Chemistry Project: Detailed Analysis of the Carbon Element
VerifiedAdded on 2023/02/01
|12
|578
|57
Project
AI Summary
This project provides a detailed exploration of the carbon element, beginning with its historical discovery and the origin of its name from the Latin word "Carbo" and French word "Charbon". It details the element's abundance in the Earth's crust and the universe, and provides general information in...
Chemical Element
Carbon
Carbon
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

History and Discovery
The term ‘Carbon’ has come from a Latin
word “Carbo” that means coal. On the other
hand, the French word “ Charbon” also stands
for charcoal.
This chemical element was discovered in the
form of charcoal and soot. In 1722,
René Antoine Ferchault de Réaumur had
reported that, the iron was transformed into
steel after absorbing an element named carbon.
In the same year, the Antoine Lavoisier
showed that diamond is a form of carbon.
The term ‘Carbon’ has come from a Latin
word “Carbo” that means coal. On the other
hand, the French word “ Charbon” also stands
for charcoal.
This chemical element was discovered in the
form of charcoal and soot. In 1722,
René Antoine Ferchault de Réaumur had
reported that, the iron was transformed into
steel after absorbing an element named carbon.
In the same year, the Antoine Lavoisier
showed that diamond is a form of carbon.

Abundance
Carbon is the 15th most
abundant element in earth’s
crust and in case of universe,
it is the fourth most
abundant element by mass.
Carbon is the 15th most
abundant element in earth’s
crust and in case of universe,
it is the fourth most
abundant element by mass.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

General Information
Name of the element- Carbon
Chemical symbol of the element- C
Atomic number of the element- 6
Atomic mass of the element- 12.0107
amu ( Peebles, 2018)
Name of the element- Carbon
Chemical symbol of the element- C
Atomic number of the element- 6
Atomic mass of the element- 12.0107
amu ( Peebles, 2018)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Continued…
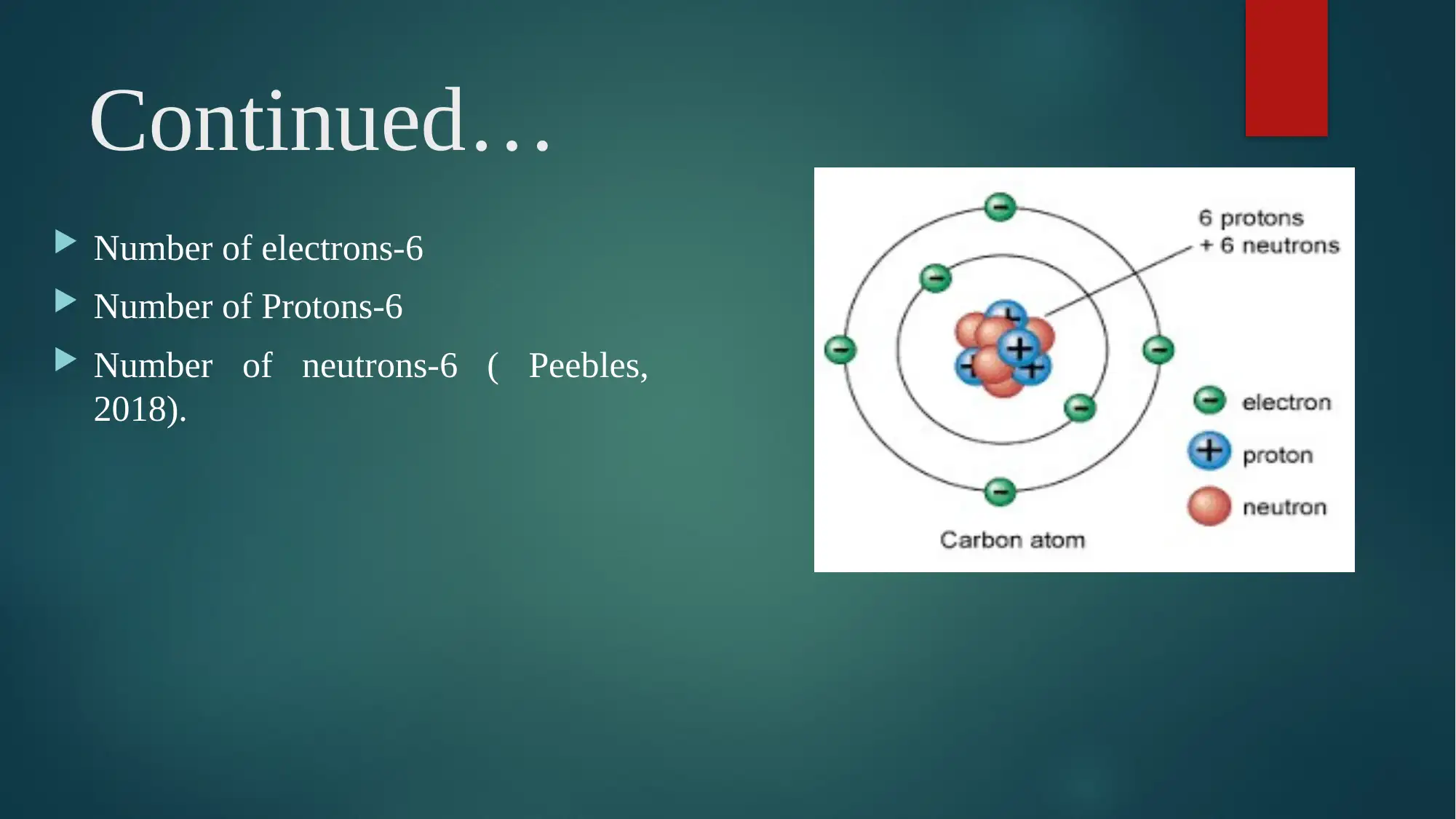
Number of electrons-6
Number of Protons-6
Number of neutrons-6 ( Peebles,
2018).
Number of electrons-6
Number of Protons-6
Number of neutrons-6 ( Peebles,
2018).
Melting and Boiling Point
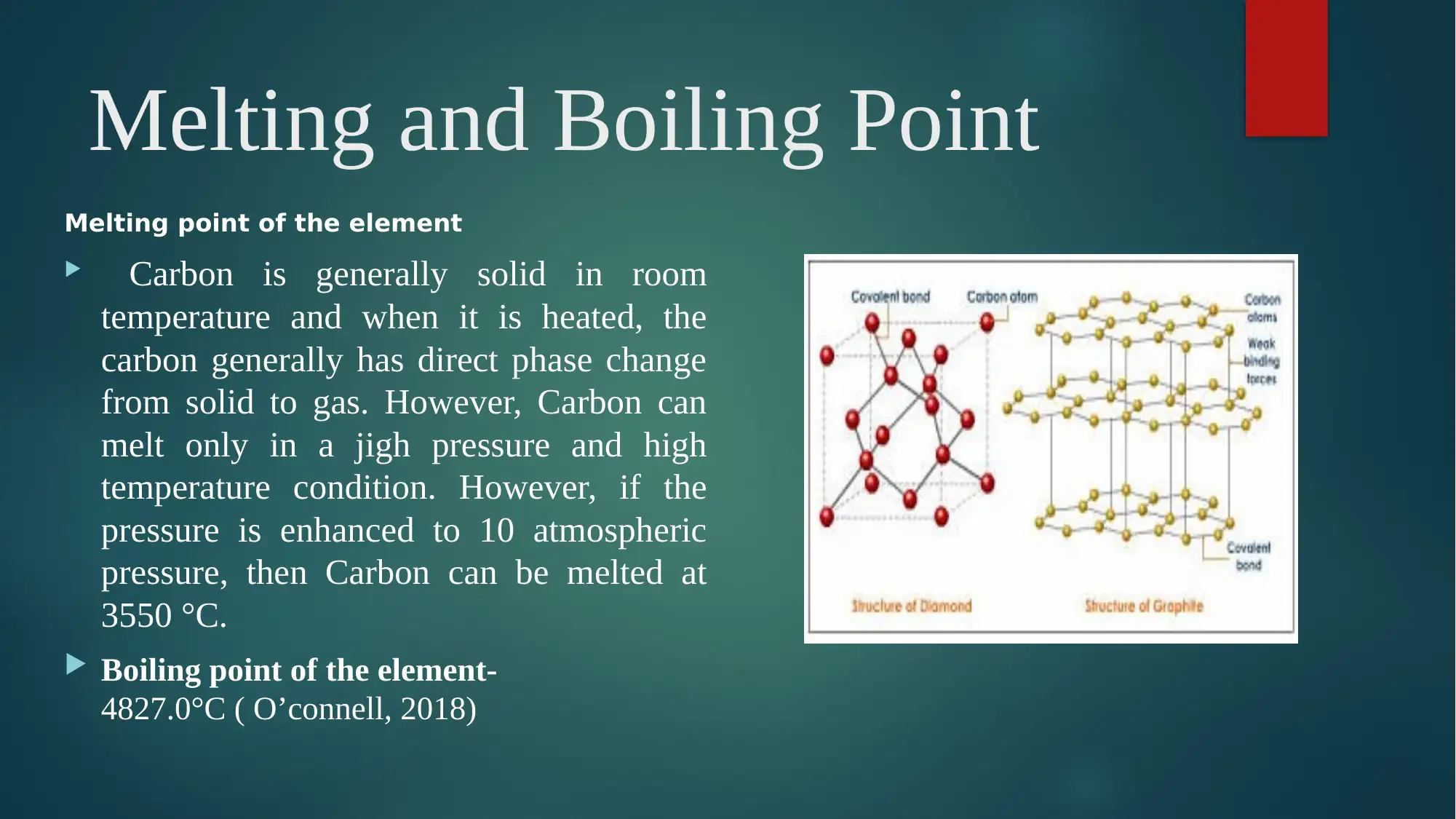
Melting point of the element
Carbon is generally solid in room
temperature and when it is heated, the
carbon generally has direct phase change
from solid to gas. However, Carbon can
melt only in a jigh pressure and high
temperature condition. However, if the
pressure is enhanced to 10 atmospheric
pressure, then Carbon can be melted at
3550 °C.
Boiling point of the element-
4827.0°C ( O’connell, 2018)
Melting point of the element
Carbon is generally solid in room
temperature and when it is heated, the
carbon generally has direct phase change
from solid to gas. However, Carbon can
melt only in a jigh pressure and high
temperature condition. However, if the
pressure is enhanced to 10 atmospheric
pressure, then Carbon can be melted at
3550 °C.
Boiling point of the element-
4827.0°C ( O’connell, 2018)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
State of the Element
State of the element at room temperature-
In room temperature, this element is solid
in nature.
Density of the element- The amorphous
state of the carbon has the density of
1.8–2.1 g/cm3. On the other hand
graphaite and diamond has the density of
almost 2.267 g/cm3 and 3.515 g/cm3
respectively.
Type of the element- Carbon is non-
metal in nature ( O’Connell, 2018).
State of the element at room temperature-
In room temperature, this element is solid
in nature.
Density of the element- The amorphous
state of the carbon has the density of
1.8–2.1 g/cm3. On the other hand
graphaite and diamond has the density of
almost 2.267 g/cm3 and 3.515 g/cm3
respectively.
Type of the element- Carbon is non-
metal in nature ( O’Connell, 2018).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Properties
Properties of the element
Carbon is an electrical conductor. For example
it can be said that, graphite is a good conductor
Carbon has different allotropes such as
diamond and graphite and properties are
different in case of different allotropic form of
the carbon,
Carbon does not reacts with water or acids.
Carbon molecule has the property to form
long chains of atoms to form a different
carbon compound ( O’Connell, 2018)
Properties of the element
Carbon is an electrical conductor. For example
it can be said that, graphite is a good conductor
Carbon has different allotropes such as
diamond and graphite and properties are
different in case of different allotropic form of
the carbon,
Carbon does not reacts with water or acids.
Carbon molecule has the property to form
long chains of atoms to form a different
carbon compound ( O’Connell, 2018)
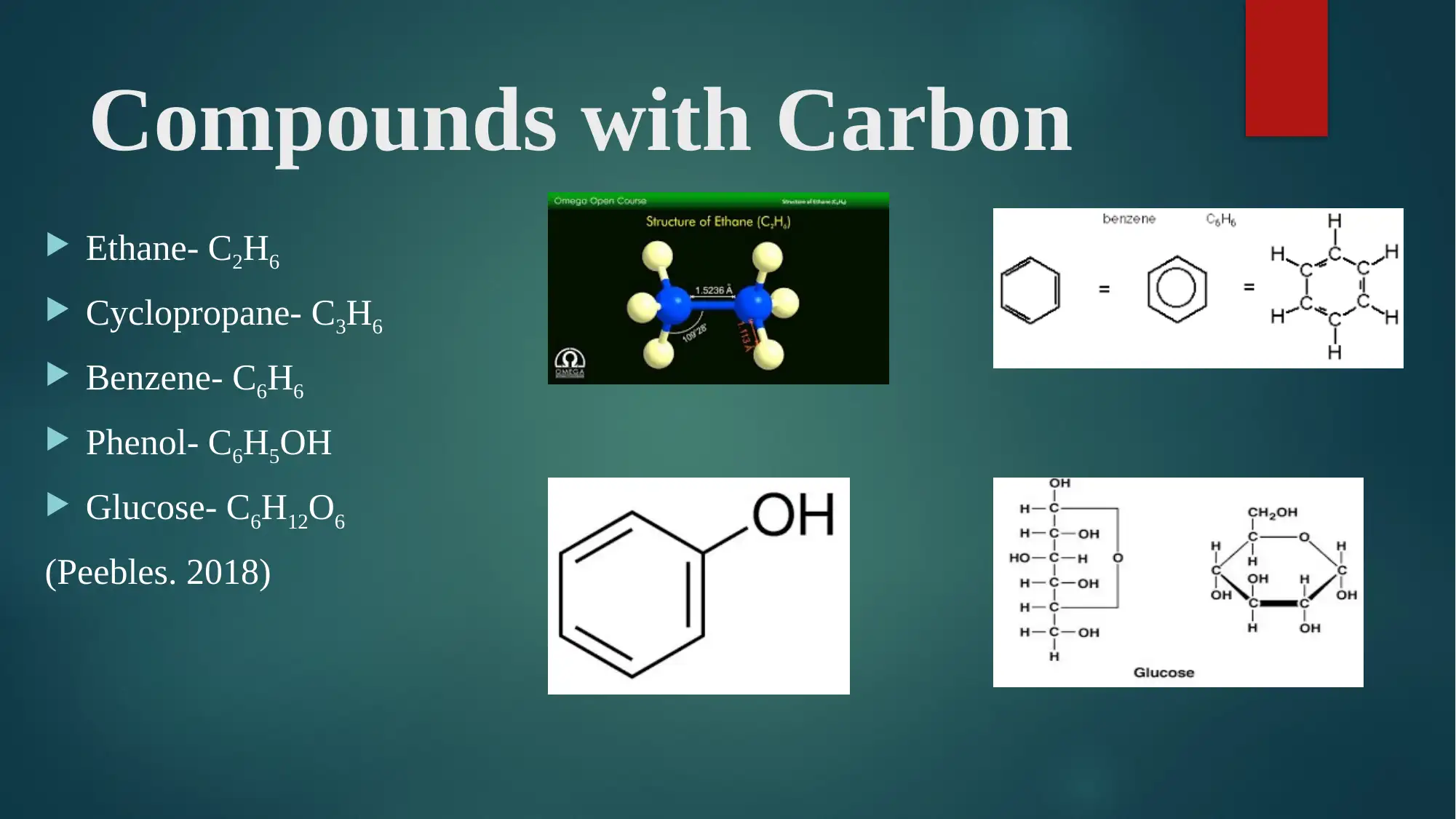
Compounds with Carbon
Ethane- C2H6
Cyclopropane- C3H6
Benzene- C6H6
Phenol- C6H5OH
Glucose- C6H12O6
(Peebles. 2018)
Ethane- C2H6
Cyclopropane- C3H6
Benzene- C6H6
Phenol- C6H5OH
Glucose- C6H12O6
(Peebles. 2018)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Uses of Carbon
Graphite, form of carbon is used as pencil
tips.
One form of carbon, coal, is used as fuel.
Diamonds are used as jewellery and also
used for drilling, cutting glass.
Carbon can be used in the manufacturing
process of fishing rods, tennis rackets.
In the filtration process of water, activated
charcoal can be used ( O’Connell, 2018).
Graphite, form of carbon is used as pencil
tips.
One form of carbon, coal, is used as fuel.
Diamonds are used as jewellery and also
used for drilling, cutting glass.
Carbon can be used in the manufacturing
process of fishing rods, tennis rackets.
In the filtration process of water, activated
charcoal can be used ( O’Connell, 2018).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
O’connell, M. J. (2018). Carbon nanotubes: properties and applications.
CRC press.
Peebles, L. H. (2018). Carbon fibers: formation, structure, and properties.
CRC Press.
O’connell, M. J. (2018). Carbon nanotubes: properties and applications.
CRC press.
Peebles, L. H. (2018). Carbon fibers: formation, structure, and properties.
CRC Press.

Thank You
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 12
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.