Chemistry Final Exam
VerifiedAdded on 2019/09/22
|5
|1489
|475
Quiz and Exam
AI Summary
This document presents a chemistry final exam with various problem types. Questions cover topics such as gas laws, solution saturation, solubility, vapor pressure, colligative properties, heat transfer, phase transitions, pH calculations, acid-base neutralization, redox reactions, and nuclear chemistry. The exam requires students to show calculations, explain concepts in their own words, and avoid plagiarism. The provided solutions demonstrate problem-solving steps and explanations for each question. The website offers past papers and solved assignments to help students prepare for their exams.
Chemistry Final Exam Version 4/7/15
Name:
Student number:
Directions: It is important that you provide answers in your own words. Please focus only on
information from the text/eBook to create your own solutions. Please do not use direct information
from an outside source (especially copying and pasting from an “answer” website). Use of direct
information from an outside source is against school policy. All answers will be checked for plagiarism.
Instances of plagiarism can result in probation or possible dismissal from the school.
Please be sure to follow all guidelines (number of sentences/showing all calculations) and to provide the
correct metric units of measure. All questions are 5 points (1 point for sentence number /units).
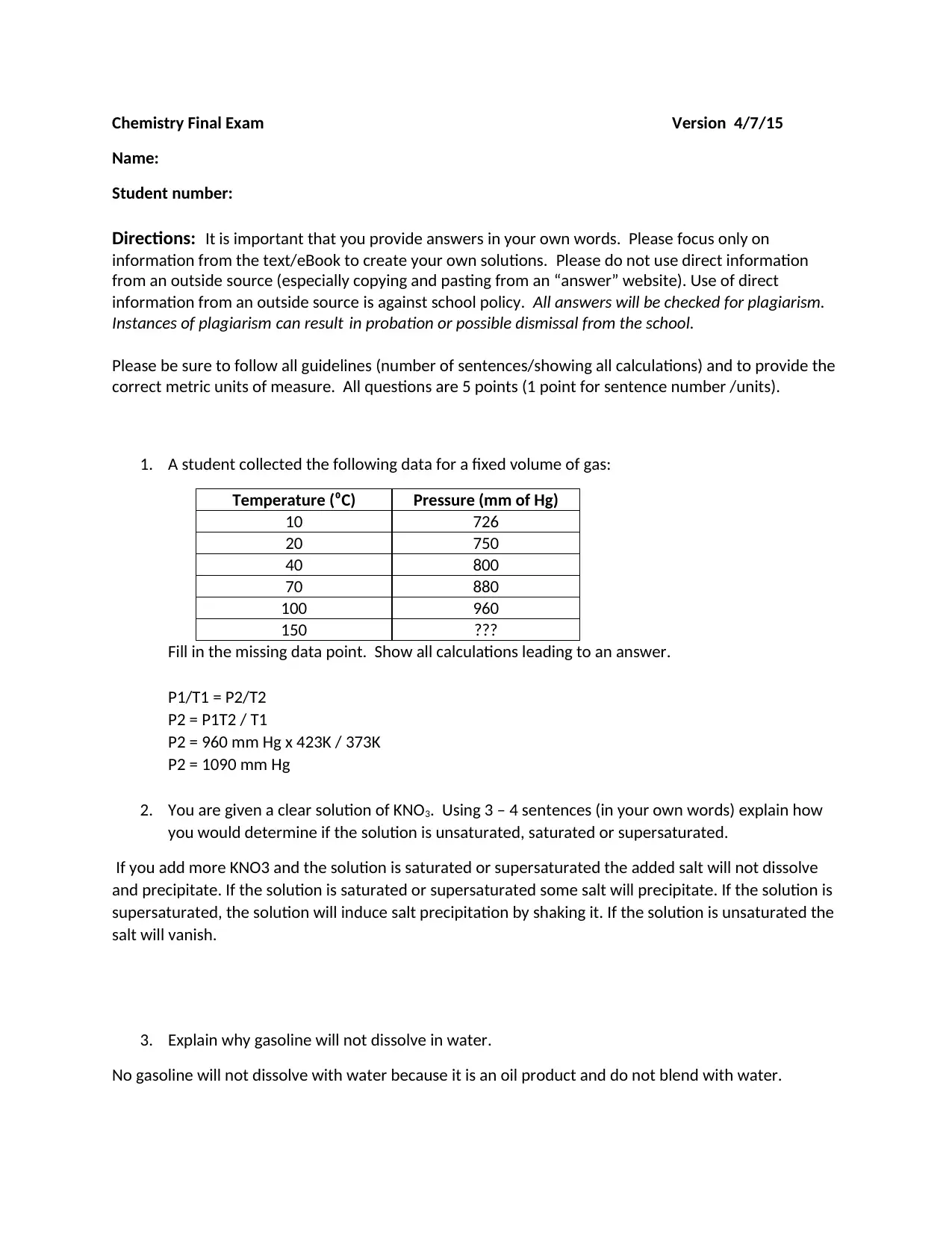
1. A student collected the following data for a fixed volume of gas:
Temperature (⁰C) Pressure (mm of Hg)
10 726
20 750
40 800
70 880
100 960
150 ???
Fill in the missing data point. Show all calculations leading to an answer.
P1/T1 = P2/T2
P2 = P1T2 / T1
P2 = 960 mm Hg x 423K / 373K
P2 = 1090 mm Hg
2. You are given a clear solution of KNO3. Using 3 – 4 sentences (in your own words) explain how
you would determine if the solution is unsaturated, saturated or supersaturated.
If you add more KNO3 and the solution is saturated or supersaturated the added salt will not dissolve
and precipitate. If the solution is saturated or supersaturated some salt will precipitate. If the solution is
supersaturated, the solution will induce salt precipitation by shaking it. If the solution is unsaturated the
salt will vanish.
3. Explain why gasoline will not dissolve in water.
No gasoline will not dissolve with water because it is an oil product and do not blend with water.
Name:
Student number:
Directions: It is important that you provide answers in your own words. Please focus only on
information from the text/eBook to create your own solutions. Please do not use direct information
from an outside source (especially copying and pasting from an “answer” website). Use of direct
information from an outside source is against school policy. All answers will be checked for plagiarism.
Instances of plagiarism can result in probation or possible dismissal from the school.
Please be sure to follow all guidelines (number of sentences/showing all calculations) and to provide the
correct metric units of measure. All questions are 5 points (1 point for sentence number /units).
1. A student collected the following data for a fixed volume of gas:
Temperature (⁰C) Pressure (mm of Hg)
10 726
20 750
40 800
70 880
100 960
150 ???
Fill in the missing data point. Show all calculations leading to an answer.
P1/T1 = P2/T2
P2 = P1T2 / T1
P2 = 960 mm Hg x 423K / 373K
P2 = 1090 mm Hg
2. You are given a clear solution of KNO3. Using 3 – 4 sentences (in your own words) explain how
you would determine if the solution is unsaturated, saturated or supersaturated.
If you add more KNO3 and the solution is saturated or supersaturated the added salt will not dissolve
and precipitate. If the solution is saturated or supersaturated some salt will precipitate. If the solution is
supersaturated, the solution will induce salt precipitation by shaking it. If the solution is unsaturated the
salt will vanish.
3. Explain why gasoline will not dissolve in water.
No gasoline will not dissolve with water because it is an oil product and do not blend with water.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4. Explain in 3 – 4 sentences (in your own words) how perspiration helps keep you cool on a hot
day.
Due to dissolution, perspiration on your skin keeps you cool. It takes thermal energy to change fluid into
a gas. Therefore, it’s on your skin, the easiest way to transfer heat is conduction from your skin to the
fluid. The skin cools down, the liquid turns to a gas, and floats away.
5. You have two sealed jars of water at the same temperature. In the first jar there is a large
amount of water. In the second jar there is a small amount of water. Using 3 -4 sentences
explain how the vapor pressure of water in the first jar compares with the vapor pressure of
water in the second jar.
6. Using 3 – 4 sentences explain (in your own words) why water expands when it freezes?
Water molecules tend to stick together to launch what are called hydrogen bonds. Therefore, Because
of the shape of the molecule, the process water molecules tend to be prone together is very open
formation. The oxygen atom is negative, and the hydrogens are positively charged. That means, there’s
quite a lot of extra empty space. When water freezes it allow to leave energy because lots of extra
chains can be made which take up more space.
7. Using your knowledge of colligative properties explain whether sodium chloride or calcium
chloride would be a more effective substance to melt the ice on a slick sidewalk. Use 3 – 4
sentences in your explanation.
Because the boiling point is higher, calcium chloride releases heat making the ice melt fast than the
sodium chloride. It would be more effective to use CaCl2 for the melting of ice because NaCl separate
into two particles and CaCl2 into three.
8. When a 2.5 mol of sugar (C12H22O11) are added to a certain amount of water the boiling point is
raised by 1 Celsius degree. If 2.5 mol of aluminum nitrate is added to the same amount of
water, by how much will the boiling point be changed? Show all calculations leading to your
answer OR use 3 – 4 sentences to explain your answer.
day.
Due to dissolution, perspiration on your skin keeps you cool. It takes thermal energy to change fluid into
a gas. Therefore, it’s on your skin, the easiest way to transfer heat is conduction from your skin to the
fluid. The skin cools down, the liquid turns to a gas, and floats away.
5. You have two sealed jars of water at the same temperature. In the first jar there is a large
amount of water. In the second jar there is a small amount of water. Using 3 -4 sentences
explain how the vapor pressure of water in the first jar compares with the vapor pressure of
water in the second jar.
6. Using 3 – 4 sentences explain (in your own words) why water expands when it freezes?
Water molecules tend to stick together to launch what are called hydrogen bonds. Therefore, Because
of the shape of the molecule, the process water molecules tend to be prone together is very open
formation. The oxygen atom is negative, and the hydrogens are positively charged. That means, there’s
quite a lot of extra empty space. When water freezes it allow to leave energy because lots of extra
chains can be made which take up more space.
7. Using your knowledge of colligative properties explain whether sodium chloride or calcium
chloride would be a more effective substance to melt the ice on a slick sidewalk. Use 3 – 4
sentences in your explanation.
Because the boiling point is higher, calcium chloride releases heat making the ice melt fast than the
sodium chloride. It would be more effective to use CaCl2 for the melting of ice because NaCl separate
into two particles and CaCl2 into three.
8. When a 2.5 mol of sugar (C12H22O11) are added to a certain amount of water the boiling point is
raised by 1 Celsius degree. If 2.5 mol of aluminum nitrate is added to the same amount of
water, by how much will the boiling point be changed? Show all calculations leading to your
answer OR use 3 – 4 sentences to explain your answer.

sugar does not detach
Al(NO3)3 will separate to produce
Al(NO3)3 → Al3+ + 3NO3-
That is 4 ions
The boiling point elevation will be 4 times that of sugar
Boiling point elevation
Al(NO3)3 solution = 1°C *4 = 4°C
9. If 5.40 kcal of heat is added to 1.00 kg of water at 100⁰C, how much steam at 100⁰C is
produced? Show all calculations leading to an answer.
Hv=2260kj/kgwater=2260kj*1kcal/4.18kj*1/kgwater
Hv= 540kcal/kgwater.
Q=m*Hv
mass=Q/Hv= 5.40kg/(540kcal/kgwater)
0.01kg = 10.0 grams’ water.
540 = 1kg x 4.18xt
10. The freezing of water at 0⁰C can be represented as follows:
H2O (l) ↔ H2O(s)
The density of liquid water is 1.00 g/cm3. The density of ice is 0.92 g/cm3. In 3 – 4 sentences
explain why applying pressure causes ice to melt.
The wire authorizes to pass through the entire block, because the pressure causes the ice to
melt. The wire's track will fill as soon as pressure is grateful. Therefore, the ice block will remain
frozen even after the wire passes completely through.
11. The Kw of water varies with temperature. Calculate the pH of water at 46⁰C with a Kw = 1.219 x
10-14. Show all calculations leading to an answer.
[H+] [OH-] = Kw.
[H+] = [OH-]
[H+] = √Kw
[H+] = √ (3.219*10^-14)
[H+] = 1.79*10^-7
pH = -log (1.79*10^-7)
pH = 6.75.
The pH of neutral water at 46°C = 6.75.
Al(NO3)3 will separate to produce
Al(NO3)3 → Al3+ + 3NO3-
That is 4 ions
The boiling point elevation will be 4 times that of sugar
Boiling point elevation
Al(NO3)3 solution = 1°C *4 = 4°C
9. If 5.40 kcal of heat is added to 1.00 kg of water at 100⁰C, how much steam at 100⁰C is
produced? Show all calculations leading to an answer.
Hv=2260kj/kgwater=2260kj*1kcal/4.18kj*1/kgwater
Hv= 540kcal/kgwater.
Q=m*Hv
mass=Q/Hv= 5.40kg/(540kcal/kgwater)
0.01kg = 10.0 grams’ water.
540 = 1kg x 4.18xt
10. The freezing of water at 0⁰C can be represented as follows:
H2O (l) ↔ H2O(s)
The density of liquid water is 1.00 g/cm3. The density of ice is 0.92 g/cm3. In 3 – 4 sentences
explain why applying pressure causes ice to melt.
The wire authorizes to pass through the entire block, because the pressure causes the ice to
melt. The wire's track will fill as soon as pressure is grateful. Therefore, the ice block will remain
frozen even after the wire passes completely through.
11. The Kw of water varies with temperature. Calculate the pH of water at 46⁰C with a Kw = 1.219 x
10-14. Show all calculations leading to an answer.
[H+] [OH-] = Kw.
[H+] = [OH-]
[H+] = √Kw
[H+] = √ (3.219*10^-14)
[H+] = 1.79*10^-7
pH = -log (1.79*10^-7)
pH = 6.75.
The pH of neutral water at 46°C = 6.75.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

12. Calculate the hydroxide ion concentration of a solution with pH = 3.25. Show all calculations
leading to an answer.
pH = -log[H+]
[H+] = 10^-pH
[H+] = 10^-3.25 = 5.62x10^-4
Kw = [H+] [OH-] = 1.00x10^-14
25C
[OH-] = Kw / [H+]
[OH-] = 1.00x10^-14 / 5.62x10^-4
[OH-] = 1.778x10^-11
1.8x10^-11M
13. What salt results from the neutralization of phosphoric acid with potassium hydroxide?
H3Po4+3KOH-K3PO4+3H2O Potassium Phosphate
14. What is the oxidation number of chlorine in Al(ClO4)3?
It would be +7 oxidation number.
the oxidation state is 3.
You have 12 O at -2 each for -24.
-24+3= -21.
+21/3 or +7 for each Cl.
15. Which element is oxidized in the following reaction:
CuO(s) + H2 (g) Cu(s) + H2O (l)
H2g
16. Why must the number of electrons lost equal the number of electrons gained in every redox
reaction? Use 3 – 4 sentences in your own words to address this question.
Conservation of Charge is the L number of electrons that must gained and also equal the number of
electrons lost. If a molecule gains an electron, another molecule must have lost it.
17. The following unbalanced equation describes the reaction that can occur when lead (II) sulfide
reacts with oxygen gas to produce lead (II) oxide and sulfur dioxide gas:
leading to an answer.
pH = -log[H+]
[H+] = 10^-pH
[H+] = 10^-3.25 = 5.62x10^-4
Kw = [H+] [OH-] = 1.00x10^-14
25C
[OH-] = Kw / [H+]
[OH-] = 1.00x10^-14 / 5.62x10^-4
[OH-] = 1.778x10^-11
1.8x10^-11M
13. What salt results from the neutralization of phosphoric acid with potassium hydroxide?
H3Po4+3KOH-K3PO4+3H2O Potassium Phosphate
14. What is the oxidation number of chlorine in Al(ClO4)3?
It would be +7 oxidation number.
the oxidation state is 3.
You have 12 O at -2 each for -24.
-24+3= -21.
+21/3 or +7 for each Cl.
15. Which element is oxidized in the following reaction:
CuO(s) + H2 (g) Cu(s) + H2O (l)
H2g
16. Why must the number of electrons lost equal the number of electrons gained in every redox
reaction? Use 3 – 4 sentences in your own words to address this question.
Conservation of Charge is the L number of electrons that must gained and also equal the number of
electrons lost. If a molecule gains an electron, another molecule must have lost it.
17. The following unbalanced equation describes the reaction that can occur when lead (II) sulfide
reacts with oxygen gas to produce lead (II) oxide and sulfur dioxide gas:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PbS + O2 PbO + SO2
Balance the equation and describe in words the electron transfer(s) that takes place.
Pb goes from +2 on the left to +2 on the right
O2 on the left goes to O on PbO with -2 and to SO2 at -2 EACH.
18. What type of radiation is emitted when chromium-51 decays into manganese-51? Show the
nuclear equation that leads you to this answer.
Chromium- 51/24
Manganese- 51/25 + Beta- 0/-1
19. A radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. What was the
identity of the original nucleus? Show the nuclear equation that leads you to this answer.
add 24+4= 28
11+2= 13
20. Iron-59 has a half-life of 45.1 days. How old is an iron nail if the Fe-59 content is 25% that of a
new sample of iron? Show all calculations leading to a solution.
25=100 e^ (-.693 t/45.1)
Ln (25) =ln (100) -.693 t/45.1
ln (25)-ln (100) =-.693 t/45.1
ln (25/100) =-.693t/45.1 t= - 45.1/.693 (ln .25)
t=90.2 days.
Balance the equation and describe in words the electron transfer(s) that takes place.
Pb goes from +2 on the left to +2 on the right
O2 on the left goes to O on PbO with -2 and to SO2 at -2 EACH.
18. What type of radiation is emitted when chromium-51 decays into manganese-51? Show the
nuclear equation that leads you to this answer.
Chromium- 51/24
Manganese- 51/25 + Beta- 0/-1
19. A radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. What was the
identity of the original nucleus? Show the nuclear equation that leads you to this answer.
add 24+4= 28
11+2= 13
20. Iron-59 has a half-life of 45.1 days. How old is an iron nail if the Fe-59 content is 25% that of a
new sample of iron? Show all calculations leading to a solution.
25=100 e^ (-.693 t/45.1)
Ln (25) =ln (100) -.693 t/45.1
ln (25)-ln (100) =-.693 t/45.1
ln (25/100) =-.693t/45.1 t= - 45.1/.693 (ln .25)
t=90.2 days.
1 out of 5
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
