Detailed Analysis of Tungstic Acid: Properties and Chemical Reactions
VerifiedAdded on 2020/05/08
|19
|4905
|257
Report
AI Summary
This report provides a comprehensive overview of Tungstic Acid, a yellow, fine-grained powder with a high degree of chemical purity. The report delves into its chemical formula (H2WO4), physical properties such as density, melting and boiling points, and its existence in both hydrated and anhydrous forms. It explores the acid's preparation methods, including reactions with strong acids, sodium tungstate, and hydrogen peroxide. Furthermore, the report details the uses of Tungstic Acid, particularly in textile industries as a dye and mordant. The chemical properties section discusses its catalytic roles in the epoxidation of alkenes, hydroxylation of olefins, and oxidation of sulfides. The report also covers the acid's role in various reactions, including the preparation of threo-1,2-glycols and the purification process. This report provides an in-depth understanding of Tungstic Acid, including its synthesis, properties, and application in various fields.

Running head: TUNGSTIC ACID 0
TUNGSTIC ACID
Name of Student
Institution Affiliation
TUNGSTIC ACID
Name of Student
Institution Affiliation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

TUNGSTIC ACID 2
Introduction
A Tungstic acid is an acid from a highly grained (fined grained) which is yellow in color
and contains an awfully narrow grained size distribution (Yarwood, 2010). This acid always has
a very high degree of the chemical purity of about 99 %. But this acid is highly reactive and is
even morphology. Because of these distinctive nature of this acid, highly sensitive, special
applications are possible areas like uniform fine chemistry, catalysis and the preparation of this
tungstic metal of a given high quality and organometallic compounds of the Tungstic acid
(Douthwaite, 2014). The figure below shows the powder which can be employed to manufacture
or prepare the Tungstic Acid.
Fig 1: Shows a powder of Tungstic employed in preparation of the Tungstic Acid (Zarina,
M.2011)
This Acid is also referred to as Tungstic (VI) Acid, Dihydrogen Wolframate tungsten. It
has a chemical formula of H2WO4 or sometimes can be written as H2O4W. This acid always
exists in a powder form and has a density of 5.5 g/mL at a temperature of the room which is 250
C (Jamwalo, 2013). This Acid referred to as hydrated forms of the tungstic trioxide WO3. The
modest form, the monohydrate is WO3.H2O, The solid structure (powder) of WO3. H2O entail of
layers of octahedrally coordinated WO5 (H2O) units which has four vertices are mutual or simply
Introduction
A Tungstic acid is an acid from a highly grained (fined grained) which is yellow in color
and contains an awfully narrow grained size distribution (Yarwood, 2010). This acid always has
a very high degree of the chemical purity of about 99 %. But this acid is highly reactive and is
even morphology. Because of these distinctive nature of this acid, highly sensitive, special
applications are possible areas like uniform fine chemistry, catalysis and the preparation of this
tungstic metal of a given high quality and organometallic compounds of the Tungstic acid
(Douthwaite, 2014). The figure below shows the powder which can be employed to manufacture
or prepare the Tungstic Acid.
Fig 1: Shows a powder of Tungstic employed in preparation of the Tungstic Acid (Zarina,
M.2011)
This Acid is also referred to as Tungstic (VI) Acid, Dihydrogen Wolframate tungsten. It
has a chemical formula of H2WO4 or sometimes can be written as H2O4W. This acid always
exists in a powder form and has a density of 5.5 g/mL at a temperature of the room which is 250
C (Jamwalo, 2013). This Acid referred to as hydrated forms of the tungstic trioxide WO3. The
modest form, the monohydrate is WO3.H2O, The solid structure (powder) of WO3. H2O entail of
layers of octahedrally coordinated WO5 (H2O) units which has four vertices are mutual or simply

TUNGSTIC ACID 3
shared. And the dihydrate contains the same layers structure with the extra water (H2O)
molecule inserted in between the layers (Enerst, 2015). The monohydrate is a yellow powder too
but it is not soluble in water.
This acid can be classically referred to as `` Acid of Wolfram. `` This acid has a
molecular mass of 249.85 g/mol, it is not soluble in water but highly soluble in ammonia and
slightly soluble in ethanol. It has a density of 5.59g/cm3, it has a melting point of 1000C and
boiling point of 14730C. Tungstic acid has a hydrogen Bond Donor of the count of four and a
hydrogen Bond Accept Count of two (Davidson G., 2015). This is a weak acid and its pH value
ranges between 4 to 5, and it has a molecular formula structure as below;
Fig 2: Showing a molecular structure of Tungstic Acid (Ebsworth, 2012)
To guarantee a justifiable amount of the raw material of this acid, a secondary source can also
be employed as a secondary material (Ebsworth, 2012). In a highly erudite recycling process, a
new highly pure tungstic acid yields from the materials which were used. There is also a chance
for recycling the products which can be done by either reprocessing tungstic products or by
buying recycled materials.
Tungstic Acid is a highly vague word used to refers to several solid prepared from the W
(VI) solution with very strong Acids. Notwithstanding an extensive literature, there is obvious
shared. And the dihydrate contains the same layers structure with the extra water (H2O)
molecule inserted in between the layers (Enerst, 2015). The monohydrate is a yellow powder too
but it is not soluble in water.
This acid can be classically referred to as `` Acid of Wolfram. `` This acid has a
molecular mass of 249.85 g/mol, it is not soluble in water but highly soluble in ammonia and
slightly soluble in ethanol. It has a density of 5.59g/cm3, it has a melting point of 1000C and
boiling point of 14730C. Tungstic acid has a hydrogen Bond Donor of the count of four and a
hydrogen Bond Accept Count of two (Davidson G., 2015). This is a weak acid and its pH value
ranges between 4 to 5, and it has a molecular formula structure as below;
Fig 2: Showing a molecular structure of Tungstic Acid (Ebsworth, 2012)
To guarantee a justifiable amount of the raw material of this acid, a secondary source can also
be employed as a secondary material (Ebsworth, 2012). In a highly erudite recycling process, a
new highly pure tungstic acid yields from the materials which were used. There is also a chance
for recycling the products which can be done by either reprocessing tungstic products or by
buying recycled materials.
Tungstic Acid is a highly vague word used to refers to several solid prepared from the W
(VI) solution with very strong Acids. Notwithstanding an extensive literature, there is obvious
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

TUNGSTIC ACID 4
uncertainty as to the identity and chemical nature of this substance (Adams, 2010). The two
compound H2WO4. H2O and H2WO4 have been categorized by x- rays diffraction and designated
as acids and a similar precipitation can be identified as WO3. A third substance WO3/H2W2O7
can be got using H2WO4 with an alkaline solution. Limiting condition can be set for precipitation
of a given compound are not determined and also the designation of the acid can be based on the
thermal stability. For the soluble W(Vi) negative charges, it only the negative charges of
Metangustate is adequately steady at a pH which is very low hence can be isolated as a free acid
(Chenglong, 2017). The acid reactions of the precipitation of tungstic acid can hence be
observed as a superficial occurrence.
Preparation of the Tungstic Acid
This acid can be obtained through the action of a strong acid on solutions of metallic
tungstate, this acid can also be prepared from the reaction which occurs between Sodium
tungstate and hydrogen carbonate (Office, 2012). Tungstic Acid can be obtained also from a pure
tungstic through a reaction between this tungstic and hydrogen peroxide. This acid is one of the
simplest one to prepare since it is only prepared in the simple three ways as seen above.
Uses
Tungstic Acid is majorly employed as a dye in so many textile industries and it can as well be
used as a mordant.
Properties of the Tungstic acid
The properties of Tungstic Acid can be discussed under three basic properties, these can
be in
uncertainty as to the identity and chemical nature of this substance (Adams, 2010). The two
compound H2WO4. H2O and H2WO4 have been categorized by x- rays diffraction and designated
as acids and a similar precipitation can be identified as WO3. A third substance WO3/H2W2O7
can be got using H2WO4 with an alkaline solution. Limiting condition can be set for precipitation
of a given compound are not determined and also the designation of the acid can be based on the
thermal stability. For the soluble W(Vi) negative charges, it only the negative charges of
Metangustate is adequately steady at a pH which is very low hence can be isolated as a free acid
(Chenglong, 2017). The acid reactions of the precipitation of tungstic acid can hence be
observed as a superficial occurrence.
Preparation of the Tungstic Acid
This acid can be obtained through the action of a strong acid on solutions of metallic
tungstate, this acid can also be prepared from the reaction which occurs between Sodium
tungstate and hydrogen carbonate (Office, 2012). Tungstic Acid can be obtained also from a pure
tungstic through a reaction between this tungstic and hydrogen peroxide. This acid is one of the
simplest one to prepare since it is only prepared in the simple three ways as seen above.
Uses
Tungstic Acid is majorly employed as a dye in so many textile industries and it can as well be
used as a mordant.
Properties of the Tungstic acid
The properties of Tungstic Acid can be discussed under three basic properties, these can
be in
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

TUNGSTIC ACID 5
i. Physical properties
ii. Chemical properties
I. Physical Properties
A tungstic acid is a yellow fined grained powder having a monohydrate (one molecule of
water), as seen above, this acid has a density of 5.5 g/mL at a temperature of the room which is
250 C. It has a molecular mass of 249.85 g/mol (Voet, 2011). it has a melting point of 1000C and
boiling point of 14730C. This acid has layers of octahedrally coordinated WO5 (H2O) units which
has four vertices. Tungstic acid is an odorless powder. This acid is almost insoluble in many
acids like sulfuric acid, nitric acid, hydrobromic acid, hydrochloric acid, dilute hydrogen
periodate, it is slightly soluble in in hydrofluoric acid and it is highly soluble in ammonia and
other alkali solutions like sodium hydroxide, calcium hydroxide (Zarina, 2011).
This acid is obtained from wolframite alkali fusion after hydrochloric decomposition of the
system and it can also be obtained in the three ways discussed above, but the main source of this
acid is from the Tungsten metal (James, 2015). Tungstic acid exists in amber, crystal ramps
which can be grained into very fine grains. There is also another form of this acid where it can
be in a white powder having two molecules of water (dehydrate) with a formula of WO3. 2H2O.
both the yellow and this white acid can produce the same salt series (Lu, 2012). The first acid to
appear during the preparation is the true acid of tungstic acid, H2WO4 (the yellow acid) while the
second one is the hydrate having the formula H2WO4.H2O. But both are not soluble in water
(Veenas, 2015). The white H2WO4. H2O is prepared by the decomposing the oxychlorides or the
tungsten pentachloride in the availability of the moist air (Bhowmik, 2014). But both the yellow
i. Physical properties
ii. Chemical properties
I. Physical Properties
A tungstic acid is a yellow fined grained powder having a monohydrate (one molecule of
water), as seen above, this acid has a density of 5.5 g/mL at a temperature of the room which is
250 C. It has a molecular mass of 249.85 g/mol (Voet, 2011). it has a melting point of 1000C and
boiling point of 14730C. This acid has layers of octahedrally coordinated WO5 (H2O) units which
has four vertices. Tungstic acid is an odorless powder. This acid is almost insoluble in many
acids like sulfuric acid, nitric acid, hydrobromic acid, hydrochloric acid, dilute hydrogen
periodate, it is slightly soluble in in hydrofluoric acid and it is highly soluble in ammonia and
other alkali solutions like sodium hydroxide, calcium hydroxide (Zarina, 2011).
This acid is obtained from wolframite alkali fusion after hydrochloric decomposition of the
system and it can also be obtained in the three ways discussed above, but the main source of this
acid is from the Tungsten metal (James, 2015). Tungstic acid exists in amber, crystal ramps
which can be grained into very fine grains. There is also another form of this acid where it can
be in a white powder having two molecules of water (dehydrate) with a formula of WO3. 2H2O.
both the yellow and this white acid can produce the same salt series (Lu, 2012). The first acid to
appear during the preparation is the true acid of tungstic acid, H2WO4 (the yellow acid) while the
second one is the hydrate having the formula H2WO4.H2O. But both are not soluble in water
(Veenas, 2015). The white H2WO4. H2O is prepared by the decomposing the oxychlorides or the
tungsten pentachloride in the availability of the moist air (Bhowmik, 2014). But both the yellow

TUNGSTIC ACID 6
and the white have a melting point of 1000C to about 1100C where they lose water and left
behind the composition of WO3. H2O the left residue does not appear to be a true hydrate on a
continued heating WO3 is obtained After that heating the results indicate that between the
dehydrate, WO3. 2H2O, and the anhydrous substance just one definite hydrate, WO3 .H2O will
exist. And this result of the physical property of melting point can be highly supported by the
help of x- rays examination (Edward, 2014). And both will exhibit a crystalline form when they
contain the water molecules but powder form when the water of crystallization is evaporated
away. And that why this yellow acid exists in powder form after evaporating its water molecule.
II. Chemical properties
Chemical properties of this tungstic acid simply deals with the how this acid is able to react with
other substances, these reactions (chemical reactions) are discussed below;
Epoxidation of Alkenes
Tungstic acid can be employed to catalyze the oxidation by peroxide of the organic
compound (alkane) to the conforming epoxides (Freedman, 2013). This acid is a fairly strong
acid the mixture reaction needs a buffering to avoid any subsequent formation of diol. The
archetypal condition of this reaction includes the following: tungstic acid at 1 mol and 1 %,
sodium acetate 2-3 mol at a pH of 4.5, hydrogen peroxide of 1.5 mol. Under the above typical
conditions, the epoxidation takes place with a full retaining of configuration for both Tran- and
cis-alkenes the reaction will increase with nucleophilicity of the double bond (Tallon, 2011).
The coordination to the catalyst by the hydroxyl group in the substrate also enriches the
epoxidation rate.
and the white have a melting point of 1000C to about 1100C where they lose water and left
behind the composition of WO3. H2O the left residue does not appear to be a true hydrate on a
continued heating WO3 is obtained After that heating the results indicate that between the
dehydrate, WO3. 2H2O, and the anhydrous substance just one definite hydrate, WO3 .H2O will
exist. And this result of the physical property of melting point can be highly supported by the
help of x- rays examination (Edward, 2014). And both will exhibit a crystalline form when they
contain the water molecules but powder form when the water of crystallization is evaporated
away. And that why this yellow acid exists in powder form after evaporating its water molecule.
II. Chemical properties
Chemical properties of this tungstic acid simply deals with the how this acid is able to react with
other substances, these reactions (chemical reactions) are discussed below;
Epoxidation of Alkenes
Tungstic acid can be employed to catalyze the oxidation by peroxide of the organic
compound (alkane) to the conforming epoxides (Freedman, 2013). This acid is a fairly strong
acid the mixture reaction needs a buffering to avoid any subsequent formation of diol. The
archetypal condition of this reaction includes the following: tungstic acid at 1 mol and 1 %,
sodium acetate 2-3 mol at a pH of 4.5, hydrogen peroxide of 1.5 mol. Under the above typical
conditions, the epoxidation takes place with a full retaining of configuration for both Tran- and
cis-alkenes the reaction will increase with nucleophilicity of the double bond (Tallon, 2011).
The coordination to the catalyst by the hydroxyl group in the substrate also enriches the
epoxidation rate.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
TUNGSTIC ACID 7
The unsaturated conjugated acid diacids epoxidized by the help of hydrogen peroxide and
sodium tungstate in water at a temperature of about 600-650C having a pH control of about 5.8
to 6.8. The usage of paratungstate, W2O112- obtained from the tungstic acid and also from the
chloride of benzyltriphenylphosphonium with a compound of formula Ph3PCH2Ph+ Cl-. The
catalyst is made in a situ Tungstic acid can be employed to help catalyze the oxidation by the
peroxide of hydrogen (hydrogen peroxide) of the sulfides to the conforming sulfones. Different
amines have been oxidized too to the corresponding oximes, hydroxamic acid, nitroso compound
and nitrones by the help of Tungstic acid (James F., 2012).
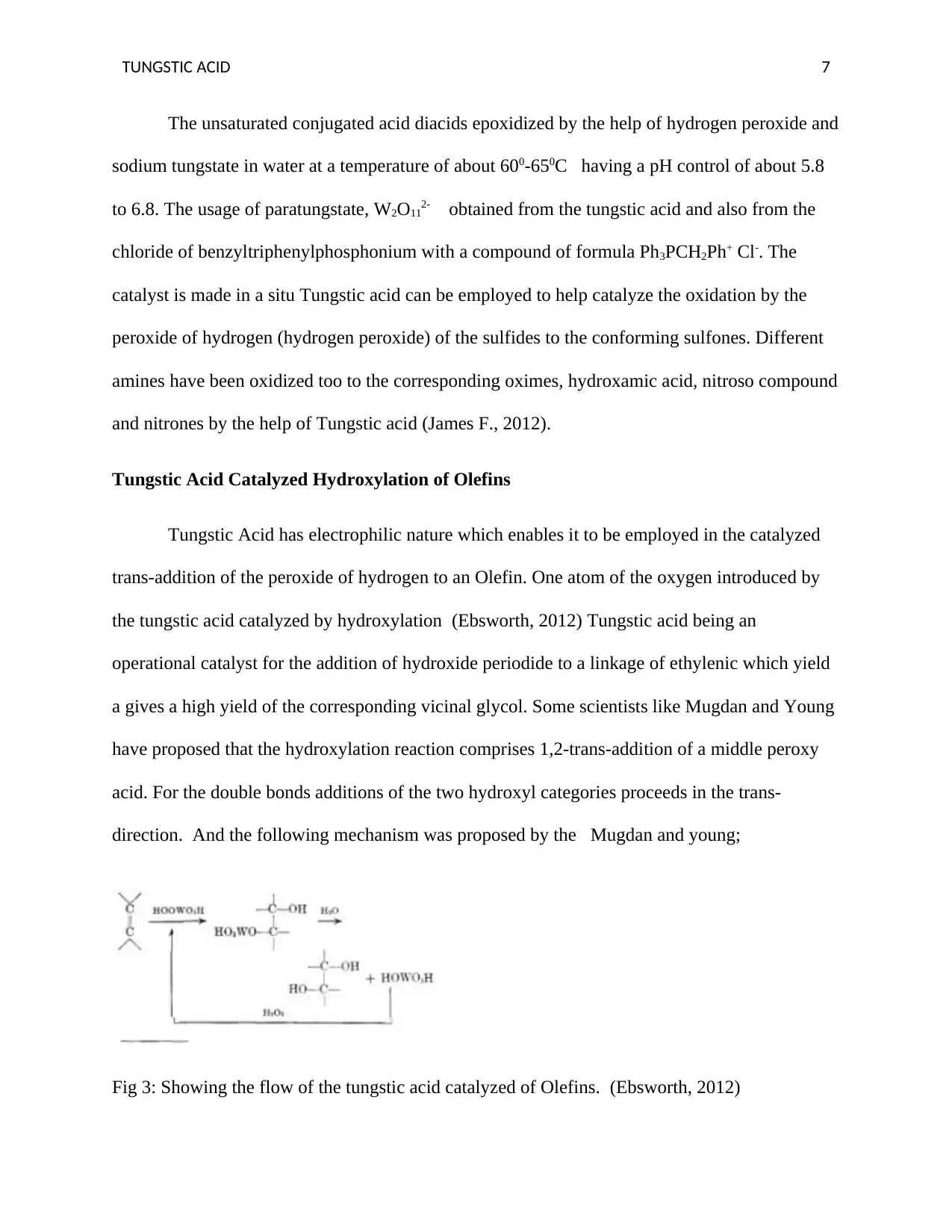
Tungstic Acid Catalyzed Hydroxylation of Olefins
Tungstic Acid has electrophilic nature which enables it to be employed in the catalyzed
trans-addition of the peroxide of hydrogen to an Olefin. One atom of the oxygen introduced by
the tungstic acid catalyzed by hydroxylation (Ebsworth, 2012) Tungstic acid being an
operational catalyst for the addition of hydroxide periodide to a linkage of ethylenic which yield
a gives a high yield of the corresponding vicinal glycol. Some scientists like Mugdan and Young
have proposed that the hydroxylation reaction comprises 1,2-trans-addition of a middle peroxy
acid. For the double bonds additions of the two hydroxyl categories proceeds in the trans-
direction. And the following mechanism was proposed by the Mugdan and young;
Fig 3: Showing the flow of the tungstic acid catalyzed of Olefins. (Ebsworth, 2012)
The unsaturated conjugated acid diacids epoxidized by the help of hydrogen peroxide and
sodium tungstate in water at a temperature of about 600-650C having a pH control of about 5.8
to 6.8. The usage of paratungstate, W2O112- obtained from the tungstic acid and also from the
chloride of benzyltriphenylphosphonium with a compound of formula Ph3PCH2Ph+ Cl-. The
catalyst is made in a situ Tungstic acid can be employed to help catalyze the oxidation by the
peroxide of hydrogen (hydrogen peroxide) of the sulfides to the conforming sulfones. Different
amines have been oxidized too to the corresponding oximes, hydroxamic acid, nitroso compound
and nitrones by the help of Tungstic acid (James F., 2012).
Tungstic Acid Catalyzed Hydroxylation of Olefins
Tungstic Acid has electrophilic nature which enables it to be employed in the catalyzed
trans-addition of the peroxide of hydrogen to an Olefin. One atom of the oxygen introduced by
the tungstic acid catalyzed by hydroxylation (Ebsworth, 2012) Tungstic acid being an
operational catalyst for the addition of hydroxide periodide to a linkage of ethylenic which yield
a gives a high yield of the corresponding vicinal glycol. Some scientists like Mugdan and Young
have proposed that the hydroxylation reaction comprises 1,2-trans-addition of a middle peroxy
acid. For the double bonds additions of the two hydroxyl categories proceeds in the trans-
direction. And the following mechanism was proposed by the Mugdan and young;
Fig 3: Showing the flow of the tungstic acid catalyzed of Olefins. (Ebsworth, 2012)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

TUNGSTIC ACID 8
Derivatives with the hydrogen peroxide tungstic acid system and direct hydroxylation of
fats.
Tungstic acid can be combined with hydrogen peroxide and used for direct hydroxylation
of fat and derivatives. The direct preparation of threo -1,2-glycols minus the isolation of the
intermediary from methyl oleate, oleic acid and oleyl alcohol by oxidation by the help of tungstic
acid system at 45 to 55 C and hydrogen peroxide of 70% minus the solvent has now been proved
to be efficient, reaction of high yield. The three- isomers are made from the intermediary
epoxides by in a hydration situ with associated inversion (Hassan, 2011). The incorporation of 2
% of the reaction of the glycol product into the methyl oleate or the oleic acid, this will help to
speeds up the chemical reaction adds and also markedly to their reproducibility Emulsion are
readily oxidized in some special conditions, but these schemes are more complex to make and
work up than it is for the oxidation of the substrate unswervingly (Wyman, 2010). Timid reaction
techniques are suggested in which an organic polyperoxytungstic acid is an operational oxidizing
agent.
A reaction where a pure tungstic acid is produced from impure alkali metal tungstate.
A tungstic acid can be produced from acidification of impure alkali or molybdate or
ammonium tungstate. This scheme is less expensive than to employ the use of the hydrochloric
acid or by use of sulfuric acid and it helps to prevent impurity of chloride for this reason
ammonium tungstate, molybdate solutions are not preferred to hydrochloric acid and sulfuric
acid. A tungsten material like a tungsten metal ore is decomposed by the help of a solution of
caustic soda to get an impure sodium tungstate solution (Na2WO4), the pH of the solution will be
Derivatives with the hydrogen peroxide tungstic acid system and direct hydroxylation of
fats.
Tungstic acid can be combined with hydrogen peroxide and used for direct hydroxylation
of fat and derivatives. The direct preparation of threo -1,2-glycols minus the isolation of the
intermediary from methyl oleate, oleic acid and oleyl alcohol by oxidation by the help of tungstic
acid system at 45 to 55 C and hydrogen peroxide of 70% minus the solvent has now been proved
to be efficient, reaction of high yield. The three- isomers are made from the intermediary
epoxides by in a hydration situ with associated inversion (Hassan, 2011). The incorporation of 2
% of the reaction of the glycol product into the methyl oleate or the oleic acid, this will help to
speeds up the chemical reaction adds and also markedly to their reproducibility Emulsion are
readily oxidized in some special conditions, but these schemes are more complex to make and
work up than it is for the oxidation of the substrate unswervingly (Wyman, 2010). Timid reaction
techniques are suggested in which an organic polyperoxytungstic acid is an operational oxidizing
agent.
A reaction where a pure tungstic acid is produced from impure alkali metal tungstate.
A tungstic acid can be produced from acidification of impure alkali or molybdate or
ammonium tungstate. This scheme is less expensive than to employ the use of the hydrochloric
acid or by use of sulfuric acid and it helps to prevent impurity of chloride for this reason
ammonium tungstate, molybdate solutions are not preferred to hydrochloric acid and sulfuric
acid. A tungsten material like a tungsten metal ore is decomposed by the help of a solution of
caustic soda to get an impure sodium tungstate solution (Na2WO4), the pH of the solution will be

TUNGSTIC ACID 9
at around 13-14 (Commission, 2012). The impurities like silicon, phosphorous and arsenic
ions. In the first step in the purification, the Na2WO4 solution is acidified to a pH of 9 with an
initially made sodium metatungstate. The quantity of sodium metatungstate to be put will
depend on the quantity of the impurities available and it is chosen in a way that the pH value of
the generated an optimum hydrolysis (precipitation) of the ions to be detached (Gmelin, 2012).
The Na5HW6O21 appears to occur as (Na) +5 (HW6O21-5). In other terms hydrogen in the
salt is not ionized, during the reaction this salt is capable to bind sodium ions, but according to
the formula, when the hydrogen is soluble only one sodium may be blind. It is more amazing and
not at all clear for those of normal skills in the art of that the polymers of this form will as well
react with the monomer salt (X) Na2 WO4 and a polymer (Y) Na5 HW6 O2. For instance; for
one liter solution having 104g/l tungsten (WO4 -2), about 43 g/l solution, 1.8 g/l of silica ( SiO2),
0.3 g/l arsenic ( ASO4- ), and 0.5 g/l phosphorous ( PO4-3) can be added to one liter of the
(Al2SO2)3 aluminum sulfate at a concentration of about 80g/l and also 30 ml of the magnesium
sulfate (MgSO4) at a concentration of roughly 300g/l.
A reaction which involves recuperating tungstic acid values from the aqueous compound.
The word tungstic acid is employed in this chemical reaction to define tungstic acid as a
catalyst chemical whatsoever its particular form it may take (Christian, 2014). In the process of
the organic epoxidation encompassing the use of peroxide of hydrogen and the tungstic acid. In
the elution from an ion-exchanged bed, the acid atom O ice catalyst occurs as the water dissolve
the sodium tungstate. Except as hereinafter several particularly distinct either in its solubility in
water or simply in terms of its actual chemical properties.
at around 13-14 (Commission, 2012). The impurities like silicon, phosphorous and arsenic
ions. In the first step in the purification, the Na2WO4 solution is acidified to a pH of 9 with an
initially made sodium metatungstate. The quantity of sodium metatungstate to be put will
depend on the quantity of the impurities available and it is chosen in a way that the pH value of
the generated an optimum hydrolysis (precipitation) of the ions to be detached (Gmelin, 2012).
The Na5HW6O21 appears to occur as (Na) +5 (HW6O21-5). In other terms hydrogen in the
salt is not ionized, during the reaction this salt is capable to bind sodium ions, but according to
the formula, when the hydrogen is soluble only one sodium may be blind. It is more amazing and
not at all clear for those of normal skills in the art of that the polymers of this form will as well
react with the monomer salt (X) Na2 WO4 and a polymer (Y) Na5 HW6 O2. For instance; for
one liter solution having 104g/l tungsten (WO4 -2), about 43 g/l solution, 1.8 g/l of silica ( SiO2),
0.3 g/l arsenic ( ASO4- ), and 0.5 g/l phosphorous ( PO4-3) can be added to one liter of the
(Al2SO2)3 aluminum sulfate at a concentration of about 80g/l and also 30 ml of the magnesium
sulfate (MgSO4) at a concentration of roughly 300g/l.
A reaction which involves recuperating tungstic acid values from the aqueous compound.
The word tungstic acid is employed in this chemical reaction to define tungstic acid as a
catalyst chemical whatsoever its particular form it may take (Christian, 2014). In the process of
the organic epoxidation encompassing the use of peroxide of hydrogen and the tungstic acid. In
the elution from an ion-exchanged bed, the acid atom O ice catalyst occurs as the water dissolve
the sodium tungstate. Except as hereinafter several particularly distinct either in its solubility in
water or simply in terms of its actual chemical properties.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

TUNGSTIC ACID 10
Recovery of this acid as a catalyst through this invention is of overall applicability
irrespective of a specific organic oxidation process where the catalyst is used. Apparently,
techniques of handling the particular chemical forms of any of the several oxidations through
using this catalyst. This is always determined best by a condition most appropriate to a given
reaction of oxidation. The interest to separate the products which are oxidized prior to
recovering this acid values will actually be pegged on a bigger degree of the steadiness of the
products which are oxidized in some beneficial conditions to the tungstic acid catalyst recovery
(Washington, 2013).
This can be easily fathomed that the available in the contemplates vention the processing
of the medium of the reaction in a way reliable with the suitable recovery of the oxidation
products produced during the reactions while taking in to account the specific techniques of
tungstic value recovery. In combination with the oxidation leading to epoxide separation of the
product erstwhile to the recovery of the catalyst may be utmost anticipated specifically when the
epoxide inclines to decompose to other products in the same reaction conditions of the catalyst
recovery.
UNIT CELL OF TUNGSTIC ACID
The unit cell is the smallest particle of any compound, for this tungstic acid having a
molecular formula as seen below;
Fig 4: showing the structure of the tungstic acid ( Freedman, M. 2013)
Recovery of this acid as a catalyst through this invention is of overall applicability
irrespective of a specific organic oxidation process where the catalyst is used. Apparently,
techniques of handling the particular chemical forms of any of the several oxidations through
using this catalyst. This is always determined best by a condition most appropriate to a given
reaction of oxidation. The interest to separate the products which are oxidized prior to
recovering this acid values will actually be pegged on a bigger degree of the steadiness of the
products which are oxidized in some beneficial conditions to the tungstic acid catalyst recovery
(Washington, 2013).
This can be easily fathomed that the available in the contemplates vention the processing
of the medium of the reaction in a way reliable with the suitable recovery of the oxidation
products produced during the reactions while taking in to account the specific techniques of
tungstic value recovery. In combination with the oxidation leading to epoxide separation of the
product erstwhile to the recovery of the catalyst may be utmost anticipated specifically when the
epoxide inclines to decompose to other products in the same reaction conditions of the catalyst
recovery.
UNIT CELL OF TUNGSTIC ACID
The unit cell is the smallest particle of any compound, for this tungstic acid having a
molecular formula as seen below;
Fig 4: showing the structure of the tungstic acid ( Freedman, M. 2013)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

TUNGSTIC ACID 11
From the above image of the molecular formula structure of the tungstic acid, it is therefore clear
that the unit cell of the tungstic acid is the W (wolfram), two atoms of Hydrogen (H2) and four
atoms of Oxygen (O4). The main component of this acid is treated to be Wolfram since this acid
is basically prepared from the Wolfram acid.
UV radiations (Ultraviolet)
Ultra Violet (UV) radiation basically is applied in the tungstic acid in it’s a preparation
stage. The monodispersed tungstic acid which is a nanoparticle solid having a mean diameter of
about 20 nanometers ( nm) can be made by using this ultraviolet light radiation of oxotungsten
citrate complicated aqueous solution. The outcomes from the Infrared spectroscopy indicates
that the oxotungsten citation complex decomposes firstly to form a citric acid and tungstic acid
under the radiations of the UV light (White, 2012). Thereafter the citric acid will undergo a
photodegradation in a tungstic acid catalysis.
The XRD outcome of this UV on tungstic acid may indicate that the attained product may
consist of the WO3. H2O and WO3. 2H2O. The time is taken to during the radiation known as
the irradiation time and the ratio of molar of the citric acid to that of the sodium tungstate will
have a very vital effect on the composition of the product. The prolonging time of radiation the
amount of the WO3.2 H2O in the whole substance will reduce but that for the WO3.H2O will
increase. For the particle size, it will increase and the size of the distribution will definitely
widen. Moreover, the size of the particles decreases and the distribution becomes very narrow.
The figure below shows the UV radiation in the preparation stages of the tungstic acid.
From the above image of the molecular formula structure of the tungstic acid, it is therefore clear
that the unit cell of the tungstic acid is the W (wolfram), two atoms of Hydrogen (H2) and four
atoms of Oxygen (O4). The main component of this acid is treated to be Wolfram since this acid
is basically prepared from the Wolfram acid.
UV radiations (Ultraviolet)
Ultra Violet (UV) radiation basically is applied in the tungstic acid in it’s a preparation
stage. The monodispersed tungstic acid which is a nanoparticle solid having a mean diameter of
about 20 nanometers ( nm) can be made by using this ultraviolet light radiation of oxotungsten
citrate complicated aqueous solution. The outcomes from the Infrared spectroscopy indicates
that the oxotungsten citation complex decomposes firstly to form a citric acid and tungstic acid
under the radiations of the UV light (White, 2012). Thereafter the citric acid will undergo a
photodegradation in a tungstic acid catalysis.
The XRD outcome of this UV on tungstic acid may indicate that the attained product may
consist of the WO3. H2O and WO3. 2H2O. The time is taken to during the radiation known as
the irradiation time and the ratio of molar of the citric acid to that of the sodium tungstate will
have a very vital effect on the composition of the product. The prolonging time of radiation the
amount of the WO3.2 H2O in the whole substance will reduce but that for the WO3.H2O will
increase. For the particle size, it will increase and the size of the distribution will definitely
widen. Moreover, the size of the particles decreases and the distribution becomes very narrow.
The figure below shows the UV radiation in the preparation stages of the tungstic acid.
TUNGSTIC ACID 12
Fig 5: UV radiation in preparation of tungstic acid (Acton, 2012).
The Nanocrystalline powders of the tungstic acid are prepared in a very controlled
chemical technique. The structure of the acid and its characteristics of the vibration can be
obtained from the Raman and the XRD spectroscopy (Acton, 2012). The X-ray phenomenon of
the Spectroscopy (XPS) scrutiny of the sample shows the availability of the oxygen vacancies
that always confirm the UV photoluminescence (PL) and absorption-emission workings. The
figure below shows the photoluminescence (PL) spectrum;
Fig 5: UV radiation in preparation of tungstic acid (Acton, 2012).
The Nanocrystalline powders of the tungstic acid are prepared in a very controlled
chemical technique. The structure of the acid and its characteristics of the vibration can be
obtained from the Raman and the XRD spectroscopy (Acton, 2012). The X-ray phenomenon of
the Spectroscopy (XPS) scrutiny of the sample shows the availability of the oxygen vacancies
that always confirm the UV photoluminescence (PL) and absorption-emission workings. The
figure below shows the photoluminescence (PL) spectrum;
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 19
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

