Detailed Chemistry Final Exam: A Thorough Assessment of Concepts
VerifiedAdded on 2023/06/13
|9
|1199
|207
Quiz and Exam
AI Summary
This document presents a solved Chemistry final exam covering various topics, including gas laws, solutions, colligative properties, thermochemistry, chemical kinetics, equilibrium, and nuclear chemistry. The exam consists of multiple-choice and problem-solving questions, with detailed solutions provided for each question. Topics covered include Gay-Lussac's Law, solution saturation, intermolecular forces, vapor pressure, hydrogen bonding, heat transfer, boiling point elevation, enthalpy, pH calculations, acid-base reactions, oxidation numbers, redox reactions, nuclear equations, and radioactive decay. The solutions demonstrate the application of key chemical principles and calculations to arrive at the correct answers.

CHEMISTRY
FINAL EXAM
Student Name
[Pick the date]
FINAL EXAM
Student Name
[Pick the date]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Question 1
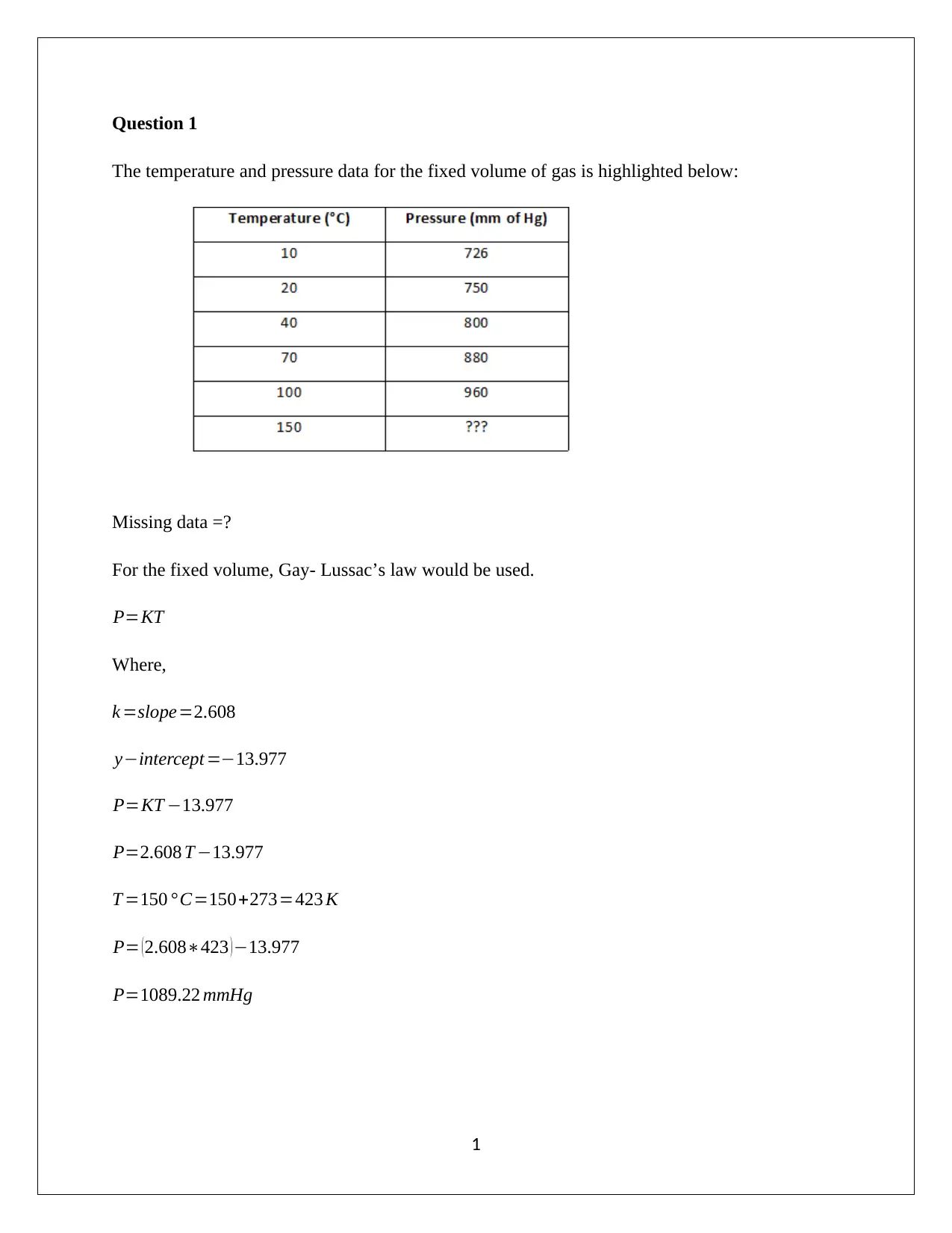
The temperature and pressure data for the fixed volume of gas is highlighted below:
Missing data =?
For the fixed volume, Gay- Lussac’s law would be used.
P=KT
Where,
k =slope=2.608
y−intercept =−13.977
P=KT −13.977
P=2.608 T −13.977
T =150 ° C=150+273=423 K
P= ( 2.608∗423 ) −13.977
P=1089.22 mmHg
1
The temperature and pressure data for the fixed volume of gas is highlighted below:
Missing data =?
For the fixed volume, Gay- Lussac’s law would be used.
P=KT
Where,
k =slope=2.608
y−intercept =−13.977
P=KT −13.977
P=2.608 T −13.977
T =150 ° C=150+273=423 K
P= ( 2.608∗423 ) −13.977
P=1089.22 mmHg
1

Question 2
It is apparent that the solution of KNO3 is a clear liquid. We will first leave the liquid to stand for
some time. We will observe whether the crystals are formed or not in the solution. If crystals are
formed, it means the solution is supersaturated. Further, if no crystals are formed, then we will
add some crystals of KNO3 in the solution. If the crystals are dissolved in the solution, then the
solution would be unsaturated. If the crystals do not dissolve, then the solution is saturated.
Question 3
Gasoline does not dissolve in water because water is a polar molecule while gasoline is non-
polar molecule. If one wants to dissolve gasoline in water, then it is essential that gasoline is in
polar form.
Question 4
Perspiration in the skin is a process by which the skin would remain cool due to the evaporation
of the sweat. Thermal energy would be received from the environment and this thermal energy
would transform the liquid (Sweat) into gas. It means the skin gets cooled and relaxed.
Question 5
The vapour pressure of the water mainly depends on the temperature of the water and the purity
of water. If the given two jars are at the same temperature and the water is having same level of
purity, then the vapour pressure in the given two jars would be the same.
Question 6
It is apparent that hydrogen is slightly positive and oxygen is slightly negative. Hence, oxygen
and hydrogen would combine together to form hydrogen bonds. When, water is allowed to freeze
2
It is apparent that the solution of KNO3 is a clear liquid. We will first leave the liquid to stand for
some time. We will observe whether the crystals are formed or not in the solution. If crystals are
formed, it means the solution is supersaturated. Further, if no crystals are formed, then we will
add some crystals of KNO3 in the solution. If the crystals are dissolved in the solution, then the
solution would be unsaturated. If the crystals do not dissolve, then the solution is saturated.
Question 3
Gasoline does not dissolve in water because water is a polar molecule while gasoline is non-
polar molecule. If one wants to dissolve gasoline in water, then it is essential that gasoline is in
polar form.
Question 4
Perspiration in the skin is a process by which the skin would remain cool due to the evaporation
of the sweat. Thermal energy would be received from the environment and this thermal energy
would transform the liquid (Sweat) into gas. It means the skin gets cooled and relaxed.
Question 5
The vapour pressure of the water mainly depends on the temperature of the water and the purity
of water. If the given two jars are at the same temperature and the water is having same level of
purity, then the vapour pressure in the given two jars would be the same.
Question 6
It is apparent that hydrogen is slightly positive and oxygen is slightly negative. Hence, oxygen
and hydrogen would combine together to form hydrogen bonds. When, water is allowed to freeze
2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

then in such cases loads of open holes like structure would form and result in ample empty
spaces in water. Hence, this empty spaces releases energy due to the formation of strong
hydrogen bonds because of freezing. Hence, the water expands when it freezes.
Question 7
In order to melt ice, heat is required. Further, it is apparent that when calcium chloride is
dissolved in water, then it is exothermic reaction and heat is produced. This generated heat
would help the ice to melt. However, there is no heat generated when sodium chloride is
dissolved in ice. Hence, calcium chloride is the best option to melt ice as compared to sodium
chloride. Moreover, dissolution of calcium chloride (3 ions) would release more ions as
compared with the ions produced by sodium chloride (2 ions).
Question 8
2.5 mole of sugar is added to water due to which boiling point increase by 1° ∁. Sugar is a non-
electrolyte and its Vant Holf factor is one.
Further,
2.5 mole of Aluminium nitrate added to water now the change in boiling point needs to be
computed.
Aluminium nitrate would form 4 ions when it gets dissolved in water. It means its Vant Holf
factor is 4. Hence, the boiling point elevation would be the 4 times of the boiling point elevation
incurred by sugar dissolution.
Aluminium nitrate added to water now the change in boiling point = 1 * 4 = 4 ° ∁
Question 9
1 cal=4.18 kj
3
spaces in water. Hence, this empty spaces releases energy due to the formation of strong
hydrogen bonds because of freezing. Hence, the water expands when it freezes.
Question 7
In order to melt ice, heat is required. Further, it is apparent that when calcium chloride is
dissolved in water, then it is exothermic reaction and heat is produced. This generated heat
would help the ice to melt. However, there is no heat generated when sodium chloride is
dissolved in ice. Hence, calcium chloride is the best option to melt ice as compared to sodium
chloride. Moreover, dissolution of calcium chloride (3 ions) would release more ions as
compared with the ions produced by sodium chloride (2 ions).
Question 8
2.5 mole of sugar is added to water due to which boiling point increase by 1° ∁. Sugar is a non-
electrolyte and its Vant Holf factor is one.
Further,
2.5 mole of Aluminium nitrate added to water now the change in boiling point needs to be
computed.
Aluminium nitrate would form 4 ions when it gets dissolved in water. It means its Vant Holf
factor is 4. Hence, the boiling point elevation would be the 4 times of the boiling point elevation
incurred by sugar dissolution.
Aluminium nitrate added to water now the change in boiling point = 1 * 4 = 4 ° ∁
Question 9
1 cal=4.18 kj
3
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Hence,
5.4 Kcal=5.4∗4.18 kJ =22.6 kJ
Steam ¿ 22.6 kJ
40.657 =0.556 moles of steam
0.556 moles of steam = 10 g of steam
Question 10
Density of ice = 0.92 g/cm3
Density of water = 1 g/cm3
92 grams of ice volume means = 92/0.92 = 100 cm3
Hence,
On melting application, the 1000 cm3 is in contrast to 92 cm3 of water.
Question 11
Kw = 1.219 x 10-14
pH of water=46 °C
¿
In case of pure water: ¿
¿
¿ ¿
¿
¿
4
5.4 Kcal=5.4∗4.18 kJ =22.6 kJ
Steam ¿ 22.6 kJ
40.657 =0.556 moles of steam
0.556 moles of steam = 10 g of steam
Question 10
Density of ice = 0.92 g/cm3
Density of water = 1 g/cm3
92 grams of ice volume means = 92/0.92 = 100 cm3
Hence,
On melting application, the 1000 cm3 is in contrast to 92 cm3 of water.
Question 11
Kw = 1.219 x 10-14
pH of water=46 °C
¿
In case of pure water: ¿
¿
¿ ¿
¿
¿
4

¿
Now,
pH=log¿ ¿
pH =6.96
Question 12
Hydroxide ion concentration of solution =?
pH of solution=3.25
¿
¿
¿
Question 13
H3 PO4 + 3 KOH → K3 PO4 +3 H2 O
Question 14
Oxidation number of chlorine in Al(ClO¿ ¿ 4)3 ¿
Al is in group III and hence, the oxidation state would be 3. Further, 3 chlorine atoms must be
having +21 in order to balance -21. Hence, +21/3 = +7 would be the oxidation number for each
Cl.
Question 15
5
Now,
pH=log¿ ¿
pH =6.96
Question 12
Hydroxide ion concentration of solution =?
pH of solution=3.25
¿
¿
¿
Question 13
H3 PO4 + 3 KOH → K3 PO4 +3 H2 O
Question 14
Oxidation number of chlorine in Al(ClO¿ ¿ 4)3 ¿
Al is in group III and hence, the oxidation state would be 3. Further, 3 chlorine atoms must be
having +21 in order to balance -21. Hence, +21/3 = +7 would be the oxidation number for each
Cl.
Question 15
5
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

CuO+ H2 → Cu+ H2 O
Element that would oxidized in the reaction ¿ Cuo
Question 16
In order to balance the equation, conservation of mass would be taken into consideration. Hence,
it is essential that in redox reactions number of electrons lost in the reaction must be equal to the
number of electrons gained in the reaction.
Question 17
Pb would go from +2 from the left side and +2 from the right side. Further, no change would
place on O2 from the left goes to o and on PbO with the -2. Similarly, for SO2 to -2 would go.
Question 18
Nuclear equation
Beta radiation (Cr 51/25) – (Management 51/25) + Beta 0/-1
Question 19
Nuclear equation
AX → 24 Na+ 4 α
Z 11 2
Here,
A = 24+4 = 28
6
Element that would oxidized in the reaction ¿ Cuo
Question 16
In order to balance the equation, conservation of mass would be taken into consideration. Hence,
it is essential that in redox reactions number of electrons lost in the reaction must be equal to the
number of electrons gained in the reaction.
Question 17
Pb would go from +2 from the left side and +2 from the right side. Further, no change would
place on O2 from the left goes to o and on PbO with the -2. Similarly, for SO2 to -2 would go.
Question 18
Nuclear equation
Beta radiation (Cr 51/25) – (Management 51/25) + Beta 0/-1
Question 19
Nuclear equation
AX → 24 Na+ 4 α
Z 11 2
Here,
A = 24+4 = 28
6
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Z=11+2=13
Question 20
100 %……………45.1………..50 %...........45.1……….25 %
Hence,
45.1+45.1=90.2 days
Question 21
It is noteworthy that liquefied gas is taken as propellant for modern aerosol. High pressure would
be applied in order to compress the gas into liquid phase. The produced vapors would create
pressure force that will make the product to go out from the can. Further, when the product level
decreases then increase amount of propellant would be consumed to maintain a constant
pressure.
Question 22
“pH is a measure of A hydrogen ion concentration.”
Question 23
“A neutralization reaction between an acid and a base always produced salt and water”
Question 24
“If the pH of a solution is 10 the solution is basic.
7
Question 20
100 %……………45.1………..50 %...........45.1……….25 %
Hence,
45.1+45.1=90.2 days
Question 21
It is noteworthy that liquefied gas is taken as propellant for modern aerosol. High pressure would
be applied in order to compress the gas into liquid phase. The produced vapors would create
pressure force that will make the product to go out from the can. Further, when the product level
decreases then increase amount of propellant would be consumed to maintain a constant
pressure.
Question 22
“pH is a measure of A hydrogen ion concentration.”
Question 23
“A neutralization reaction between an acid and a base always produced salt and water”
Question 24
“If the pH of a solution is 10 the solution is basic.
7

Question 25
“Enthalpy heat content of a system at constant pressure.”
Question 26
“Energy ability to do work”
Question 27
“Entropy Disorder”
Question 28
“Kinetic energy is energy of motion.”
Question 29
“Thermo Chemistry is study of energy changes that occurred during chemic reaction and
changes in states.”
Question 29
“Calorie is the amount of heat required to raise T of 1 g of pure water 1degree Celsius”
8
“Enthalpy heat content of a system at constant pressure.”
Question 26
“Energy ability to do work”
Question 27
“Entropy Disorder”
Question 28
“Kinetic energy is energy of motion.”
Question 29
“Thermo Chemistry is study of energy changes that occurred during chemic reaction and
changes in states.”
Question 29
“Calorie is the amount of heat required to raise T of 1 g of pure water 1degree Celsius”
8
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.