.338 20.00 97.00 344.388 340.710 19.00 100.00
Butan-1-ol 345.006 342.791 21.00 99.00 342.741 339.581 20.00 98.00
Pentan-1-ol 337.923 336.961 20.00 78.00 336.961 334.142 21.00 70.00
Hexan-1-ol 345.985 345.425 20.00 75.00 335.017 334.871 20.00 71.00
Qualitative Observations
Some of the qualitative observations that I observed in this experiment include:
the inability to achieve complete combustion of the alcohols whose combustibility is
being analysed.
This was characterized by the occurrence of a yellow flame
The flame also caused soot to be formed in this process as the fuel was not able to
get enough oxygen to burn down completely combust the fuel and use it efficiently
and release the largest amount of energy.
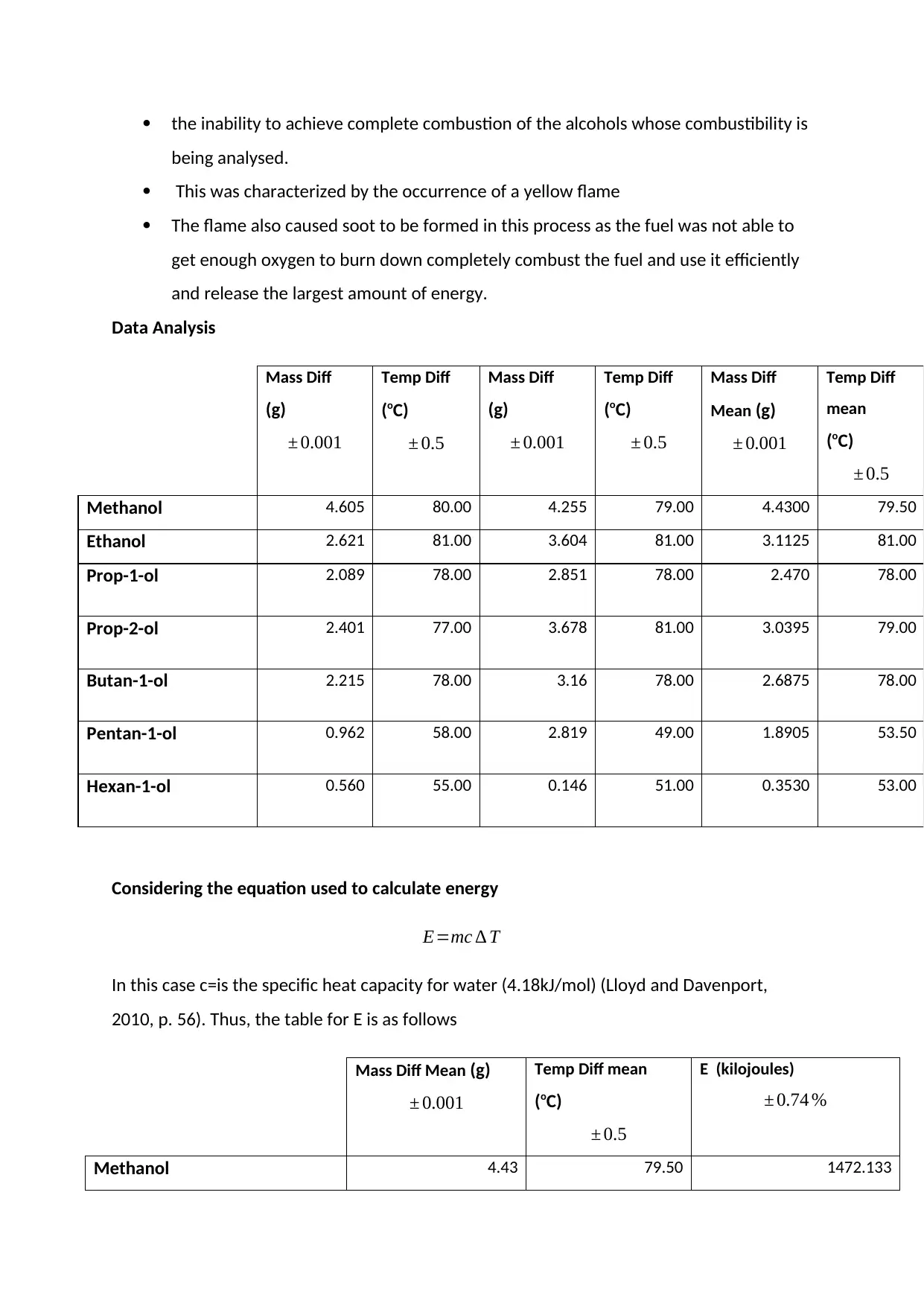
Data Analysis
Mass Diff
(g)
± 0.001
Temp Diff
(oC)
± 0.5
Mass Diff
(g)
± 0.001
Temp Diff
(oC)
± 0.5
Mass Diff
Mean (g)
± 0.001
Temp Diff
mean
(oC)
± 0.5
Methanol 4.605 80.00 4.255 79.00 4.4300 79.50
Ethanol 2.621 81.00 3.604 81.00 3.1125 81.00
Prop-1-ol 2.089 78.00 2.851 78.00 2.470 78.00
Prop-2-ol 2.401 77.00 3.678 81.00 3.0395 79.00
Butan-1-ol 2.215 78.00 3.16 78.00 2.6875 78.00
Pentan-1-ol 0.962 58.00 2.819 49.00 1.8905 53.50
Hexan-1-ol 0.560 55.00 0.146 51.00 0.3530 53.00
Considering the equation used to calculate energy
E=mc ∆ T
In this case c=is the specific heat capacity for water (4.18kJ/mol) (Lloyd and Davenport,
2010, p. 56). Thus, the table for E is as follows
Mass Diff Mean (g)
± 0.001
Temp Diff mean
(oC)
± 0.5
E (kilojoules)
± 0.74 %
Methanol 4.43 79.50 1472.133
being analysed.
This was characterized by the occurrence of a yellow flame
The flame also caused soot to be formed in this process as the fuel was not able to
get enough oxygen to burn down completely combust the fuel and use it efficiently
and release the largest amount of energy.
Data Analysis
Mass Diff
(g)
± 0.001
Temp Diff
(oC)
± 0.5
Mass Diff
(g)
± 0.001
Temp Diff
(oC)
± 0.5
Mass Diff
Mean (g)
± 0.001
Temp Diff
mean
(oC)
± 0.5
Methanol 4.605 80.00 4.255 79.00 4.4300 79.50
Ethanol 2.621 81.00 3.604 81.00 3.1125 81.00
Prop-1-ol 2.089 78.00 2.851 78.00 2.470 78.00
Prop-2-ol 2.401 77.00 3.678 81.00 3.0395 79.00
Butan-1-ol 2.215 78.00 3.16 78.00 2.6875 78.00
Pentan-1-ol 0.962 58.00 2.819 49.00 1.8905 53.50
Hexan-1-ol 0.560 55.00 0.146 51.00 0.3530 53.00
Considering the equation used to calculate energy
E=mc ∆ T
In this case c=is the specific heat capacity for water (4.18kJ/mol) (Lloyd and Davenport,
2010, p. 56). Thus, the table for E is as follows
Mass Diff Mean (g)
± 0.001
Temp Diff mean
(oC)
± 0.5
E (kilojoules)
± 0.74 %
Methanol 4.43 79.50 1472.133
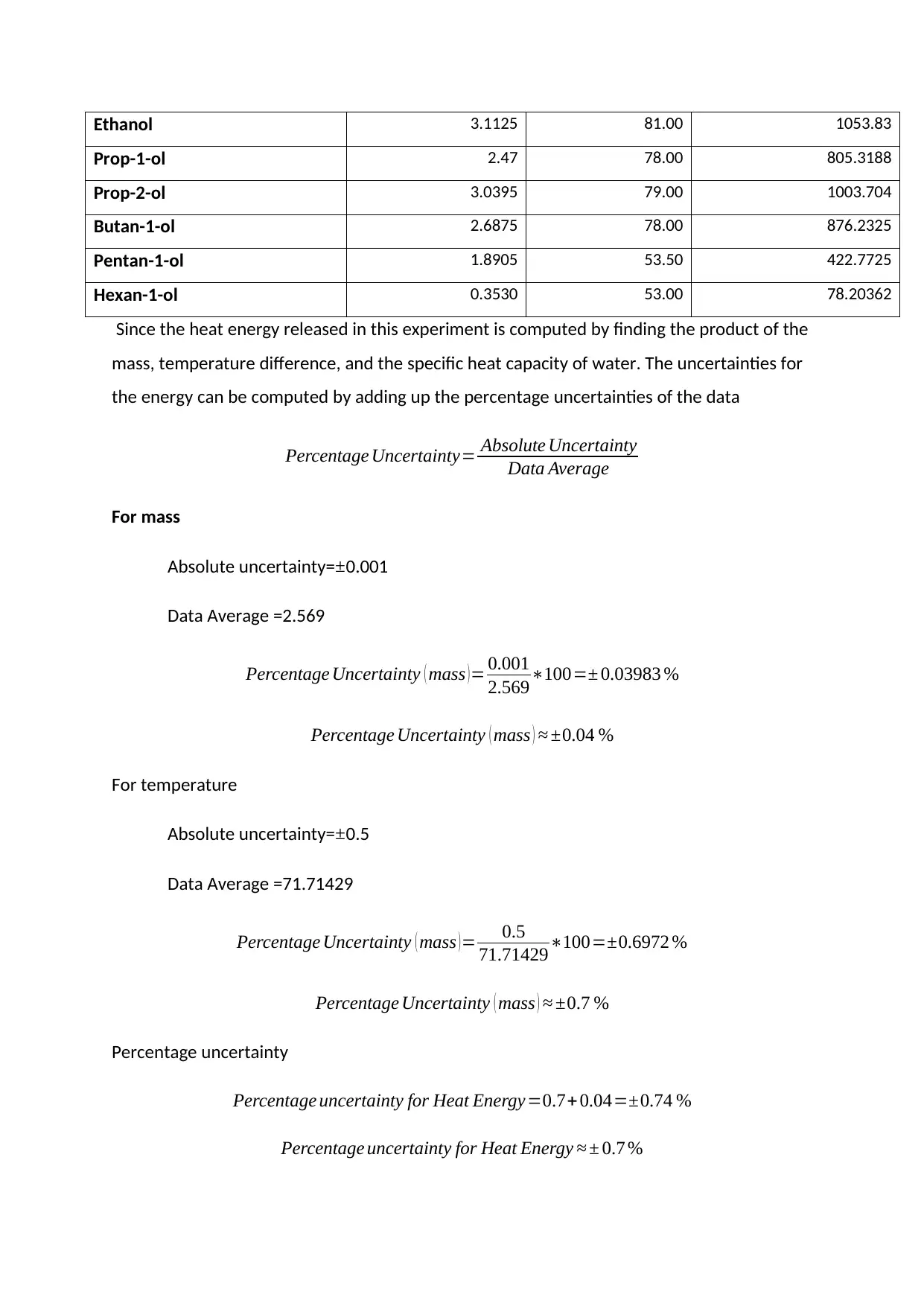
Ethanol 3.1125 81.00 1053.83
Prop-1-ol 2.47 78.00 805.3188
Prop-2-ol 3.0395 79.00 1003.704
Butan-1-ol 2.6875 78.00 876.2325
Pentan-1-ol 1.8905 53.50 422.7725
Hexan-1-ol 0.3530 53.00 78.20362
Since the heat energy released in this experiment is computed by finding the product of the
mass, temperature difference, and the specific heat capacity of water. The uncertainties for
the energy can be computed by adding up the percentage uncertainties of the data
Percentage Uncertainty= Absolute Uncertainty
Data Average
For mass
Absolute uncertainty=±0.001
Data Average =2.569
Percentage Uncertainty ( mass )= 0.001
2.569∗100=± 0.03983 %
Percentage Uncertainty ( mass ) ≈ ±0.04 %
For temperature
Absolute uncertainty= ±0.5
Data Average =71.71429
Percentage Uncertainty ( mass )= 0.5
71.71429∗100=±0.6972 %
Percentage Uncertainty ( mass ) ≈ ±0.7 %
Percentage uncertainty
Percentage uncertainty for Heat Energy=0.7+ 0.04=±0.74 %
Percentage uncertainty for Heat Energy ≈ ± 0.7 %
Prop-1-ol 2.47 78.00 805.3188
Prop-2-ol 3.0395 79.00 1003.704
Butan-1-ol 2.6875 78.00 876.2325
Pentan-1-ol 1.8905 53.50 422.7725
Hexan-1-ol 0.3530 53.00 78.20362
Since the heat energy released in this experiment is computed by finding the product of the
mass, temperature difference, and the specific heat capacity of water. The uncertainties for
the energy can be computed by adding up the percentage uncertainties of the data
Percentage Uncertainty= Absolute Uncertainty
Data Average
For mass
Absolute uncertainty=±0.001
Data Average =2.569
Percentage Uncertainty ( mass )= 0.001
2.569∗100=± 0.03983 %
Percentage Uncertainty ( mass ) ≈ ±0.04 %
For temperature
Absolute uncertainty= ±0.5
Data Average =71.71429
Percentage Uncertainty ( mass )= 0.5
71.71429∗100=±0.6972 %
Percentage Uncertainty ( mass ) ≈ ±0.7 %
Percentage uncertainty
Percentage uncertainty for Heat Energy=0.7+ 0.04=±0.74 %
Percentage uncertainty for Heat Energy ≈ ± 0.7 %
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
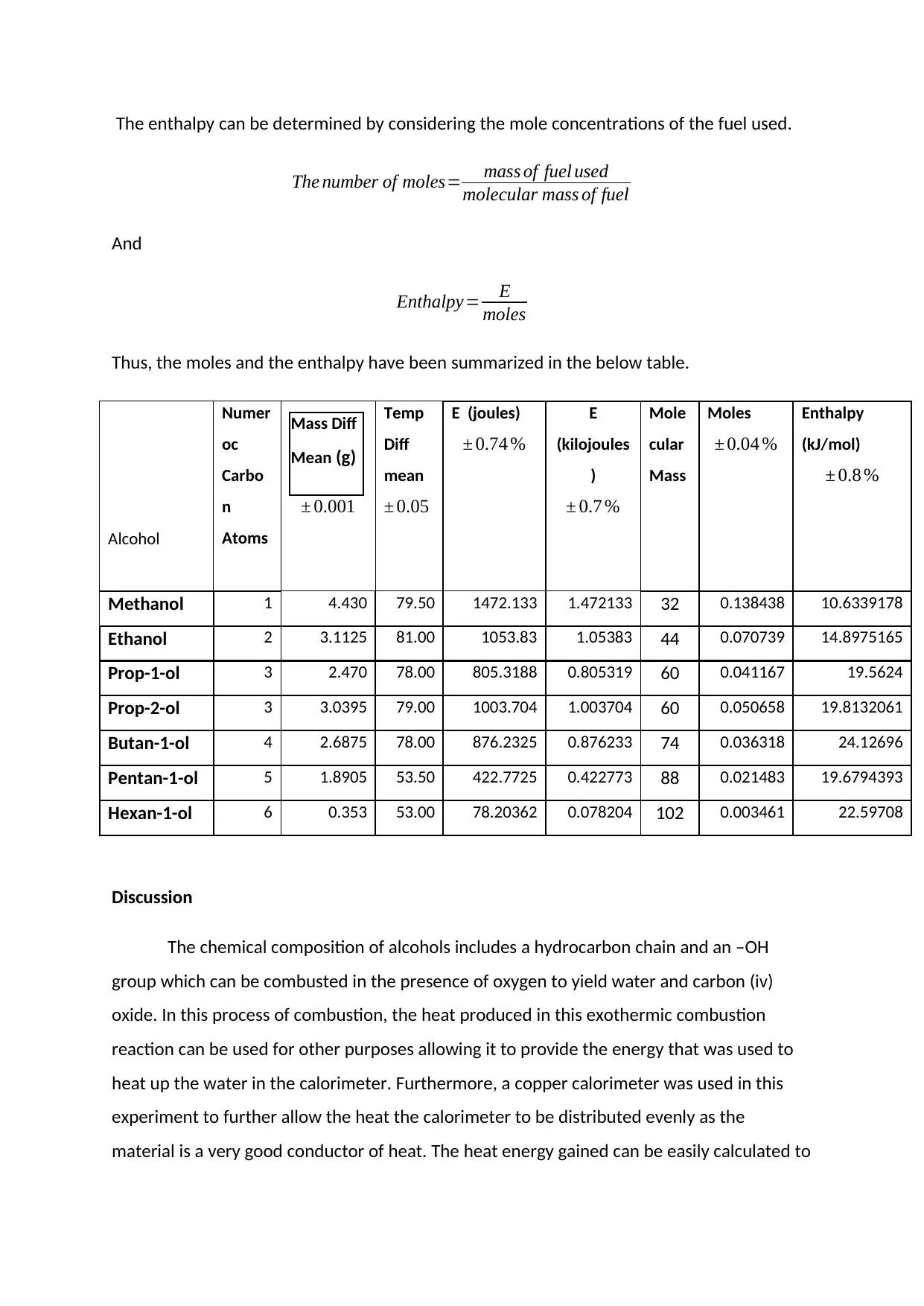
The enthalpy can be determined by considering the mole concentrations of the fuel used.
The number of moles= mass of fuel used
molecular mass of fuel
And
Enthalpy= E
moles
Thus, the moles and the enthalpy have been summarized in the below table.
Alcohol
Numer
oc
Carbo
n
Atoms
Mass Diff
Mean (g)
± 0.001
Temp
Diff
mean
± 0.05
E (joules)
± 0.74 %
E
(kilojoules
)
± 0.7 %
Mole
cular
Mass
Moles
± 0.04 %
Enthalpy
(kJ/mol)
± 0.8 %
Methanol 1 4.430 79.50 1472.133 1.472133 32 0.138438 10.6339178
Ethanol 2 3.1125 81.00 1053.83 1.05383 44 0.070739 14.8975165
Prop-1-ol 3 2.470 78.00 805.3188 0.805319 60 0.041167 19.5624
Prop-2-ol 3 3.0395 79.00 1003.704 1.003704 60 0.050658 19.8132061
Butan-1-ol 4 2.6875 78.00 876.2325 0.876233 74 0.036318 24.12696
Pentan-1-ol 5 1.8905 53.50 422.7725 0.422773 88 0.021483 19.6794393
Hexan-1-ol 6 0.353 53.00 78.20362 0.078204 102 0.003461 22.59708
Discussion
The chemical composition of alcohols includes a hydrocarbon chain and an –OH
group which can be combusted in the presence of oxygen to yield water and carbon (iv)
oxide. In this process of combustion, the heat produced in this exothermic combustion
reaction can be used for other purposes allowing it to provide the energy that was used to
heat up the water in the calorimeter. Furthermore, a copper calorimeter was used in this
experiment to further allow the heat the calorimeter to be distributed evenly as the
material is a very good conductor of heat. The heat energy gained can be easily calculated to
The number of moles= mass of fuel used
molecular mass of fuel
And
Enthalpy= E
moles
Thus, the moles and the enthalpy have been summarized in the below table.
Alcohol
Numer
oc
Carbo
n
Atoms
Mass Diff
Mean (g)
± 0.001
Temp
Diff
mean
± 0.05
E (joules)
± 0.74 %
E
(kilojoules
)
± 0.7 %
Mole
cular
Mass
Moles
± 0.04 %
Enthalpy
(kJ/mol)
± 0.8 %
Methanol 1 4.430 79.50 1472.133 1.472133 32 0.138438 10.6339178
Ethanol 2 3.1125 81.00 1053.83 1.05383 44 0.070739 14.8975165
Prop-1-ol 3 2.470 78.00 805.3188 0.805319 60 0.041167 19.5624
Prop-2-ol 3 3.0395 79.00 1003.704 1.003704 60 0.050658 19.8132061
Butan-1-ol 4 2.6875 78.00 876.2325 0.876233 74 0.036318 24.12696
Pentan-1-ol 5 1.8905 53.50 422.7725 0.422773 88 0.021483 19.6794393
Hexan-1-ol 6 0.353 53.00 78.20362 0.078204 102 0.003461 22.59708
Discussion
The chemical composition of alcohols includes a hydrocarbon chain and an –OH
group which can be combusted in the presence of oxygen to yield water and carbon (iv)
oxide. In this process of combustion, the heat produced in this exothermic combustion
reaction can be used for other purposes allowing it to provide the energy that was used to
heat up the water in the calorimeter. Furthermore, a copper calorimeter was used in this
experiment to further allow the heat the calorimeter to be distributed evenly as the
material is a very good conductor of heat. The heat energy gained can be easily calculated to

establish the energy utilized to bring the water in the calorimeter to a boil. However, the
suitability of a compound to being a good fuel is largely determined by the enthalpy of that
compound. Enthalpy is a term used to define the amount of heat energy that will be yielded
when one mole of a substance is combusted in the presence of oxygen (Wiberg, Crocker,
and Morgan, 2016, p. 2147). A higher the enthalpy of a compound thus implies that the
compound will generate a large amount of energy when a mole of the compound is
combusted. The results of this experiment show that propanol and pentanol are the best
alcohols to be used as fuel as they tend to emit 25 and 28 kilojoules for every mole of the
compounds combusted.
Further, in the case of propan-1ol and propan-2-ol, the latter is seen to yield a higher
enthalpy than the former regardless of the two compounds having the same molecular mass
and number of carbons. This observation can be attributed to the fact that the propan-1-ol
heated the water to boiling point faster than propan-2-ol. This implies that the molecular
structure of propan-2-ol has a structure that is different from that of propan-1-ol in terms of
the bonds and van der Waals forces that the two alcohols have. While enthalpy is
determined by the types and number of atoms that make up the molecular composition of
the compound, the molecular structure of the compound also plays very critical but
secondary role.
The errors that were experienced during this experiment were caused by the changes in the
environment of the experiment as temperature and pressure dissipation are not easy to
control. There might have also been some systematic errors associated with reading the
apparatus and carrying out the experiment as was required to the letter. Finally, errors
could also have resulted from random errors that occurs as the fuel was allowed to
evaporate during measurement of the weight resulting in errors (Rakopoulos, Rakopoulos,
Papagiannakis, and Kyritsis, 2011, p.1856). These errors can be reduced by completely
controlling the experiment through insulating it and placing air foams around the setup of
the experiment to prevent the high rates of heat loss.
Evaluation
The results I obtained show a trend in the enthalpies obtained as can be summarized in the
below graphs.
suitability of a compound to being a good fuel is largely determined by the enthalpy of that
compound. Enthalpy is a term used to define the amount of heat energy that will be yielded
when one mole of a substance is combusted in the presence of oxygen (Wiberg, Crocker,
and Morgan, 2016, p. 2147). A higher the enthalpy of a compound thus implies that the
compound will generate a large amount of energy when a mole of the compound is
combusted. The results of this experiment show that propanol and pentanol are the best
alcohols to be used as fuel as they tend to emit 25 and 28 kilojoules for every mole of the
compounds combusted.
Further, in the case of propan-1ol and propan-2-ol, the latter is seen to yield a higher
enthalpy than the former regardless of the two compounds having the same molecular mass
and number of carbons. This observation can be attributed to the fact that the propan-1-ol
heated the water to boiling point faster than propan-2-ol. This implies that the molecular
structure of propan-2-ol has a structure that is different from that of propan-1-ol in terms of
the bonds and van der Waals forces that the two alcohols have. While enthalpy is
determined by the types and number of atoms that make up the molecular composition of
the compound, the molecular structure of the compound also plays very critical but
secondary role.
The errors that were experienced during this experiment were caused by the changes in the
environment of the experiment as temperature and pressure dissipation are not easy to
control. There might have also been some systematic errors associated with reading the
apparatus and carrying out the experiment as was required to the letter. Finally, errors
could also have resulted from random errors that occurs as the fuel was allowed to
evaporate during measurement of the weight resulting in errors (Rakopoulos, Rakopoulos,
Papagiannakis, and Kyritsis, 2011, p.1856). These errors can be reduced by completely
controlling the experiment through insulating it and placing air foams around the setup of
the experiment to prevent the high rates of heat loss.
Evaluation
The results I obtained show a trend in the enthalpies obtained as can be summarized in the
below graphs.
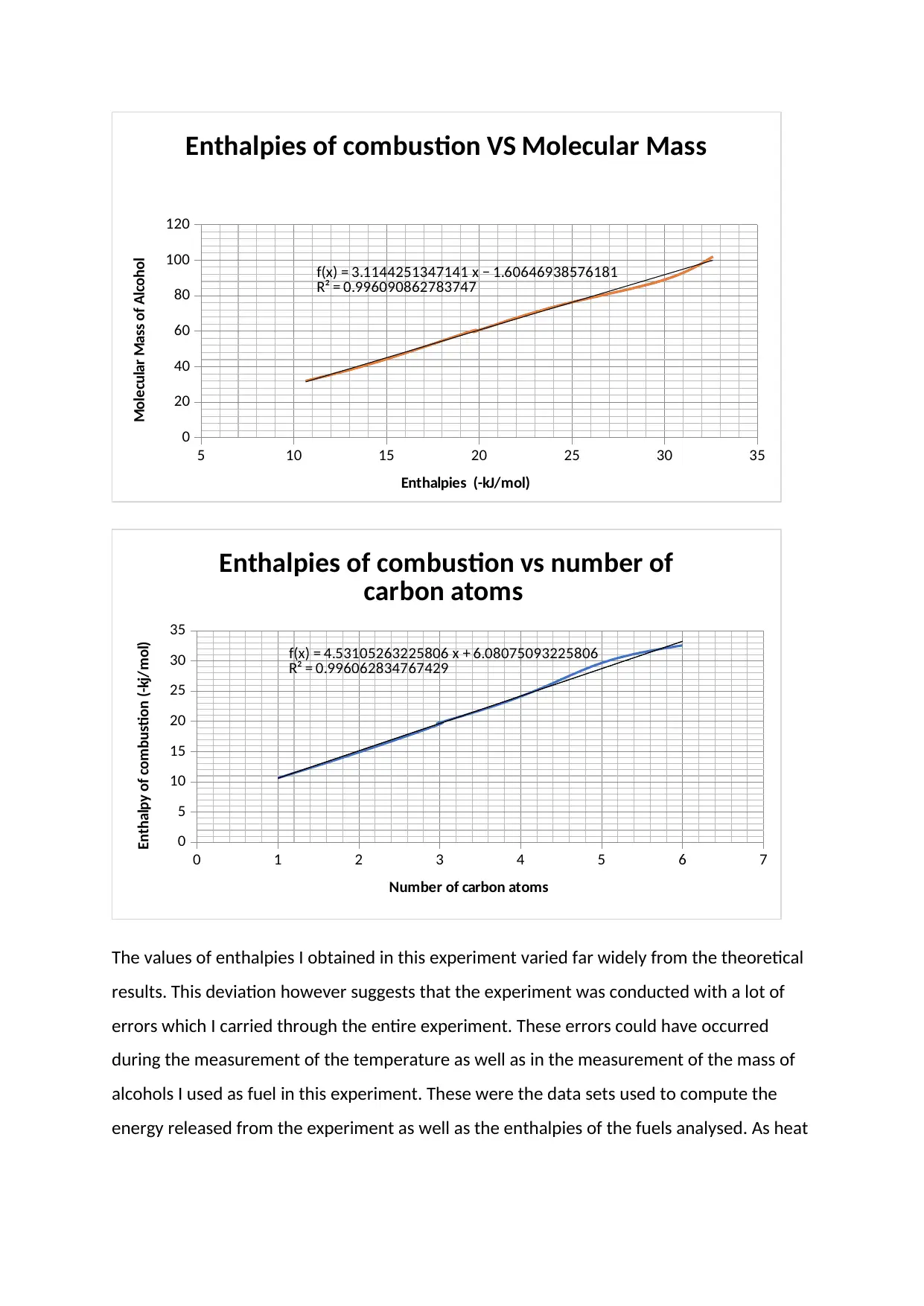
5 10 15 20 25 30 35
0
20
40
60
80
100
120
f(x) = 3.1144251347141 x − 1.60646938576181
R² = 0.996090862783747
Enthalpies of combustion VS Molecular Mass
Enthalpies (-kJ/mol)
Molecular Mass of Alcohol
0 1 2 3 4 5 6 7
0
5
10
15
20
25
30
35
f(x) = 4.53105263225806 x + 6.08075093225806
R² = 0.996062834767429
Enthalpies of combustion vs number of
carbon atoms
Number of carbon atoms
Enthalpy of combustion (-kj/mol)
The values of enthalpies I obtained in this experiment varied far widely from the theoretical
results. This deviation however suggests that the experiment was conducted with a lot of
errors which I carried through the entire experiment. These errors could have occurred
during the measurement of the temperature as well as in the measurement of the mass of
alcohols I used as fuel in this experiment. These were the data sets used to compute the
energy released from the experiment as well as the enthalpies of the fuels analysed. As heat
0
20
40
60
80
100
120
f(x) = 3.1144251347141 x − 1.60646938576181
R² = 0.996090862783747
Enthalpies of combustion VS Molecular Mass
Enthalpies (-kJ/mol)
Molecular Mass of Alcohol
0 1 2 3 4 5 6 7
0
5
10
15
20
25
30
35
f(x) = 4.53105263225806 x + 6.08075093225806
R² = 0.996062834767429
Enthalpies of combustion vs number of
carbon atoms
Number of carbon atoms
Enthalpy of combustion (-kj/mol)
The values of enthalpies I obtained in this experiment varied far widely from the theoretical
results. This deviation however suggests that the experiment was conducted with a lot of
errors which I carried through the entire experiment. These errors could have occurred
during the measurement of the temperature as well as in the measurement of the mass of
alcohols I used as fuel in this experiment. These were the data sets used to compute the
energy released from the experiment as well as the enthalpies of the fuels analysed. As heat
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
energy was being lost to the surrounding during the exercise, the temperatures recorded
were lower than the expected values (Qi, et al., 2011, p. 1679).
The lost heat energy can be incorporated or considered by calculating the
uncertainty level of the results from the manner in which the important parameters were
arrived at. The uncertainty in this experiment was obtained as follows:
%age error enthalpty calculation= percentage uncertainty
100 ∗computed value
Since the net uncertainties for energy and enthalpies were obtained from computing the
absolute and percentage uncertainties in previous sections the percentage error values
were found to be:
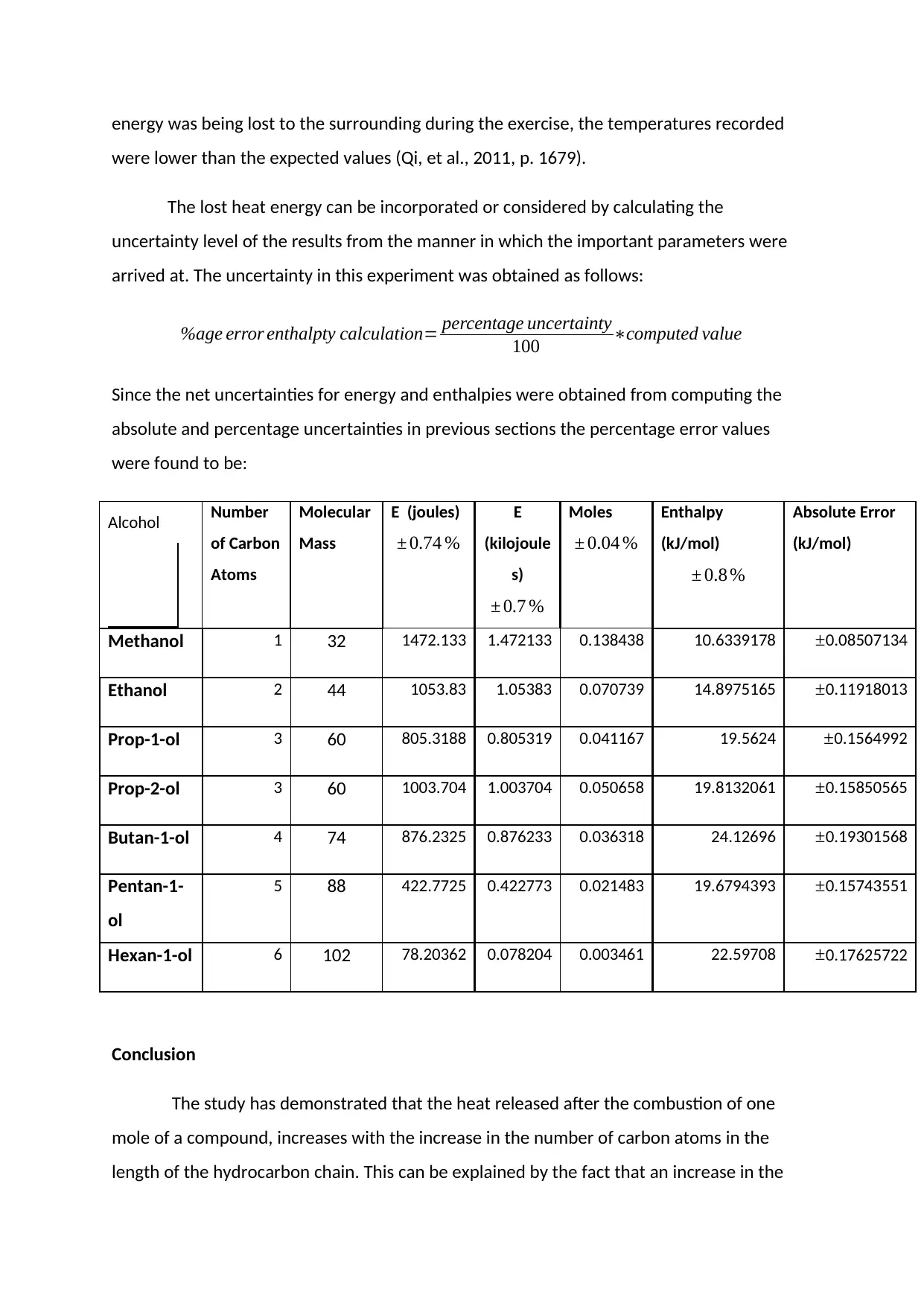
Alcohol Number
of Carbon
Atoms
Molecular
Mass
E (joules)
± 0.74 %
E
(kilojoule
s)
± 0.7 %
Moles
± 0.04 %
Enthalpy
(kJ/mol)
± 0.8 %
Absolute Error
(kJ/mol)
Methanol 1 32 1472.133 1.472133 0.138438 10.6339178 ±0.08507134
Ethanol 2 44 1053.83 1.05383 0.070739 14.8975165 ±0.11918013
Prop-1-ol 3 60 805.3188 0.805319 0.041167 19.5624 ±0.1564992
Prop-2-ol 3 60 1003.704 1.003704 0.050658 19.8132061 ±0.15850565
Butan-1-ol 4 74 876.2325 0.876233 0.036318 24.12696 ±0.19301568
Pentan-1-
ol
5 88 422.7725 0.422773 0.021483 19.6794393 ±0.15743551
Hexan-1-ol 6 102 78.20362 0.078204 0.003461 22.59708 ±0.17625722
Conclusion
The study has demonstrated that the heat released after the combustion of one
mole of a compound, increases with the increase in the number of carbon atoms in the
length of the hydrocarbon chain. This can be explained by the fact that an increase in the
were lower than the expected values (Qi, et al., 2011, p. 1679).
The lost heat energy can be incorporated or considered by calculating the
uncertainty level of the results from the manner in which the important parameters were
arrived at. The uncertainty in this experiment was obtained as follows:
%age error enthalpty calculation= percentage uncertainty
100 ∗computed value
Since the net uncertainties for energy and enthalpies were obtained from computing the
absolute and percentage uncertainties in previous sections the percentage error values
were found to be:
Alcohol Number
of Carbon
Atoms
Molecular
Mass
E (joules)
± 0.74 %
E
(kilojoule
s)
± 0.7 %
Moles
± 0.04 %
Enthalpy
(kJ/mol)
± 0.8 %
Absolute Error
(kJ/mol)
Methanol 1 32 1472.133 1.472133 0.138438 10.6339178 ±0.08507134
Ethanol 2 44 1053.83 1.05383 0.070739 14.8975165 ±0.11918013
Prop-1-ol 3 60 805.3188 0.805319 0.041167 19.5624 ±0.1564992
Prop-2-ol 3 60 1003.704 1.003704 0.050658 19.8132061 ±0.15850565
Butan-1-ol 4 74 876.2325 0.876233 0.036318 24.12696 ±0.19301568
Pentan-1-
ol
5 88 422.7725 0.422773 0.021483 19.6794393 ±0.15743551
Hexan-1-ol 6 102 78.20362 0.078204 0.003461 22.59708 ±0.17625722
Conclusion
The study has demonstrated that the heat released after the combustion of one
mole of a compound, increases with the increase in the number of carbon atoms in the
length of the hydrocarbon chain. This can be explained by the fact that an increase in the

number of carbon atoms in a hydrocarbon chain tends to increase the size of the fuel
particle. An increase in the carbon atoms translates into an increase in the molecular mass
of the compound which in turn affects the van der Waal’s forces and molecular bonds
joining the long chains. This is depicted in the graph that was obtained after the results
were plotted. This increase however was not observed to be a linear one.
Further Investigation
When I will be conducting further investigations, the impact of heat losses from the burner
and from the calorimeter ought to be considered. Further insulating the experiment is also
recommended to ensure that the heat losses have been completely reduced. The heat
losses can for instance be minimized through insulating the experiment surrounding using
an air foam around the experiment set up and through insulating the copper calorimeter.
The alcohols will also have to be burnt in the presence of oxygen to ensure that they are
fully combusted for more accurate results an even accurate inference of the experiment as
more energy will be release during complete combustion. The thermometer I used in the
experiment can also be substituted with an electronic thermometer that will enable the
errors that I encountered to be reduced a great deal due to the precision of reading the
temperatures. If I had more time the experiment would have been conducted in a
controlled surrounding such that the impact of air pressure and temperature are minimized
to the very least.
particle. An increase in the carbon atoms translates into an increase in the molecular mass
of the compound which in turn affects the van der Waal’s forces and molecular bonds
joining the long chains. This is depicted in the graph that was obtained after the results
were plotted. This increase however was not observed to be a linear one.
Further Investigation
When I will be conducting further investigations, the impact of heat losses from the burner
and from the calorimeter ought to be considered. Further insulating the experiment is also
recommended to ensure that the heat losses have been completely reduced. The heat
losses can for instance be minimized through insulating the experiment surrounding using
an air foam around the experiment set up and through insulating the copper calorimeter.
The alcohols will also have to be burnt in the presence of oxygen to ensure that they are
fully combusted for more accurate results an even accurate inference of the experiment as
more energy will be release during complete combustion. The thermometer I used in the
experiment can also be substituted with an electronic thermometer that will enable the
errors that I encountered to be reduced a great deal due to the precision of reading the
temperatures. If I had more time the experiment would have been conducted in a
controlled surrounding such that the impact of air pressure and temperature are minimized
to the very least.

References
Anderson, R.B., 2016. Thermodynamics of the hydrogenation of oxides of carbon. The
Journal of Physical Chemistry, 90(20), pp.4806-4810.
Bruno, T.J. and Smith, B.L., 2016. Enthalpy of combustion of fuels as a function of distillate
cut: application of an advanced distillation curve method. Energy & fuels, 20(5), pp.2109-
2116.
Lloyd, W.G. and Davenport, D.A., 2010. Applying thermodynamics to fossil fuels: Heats of
combustion from elemental compositions. Journal of chemical education, 57(1), p.56.
Qi, D.H., Chen, H., Geng, L.M., Bian, Y.Z. and Ren, X.C., 2010. Performance and combustion
characteristics of biodiesel–diesel–methanol blend fuelled engine. Applied Energy, 87(5),
pp.1679-1686.
Rakopoulos, D.C., Rakopoulos, C.D., Papagiannakis, R.G. and Kyritsis, D.C., 2011. Combustion
heat release analysis of ethanol or n-butanol diesel fuel blends in heavy-duty DI diesel
engine. Fuel, 90(5), pp.1855-1867.
Thornton, W.M., 2017. XV. The relation of oxygen to the heat of combustion of organic
compounds. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of
Science, 33(194), pp.196-203.
Wiberg, K.B., Crocker, L.S. and Morgan, K.M., 2011. Thermochemical studies of carbonyl
compounds. 5. Enthalpies of reduction of carbonyl groups. Journal of the American
Chemical Society, 113(9), pp.3447-3450.
Anderson, R.B., 2016. Thermodynamics of the hydrogenation of oxides of carbon. The
Journal of Physical Chemistry, 90(20), pp.4806-4810.
Bruno, T.J. and Smith, B.L., 2016. Enthalpy of combustion of fuels as a function of distillate
cut: application of an advanced distillation curve method. Energy & fuels, 20(5), pp.2109-
2116.
Lloyd, W.G. and Davenport, D.A., 2010. Applying thermodynamics to fossil fuels: Heats of
combustion from elemental compositions. Journal of chemical education, 57(1), p.56.
Qi, D.H., Chen, H., Geng, L.M., Bian, Y.Z. and Ren, X.C., 2010. Performance and combustion
characteristics of biodiesel–diesel–methanol blend fuelled engine. Applied Energy, 87(5),
pp.1679-1686.
Rakopoulos, D.C., Rakopoulos, C.D., Papagiannakis, R.G. and Kyritsis, D.C., 2011. Combustion
heat release analysis of ethanol or n-butanol diesel fuel blends in heavy-duty DI diesel
engine. Fuel, 90(5), pp.1855-1867.
Thornton, W.M., 2017. XV. The relation of oxygen to the heat of combustion of organic
compounds. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of
Science, 33(194), pp.196-203.
Wiberg, K.B., Crocker, L.S. and Morgan, K.M., 2011. Thermochemical studies of carbonyl
compounds. 5. Enthalpies of reduction of carbonyl groups. Journal of the American
Chemical Society, 113(9), pp.3447-3450.
1 out of 13
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.

