Chemistry for Biologists 4LMS0006-0902 Assignment
VerifiedAdded on 2023/06/16
|7
|1384
|300
AI Summary
Solve Chemistry for Biologists 4LMS0006-0902 Assignment with UV-Vis Absorption Spectrum of different plant pigments. Answer all questions with clear calculations and units. Get a calibration curve for Phycocyanin using four standards and a blank.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: CHEMISTRY FOR BIOLOGISTS 4LMS0006-0902 ASSIGNMENT
1
Chemistry for Biologists 4LMS0006-0902 Assignment
Professor’s Name:
Name:
Date:
1
Chemistry for Biologists 4LMS0006-0902 Assignment
Professor’s Name:
Name:
Date:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
CHEMISTRY FOR BIOLOGISTS 4LMS0006-0902 ASSIGNMENT
2
Part 2 of 2
Instruction for candidates
Answer all questions. Show all steps of your calculations and show all units! You can handwrite
your answers and scan them in if you prefer, but they must be readable. Your completed
assignment should be submitted as a Word document via the assignment portal on the
Chemistry for Biologists module website. Total marks are out of 100.
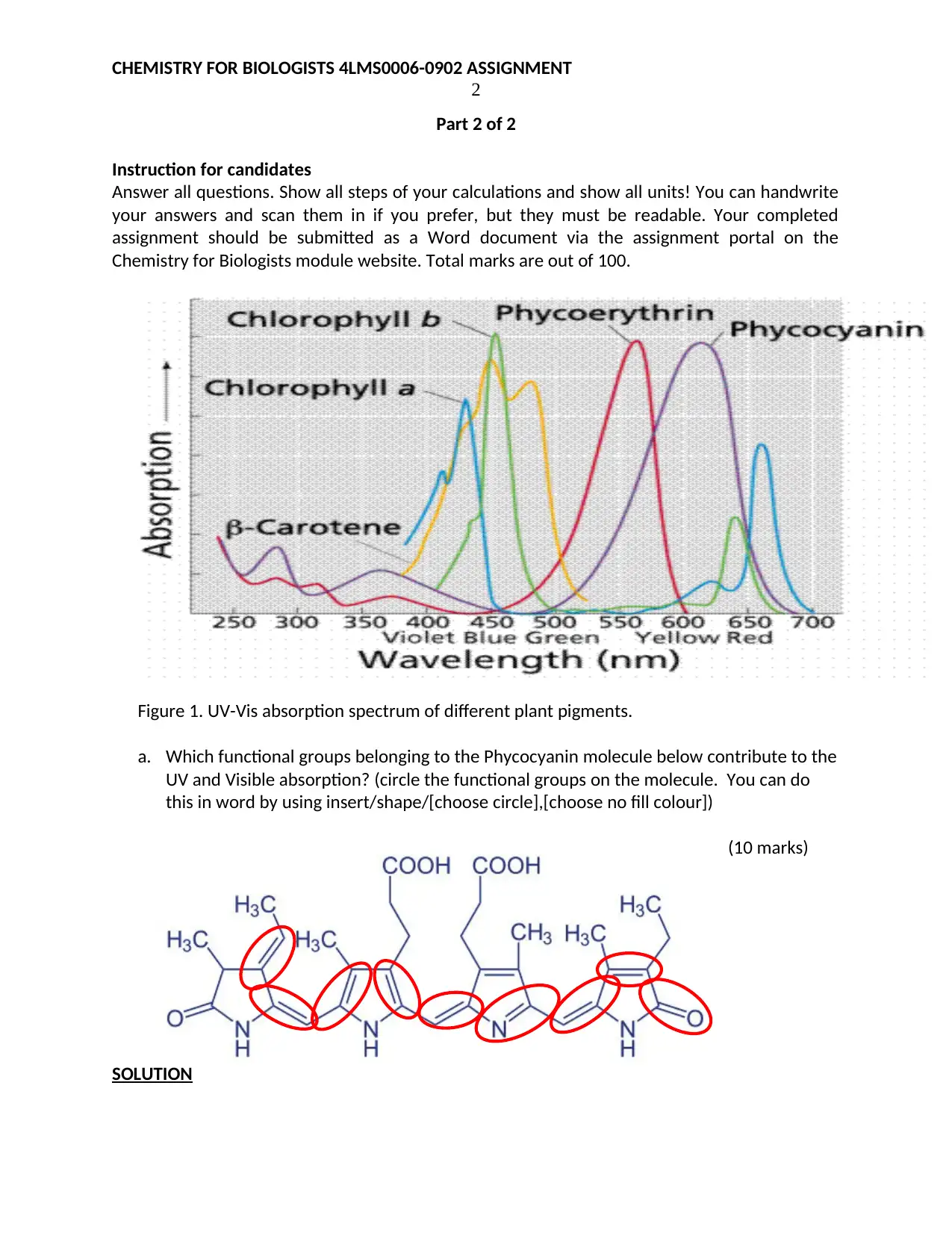
Figure 1. UV-Vis absorption spectrum of different plant pigments.
a. Which functional groups belonging to the Phycocyanin molecule below contribute to the
UV and Visible absorption? (circle the functional groups on the molecule. You can do
this in word by using insert/shape/[choose circle],[choose no fill colour])
(10 marks)
SOLUTION
2
Part 2 of 2
Instruction for candidates
Answer all questions. Show all steps of your calculations and show all units! You can handwrite
your answers and scan them in if you prefer, but they must be readable. Your completed
assignment should be submitted as a Word document via the assignment portal on the
Chemistry for Biologists module website. Total marks are out of 100.
Figure 1. UV-Vis absorption spectrum of different plant pigments.
a. Which functional groups belonging to the Phycocyanin molecule below contribute to the
UV and Visible absorption? (circle the functional groups on the molecule. You can do
this in word by using insert/shape/[choose circle],[choose no fill colour])
(10 marks)
SOLUTION
CHEMISTRY FOR BIOLOGISTS 4LMS0006-0902 ASSIGNMENT
3
The molecule contains an extended system of conjugated double bonds that are responsible for
UV-VIS absorption. The absorption maximum wavelength can be predicted for phycocyanin
using the woodward-Fieser rules as follows.
The primary chromophore is a five membered ring enone. It has 1 alkyl substituent on the α-
carbon (-C2H5) and 1 alkyl substituent on the β-carbon. It has seven double bonds extending
conjugation. There are 10 alkyl or ring residues at positions higher than γ (on the extended
conjugated system) and 5 exocyclic double bonds
The Base value: 5-membered ring enone 202 nm
Increments: α-alkyl 5 nm
β-alkyl 12 nm
γ- Or higher = 10 x 18 nm 180 nm
Double bonds extending conjugation = 7x 30 nm 210 nm
Exocyclic double bonds = 5 x 5 nm 25 nm
Predicted absorption peak wavelength 635 nm
The peak predicted by Woodward-Fieser rules is close to the peak of the given uv-vis spectrum
of phycocyanin therefore the chromophoric group responsible for its spectrum is the
conjugated pi-system of double bonds.
b. Identity the best wavelengths, using Figure 1, that can be used for quantifying
Phycocyanin and Phycoerythrin via UV-Vis spectroscopy if (a) they are separated and (b)
if they are present in a mixture. Fill in the table below appropriately.
(10 marks)
Substance (a) Separated (b) In Mixture
Phycocyanin 620 nm 600 nm
Phycoerythrin 560 nm 550 nm
c. Using Excel, plot a calibration curve for Phycocyanin using four standards and a blank.
Plot the calibration curve in concentration units of mg/ml. The absorbance readings of
these solutions at the best wavelength are as follows:
0 mM = 0.004 Abs
1.2 mM = 0.230 Abs
2.4 mM = 0.477 Abs
3.1 mM = 0.710 Abs
3.8 mM = 0.950 Abs
SOLUTION
From the structure given, the molar mass of phycocyanin can be calculated. It has 33 carbon
atoms, 38 hydrogen atoms, 16 oxygen atoms and 4 nitrogen atoms. The molecular formula
is C33H38O6N4.
Molar mass = 33(12) + 38 (1) + 6 (16) + 4 (14) = 586 g/mol.
Thus, 1mM is equivalent to 0.586mg/ml.
The concentrations of the standards were converted to mg/ml and then used for plotting
the calibration curve. The absorbance values were corrected to eliminate background
absorption by the blank. For each standard, the blank absorbance value was subtracted
3
The molecule contains an extended system of conjugated double bonds that are responsible for
UV-VIS absorption. The absorption maximum wavelength can be predicted for phycocyanin
using the woodward-Fieser rules as follows.
The primary chromophore is a five membered ring enone. It has 1 alkyl substituent on the α-
carbon (-C2H5) and 1 alkyl substituent on the β-carbon. It has seven double bonds extending
conjugation. There are 10 alkyl or ring residues at positions higher than γ (on the extended
conjugated system) and 5 exocyclic double bonds
The Base value: 5-membered ring enone 202 nm
Increments: α-alkyl 5 nm
β-alkyl 12 nm
γ- Or higher = 10 x 18 nm 180 nm
Double bonds extending conjugation = 7x 30 nm 210 nm
Exocyclic double bonds = 5 x 5 nm 25 nm
Predicted absorption peak wavelength 635 nm
The peak predicted by Woodward-Fieser rules is close to the peak of the given uv-vis spectrum
of phycocyanin therefore the chromophoric group responsible for its spectrum is the
conjugated pi-system of double bonds.
b. Identity the best wavelengths, using Figure 1, that can be used for quantifying
Phycocyanin and Phycoerythrin via UV-Vis spectroscopy if (a) they are separated and (b)
if they are present in a mixture. Fill in the table below appropriately.
(10 marks)
Substance (a) Separated (b) In Mixture
Phycocyanin 620 nm 600 nm
Phycoerythrin 560 nm 550 nm
c. Using Excel, plot a calibration curve for Phycocyanin using four standards and a blank.
Plot the calibration curve in concentration units of mg/ml. The absorbance readings of
these solutions at the best wavelength are as follows:
0 mM = 0.004 Abs
1.2 mM = 0.230 Abs
2.4 mM = 0.477 Abs
3.1 mM = 0.710 Abs
3.8 mM = 0.950 Abs
SOLUTION
From the structure given, the molar mass of phycocyanin can be calculated. It has 33 carbon
atoms, 38 hydrogen atoms, 16 oxygen atoms and 4 nitrogen atoms. The molecular formula
is C33H38O6N4.
Molar mass = 33(12) + 38 (1) + 6 (16) + 4 (14) = 586 g/mol.
Thus, 1mM is equivalent to 0.586mg/ml.
The concentrations of the standards were converted to mg/ml and then used for plotting
the calibration curve. The absorbance values were corrected to eliminate background
absorption by the blank. For each standard, the blank absorbance value was subtracted
CHEMISTRY FOR BIOLOGISTS 4LMS0006-0902 ASSIGNMENT
4
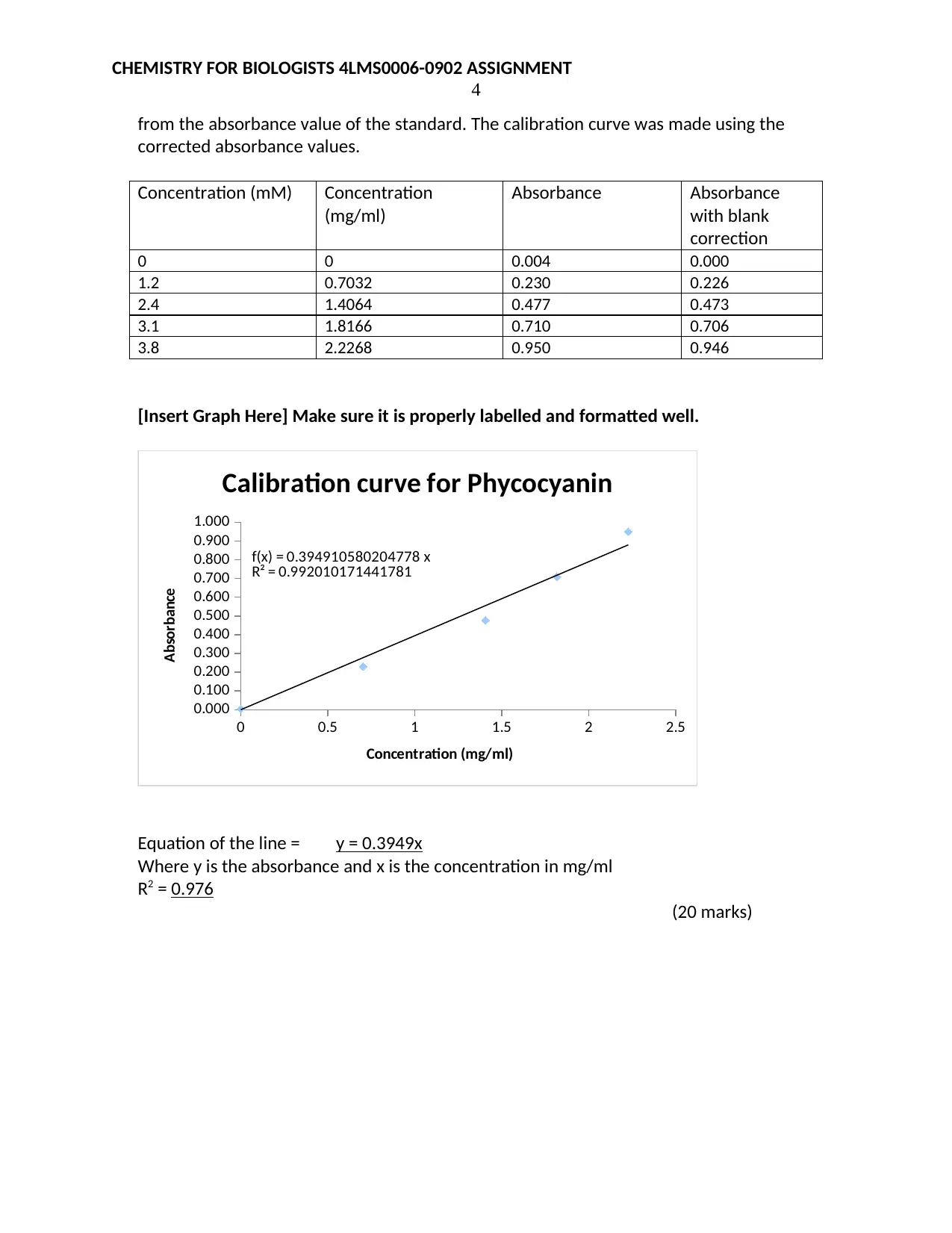
from the absorbance value of the standard. The calibration curve was made using the
corrected absorbance values.
Concentration (mM) Concentration
(mg/ml)
Absorbance Absorbance
with blank
correction
0 0 0.004 0.000
1.2 0.7032 0.230 0.226
2.4 1.4064 0.477 0.473
3.1 1.8166 0.710 0.706
3.8 2.2268 0.950 0.946
[Insert Graph Here] Make sure it is properly labelled and formatted well.
0 0.5 1 1.5 2 2.5
0.000
0.100
0.200
0.300
0.400
0.500
0.600
0.700
0.800
0.900
1.000
f(x) = 0.394910580204778 x
R² = 0.992010171441781
Calibration curve for Phycocyanin
Concentration (mg/ml)
Absorbance
Equation of the line = y = 0.3949x
Where y is the absorbance and x is the concentration in mg/ml
R2 = 0.976
(20 marks)
4
from the absorbance value of the standard. The calibration curve was made using the
corrected absorbance values.
Concentration (mM) Concentration
(mg/ml)
Absorbance Absorbance
with blank
correction
0 0 0.004 0.000
1.2 0.7032 0.230 0.226
2.4 1.4064 0.477 0.473
3.1 1.8166 0.710 0.706
3.8 2.2268 0.950 0.946
[Insert Graph Here] Make sure it is properly labelled and formatted well.
0 0.5 1 1.5 2 2.5
0.000
0.100
0.200
0.300
0.400
0.500
0.600
0.700
0.800
0.900
1.000
f(x) = 0.394910580204778 x
R² = 0.992010171441781
Calibration curve for Phycocyanin
Concentration (mg/ml)
Absorbance
Equation of the line = y = 0.3949x
Where y is the absorbance and x is the concentration in mg/ml
R2 = 0.976
(20 marks)
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

CHEMISTRY FOR BIOLOGISTS 4LMS0006-0902 ASSIGNMENT
5
d. Calculate the molar absorptivity (ε) for Phycocyanin.
SOLUTION
Beer-Lamberts law gives the relationship between absorbance and concentration as follows:
A = ϵcL
Where A= the absorbance,
ϵ = molar absorptivity,
c = molar concentration and
L = path length of light in centimetres
The path length of most UV-VIS cells is usually 1 cm therefore; the molar absorptivity is the
gradient of a graph of absorbance versus concentration. Its units when molar concentrations
are used should be M-1cm-1
From the calibration graph obtained, the gradient is 0.3949. However, the concentration
units are mg/ml. therefore the absorptivity value from this graph is 0.3949 mlmg-1cm-1
This can be converted to M-1cm-1 units using appropriate unit conversions as follows:
molar absorptivity =0.3949 ml
mgcm × 1 L
1000 ml × 1000 mg
g × 586 g
mol
¿ 231.4114 L/molcm
¿ 231.4 M−1 cm−1
molar absorptivity ¿ 231.4 M−1 cm−1
_________________________________________________________
(10 marks)
e. You want to determine the amount of Phycocyanin in a 500 mg herbal tablet. Before
analysis by UV-Vis spectroscopy, five Phycocyanin tablets (each 500 mg) were crushed in
a pestle and mortar. 1.2 g of the crushed tablets were then dissolved in 100 ml of
ethanol. 50 ml of the solution was filtered to remove undissolved particles. 2 ml of the
filtered solution was then diluted by a factor of 10 (i.e., dilution factor is 10). The diluted
sample was analysed using UV-Vis spectroscopy and had an absorbance of 0.324. Show
each calculation step clearly.
What was the amount in mg of Phycocyanin in a 500 mg tablet?
What is the percent (%w/w) of Phycocyanin in a tablet?
SOLUTION
Method 1: using the molar absorptivity
From beers lambert’s law, A = ϵcl
A/ϵl = c
But we know that ϵ =231.4 M−1 cm−1 (calculated from the calibration curve)
5
d. Calculate the molar absorptivity (ε) for Phycocyanin.
SOLUTION
Beer-Lamberts law gives the relationship between absorbance and concentration as follows:
A = ϵcL
Where A= the absorbance,
ϵ = molar absorptivity,
c = molar concentration and
L = path length of light in centimetres
The path length of most UV-VIS cells is usually 1 cm therefore; the molar absorptivity is the
gradient of a graph of absorbance versus concentration. Its units when molar concentrations
are used should be M-1cm-1
From the calibration graph obtained, the gradient is 0.3949. However, the concentration
units are mg/ml. therefore the absorptivity value from this graph is 0.3949 mlmg-1cm-1
This can be converted to M-1cm-1 units using appropriate unit conversions as follows:
molar absorptivity =0.3949 ml
mgcm × 1 L
1000 ml × 1000 mg
g × 586 g
mol
¿ 231.4114 L/molcm
¿ 231.4 M−1 cm−1
molar absorptivity ¿ 231.4 M−1 cm−1
_________________________________________________________
(10 marks)
e. You want to determine the amount of Phycocyanin in a 500 mg herbal tablet. Before
analysis by UV-Vis spectroscopy, five Phycocyanin tablets (each 500 mg) were crushed in
a pestle and mortar. 1.2 g of the crushed tablets were then dissolved in 100 ml of
ethanol. 50 ml of the solution was filtered to remove undissolved particles. 2 ml of the
filtered solution was then diluted by a factor of 10 (i.e., dilution factor is 10). The diluted
sample was analysed using UV-Vis spectroscopy and had an absorbance of 0.324. Show
each calculation step clearly.
What was the amount in mg of Phycocyanin in a 500 mg tablet?
What is the percent (%w/w) of Phycocyanin in a tablet?
SOLUTION
Method 1: using the molar absorptivity
From beers lambert’s law, A = ϵcl
A/ϵl = c
But we know that ϵ =231.4 M−1 cm−1 (calculated from the calibration curve)

CHEMISTRY FOR BIOLOGISTS 4LMS0006-0902 ASSIGNMENT
6
Assuming that path length of light is 1 cm, the concentration for any sample is obtained
by dividing its absorbance with the molar absorptivity.
Concentration of the diluted sample = 0.324
231.4 =1.400173 ×10−3 M
But 1M phycoyanin =586mg/ml
Concentration of diluted sample in mg/ml = 0.8205mg/ml
Concentration of undiluted sample = 8.205 mg/ml
1.2 g of tablet was dissolved to make 100ml solution. Concentration of this solution was
8.205 mg/ml. therefore, the amount of phycocyanin in this solution =
100 ml × 8.205 mg
ml =820.5 mg
1.2g tablet contains 820.5 mg. how about 500 mg (0.5g) tablets?
¿ 0.5 g
1.2 g ×820.5 mg
¿ 341.9 mg
%w/w of phycocyanin in the tablet = 341.9 mg
500 mg ×100 %=68.38 %
Method 2: using the linear equation of the calibration curve
y = 0.3949x
0.324=0.3949 x
x=0.8205 mg/ml
But the dilution factor was 10. The concentration of undiluted sample = 8.205 mg /ml
100ml solution was made from 1.2 g of tablet. The amount of phycocyanin in this solution =
820.5 mg
1.2 g of tablet contains 820.5 mg. how about 500 mg (=0.5 g) of tablet?
0.5 g
1.2 g ×820.5 mg=341.9mg
%w/w of phycocyanin in the tablet = 341.9 mg
500 mg ×100 %=68.38 %
A 500 mg tablet contains 341.9 mg of phycocyanin. This is equivalent to 68.38% (w/w) of
phycocyanin.
(50 marks)
6
Assuming that path length of light is 1 cm, the concentration for any sample is obtained
by dividing its absorbance with the molar absorptivity.
Concentration of the diluted sample = 0.324
231.4 =1.400173 ×10−3 M
But 1M phycoyanin =586mg/ml
Concentration of diluted sample in mg/ml = 0.8205mg/ml
Concentration of undiluted sample = 8.205 mg/ml
1.2 g of tablet was dissolved to make 100ml solution. Concentration of this solution was
8.205 mg/ml. therefore, the amount of phycocyanin in this solution =
100 ml × 8.205 mg
ml =820.5 mg
1.2g tablet contains 820.5 mg. how about 500 mg (0.5g) tablets?
¿ 0.5 g
1.2 g ×820.5 mg
¿ 341.9 mg
%w/w of phycocyanin in the tablet = 341.9 mg
500 mg ×100 %=68.38 %
Method 2: using the linear equation of the calibration curve
y = 0.3949x
0.324=0.3949 x
x=0.8205 mg/ml
But the dilution factor was 10. The concentration of undiluted sample = 8.205 mg /ml
100ml solution was made from 1.2 g of tablet. The amount of phycocyanin in this solution =
820.5 mg
1.2 g of tablet contains 820.5 mg. how about 500 mg (=0.5 g) of tablet?
0.5 g
1.2 g ×820.5 mg=341.9mg
%w/w of phycocyanin in the tablet = 341.9 mg
500 mg ×100 %=68.38 %
A 500 mg tablet contains 341.9 mg of phycocyanin. This is equivalent to 68.38% (w/w) of
phycocyanin.
(50 marks)

CHEMISTRY FOR BIOLOGISTS 4LMS0006-0902 ASSIGNMENT
7
References
Kalsi, P.S., 2007. Spectroscopy of organic compounds. New Age International
Sharma, Y.R., 2007. Elementary organic spectroscopy. S. Chand Publishing.
7
References
Kalsi, P.S., 2007. Spectroscopy of organic compounds. New Age International
Sharma, Y.R., 2007. Elementary organic spectroscopy. S. Chand Publishing.
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.