Chemistry Assignment Report
VerifiedAdded on 2022/08/10
|23
|3385
|20
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

RUNNING HEAD: CHEMISTRY ASSIGNMENT
Chemistry Assignment
Institution’s Affiliation
Student Name
Date
Chemistry Assignment
Institution’s Affiliation
Student Name
Date
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

RUNNING HEAD: CHEMISTRY ASSIGNMENT
Part 1: Assign point groups to the following. Use the most symmetric conformation available
for your assignment .Indicate if the structure is chiral (i.e., non-superimposable with its
mirror image)(12 points:2 points each)
Answers
(a) Metallocene
Point group = D5d
The structural is achiral molecule
(b) Dimethylsulfoxide
Point group = C2V
The structural is achiral molecule
(c) Cl4-Cyclooctatetraene
Point group=C8
The structural is achiral molecule
(d) Acetonitrile
Point group = C∞V
The structural is achiral molecule
(e) Tungsten Hexachloride
Point group =Oh
The structural is achiral molecule
(f) Extended Metal Atom Chain
Point group = C2V
The structural is achiral molecule
Part 1: Assign point groups to the following. Use the most symmetric conformation available
for your assignment .Indicate if the structure is chiral (i.e., non-superimposable with its
mirror image)(12 points:2 points each)
Answers
(a) Metallocene
Point group = D5d
The structural is achiral molecule
(b) Dimethylsulfoxide
Point group = C2V
The structural is achiral molecule
(c) Cl4-Cyclooctatetraene
Point group=C8
The structural is achiral molecule
(d) Acetonitrile
Point group = C∞V
The structural is achiral molecule
(e) Tungsten Hexachloride
Point group =Oh
The structural is achiral molecule
(f) Extended Metal Atom Chain
Point group = C2V
The structural is achiral molecule
RUNNING HEAD: CHEMISTRY ASSIGNMENT
Part 2. Vibrational Spectroscopy.
The molecule C2H2F2, shown in the figure, belongs to the C2v point group.
l .Determine the number of degrees of freedom for C2H2F2 (2 points).
Answers
The molecule is non-linear Therefore the degree of freedom is
3N-6 where N is Number is the number of atoms
(3x6)-6=18-6=12
# of degrees of freedom = 12
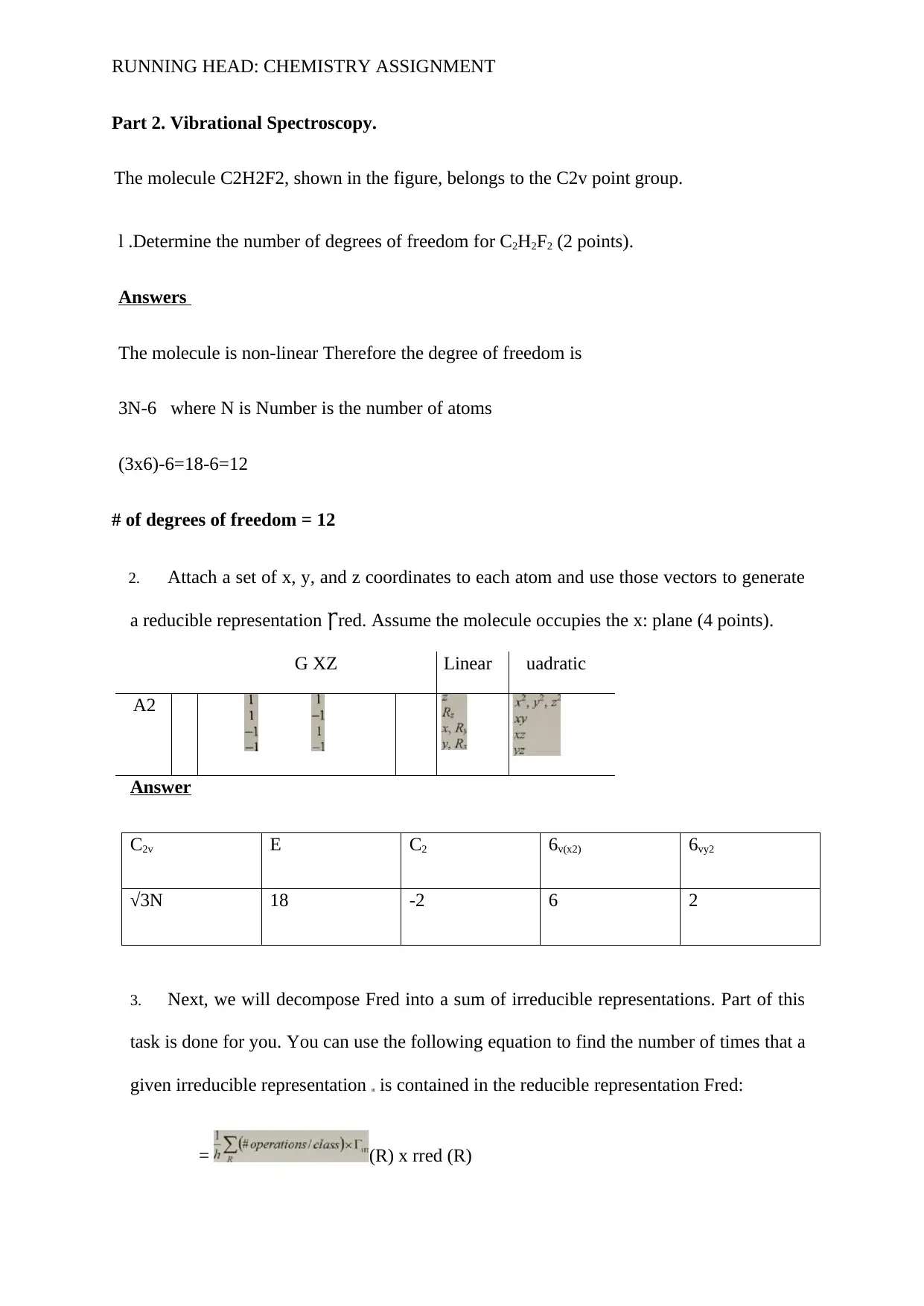
2. Attach a set of x, y, and z coordinates to each atom and use those vectors to generate
a reducible representation red. Assume the molecule occupies the x: plane (4 points).Ꞅ
G XZ Linear uadratic
A2
Answer
C2v E C2 6v(x2) 6vy2
√3N 18 -2 6 2
3. Next, we will decompose Fred into a sum of irreducible representations. Part of this
task is done for you. You can use the following equation to find the number of times that a
given irreducible representation is contained in the reducible representation Fred:
= (R) x rred (R)
Part 2. Vibrational Spectroscopy.
The molecule C2H2F2, shown in the figure, belongs to the C2v point group.
l .Determine the number of degrees of freedom for C2H2F2 (2 points).
Answers
The molecule is non-linear Therefore the degree of freedom is
3N-6 where N is Number is the number of atoms
(3x6)-6=18-6=12
# of degrees of freedom = 12
2. Attach a set of x, y, and z coordinates to each atom and use those vectors to generate
a reducible representation red. Assume the molecule occupies the x: plane (4 points).Ꞅ
G XZ Linear uadratic
A2
Answer
C2v E C2 6v(x2) 6vy2
√3N 18 -2 6 2
3. Next, we will decompose Fred into a sum of irreducible representations. Part of this
task is done for you. You can use the following equation to find the number of times that a
given irreducible representation is contained in the reducible representation Fred:
= (R) x rred (R)
RUNNING HEAD: CHEMISTRY ASSIGNMENT
Where h is the order (i.e., total number of symmetry operations) of the point group and
the sum is taken over all symmetry operations R of the group. These yields:
rred 6 Al + X A2 + y Bl + 4 B2
Determine x and y (8 points):
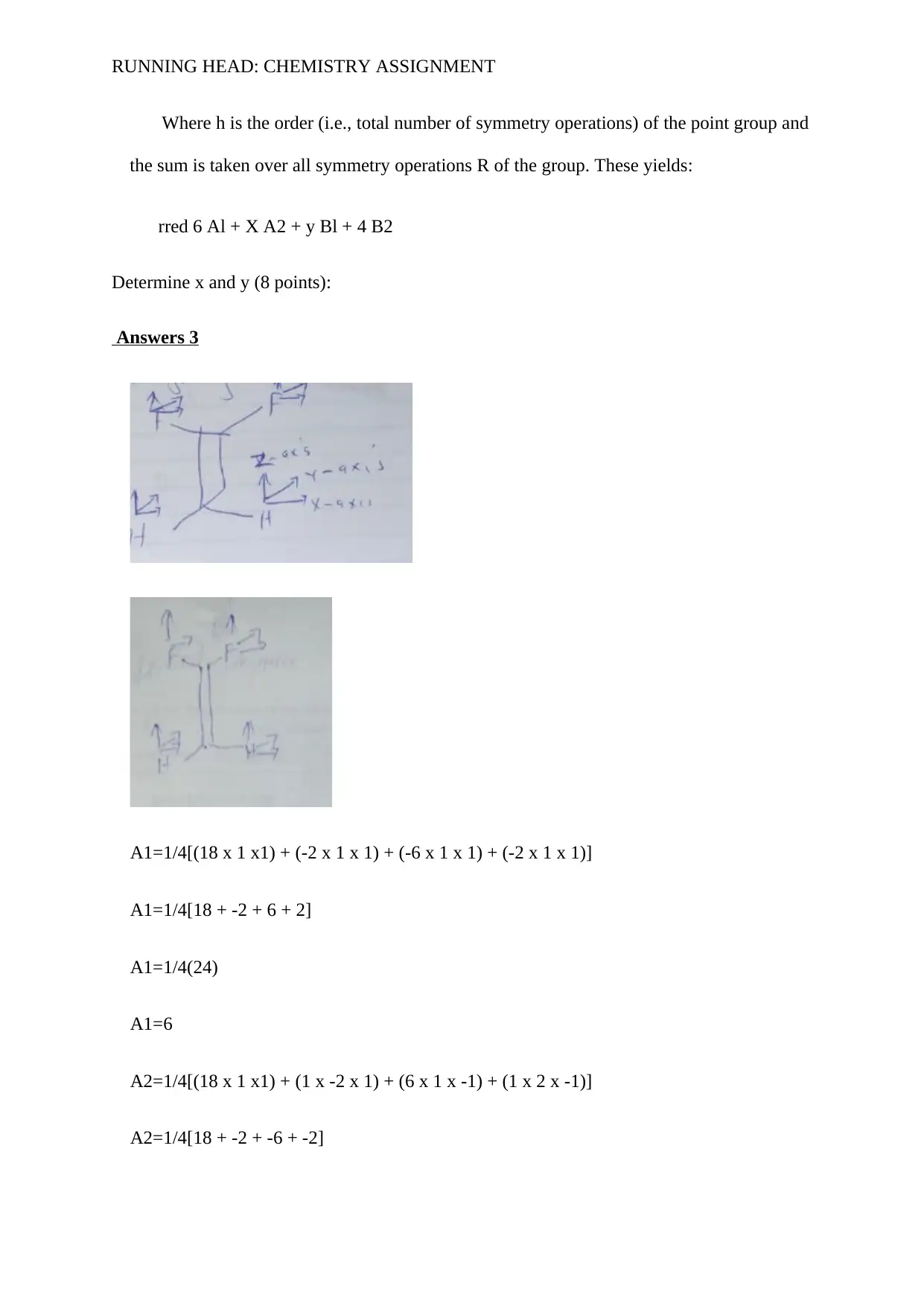
Answers 3
A1=1/4[(18 x 1 x1) + (-2 x 1 x 1) + (-6 x 1 x 1) + (-2 x 1 x 1)]
A1=1/4[18 + -2 + 6 + 2]
A1=1/4(24)
A1=6
A2=1/4[(18 x 1 x1) + (1 x -2 x 1) + (6 x 1 x -1) + (1 x 2 x -1)]
A2=1/4[18 + -2 + -6 + -2]
Where h is the order (i.e., total number of symmetry operations) of the point group and
the sum is taken over all symmetry operations R of the group. These yields:
rred 6 Al + X A2 + y Bl + 4 B2
Determine x and y (8 points):
Answers 3
A1=1/4[(18 x 1 x1) + (-2 x 1 x 1) + (-6 x 1 x 1) + (-2 x 1 x 1)]
A1=1/4[18 + -2 + 6 + 2]
A1=1/4(24)
A1=6
A2=1/4[(18 x 1 x1) + (1 x -2 x 1) + (6 x 1 x -1) + (1 x 2 x -1)]
A2=1/4[18 + -2 + -6 + -2]
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

RUNNING HEAD: CHEMISTRY ASSIGNMENT
A2=1/4(8)
A2=2
B1=1/4[(18 x 1 x1) + (-1 x -2 x 1) + (6 x 1 x 1) + (-2 x -1 x 1)]
B1=1/4[18 + 2 + 6 +-2]
B1=1/4(24)
B1=6
B2=1/4[(18 x 1 x1) + (-2 x -1 x 1) + (6 x -1 x 1) + (2x 1 x 1)]
B2=1/4[18 + 2 + -6 + 2]
B2=1/4(16)
B2=4
√3N =6A1 + 2A2 + 6B1 +4B2
Hence x=2
y=6
(4) Separate vibrations from translations and rotations (6points)
Now we want to determine the symmetries of all vibrational modes, which can be achieved
by substracting translations and rotations from Tred
Answers
√3N =6A1 + 2A2 + 6B1 +4B2
rans = A1 +B1 + B2Ꞅ
A2=1/4(8)
A2=2
B1=1/4[(18 x 1 x1) + (-1 x -2 x 1) + (6 x 1 x 1) + (-2 x -1 x 1)]
B1=1/4[18 + 2 + 6 +-2]
B1=1/4(24)
B1=6
B2=1/4[(18 x 1 x1) + (-2 x -1 x 1) + (6 x -1 x 1) + (2x 1 x 1)]
B2=1/4[18 + 2 + -6 + 2]
B2=1/4(16)
B2=4
√3N =6A1 + 2A2 + 6B1 +4B2
Hence x=2
y=6
(4) Separate vibrations from translations and rotations (6points)
Now we want to determine the symmetries of all vibrational modes, which can be achieved
by substracting translations and rotations from Tred
Answers
√3N =6A1 + 2A2 + 6B1 +4B2
rans = A1 +B1 + B2Ꞅ

RUNNING HEAD: CHEMISTRY ASSIGNMENT
rot= A2 + B1 + B2Ꞅ
vib=5A1 + A2 + 4B1 + 2B2Ꞅ
(5) Determine infrared (IR) and Raman active Vibrational modes (10 point)
Use the C2vcharacter table to determine whichof the vibrational modes of C2H2F2 are
infrared (IR) active .By combining this information with your answer in question 4,Predict
how many bands you would observe intheIRspectrum of C2H2F2
Symmetry labels of IR active modes:
Answers
Vibration of the molecular is represented as
Vibration will be infrared active if its normal mode belong to one of the irreducible
representative corresponding to x,y,z coordinate in the character table
Avibration will be Raman active if there are changes in polarizability tension
Raman active on transformation represented in quadratic function eg xy,x2,z2
All the 2 vibration are
-Raman active
rot= A2 + B1 + B2Ꞅ
vib=5A1 + A2 + 4B1 + 2B2Ꞅ
(5) Determine infrared (IR) and Raman active Vibrational modes (10 point)
Use the C2vcharacter table to determine whichof the vibrational modes of C2H2F2 are
infrared (IR) active .By combining this information with your answer in question 4,Predict
how many bands you would observe intheIRspectrum of C2H2F2
Symmetry labels of IR active modes:
Answers
Vibration of the molecular is represented as
Vibration will be infrared active if its normal mode belong to one of the irreducible
representative corresponding to x,y,z coordinate in the character table
Avibration will be Raman active if there are changes in polarizability tension
Raman active on transformation represented in quadratic function eg xy,x2,z2
All the 2 vibration are
-Raman active
RUNNING HEAD: CHEMISTRY ASSIGNMENT
Infrared vibration
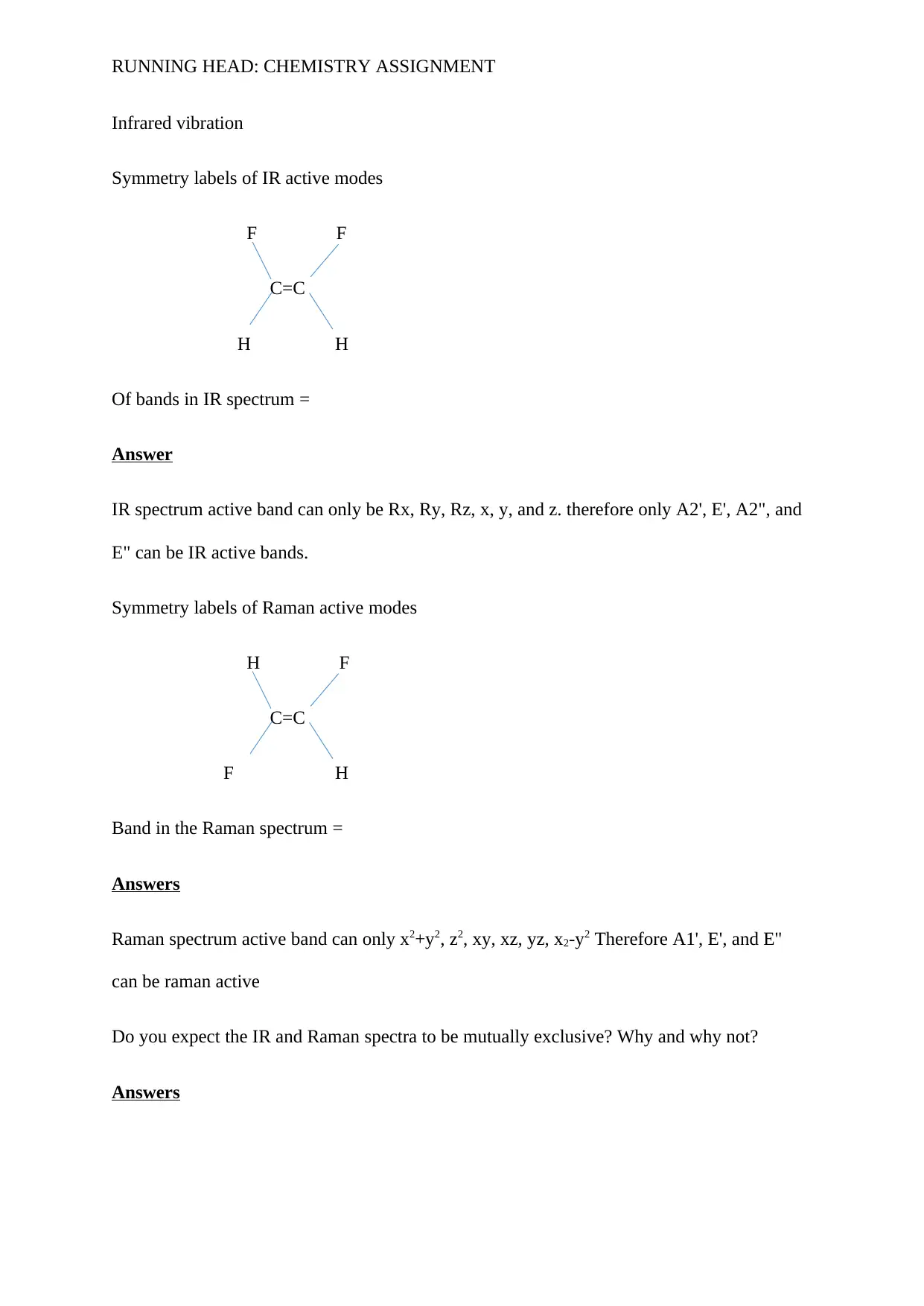
Symmetry labels of IR active modes
F F
C=C
H H
Of bands in IR spectrum =
Answer
IR spectrum active band can only be Rx, Ry, Rz, x, y, and z. therefore only A2', E', A2", and
E" can be IR active bands.
Symmetry labels of Raman active modes
H F
C=C
F H
Band in the Raman spectrum =
Answers
Raman spectrum active band can only x2+y2, z2, xy, xz, yz, x2-y2 Therefore A1', E', and E"
can be raman active
Do you expect the IR and Raman spectra to be mutually exclusive? Why and why not?
Answers
Infrared vibration
Symmetry labels of IR active modes
F F
C=C
H H
Of bands in IR spectrum =
Answer
IR spectrum active band can only be Rx, Ry, Rz, x, y, and z. therefore only A2', E', A2", and
E" can be IR active bands.
Symmetry labels of Raman active modes
H F
C=C
F H
Band in the Raman spectrum =
Answers
Raman spectrum active band can only x2+y2, z2, xy, xz, yz, x2-y2 Therefore A1', E', and E"
can be raman active
Do you expect the IR and Raman spectra to be mutually exclusive? Why and why not?
Answers
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

RUNNING HEAD: CHEMISTRY ASSIGNMENT
Raman and IR are mutually exclusive, bonds that are IR-active are not Raman-active or vice
versa. Raman spectroscopy is often c onsidered as complementary to IR spectroscopy.
(6) NMR spectroscopy (6 points)
How many signals do you expect to find in the following NMR spectra of C2H2F2?
Answers
1H NMR Spectrum =2 signals
13C NMR Spectrum =2 signals
19F NMR Spectrum = None (There are no 19 F-NMR)
Raman and IR are mutually exclusive, bonds that are IR-active are not Raman-active or vice
versa. Raman spectroscopy is often c onsidered as complementary to IR spectroscopy.
(6) NMR spectroscopy (6 points)
How many signals do you expect to find in the following NMR spectra of C2H2F2?
Answers
1H NMR Spectrum =2 signals
13C NMR Spectrum =2 signals
19F NMR Spectrum = None (There are no 19 F-NMR)

RUNNING HEAD: CHEMISTRY ASSIGNMENT
Part 3. Atomic Theory
1. (a) Write the valence shell configuration for the cobalt atom, Co (2 points).
Answers
Electronic configuration is the arrangement of electrons on the energy level.
Valence electron configuration of cobalt atom is
2:8:15:2
And energy level electron configuration is [Ar] 3d7 4s
(b) How many unpaired paired electrons does the Co atom have. What value of S does
this correspond to? What is the spin multiplicity of Co? (Remember that S = ⅀ ms and
that spin multiplicity is 2S + l.) (6 points)
Answers
Cobalt has three unpaired pair of electrons
S= 4s2
The charge and multiplicity system for cobalt is (+1, 7)
Co2+ has three unpaired electrons resulting in a spin multiplicity of 2(1/2(6) +1 = 7
for the system
For cobalt 4s orbital
(1s) 2 (2s, 2p) 8 (3s, 3p) 8 (3d) 7 (4s, 4p) 2-1
SCo = 2 x 1.00+8 x 1.00+8 x 0.85+7 x 0.85+1 x 0.35 =23.1
δ= 3.9
Part 3. Atomic Theory
1. (a) Write the valence shell configuration for the cobalt atom, Co (2 points).
Answers
Electronic configuration is the arrangement of electrons on the energy level.
Valence electron configuration of cobalt atom is
2:8:15:2
And energy level electron configuration is [Ar] 3d7 4s
(b) How many unpaired paired electrons does the Co atom have. What value of S does
this correspond to? What is the spin multiplicity of Co? (Remember that S = ⅀ ms and
that spin multiplicity is 2S + l.) (6 points)
Answers
Cobalt has three unpaired pair of electrons
S= 4s2
The charge and multiplicity system for cobalt is (+1, 7)
Co2+ has three unpaired electrons resulting in a spin multiplicity of 2(1/2(6) +1 = 7
for the system
For cobalt 4s orbital
(1s) 2 (2s, 2p) 8 (3s, 3p) 8 (3d) 7 (4s, 4p) 2-1
SCo = 2 x 1.00+8 x 1.00+8 x 0.85+7 x 0.85+1 x 0.35 =23.1
δ= 3.9

RUNNING HEAD: CHEMISTRY ASSIGNMENT
(c) Use Slater's rules to determine the shielding constant, G, and the effective nuclear
charge, Z*, for the outermost 4s and 3d electrons in the Co atom. Estimate the
energies of these orbitals (12 points).
Answers
Z¿ = 23.1
Energy of cobalt 4s orbital is 760.4Kj
For cobalt 3d orbital
SCo = 2 x 1.00+8 x 1.00+8 x 0.85+7 x 0.85=22.75
δ= 3.9
Z¿ = 27
Energy of cobalt 3d orbital is 748.48kJ
2. Write the valence shell configuration for the C03+ ion and rationalize this
configuration in terms of effective nuclear charge and the relative energies of the
valence orbitals (2 points).
The valence shell configuration for cobalt (III) ion, (Co3+¿ ¿is [Ar] 4s3d5
The relative energy is 754kJ
(c) Use Slater's rules to determine the shielding constant, G, and the effective nuclear
charge, Z*, for the outermost 4s and 3d electrons in the Co atom. Estimate the
energies of these orbitals (12 points).
Answers
Z¿ = 23.1
Energy of cobalt 4s orbital is 760.4Kj
For cobalt 3d orbital
SCo = 2 x 1.00+8 x 1.00+8 x 0.85+7 x 0.85=22.75
δ= 3.9
Z¿ = 27
Energy of cobalt 3d orbital is 748.48kJ
2. Write the valence shell configuration for the C03+ ion and rationalize this
configuration in terms of effective nuclear charge and the relative energies of the
valence orbitals (2 points).
The valence shell configuration for cobalt (III) ion, (Co3+¿ ¿is [Ar] 4s3d5
The relative energy is 754kJ
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

RUNNING HEAD: CHEMISTRY ASSIGNMENT
Part 4. Molecular Orbital Theory
1. The two diatomic molecules HF and HCL have remarkably different chemical
properties that may be explained by differences in bonding
(A) Using a common energy scale provided below, draw molecular orbital diagrams for
the HF and HCL molecules. Fill in the appropriate number of electrons in your
diagram and labelled all the orbitals (6 points)
Answers
Find the attached pictures of diagrams
(B) HF is a much weaker acid than HCL .Use your understanding of orbital overlap and
bonding principles to explain this differences. Be sure to make references to your MO
diagrams in your answer (2 points)
Answers
The reason;
It’s because of strong affinity of Fluorine for Hydrogen so it strongly holds Hydrogen
and don’t lose it easily. As in HCl due to lesser H-Cl bond dissociation enthalpy as
compared to HF thus HCl is a stronger acid than HF. The bonding integral of 1s and a
2p orbital of Fluorine is larger and so the sigma bond on the HF is stronger whereas
between 1s and 3p the bond gets weaker as the bonding integral is smaller for HCl.
This makes HF weaker compared to HCl.
2. Consider the linear triatomic molecules HeH2 and BeH2 ,having either He or Be as
the central atom.HeH2 is not stable but BeH2 is stable. If we compare the bonding
properties of the two molecules using molecular orbital theory. Then it should be
possible to explain the difference in their stability.
Part 4. Molecular Orbital Theory
1. The two diatomic molecules HF and HCL have remarkably different chemical
properties that may be explained by differences in bonding
(A) Using a common energy scale provided below, draw molecular orbital diagrams for
the HF and HCL molecules. Fill in the appropriate number of electrons in your
diagram and labelled all the orbitals (6 points)
Answers
Find the attached pictures of diagrams
(B) HF is a much weaker acid than HCL .Use your understanding of orbital overlap and
bonding principles to explain this differences. Be sure to make references to your MO
diagrams in your answer (2 points)
Answers
The reason;
It’s because of strong affinity of Fluorine for Hydrogen so it strongly holds Hydrogen
and don’t lose it easily. As in HCl due to lesser H-Cl bond dissociation enthalpy as
compared to HF thus HCl is a stronger acid than HF. The bonding integral of 1s and a
2p orbital of Fluorine is larger and so the sigma bond on the HF is stronger whereas
between 1s and 3p the bond gets weaker as the bonding integral is smaller for HCl.
This makes HF weaker compared to HCl.
2. Consider the linear triatomic molecules HeH2 and BeH2 ,having either He or Be as
the central atom.HeH2 is not stable but BeH2 is stable. If we compare the bonding
properties of the two molecules using molecular orbital theory. Then it should be
possible to explain the difference in their stability.

RUNNING HEAD: CHEMISTRY ASSIGNMENT
(A) The Hydrogen group orbitals (SALCs) will be the same for both molecules.Draw the
complete set of SALCs based on the H atom and label their symmetry based on the
D4h point group (4 points)
Answer
Find the attached pictures of diagrams
(B) List the valence orbitals of He and Be along with symmetries in D4h.Which ones can
nteract with the hydrogen SALCs? (4points)
Answers
He……………1S2
Single electron is found in a 1s orbital. It is also the only valence orbital.
He has only one valance orbital
D4H symmetries…………..A1g
Hydrogen SALC can interact with He of the appropriate symmetry
Be…………….1S2 2S2 meaning it has two valency orbital
D4H symmetries…………..A1g, B1g and Eu
Hydrogen SALC can interact with Be AOs of the appropriate symmetry to form
LCAO-MOs.
(C) Using the common energy scale below, draw molecular orbital diagrams
Answers
Find the attached pictures of diagrams
(D) Use the diagrams that you construct in part (C) to explain why BeH2 is stable but
HeH2 is not (2 points)
(A) The Hydrogen group orbitals (SALCs) will be the same for both molecules.Draw the
complete set of SALCs based on the H atom and label their symmetry based on the
D4h point group (4 points)
Answer
Find the attached pictures of diagrams
(B) List the valence orbitals of He and Be along with symmetries in D4h.Which ones can
nteract with the hydrogen SALCs? (4points)
Answers
He……………1S2
Single electron is found in a 1s orbital. It is also the only valence orbital.
He has only one valance orbital
D4H symmetries…………..A1g
Hydrogen SALC can interact with He of the appropriate symmetry
Be…………….1S2 2S2 meaning it has two valency orbital
D4H symmetries…………..A1g, B1g and Eu
Hydrogen SALC can interact with Be AOs of the appropriate symmetry to form
LCAO-MOs.
(C) Using the common energy scale below, draw molecular orbital diagrams
Answers
Find the attached pictures of diagrams
(D) Use the diagrams that you construct in part (C) to explain why BeH2 is stable but
HeH2 is not (2 points)

RUNNING HEAD: CHEMISTRY ASSIGNMENT
Answers
BeH2 is stable because it contains symmetrical distribution of electrons compared to
HeH2 whose orbital have maximum number of exchanges.
(E) Can BeH2 be a bent molecule? Bent Be H2 would belong to the C2v point group.
Derive an MOdiagram for C2v BeH2 compare it to Dch diagram. Which orbital
become stabilized/destabilized upon bending, and how might these changes contribute
to the total Energy? (6 points)
Answers
No BeH2 is linear molecules .Beh² molecule Walsh diagram is shown below.
Consider (2og) orbital which is constructed from atomic wave function that are
everywhere positive and hence on bending there will be increase in overlap since the
two H- atoms wave function will overlap to slightly greater extent. The energy is 2°g
is lowered somewhat. It is now labelled as 2a¹. In contrast the energy of the increases
in bending. This is because the sign of wave function changes of overlap and H-atoms
will be to lesser extent .Electronic configuration of BeH² in linear form (2óg)², (1óu)²
whereas in bent form it is (2a1)², (1b2)². Since1b2 loses more energy than 2a1 gains;
BeH2 is linear, not bent.
Answers
BeH2 is stable because it contains symmetrical distribution of electrons compared to
HeH2 whose orbital have maximum number of exchanges.
(E) Can BeH2 be a bent molecule? Bent Be H2 would belong to the C2v point group.
Derive an MOdiagram for C2v BeH2 compare it to Dch diagram. Which orbital
become stabilized/destabilized upon bending, and how might these changes contribute
to the total Energy? (6 points)
Answers
No BeH2 is linear molecules .Beh² molecule Walsh diagram is shown below.
Consider (2og) orbital which is constructed from atomic wave function that are
everywhere positive and hence on bending there will be increase in overlap since the
two H- atoms wave function will overlap to slightly greater extent. The energy is 2°g
is lowered somewhat. It is now labelled as 2a¹. In contrast the energy of the increases
in bending. This is because the sign of wave function changes of overlap and H-atoms
will be to lesser extent .Electronic configuration of BeH² in linear form (2óg)², (1óu)²
whereas in bent form it is (2a1)², (1b2)². Since1b2 loses more energy than 2a1 gains;
BeH2 is linear, not bent.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

RUNNING HEAD: CHEMISTRY ASSIGNMENT
Vibrational Spectroscopy: CHEM 511 – Inorganic Chemistry – spring 2020
Problem Set
1. In nuclear magnetic resonance (NMR) spectroscopy, all atoms in a molecule that are
related to each other by symmetry show only one distinct NMR signal at the same chemical
shift.
(a) Fullerenes. This is an amazing class of carbon compounds that have geodesic shapes.
Below is a depiction of the structures of several fullerenes, with their point group assignment.
Predict how many chemically distinct 13C signals each compound should display in its 13C
NMR spectrum.
(b) Are any of these molecules chiral? If so, explain why.
Answers For (a) and (b)
C60 (ln) Buckminsterfullerene
Give one signal in the NMR spectrum.
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C70 (D5h) Fullerene
Five NMR peakswere predicted
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C76(D2) Fullerene
Vibrational Spectroscopy: CHEM 511 – Inorganic Chemistry – spring 2020
Problem Set
1. In nuclear magnetic resonance (NMR) spectroscopy, all atoms in a molecule that are
related to each other by symmetry show only one distinct NMR signal at the same chemical
shift.
(a) Fullerenes. This is an amazing class of carbon compounds that have geodesic shapes.
Below is a depiction of the structures of several fullerenes, with their point group assignment.
Predict how many chemically distinct 13C signals each compound should display in its 13C
NMR spectrum.
(b) Are any of these molecules chiral? If so, explain why.
Answers For (a) and (b)
C60 (ln) Buckminsterfullerene
Give one signal in the NMR spectrum.
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C70 (D5h) Fullerene
Five NMR peakswere predicted
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C76(D2) Fullerene

RUNNING HEAD: CHEMISTRY ASSIGNMENT
19 NMR peaks were reported with similar strength, experimentally. The 19 peaks form five
classes from downfield to upfield, including 1, 6, 8, 3 and 1 peaks. Those five groups and the
spectral period were well reproduced in the NMR spectrum predicted
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C78:1 (D3)
Has 13 NMR peaks of the same intensity, which can be divided into four groups with 2, 2, 7
and 2 peaks from down to upfield.
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C78:2(C2v)
Has 21 NMR peaks of thethe ratio of full-intensity peaks: half-intensity peaks is 18:3
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C78:3(C2v)
Has 22 NMR peaks of thethe ratio of full-intensity peaks: half-intensity peaks is 17:5
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable Molecular is a chiral because it has IPR satisfying structure
C80:1(D5d)
Shows three full-intensity peaks and two half-intensity peaks.The molecular is chiral because
from the asymmetric the mirror image of the structure and structure are not superimposable
C80:2(D2)
19 NMR peaks were reported with similar strength, experimentally. The 19 peaks form five
classes from downfield to upfield, including 1, 6, 8, 3 and 1 peaks. Those five groups and the
spectral period were well reproduced in the NMR spectrum predicted
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C78:1 (D3)
Has 13 NMR peaks of the same intensity, which can be divided into four groups with 2, 2, 7
and 2 peaks from down to upfield.
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C78:2(C2v)
Has 21 NMR peaks of thethe ratio of full-intensity peaks: half-intensity peaks is 18:3
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C78:3(C2v)
Has 22 NMR peaks of thethe ratio of full-intensity peaks: half-intensity peaks is 17:5
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable Molecular is a chiral because it has IPR satisfying structure
C80:1(D5d)
Shows three full-intensity peaks and two half-intensity peaks.The molecular is chiral because
from the asymmetric the mirror image of the structure and structure are not superimposable
C80:2(D2)

RUNNING HEAD: CHEMISTRY ASSIGNMENT
Shows three full-intensity peaks and two half-intensity peaks.The molecular is chiral because
from the asymmetric the mirror image of the structure and structure are not superimposable
C82:3(C2)
Symmetry was not determinable
41-resonance intensity of chemical shift
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C84 22(D2)
Cannot be determined.The molecular is chiral because from the asymmetric the mirror image
of the structure and structure are not superimposable
C84:23(D2d)
Cannot be determined .The molecular is chiral because from the asymmetric the mirror image
of the structure and structure are not superimposable
2. Vibrational Spectroscopy: Consider the carbonate ion, (CO3)2–. Consider the carbonate ion,
(CO3)2–.
Shows three full-intensity peaks and two half-intensity peaks.The molecular is chiral because
from the asymmetric the mirror image of the structure and structure are not superimposable
C82:3(C2)
Symmetry was not determinable
41-resonance intensity of chemical shift
The molecular is chiral because from the asymmetric the mirror image of the structure and
structure are not superimposable
C84 22(D2)
Cannot be determined.The molecular is chiral because from the asymmetric the mirror image
of the structure and structure are not superimposable
C84:23(D2d)
Cannot be determined .The molecular is chiral because from the asymmetric the mirror image
of the structure and structure are not superimposable
2. Vibrational Spectroscopy: Consider the carbonate ion, (CO3)2–. Consider the carbonate ion,
(CO3)2–.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

RUNNING HEAD: CHEMISTRY ASSIGNMENT
(a) How many degrees of freedom does the (CO3)2– ion have?
Answer
(CO3)2– ion is non-linear molecule degree of freedom of non-linear molecules
is given by
3N-6
Where N is a number of atoms
3(4)-6=6
Degrees of freedom does the (CO3)2– = 6
(b) Use all the degrees of freedom of the (CO3)2– ion as the basis for a
representation (ΓDOF) of the D3h point group (I’ve filled in one of
the characters for you – think carefully about how I determined that
value!):
Answer
E = 12
2C3 = -1
(a) How many degrees of freedom does the (CO3)2– ion have?
Answer
(CO3)2– ion is non-linear molecule degree of freedom of non-linear molecules
is given by
3N-6
Where N is a number of atoms
3(4)-6=6
Degrees of freedom does the (CO3)2– = 6
(b) Use all the degrees of freedom of the (CO3)2– ion as the basis for a
representation (ΓDOF) of the D3h point group (I’ve filled in one of
the characters for you – think carefully about how I determined that
value!):
Answer
E = 12
2C3 = -1

RUNNING HEAD: CHEMISTRY ASSIGNMENT
3C2 =-2
σh =4
2S3 =-1
3σv =+2
(c) Reduce ΓDOF into its component normal modes (irreducible representations).
Answer
4E', A1'', 3A''
(d) Identify which normal modes correspond to translations, rotations,
and vibrations of the molecule. Which vibrations are IR active, and
which are Raman active?
Answers
transꞄ 2E1
rot -Ꞅ A1, 2E1
vibꞄ Aσ1 + E1
(e) Use the three C–O vectors of the (CO3)2– ion as a representation of
D3h and identify the IR and Raman active C–O stretching modes of the
ion.
Answer
3C2 =-2
σh =4
2S3 =-1
3σv =+2
(c) Reduce ΓDOF into its component normal modes (irreducible representations).
Answer
4E', A1'', 3A''
(d) Identify which normal modes correspond to translations, rotations,
and vibrations of the molecule. Which vibrations are IR active, and
which are Raman active?
Answers
transꞄ 2E1
rot -Ꞅ A1, 2E1
vibꞄ Aσ1 + E1
(e) Use the three C–O vectors of the (CO3)2– ion as a representation of
D3h and identify the IR and Raman active C–O stretching modes of the
ion.
Answer

RUNNING HEAD: CHEMISTRY ASSIGNMENT
Irreducible representation of C-O bond
C3v E C3 3σv
┌ 4 1 2
A1 1 1 1 z
Rx
(x,y),(Rx,Ry)
X2 + y2 ,z2
(x2-y2,xy),(xz,yz)A2 1 1 -1
E 2 1 0
2A 1 + B 1
IR active ( x,y,z): A1 and B1 (3 peaks predicted) and Three v(CO)
absorption are expected for A
Raman active (quadratic): A1 and B1 (3 peaks predicted) Three v (CO)
absorption are expected for A
(f) Is it possible to distinguish between the planar D3h structure of
carbonate and a hypothetical pyramidal (C3v) structure by examining the
C–O stretching region of the IR and Raman spectrum of carbonate?
Answer
Yes, is it possible to distinguish between the planar D3h structure of
carbonate and a hypothetical pyramidal (C3v) structure by examining the
C–O stretching region of the IR and Raman spectrum of carbonate?
3. Give the characteristic valence shell electron configuration for the following
periodic groups (e.g., ns1 for the alkali metals).
(a) Group 16 (O family)
Irreducible representation of C-O bond
C3v E C3 3σv
┌ 4 1 2
A1 1 1 1 z
Rx
(x,y),(Rx,Ry)
X2 + y2 ,z2
(x2-y2,xy),(xz,yz)A2 1 1 -1
E 2 1 0
2A 1 + B 1
IR active ( x,y,z): A1 and B1 (3 peaks predicted) and Three v(CO)
absorption are expected for A
Raman active (quadratic): A1 and B1 (3 peaks predicted) Three v (CO)
absorption are expected for A
(f) Is it possible to distinguish between the planar D3h structure of
carbonate and a hypothetical pyramidal (C3v) structure by examining the
C–O stretching region of the IR and Raman spectrum of carbonate?
Answer
Yes, is it possible to distinguish between the planar D3h structure of
carbonate and a hypothetical pyramidal (C3v) structure by examining the
C–O stretching region of the IR and Raman spectrum of carbonate?
3. Give the characteristic valence shell electron configuration for the following
periodic groups (e.g., ns1 for the alkali metals).
(a) Group 16 (O family)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

RUNNING HEAD: CHEMISTRY ASSIGNMENT
ns2np4
(b) Halogens (F family)
ns2np5
(c) Group 14 (C family)
ns2np2
5. Write the electron configuration including the appropriate noble gas core
(e.g., [He]2s22p5 for F) for P, Fe, Ga, Rh, Pr, and I.
Answers
P = [Ne] 3s23p3
Fe = [Ar] 3d64s24po
Ga= [Ar] 3d104s24p1
Rh = [Kr] 4d85s1
Pr = [Xe] 4f36s2
I = [Kr] 4d105s25p5
ns2np4
(b) Halogens (F family)
ns2np5
(c) Group 14 (C family)
ns2np2
5. Write the electron configuration including the appropriate noble gas core
(e.g., [He]2s22p5 for F) for P, Fe, Ga, Rh, Pr, and I.
Answers
P = [Ne] 3s23p3
Fe = [Ar] 3d64s24po
Ga= [Ar] 3d104s24p1
Rh = [Kr] 4d85s1
Pr = [Xe] 4f36s2
I = [Kr] 4d105s25p5

RUNNING HEAD: CHEMISTRY ASSIGNMENT
6. Write the electron configuration beyond the noble gas core, and give the number of
unpaired electrons for Ce3+, Fe3+, Rh3+, Cr2+, Mo2+, Ge2+, S2–, Tm3+, Sc3+, Al3+, and
Sm2+.
Answers
1. Ce3+: [Xe] 4f15d16s2 has 3 unpaired electrons.
2. Fe3+: [Ar] 3d64S2 has 3 unpaired electrons.
3. Rh3+: [Kr] 4d6 has3 unpaired electrons.
4. Cr2+: [Ar] 3d6 has 2 unpaired electrons.
5. Mo2+: [Kr] 4d3 has 2 unpaired electrons.
6. Ge2+: [Ar] 4s23d10 has 2 unpaired electrons.
7. S2- : [Ne] 3s23p4 has 2 unpaired electrons
8. Tm3+: [Xe] 4f13 6s2 has 3 unpaired electrons.
9. Sc3+: [Ar] 3d14s2 has 3 unpaired electrons.
10. Al3+: [He] 2s22p6 has 3 unpaired electrons.
11. Sm2+: [Xe] 4f6 has 2 unpaired electrons.
7. Use Slater’s rules to calculate Z* for the outermost electrons in following
series of atoms:
(a) Mn,
6. Write the electron configuration beyond the noble gas core, and give the number of
unpaired electrons for Ce3+, Fe3+, Rh3+, Cr2+, Mo2+, Ge2+, S2–, Tm3+, Sc3+, Al3+, and
Sm2+.
Answers
1. Ce3+: [Xe] 4f15d16s2 has 3 unpaired electrons.
2. Fe3+: [Ar] 3d64S2 has 3 unpaired electrons.
3. Rh3+: [Kr] 4d6 has3 unpaired electrons.
4. Cr2+: [Ar] 3d6 has 2 unpaired electrons.
5. Mo2+: [Kr] 4d3 has 2 unpaired electrons.
6. Ge2+: [Ar] 4s23d10 has 2 unpaired electrons.
7. S2- : [Ne] 3s23p4 has 2 unpaired electrons
8. Tm3+: [Xe] 4f13 6s2 has 3 unpaired electrons.
9. Sc3+: [Ar] 3d14s2 has 3 unpaired electrons.
10. Al3+: [He] 2s22p6 has 3 unpaired electrons.
11. Sm2+: [Xe] 4f6 has 2 unpaired electrons.
7. Use Slater’s rules to calculate Z* for the outermost electrons in following
series of atoms:
(a) Mn,

RUNNING HEAD: CHEMISTRY ASSIGNMENT
1s22s22p63s23p63d54s2
Zeff: 3.6
Fe
(1s)2 (2s,2p)8 (3s,3p)8 (3d)6 (4s,4p)2-1
SFe = 2 x 1.00+8 x 1.00+8 x 0.85+6 x 0.85+1 x 0.35 =22.25
Zeff = 26-22.25= 3.75
Co,
(1s)2 (2s,2p)8 (3s,3p)8 (3d)7 (4s,4p)2-1
SCo = 2 x 1.00+8 x 1.00+8 x 0.85+7 x 0.85+1 x 0.35 =23.1
Zeff = 27-23.1 = 3.9
Ni
(1s)2 (2s,2p)8 (3s,3p)8 (3d)8 (4s,4p)2-1
SNi = 2 x 1.00+8 x 1.00+8 x 0.85+8 x 0.85+1 x 0.35 =23.95
Zeff = 28-23.95 = 4.05
(b) Cr,
Zeff(Cr ,3d)=3.2522
Mo
Zeff(Mo ,4d)=2.8481
W
1s22s22p63s23p63d54s2
Zeff: 3.6
Fe
(1s)2 (2s,2p)8 (3s,3p)8 (3d)6 (4s,4p)2-1
SFe = 2 x 1.00+8 x 1.00+8 x 0.85+6 x 0.85+1 x 0.35 =22.25
Zeff = 26-22.25= 3.75
Co,
(1s)2 (2s,2p)8 (3s,3p)8 (3d)7 (4s,4p)2-1
SCo = 2 x 1.00+8 x 1.00+8 x 0.85+7 x 0.85+1 x 0.35 =23.1
Zeff = 27-23.1 = 3.9
Ni
(1s)2 (2s,2p)8 (3s,3p)8 (3d)8 (4s,4p)2-1
SNi = 2 x 1.00+8 x 1.00+8 x 0.85+8 x 0.85+1 x 0.35 =23.95
Zeff = 28-23.95 = 4.05
(b) Cr,
Zeff(Cr ,3d)=3.2522
Mo
Zeff(Mo ,4d)=2.8481
W
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

RUNNING HEAD: CHEMISTRY ASSIGNMENT
1s22s22p63s23p63d104s24p64d105s25p64f145d46s2
Zeff: 40.85
2
1s22s22p63s23p63d104s24p64d105s25p64f145d46s2
Zeff: 40.85
2
1 out of 23
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.




