Effect of Temperature on Enzyme Activity
Added on 2023-01-11
12 Pages1992 Words37 Views
CHEMISTRY ASSIGMENT
By Name
Course
Instructor
Institution
Location
Date
By Name
Course
Instructor
Institution
Location
Date

Introduction
Enzymes are frequently depicted as 'organic impetuses which increment the rate of reaction of a
bio-synthetic reaction' (David Nelson et. al 2008). Enzymes enhance the rate speed of different
reaction that happen in a natural system, for example, the mammalian stomach related
framework. Proteins can have capacities including exchange, amalgamation or breakdown of
particles. Mention that catalysts are proteins which accelerate the rate of response without being
spent themselves for example they are reusable. Catalysts have an unmistakable dynamic site
which is corresponding to a particular substrate 3 dimensional structure (Küchler et al., 2016).
The explicitness is because of the reciprocal hydrophilic/hydrophobic charge, electrical charge
and state of dynamic site on the protein (Bansode, Hardikar and Rathod, 2017). The specificity
of an enzyme with a particular substrate creates a chemical substrate complex (ES).The rate at
which the protein substrate complex is framed is drastically expanded or diminished in changes
in substrate focus, temperature, pH and nearness of an aggressive inhibitor the impact of these 4
factors on the catalyst action is known as protein energy (Jeremy M. Berg etal 2006).Enzymes
work on the idea of change states. Progress condition of proteins is where the substrate is not a
yet an item and not a substrate (von Sperber et al., 2017). Enzymes decrease this stage .The
contrast between the free vitality of the reactants and the free energy of the progress state is the
activation energy (Ea).The least vitality required for a triumph full response to happen. Catalysts
accelerate the rate of response by bringing down the initiation energy hindrance (Kamp et al.,
2018).
This examination concerns the impact of temperature on the rate of response. I would exepcet to
find that an expansion in temperature would result in an increment in the rate of response. The
explanation behind this wonder is that as there is increment in dynamic vitality being connected
Enzymes are frequently depicted as 'organic impetuses which increment the rate of reaction of a
bio-synthetic reaction' (David Nelson et. al 2008). Enzymes enhance the rate speed of different
reaction that happen in a natural system, for example, the mammalian stomach related
framework. Proteins can have capacities including exchange, amalgamation or breakdown of
particles. Mention that catalysts are proteins which accelerate the rate of response without being
spent themselves for example they are reusable. Catalysts have an unmistakable dynamic site
which is corresponding to a particular substrate 3 dimensional structure (Küchler et al., 2016).
The explicitness is because of the reciprocal hydrophilic/hydrophobic charge, electrical charge
and state of dynamic site on the protein (Bansode, Hardikar and Rathod, 2017). The specificity
of an enzyme with a particular substrate creates a chemical substrate complex (ES).The rate at
which the protein substrate complex is framed is drastically expanded or diminished in changes
in substrate focus, temperature, pH and nearness of an aggressive inhibitor the impact of these 4
factors on the catalyst action is known as protein energy (Jeremy M. Berg etal 2006).Enzymes
work on the idea of change states. Progress condition of proteins is where the substrate is not a
yet an item and not a substrate (von Sperber et al., 2017). Enzymes decrease this stage .The
contrast between the free vitality of the reactants and the free energy of the progress state is the
activation energy (Ea).The least vitality required for a triumph full response to happen. Catalysts
accelerate the rate of response by bringing down the initiation energy hindrance (Kamp et al.,
2018).
This examination concerns the impact of temperature on the rate of response. I would exepcet to
find that an expansion in temperature would result in an increment in the rate of response. The
explanation behind this wonder is that as there is increment in dynamic vitality being connected

to chemicals and substrates it expands the odds of crash happening so more item (PNP) is shaped
per unit time. Anyway I likewise trust that temperatures over 50 - 70 degrees Celsius would
denature the proteins dynamic site and the 3 dimensional structures. At this stage the compound
won't be integral fit as a fiddle to the substrate (Khan, Jadhav and Rathod, 2015).
This would imply that no response can be finished so the rate of response will diminish. State
that the catalyst will have an ideal temperature at which the ES buildings and items are made at
the quickest speed. The expansion in temperature increment the measure of atoms which have
higher vitality than the Ea hindrance this thus builds the measure of particles which can respond
expanding the rate of response or starting speed. I trust the ideal temperature is going to extend
between 20-40 degrees Celsius (Juneidi et al., 2017).
Aim
To determine the rate constants for the -chymotrypsin catalysed hydrolysis of an ester
Methods
The 10 mL of 3.8 x 10-3M arrangement of substrate, 4-nitrophenyl trimethylacetate, and
acetonitrile was moreover added and weakened to make a stock arrangement. After 50 mg of
alpha chymotrypsin was broken down in a 1.0 mL of 4.6 pH acetic acid derivation cushion. The
TRISS powder was weighed for 0.12114 grams and was put in a 200 mL container and 100 mL
of deionized water was utilized to break up the TRIS powder; after when all powder is broken up
HCL was included and estimated with a pH meter until pH stretched around 8 pH (P1). Later 10
mL of 4-Nitrophenol Solution (2.8 x 10-5 M) was set up in the TRIS cushion that was arranged
already, as the arrangement will be utilized to decide the molar absorptivity. Thereafter, the
spectrophotometer was set up at 400 nm (Which P1 is hydrolysis item); the TRIS support was
per unit time. Anyway I likewise trust that temperatures over 50 - 70 degrees Celsius would
denature the proteins dynamic site and the 3 dimensional structures. At this stage the compound
won't be integral fit as a fiddle to the substrate (Khan, Jadhav and Rathod, 2015).
This would imply that no response can be finished so the rate of response will diminish. State
that the catalyst will have an ideal temperature at which the ES buildings and items are made at
the quickest speed. The expansion in temperature increment the measure of atoms which have
higher vitality than the Ea hindrance this thus builds the measure of particles which can respond
expanding the rate of response or starting speed. I trust the ideal temperature is going to extend
between 20-40 degrees Celsius (Juneidi et al., 2017).
Aim
To determine the rate constants for the -chymotrypsin catalysed hydrolysis of an ester
Methods
The 10 mL of 3.8 x 10-3M arrangement of substrate, 4-nitrophenyl trimethylacetate, and
acetonitrile was moreover added and weakened to make a stock arrangement. After 50 mg of
alpha chymotrypsin was broken down in a 1.0 mL of 4.6 pH acetic acid derivation cushion. The
TRISS powder was weighed for 0.12114 grams and was put in a 200 mL container and 100 mL
of deionized water was utilized to break up the TRIS powder; after when all powder is broken up
HCL was included and estimated with a pH meter until pH stretched around 8 pH (P1). Later 10
mL of 4-Nitrophenol Solution (2.8 x 10-5 M) was set up in the TRIS cushion that was arranged
already, as the arrangement will be utilized to decide the molar absorptivity. Thereafter, the
spectrophotometer was set up at 400 nm (Which P1 is hydrolysis item); the TRIS support was

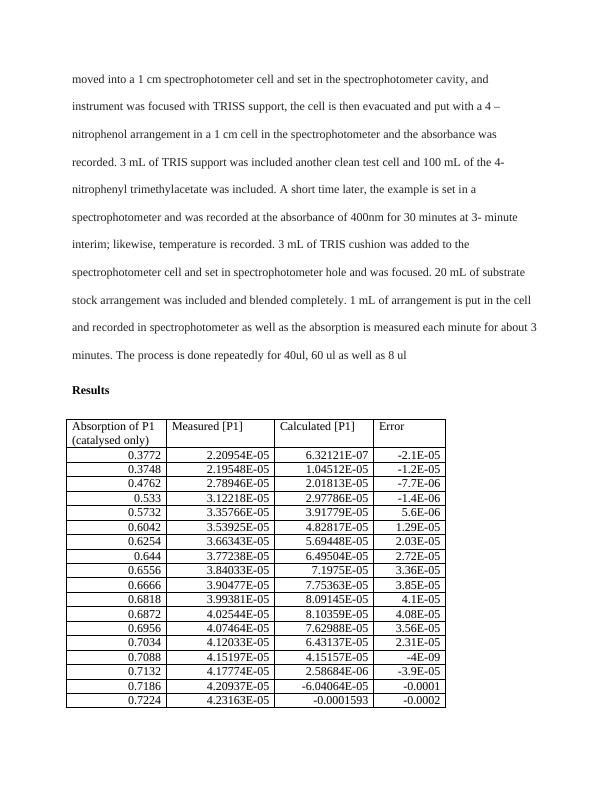
moved into a 1 cm spectrophotometer cell and set in the spectrophotometer cavity, and
instrument was focused with TRISS support, the cell is then evacuated and put with a 4 –
nitrophenol arrangement in a 1 cm cell in the spectrophotometer and the absorbance was
recorded. 3 mL of TRIS support was included another clean test cell and 100 mL of the 4-
nitrophenyl trimethylacetate was included. A short time later, the example is set in a
spectrophotometer and was recorded at the absorbance of 400nm for 30 minutes at 3- minute
interim; likewise, temperature is recorded. 3 mL of TRIS cushion was added to the
spectrophotometer cell and set in spectrophotometer hole and was focused. 20 mL of substrate
stock arrangement was included and blended completely. 1 mL of arrangement is put in the cell
and recorded in spectrophotometer as well as the absorption is measured each minute for about 3
minutes. The process is done repeatedly for 40ul, 60 ul as well as 8 ul
Results
Absorption of P1
(catalysed only)
Measured [P1] Calculated [P1] Error
0.3772 2.20954E-05 6.32121E-07 -2.1E-05
0.3748 2.19548E-05 1.04512E-05 -1.2E-05
0.4762 2.78946E-05 2.01813E-05 -7.7E-06
0.533 3.12218E-05 2.97786E-05 -1.4E-06
0.5732 3.35766E-05 3.91779E-05 5.6E-06
0.6042 3.53925E-05 4.82817E-05 1.29E-05
0.6254 3.66343E-05 5.69448E-05 2.03E-05
0.644 3.77238E-05 6.49504E-05 2.72E-05
0.6556 3.84033E-05 7.1975E-05 3.36E-05
0.6666 3.90477E-05 7.75363E-05 3.85E-05
0.6818 3.99381E-05 8.09145E-05 4.1E-05
0.6872 4.02544E-05 8.10359E-05 4.08E-05
0.6956 4.07464E-05 7.62988E-05 3.56E-05
0.7034 4.12033E-05 6.43137E-05 2.31E-05
0.7088 4.15197E-05 4.15157E-05 -4E-09
0.7132 4.17774E-05 2.58684E-06 -3.9E-05
0.7186 4.20937E-05 -6.04064E-05 -0.0001
0.7224 4.23163E-05 -0.0001593 -0.0002
instrument was focused with TRISS support, the cell is then evacuated and put with a 4 –
nitrophenol arrangement in a 1 cm cell in the spectrophotometer and the absorbance was
recorded. 3 mL of TRIS support was included another clean test cell and 100 mL of the 4-
nitrophenyl trimethylacetate was included. A short time later, the example is set in a
spectrophotometer and was recorded at the absorbance of 400nm for 30 minutes at 3- minute
interim; likewise, temperature is recorded. 3 mL of TRIS cushion was added to the
spectrophotometer cell and set in spectrophotometer hole and was focused. 20 mL of substrate
stock arrangement was included and blended completely. 1 mL of arrangement is put in the cell
and recorded in spectrophotometer as well as the absorption is measured each minute for about 3
minutes. The process is done repeatedly for 40ul, 60 ul as well as 8 ul
Results
Absorption of P1
(catalysed only)
Measured [P1] Calculated [P1] Error
0.3772 2.20954E-05 6.32121E-07 -2.1E-05
0.3748 2.19548E-05 1.04512E-05 -1.2E-05
0.4762 2.78946E-05 2.01813E-05 -7.7E-06
0.533 3.12218E-05 2.97786E-05 -1.4E-06
0.5732 3.35766E-05 3.91779E-05 5.6E-06
0.6042 3.53925E-05 4.82817E-05 1.29E-05
0.6254 3.66343E-05 5.69448E-05 2.03E-05
0.644 3.77238E-05 6.49504E-05 2.72E-05
0.6556 3.84033E-05 7.1975E-05 3.36E-05
0.6666 3.90477E-05 7.75363E-05 3.85E-05
0.6818 3.99381E-05 8.09145E-05 4.1E-05
0.6872 4.02544E-05 8.10359E-05 4.08E-05
0.6956 4.07464E-05 7.62988E-05 3.56E-05
0.7034 4.12033E-05 6.43137E-05 2.31E-05
0.7088 4.15197E-05 4.15157E-05 -4E-09
0.7132 4.17774E-05 2.58684E-06 -3.9E-05
0.7186 4.20937E-05 -6.04064E-05 -0.0001
0.7224 4.23163E-05 -0.0001593 -0.0002
End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Report on Inversion of Sucrose (pdf)lg...
|9
|1315
|118
Enzymes: Structure, Function, Activation Energy, Lock and Key Model, Induced Fit Model, and External Factorslg...
|7
|745
|134
Analysis of Alcohol Dehydrogenase Kinetics under Varied Temperature Conditionslg...
|18
|3406
|237
