Chemistry of Organic Compounds
VerifiedAdded on 2020/06/04
|20
|3522
|284
AI Summary
This chemistry assignment delves into the properties and reactions of various organic compounds. It explores the structure of water, lactic acid, and valine, explaining their bonding and spatial arrangement. The assignment also examines reactions involving pent-2-ene, 2,2-dimethylpropan-2-ol, and propanoic acid, illustrating how structural formulae relate to chemical transformations. Lastly, it discusses isomerism in compounds like butanal, 4-hydroxybut-1-ene, 2-amino-3-methylbutanoic acid, and 2-aminopropanoic acid.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.
Chemistry for applied biology
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Table of Contents
TASK 1 (AC: 1.1, 1.2, 1.3)..............................................................................................................1
A) Physical properties of substances...........................................................................................1
B) Enthalpy changes .................................................................................................................5
C) Factors affecting the rate of sucrose break down reaction ....................................................6
TASK 2 (AC: 2.1, 2.2, 2.3, 2.4).......................................................................................................6
1) ................................................................................................................................................6
a) Enthalpy of formation and enthalpy change for the reaction..................................................6
b) Values of ΔSƟ at 298K..........................................................................................................7
c) Values of SƟ for all other substances and ΔSƟ for the system............................................7
d) ΔSƟ and ΔGƟ ......................................................................................................................7
e) Feasibility of the process and the assumptions.......................................................................7
2) Redox Reactions.....................................................................................................................7
3) Using oxidation numbers for deciding the redox nature of the reaction or process...............8
4) Feasibility of the process........................................................................................................9
TASK 3 (AC: 3.1, 3.2, 3.3, 3.4).......................................................................................................9
A) Experimental work on equilibrium system............................................................................9
B) Shift in equilibrium position for favouring the formation of products................................10
C) Monoprotic weak acid..........................................................................................................10
TASK 4 (AC: 4.1,4.2, 4.3, 4.4, 4.5)...............................................................................................11
A) Relation of shapes and bonding in molecules .....................................................................11
B) Structures and systematic names .........................................................................................11
C) Reactions and their relation to structural formulae .............................................................12
D) Isomerism displayed ...........................................................................................................13
TASK 1 (AC: 1.1, 1.2, 1.3)..............................................................................................................1
A) Physical properties of substances...........................................................................................1
B) Enthalpy changes .................................................................................................................5
C) Factors affecting the rate of sucrose break down reaction ....................................................6
TASK 2 (AC: 2.1, 2.2, 2.3, 2.4).......................................................................................................6
1) ................................................................................................................................................6
a) Enthalpy of formation and enthalpy change for the reaction..................................................6
b) Values of ΔSƟ at 298K..........................................................................................................7
c) Values of SƟ for all other substances and ΔSƟ for the system............................................7
d) ΔSƟ and ΔGƟ ......................................................................................................................7
e) Feasibility of the process and the assumptions.......................................................................7
2) Redox Reactions.....................................................................................................................7
3) Using oxidation numbers for deciding the redox nature of the reaction or process...............8
4) Feasibility of the process........................................................................................................9
TASK 3 (AC: 3.1, 3.2, 3.3, 3.4).......................................................................................................9
A) Experimental work on equilibrium system............................................................................9
B) Shift in equilibrium position for favouring the formation of products................................10
C) Monoprotic weak acid..........................................................................................................10
TASK 4 (AC: 4.1,4.2, 4.3, 4.4, 4.5)...............................................................................................11
A) Relation of shapes and bonding in molecules .....................................................................11
B) Structures and systematic names .........................................................................................11
C) Reactions and their relation to structural formulae .............................................................12
D) Isomerism displayed ...........................................................................................................13


TASK 1 (AC: 1.1, 1.2, 1.3)
A) Physical properties of substances
a) Water
The molecular formula for water is H2O and the following is the structural formula
depicting the bonds and molecular arrangement of the molecules of water.
The shape of this molecular is contemplated as bent shape and bonds formed in this
structure are covalent bonds. These are quite strong but have weak force of attraction. This
property relates to the flexibility of water in having no specific shape. The fluidity is acquired
due to this nature of the bonds. There are two lone pairs denoted over oxygen. Water is found in
liquid form. Its gaseous form is known as water vapour while the solid version is ice. Water has
three forms which includes liquid, solid (ice) and gaseous as water vapour. The boiling point of
water is 100°C and freezing point is 0°C. The cohesiveness for water is very high while
viscosity is low because it has high flowing capacity.
b) Methane
The chemical formula of methane is CH4 and the following is the structural formula:
1
Illustration 1: Structural formula of
water
A) Physical properties of substances
a) Water
The molecular formula for water is H2O and the following is the structural formula
depicting the bonds and molecular arrangement of the molecules of water.
The shape of this molecular is contemplated as bent shape and bonds formed in this
structure are covalent bonds. These are quite strong but have weak force of attraction. This
property relates to the flexibility of water in having no specific shape. The fluidity is acquired
due to this nature of the bonds. There are two lone pairs denoted over oxygen. Water is found in
liquid form. Its gaseous form is known as water vapour while the solid version is ice. Water has
three forms which includes liquid, solid (ice) and gaseous as water vapour. The boiling point of
water is 100°C and freezing point is 0°C. The cohesiveness for water is very high while
viscosity is low because it has high flowing capacity.
b) Methane
The chemical formula of methane is CH4 and the following is the structural formula:
1
Illustration 1: Structural formula of
water
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
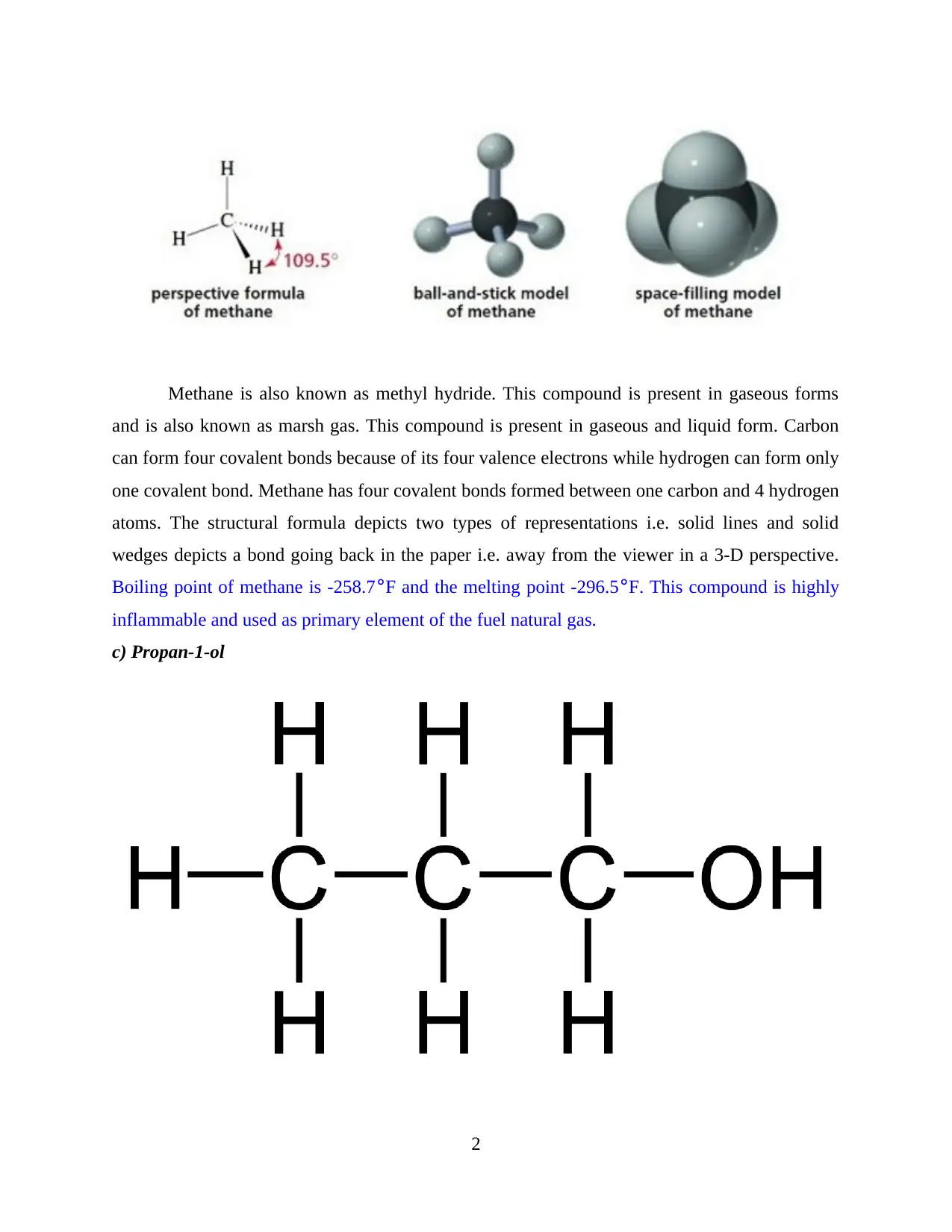
Methane is also known as methyl hydride. This compound is present in gaseous forms
and is also known as marsh gas. This compound is present in gaseous and liquid form. Carbon
can form four covalent bonds because of its four valence electrons while hydrogen can form only
one covalent bond. Methane has four covalent bonds formed between one carbon and 4 hydrogen
atoms. The structural formula depicts two types of representations i.e. solid lines and solid
wedges depicts a bond going back in the paper i.e. away from the viewer in a 3-D perspective.
Boiling point of methane is -258.7°F and the melting point -296.5°F. This compound is highly
inflammable and used as primary element of the fuel natural gas.
c) Propan-1-ol
2
and is also known as marsh gas. This compound is present in gaseous and liquid form. Carbon
can form four covalent bonds because of its four valence electrons while hydrogen can form only
one covalent bond. Methane has four covalent bonds formed between one carbon and 4 hydrogen
atoms. The structural formula depicts two types of representations i.e. solid lines and solid
wedges depicts a bond going back in the paper i.e. away from the viewer in a 3-D perspective.
Boiling point of methane is -258.7°F and the melting point -296.5°F. This compound is highly
inflammable and used as primary element of the fuel natural gas.
c) Propan-1-ol
2
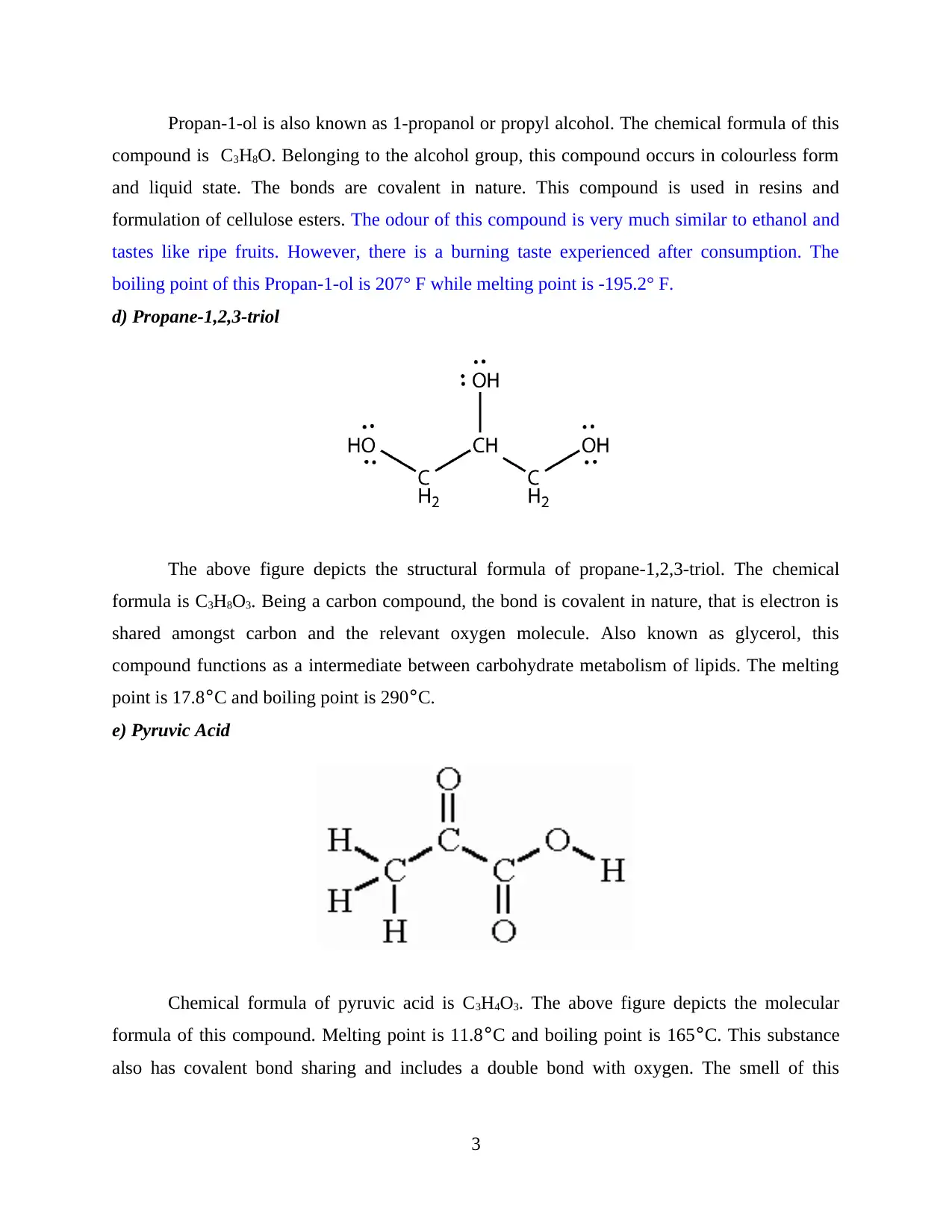
Propan-1-ol is also known as 1-propanol or propyl alcohol. The chemical formula of this
compound is C3H8O. Belonging to the alcohol group, this compound occurs in colourless form
and liquid state. The bonds are covalent in nature. This compound is used in resins and
formulation of cellulose esters. The odour of this compound is very much similar to ethanol and
tastes like ripe fruits. However, there is a burning taste experienced after consumption. The
boiling point of this Propan-1-ol is 207° F while melting point is -195.2° F.
d) Propane-1,2,3-triol
The above figure depicts the structural formula of propane-1,2,3-triol. The chemical
formula is C3H8O3. Being a carbon compound, the bond is covalent in nature, that is electron is
shared amongst carbon and the relevant oxygen molecule. Also known as glycerol, this
compound functions as a intermediate between carbohydrate metabolism of lipids. The melting
point is 17.8°C and boiling point is 290°C.
e) Pyruvic Acid
Chemical formula of pyruvic acid is C3H4O3. The above figure depicts the molecular
formula of this compound. Melting point is 11.8°C and boiling point is 165°C. This substance
also has covalent bond sharing and includes a double bond with oxygen. The smell of this
3
compound is C3H8O. Belonging to the alcohol group, this compound occurs in colourless form
and liquid state. The bonds are covalent in nature. This compound is used in resins and
formulation of cellulose esters. The odour of this compound is very much similar to ethanol and
tastes like ripe fruits. However, there is a burning taste experienced after consumption. The
boiling point of this Propan-1-ol is 207° F while melting point is -195.2° F.
d) Propane-1,2,3-triol
The above figure depicts the structural formula of propane-1,2,3-triol. The chemical
formula is C3H8O3. Being a carbon compound, the bond is covalent in nature, that is electron is
shared amongst carbon and the relevant oxygen molecule. Also known as glycerol, this
compound functions as a intermediate between carbohydrate metabolism of lipids. The melting
point is 17.8°C and boiling point is 290°C.
e) Pyruvic Acid
Chemical formula of pyruvic acid is C3H4O3. The above figure depicts the molecular
formula of this compound. Melting point is 11.8°C and boiling point is 165°C. This substance
also has covalent bond sharing and includes a double bond with oxygen. The smell of this
3
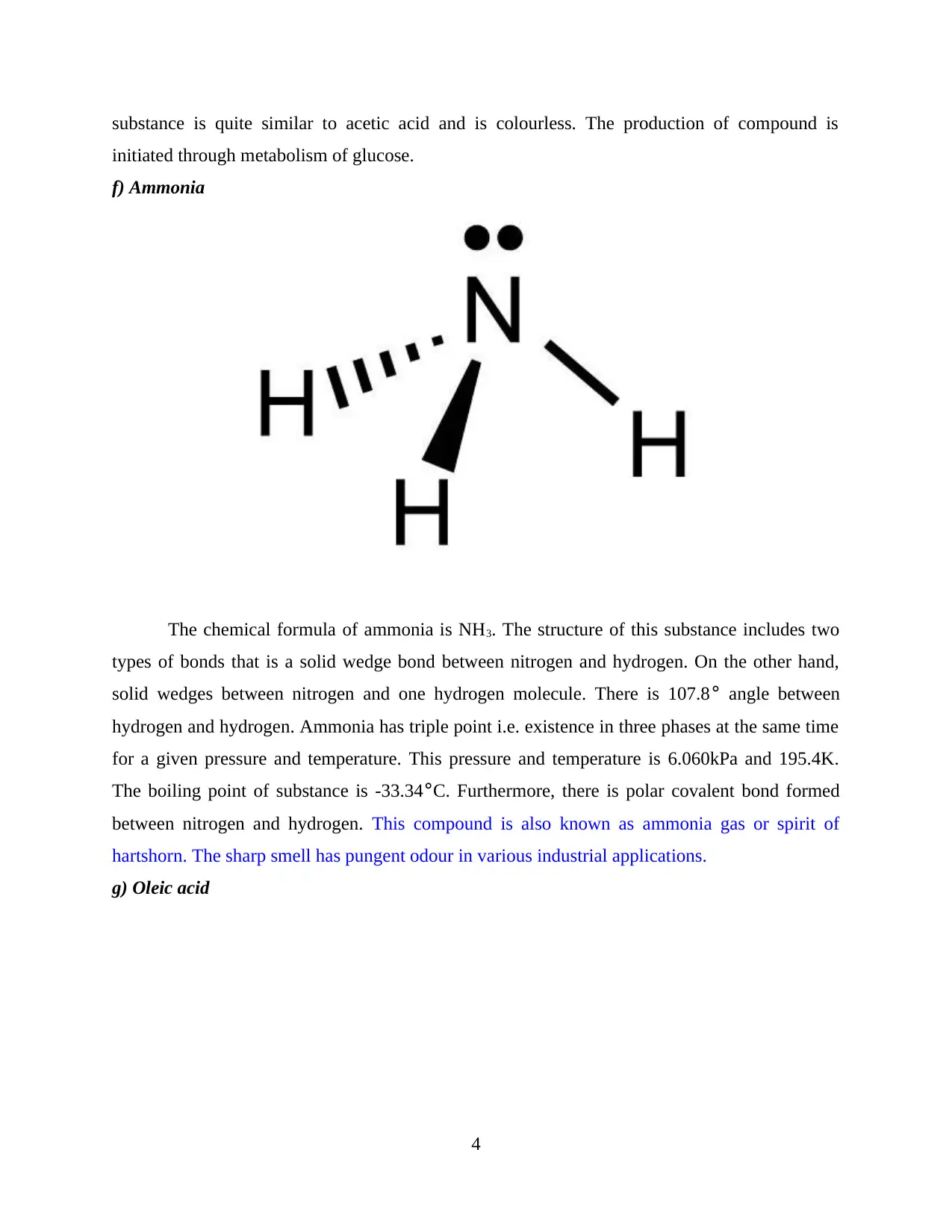
substance is quite similar to acetic acid and is colourless. The production of compound is
initiated through metabolism of glucose.
f) Ammonia
The chemical formula of ammonia is NH3. The structure of this substance includes two
types of bonds that is a solid wedge bond between nitrogen and hydrogen. On the other hand,
solid wedges between nitrogen and one hydrogen molecule. There is 107.8° angle between
hydrogen and hydrogen. Ammonia has triple point i.e. existence in three phases at the same time
for a given pressure and temperature. This pressure and temperature is 6.060kPa and 195.4K.
The boiling point of substance is -33.34°C. Furthermore, there is polar covalent bond formed
between nitrogen and hydrogen. This compound is also known as ammonia gas or spirit of
hartshorn. The sharp smell has pungent odour in various industrial applications.
g) Oleic acid
4
initiated through metabolism of glucose.
f) Ammonia
The chemical formula of ammonia is NH3. The structure of this substance includes two
types of bonds that is a solid wedge bond between nitrogen and hydrogen. On the other hand,
solid wedges between nitrogen and one hydrogen molecule. There is 107.8° angle between
hydrogen and hydrogen. Ammonia has triple point i.e. existence in three phases at the same time
for a given pressure and temperature. This pressure and temperature is 6.060kPa and 195.4K.
The boiling point of substance is -33.34°C. Furthermore, there is polar covalent bond formed
between nitrogen and hydrogen. This compound is also known as ammonia gas or spirit of
hartshorn. The sharp smell has pungent odour in various industrial applications.
g) Oleic acid
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
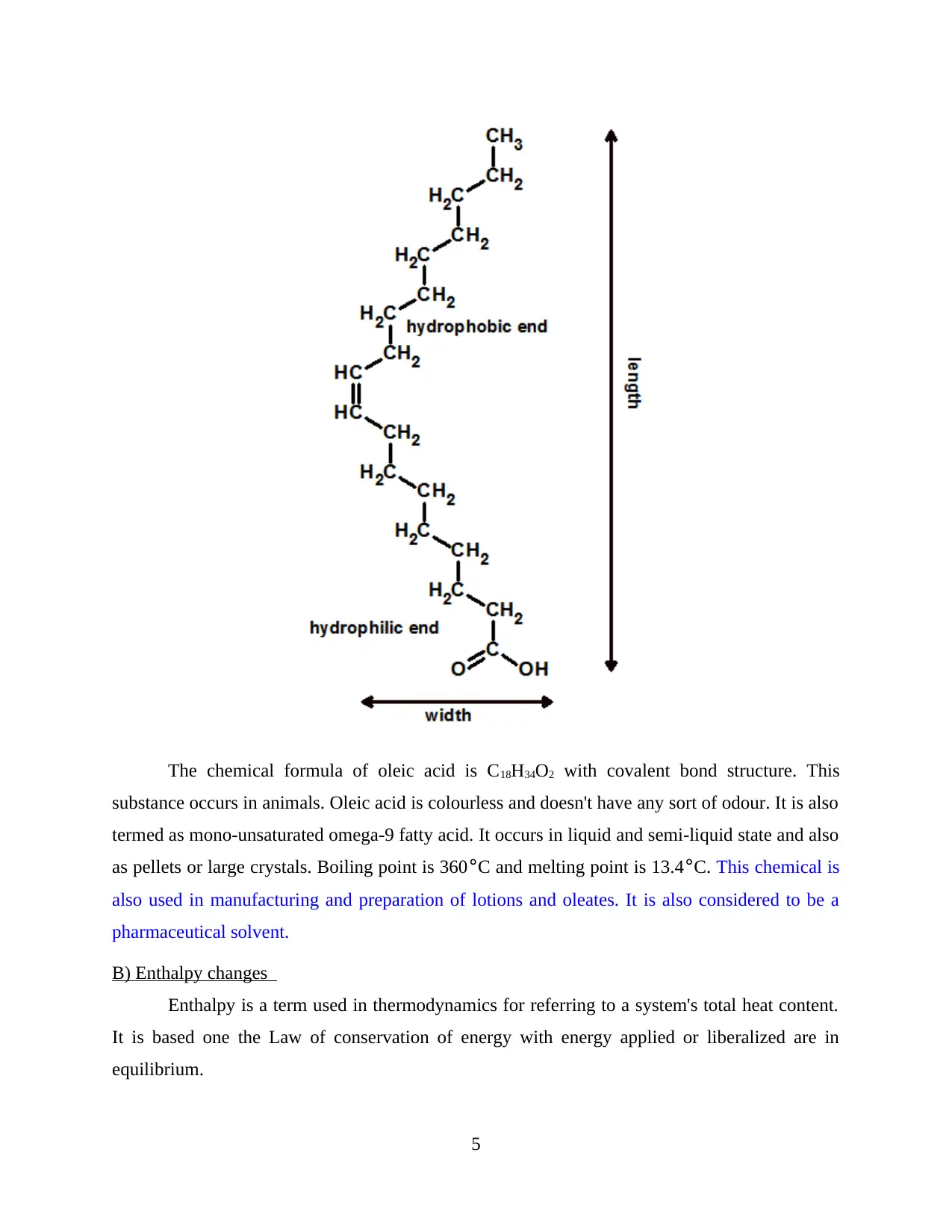
The chemical formula of oleic acid is C18H34O2 with covalent bond structure. This
substance occurs in animals. Oleic acid is colourless and doesn't have any sort of odour. It is also
termed as mono-unsaturated omega-9 fatty acid. It occurs in liquid and semi-liquid state and also
as pellets or large crystals. Boiling point is 360°C and melting point is 13.4°C. This chemical is
also used in manufacturing and preparation of lotions and oleates. It is also considered to be a
pharmaceutical solvent.
B) Enthalpy changes
Enthalpy is a term used in thermodynamics for referring to a system's total heat content.
It is based one the Law of conservation of energy with energy applied or liberalized are in
equilibrium.
5
substance occurs in animals. Oleic acid is colourless and doesn't have any sort of odour. It is also
termed as mono-unsaturated omega-9 fatty acid. It occurs in liquid and semi-liquid state and also
as pellets or large crystals. Boiling point is 360°C and melting point is 13.4°C. This chemical is
also used in manufacturing and preparation of lotions and oleates. It is also considered to be a
pharmaceutical solvent.
B) Enthalpy changes
Enthalpy is a term used in thermodynamics for referring to a system's total heat content.
It is based one the Law of conservation of energy with energy applied or liberalized are in
equilibrium.
5

Enthalpy of dissociation: The change in enthalpy which takes place during the
breakdown or cleavage of a bond in a homo-lytic reaction is known as enthalpy of dissociation.
The chemical reactions in which new compounds are formed or certain elements are displaced
have bond dissociation enthalpies. However, this type of enthalpy change doesn't take place in
diatomic molecules.
Enthalpy of solution: Also known as the heat of solution, this enthalpy is completely
linked with mixing or dissolution of a particular substance on application of constant pressure.
The solvent and substance are constantly dissolving because of certain pressure. The heat
evolved or absorbed in this process is referred to as enthalpy of solution. The temperature is
mostly kept constant.
Lattice enthalpy: There are certain forces active in the lattice structure of a solid i.e.
between different ions. The strength of these forces is measured in the form of lattice enthalpy.
Ionic compounds are formed within standard atmospheric conditions and there is exchange of
charged ions which further helps in formation of the lattice.
Enthalpy of hydration: The energy released during the formation of ionic bonds and
water molecules is known as enthalpy of hydration. The term hydration depicts that a particular
compound or substance is introduced in water. For instance: dissolving copper sulphate in water
results in production of enthalpy of hydration.
Enthalpy profile for oxidation of ethanol is provided as follows:
6
breakdown or cleavage of a bond in a homo-lytic reaction is known as enthalpy of dissociation.
The chemical reactions in which new compounds are formed or certain elements are displaced
have bond dissociation enthalpies. However, this type of enthalpy change doesn't take place in
diatomic molecules.
Enthalpy of solution: Also known as the heat of solution, this enthalpy is completely
linked with mixing or dissolution of a particular substance on application of constant pressure.
The solvent and substance are constantly dissolving because of certain pressure. The heat
evolved or absorbed in this process is referred to as enthalpy of solution. The temperature is
mostly kept constant.
Lattice enthalpy: There are certain forces active in the lattice structure of a solid i.e.
between different ions. The strength of these forces is measured in the form of lattice enthalpy.
Ionic compounds are formed within standard atmospheric conditions and there is exchange of
charged ions which further helps in formation of the lattice.
Enthalpy of hydration: The energy released during the formation of ionic bonds and
water molecules is known as enthalpy of hydration. The term hydration depicts that a particular
compound or substance is introduced in water. For instance: dissolving copper sulphate in water
results in production of enthalpy of hydration.
Enthalpy profile for oxidation of ethanol is provided as follows:
6

The above reaction depicts formation of ethanoic acid after oxidation of ethanol. The
entire process is exothermic and following is the chemical reaction
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
The production of acetic acid is a result of oxidation of alcohol. This reaction is a
exothermic reaction.
Enthalpy of formation is considered as the enthalpy change which occurs when a substance is
formed from certain components. In the oxidation of ethanol, a new substance is formed which is
ethanoic acid and water is released.
C) Factors affecting the rate of sucrose break down reaction
The breakdown of sucrose into glucose and fructose is also known as hydrolysis of
sucrose. The speed of this reaction is affected by following factors:
Concentration of the enzyme
Atmospheric conditions in which the reaction is taking place.
Supply of oxygen in the body.
If the enzyme is not produced in sufficient forms then the reaction rate will be
simultaneously affected. On the other hand, if there is no proper supply of oxygen in the body
7
entire process is exothermic and following is the chemical reaction
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
The production of acetic acid is a result of oxidation of alcohol. This reaction is a
exothermic reaction.
Enthalpy of formation is considered as the enthalpy change which occurs when a substance is
formed from certain components. In the oxidation of ethanol, a new substance is formed which is
ethanoic acid and water is released.
C) Factors affecting the rate of sucrose break down reaction
The breakdown of sucrose into glucose and fructose is also known as hydrolysis of
sucrose. The speed of this reaction is affected by following factors:
Concentration of the enzyme
Atmospheric conditions in which the reaction is taking place.
Supply of oxygen in the body.
If the enzyme is not produced in sufficient forms then the reaction rate will be
simultaneously affected. On the other hand, if there is no proper supply of oxygen in the body
7
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

then reaction rate is affected. The break down process always requires certain amount of energy
so that reaction can be facilitated. When body gets more oxygenated blood then significant
energy is supplied to such reactions of breakdown of sucrose. Furthermore, the concentration of
enzyme when present in excess amount results in slower reaction while of the concentration is
reduced then reaction will not be possible. This implies body will not gain any benefits from
sucrose breakdown which further interrupts other depending processes and reactions.
TASK 2 (AC: 2.1, 2.2, 2.3, 2.4)
1)
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
a) Enthalpy of formation and enthalpy change for the reaction
The enthalpy of formation and enthalpy change for the reaction of oxidation of ethanol is
depicted as follows:
The enthalpy of solution = 17.87kJ mol-1
Enthalpy change ∆H = cm∆T;
∆T = change in temperature during the reaction
Moles of water molecules produced = 1 mole
Enthalpy change for one mole of water = -8.54 kJ
Neutralisation enthalpy = -56.9 kJ/mol
c = 4.18 kJ kg-1 °C-1
m = 1 kg
∆H = -627 kJ/mol
The enthalpy of formation when considering oxidation of ethanol is provided as follows:
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
∆fH = Sum (∆fH for products) – Sum (∆fH for reactants)
∆fH is the enthalpy as per the thermodynamic tables.
∆fH for ethanol = -1058 kJ/mol
∆fH for Oxygen = 0
∆fH for acetic acid = -483.5 kJ/mol
∆fH for water = -285.9 kJ/mol
Substituting the values as per the formula:
8
so that reaction can be facilitated. When body gets more oxygenated blood then significant
energy is supplied to such reactions of breakdown of sucrose. Furthermore, the concentration of
enzyme when present in excess amount results in slower reaction while of the concentration is
reduced then reaction will not be possible. This implies body will not gain any benefits from
sucrose breakdown which further interrupts other depending processes and reactions.
TASK 2 (AC: 2.1, 2.2, 2.3, 2.4)
1)
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
a) Enthalpy of formation and enthalpy change for the reaction
The enthalpy of formation and enthalpy change for the reaction of oxidation of ethanol is
depicted as follows:
The enthalpy of solution = 17.87kJ mol-1
Enthalpy change ∆H = cm∆T;
∆T = change in temperature during the reaction
Moles of water molecules produced = 1 mole
Enthalpy change for one mole of water = -8.54 kJ
Neutralisation enthalpy = -56.9 kJ/mol
c = 4.18 kJ kg-1 °C-1
m = 1 kg
∆H = -627 kJ/mol
The enthalpy of formation when considering oxidation of ethanol is provided as follows:
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
∆fH = Sum (∆fH for products) – Sum (∆fH for reactants)
∆fH is the enthalpy as per the thermodynamic tables.
∆fH for ethanol = -1058 kJ/mol
∆fH for Oxygen = 0
∆fH for acetic acid = -483.5 kJ/mol
∆fH for water = -285.9 kJ/mol
Substituting the values as per the formula:
8

∆fH = (-285.9 – 483.5 ) - (-1058)
∆fH = 1058 – 769.4
∆fH = 288.6 kJ/mol is the enthalpy of formation
b) Values of ΔSƟ at 298K
The molecular entropy of the initial reaction that is at the reactant end is 365.7 J/(mol K)
and the final value of S is 229.9 J/(mol K).
c) Values of SƟ for all other substances and ΔSƟ for the system
Molecular and total entropy for ethanol is 160.7 J/(mol K) and oxygen is 205 J/(mol K).
This can also be considered as initial values and the following are final values.
Acetic acid has molecular entropy as 160 J/(mol K) and water has molecular entropy as
69.91 J/(mol K)
Hence, SƟ for C2H4O2 is 160 J/(mol K) and for H2O is 69.91 J/(mol K)
ΔSƟ = 229.9-365.7
= -135.8 J/(mol K)
The value of ΔSƟ at 298 K is -135.8 J/(mol K) for the entire system
d) ΔSƟ and ΔGƟ
ΔGƟ = ΔHƟ – T ΔSƟsystem
Here, delta G is known as Gibbs energy or Gibbs free energy
ΔGƟ = -627- 56.9
ΔGƟ = -683.9 kJ/mol (exergonic)
e) Feasibility of the process and the assumptions
The assumptions for this reaction include standard values of specific heats and the
standard temperature at which reaction takes place. Further, the molecular entropies for all the
substances have been taken as per the standards. The feasibility of process is quite large and
there is sufficient production of energy which means the reaction is exothermic.
2) Redox Reactions
Following are the displacement reactions between chlorine and bromine
Cl2(aq) + 2KBr(aq) ==> 2KCl(aq) + Br2(aq)__________(i)
Cl2(aq) + 2Br–(aq) ==> 2Cl–(aq) + Br2(aq)_________________(1)
The below produced reactions are the half reactions for displacement of ions:
9
∆fH = 1058 – 769.4
∆fH = 288.6 kJ/mol is the enthalpy of formation
b) Values of ΔSƟ at 298K
The molecular entropy of the initial reaction that is at the reactant end is 365.7 J/(mol K)
and the final value of S is 229.9 J/(mol K).
c) Values of SƟ for all other substances and ΔSƟ for the system
Molecular and total entropy for ethanol is 160.7 J/(mol K) and oxygen is 205 J/(mol K).
This can also be considered as initial values and the following are final values.
Acetic acid has molecular entropy as 160 J/(mol K) and water has molecular entropy as
69.91 J/(mol K)
Hence, SƟ for C2H4O2 is 160 J/(mol K) and for H2O is 69.91 J/(mol K)
ΔSƟ = 229.9-365.7
= -135.8 J/(mol K)
The value of ΔSƟ at 298 K is -135.8 J/(mol K) for the entire system
d) ΔSƟ and ΔGƟ
ΔGƟ = ΔHƟ – T ΔSƟsystem
Here, delta G is known as Gibbs energy or Gibbs free energy
ΔGƟ = -627- 56.9
ΔGƟ = -683.9 kJ/mol (exergonic)
e) Feasibility of the process and the assumptions
The assumptions for this reaction include standard values of specific heats and the
standard temperature at which reaction takes place. Further, the molecular entropies for all the
substances have been taken as per the standards. The feasibility of process is quite large and
there is sufficient production of energy which means the reaction is exothermic.
2) Redox Reactions
Following are the displacement reactions between chlorine and bromine
Cl2(aq) + 2KBr(aq) ==> 2KCl(aq) + Br2(aq)__________(i)
Cl2(aq) + 2Br–(aq) ==> 2Cl–(aq) + Br2(aq)_________________(1)
The below produced reactions are the half reactions for displacement of ions:
9

Equation (1) depicts chlorine gaining ions and Bromine has lost electrons. Hence, the gaining of
electrons is reduction while loosing of electrons by Bromine is an oxidation process. The overall
reaction (i) known as redox reaction.
3) Using oxidation numbers for deciding the redox nature of the reaction or process
Oxidation number is contemplated as the charge which is acquired by atom when
considered to be an ion. For the reactions :
C + 4HNO3 CO➝ 2 + 4NO2 + 2H2 O
0 +1 -1 +2 -2 +2 -2
In the above reaction, carbon is getting oxidised i.e. from 0 to +2 while nitrate ion is
reduced to nitric ion. Hence, the above reaction is termed as redox reaction.
NH4 + + CO3 2− NH➝ 3 + HCO3 −
+1 -2 -1 +1 -1
In the above reaction, the ammonium compound is reduced to ammonia i.e. from +1 to -1
and the carbonate ion is oxidised from -2 to -1. This determines that the above reaction is also
redox because there is reduction of ammonium and oxidation of the carbonate ion.
5NO2 − + 2MnO4 − + 6H+ 5NO➝ 3 − + 2Mn2+ + 3H2 O
-2 +2 +4 -4 +6 -3 +3 +2 +2 -2
The above reaction has oxidation of nitric ion into nitrate. On the other hand, MnO4 − ion
is getting reduced with the Hydrogen ions provided. This reaction depicts simultaneous oxidation
and reduction of two compounds. Hence, it is a redox reaction.
10
electrons is reduction while loosing of electrons by Bromine is an oxidation process. The overall
reaction (i) known as redox reaction.
3) Using oxidation numbers for deciding the redox nature of the reaction or process
Oxidation number is contemplated as the charge which is acquired by atom when
considered to be an ion. For the reactions :
C + 4HNO3 CO➝ 2 + 4NO2 + 2H2 O
0 +1 -1 +2 -2 +2 -2
In the above reaction, carbon is getting oxidised i.e. from 0 to +2 while nitrate ion is
reduced to nitric ion. Hence, the above reaction is termed as redox reaction.
NH4 + + CO3 2− NH➝ 3 + HCO3 −
+1 -2 -1 +1 -1
In the above reaction, the ammonium compound is reduced to ammonia i.e. from +1 to -1
and the carbonate ion is oxidised from -2 to -1. This determines that the above reaction is also
redox because there is reduction of ammonium and oxidation of the carbonate ion.
5NO2 − + 2MnO4 − + 6H+ 5NO➝ 3 − + 2Mn2+ + 3H2 O
-2 +2 +4 -4 +6 -3 +3 +2 +2 -2
The above reaction has oxidation of nitric ion into nitrate. On the other hand, MnO4 − ion
is getting reduced with the Hydrogen ions provided. This reaction depicts simultaneous oxidation
and reduction of two compounds. Hence, it is a redox reaction.
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
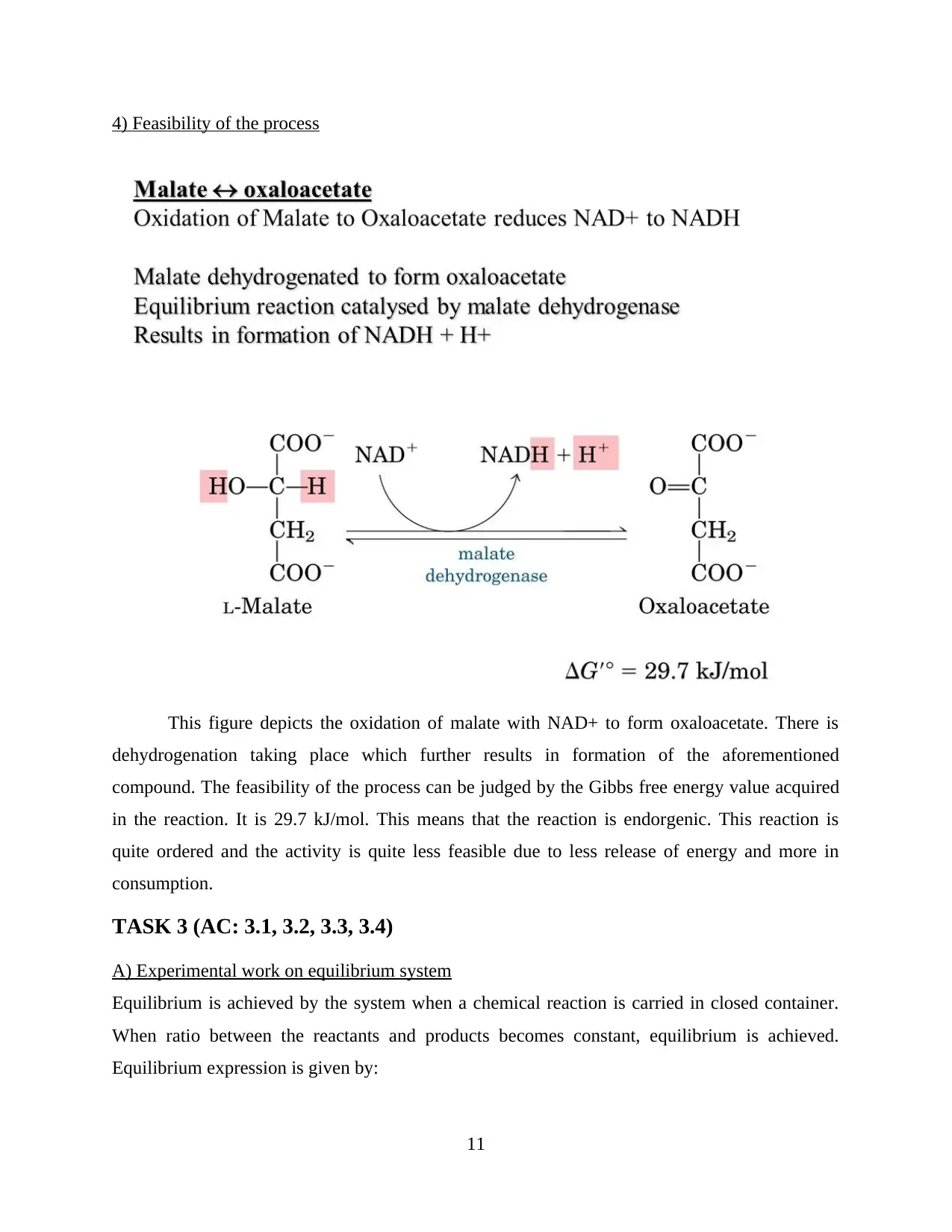
4) Feasibility of the process
This figure depicts the oxidation of malate with NAD+ to form oxaloacetate. There is
dehydrogenation taking place which further results in formation of the aforementioned
compound. The feasibility of the process can be judged by the Gibbs free energy value acquired
in the reaction. It is 29.7 kJ/mol. This means that the reaction is endorgenic. This reaction is
quite ordered and the activity is quite less feasible due to less release of energy and more in
consumption.
TASK 3 (AC: 3.1, 3.2, 3.3, 3.4)
A) Experimental work on equilibrium system
Equilibrium is achieved by the system when a chemical reaction is carried in closed container.
When ratio between the reactants and products becomes constant, equilibrium is achieved.
Equilibrium expression is given by:
11
This figure depicts the oxidation of malate with NAD+ to form oxaloacetate. There is
dehydrogenation taking place which further results in formation of the aforementioned
compound. The feasibility of the process can be judged by the Gibbs free energy value acquired
in the reaction. It is 29.7 kJ/mol. This means that the reaction is endorgenic. This reaction is
quite ordered and the activity is quite less feasible due to less release of energy and more in
consumption.
TASK 3 (AC: 3.1, 3.2, 3.3, 3.4)
A) Experimental work on equilibrium system
Equilibrium is achieved by the system when a chemical reaction is carried in closed container.
When ratio between the reactants and products becomes constant, equilibrium is achieved.
Equilibrium expression is given by:
11

here, K= equilibrium constant
[A],[B] etc.= molar concentrations of A, B etc.
l, m are the coefficients of the reaction which is balanced.
B) Shift in equilibrium position for favouring the formation of products
2SO2 (g)+O2(g)-> 2SO3(g)
Sulphur dioxide + oxygen → sulphur trioxide
Gibbs free energy = -379.1 kj/mol
Equilibrium constant:
Density of Sulphur dioxide is 0.002619 g/cm3.
Density of oxygen is: 0.001429 g/cm3
Density of sulphur trioxide: 1.97 g/cm3
Oxygen is odourless. Sulphur dioxide and oxygen are present in gaseous form and sulphur
trioxide is present in liquid state.
Le Chatelier's Principle
When introduction of change is made into equation, it leads to change into equilibrium.
To control the change, equilibrium position moves. Suppose we have following equation:
A + 2B → C+D
This equation comprises four different elements.
When conditions are changed by increasing concentration of A, then according to the
principle equilibrium state will move to counteract the modification. It will change in such a way
that decreases A's concentration by making reaction with B element and result of C+ D will get
produced.
12
[A],[B] etc.= molar concentrations of A, B etc.
l, m are the coefficients of the reaction which is balanced.
B) Shift in equilibrium position for favouring the formation of products
2SO2 (g)+O2(g)-> 2SO3(g)
Sulphur dioxide + oxygen → sulphur trioxide
Gibbs free energy = -379.1 kj/mol
Equilibrium constant:
Density of Sulphur dioxide is 0.002619 g/cm3.
Density of oxygen is: 0.001429 g/cm3
Density of sulphur trioxide: 1.97 g/cm3
Oxygen is odourless. Sulphur dioxide and oxygen are present in gaseous form and sulphur
trioxide is present in liquid state.
Le Chatelier's Principle
When introduction of change is made into equation, it leads to change into equilibrium.
To control the change, equilibrium position moves. Suppose we have following equation:
A + 2B → C+D
This equation comprises four different elements.
When conditions are changed by increasing concentration of A, then according to the
principle equilibrium state will move to counteract the modification. It will change in such a way
that decreases A's concentration by making reaction with B element and result of C+ D will get
produced.
12

When conditions are changed that is now concentration of A decreases then in these situations
C+D will react to form A again which has been removed.
C) Monoprotic weak acid
The monoprotic acid considered for these questions is pyruvic acid. It has chemical
formula as CH3COCOOH. The dissociation reaction is produced as follows:
CH3COCOOH CH➝ 3COCOO- + H+
The following equation represents the acid dissociation constant at 298 K
Ka = [ CH3COCOO-] [H+] / [ CH3COCOOH ]
TASK 4 (AC: 4.1,4.2, 4.3, 4.4, 4.5)
A) Relation of shapes and bonding in molecules
Molecular formula of propan-1-ol is : C3H8O It is a colourless liquid which is a primary
alcohol.
Molecular formula of but-2-ene is: C4H8.
Molecular formula of ethanal is: C2H4O. It is an aldehyde which is colourless and volatile
that has pungent smell.
Molecular formula of phenol is : C6H6O . It is also known as carbolic acid which is a
fragrant organic compound.
13
C+D will react to form A again which has been removed.
C) Monoprotic weak acid
The monoprotic acid considered for these questions is pyruvic acid. It has chemical
formula as CH3COCOOH. The dissociation reaction is produced as follows:
CH3COCOOH CH➝ 3COCOO- + H+
The following equation represents the acid dissociation constant at 298 K
Ka = [ CH3COCOO-] [H+] / [ CH3COCOOH ]
TASK 4 (AC: 4.1,4.2, 4.3, 4.4, 4.5)
A) Relation of shapes and bonding in molecules
Molecular formula of propan-1-ol is : C3H8O It is a colourless liquid which is a primary
alcohol.
Molecular formula of but-2-ene is: C4H8.
Molecular formula of ethanal is: C2H4O. It is an aldehyde which is colourless and volatile
that has pungent smell.
Molecular formula of phenol is : C6H6O . It is also known as carbolic acid which is a
fragrant organic compound.
13
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Carboxyl carbon atoms is sp2 hybridised and it forms three sigma bonds. Fourth valence electron
of carbon remains in p-orbit and results into formation of π- bond with oxygen. It overlaps with
oxygen of p-orbit. Oxygen atom also has two pairs of electrons which are not bonded.
Angle between them is approximately 120° of a trigonal co-planar structure.
B) Structures and systematic names
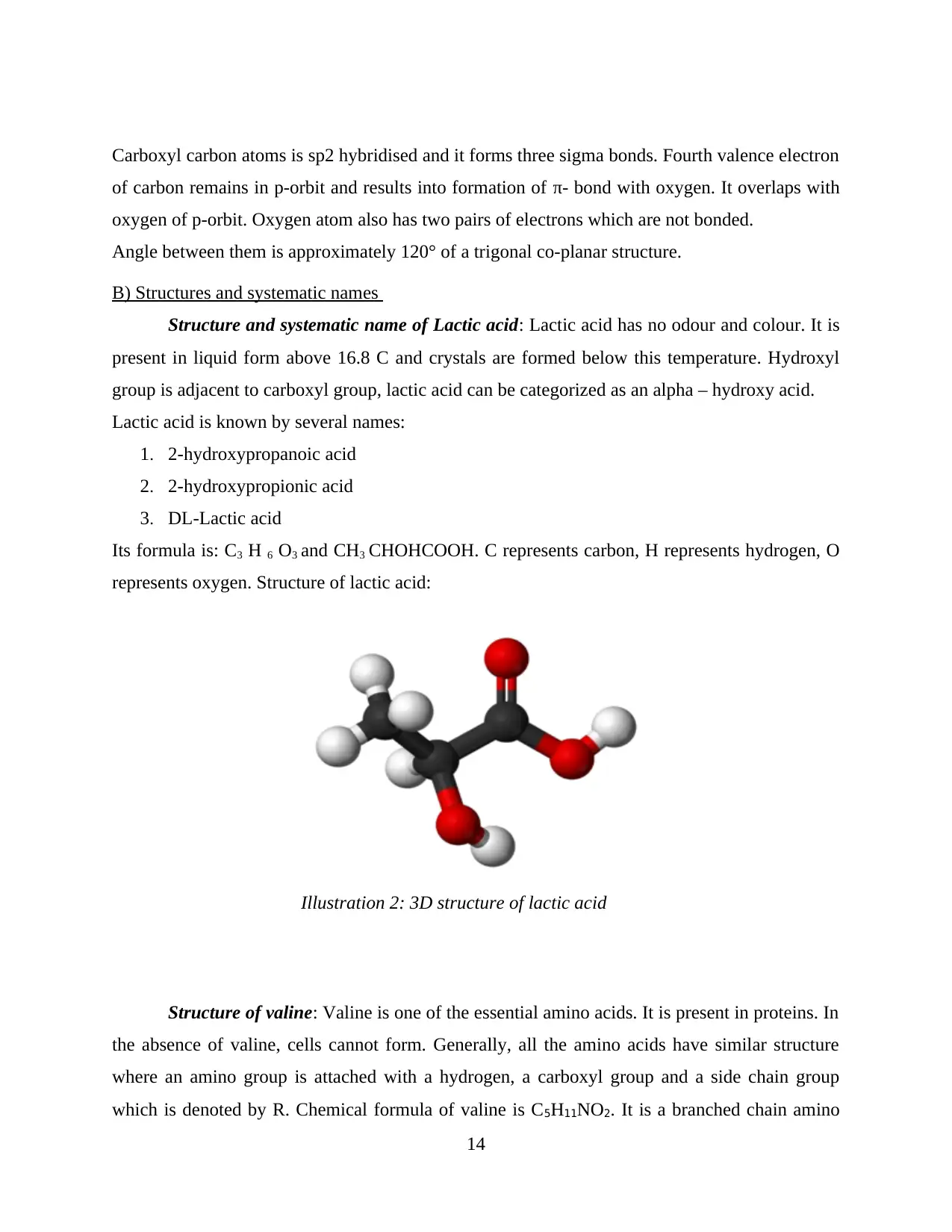
Structure and systematic name of Lactic acid: Lactic acid has no odour and colour. It is
present in liquid form above 16.8 C and crystals are formed below this temperature. Hydroxyl
group is adjacent to carboxyl group, lactic acid can be categorized as an alpha – hydroxy acid.
Lactic acid is known by several names:
1. 2-hydroxypropanoic acid
2. 2-hydroxypropionic acid
3. DL-Lactic acid
Its formula is: C3 H 6 O3 and CH3 CHOHCOOH. C represents carbon, H represents hydrogen, O
represents oxygen. Structure of lactic acid:
Structure of valine: Valine is one of the essential amino acids. It is present in proteins. In
the absence of valine, cells cannot form. Generally, all the amino acids have similar structure
where an amino group is attached with a hydrogen, a carboxyl group and a side chain group
which is denoted by R. Chemical formula of valine is C5H11NO2. It is a branched chain amino
14
Illustration 2: 3D structure of lactic acid
of carbon remains in p-orbit and results into formation of π- bond with oxygen. It overlaps with
oxygen of p-orbit. Oxygen atom also has two pairs of electrons which are not bonded.
Angle between them is approximately 120° of a trigonal co-planar structure.
B) Structures and systematic names
Structure and systematic name of Lactic acid: Lactic acid has no odour and colour. It is
present in liquid form above 16.8 C and crystals are formed below this temperature. Hydroxyl
group is adjacent to carboxyl group, lactic acid can be categorized as an alpha – hydroxy acid.
Lactic acid is known by several names:
1. 2-hydroxypropanoic acid
2. 2-hydroxypropionic acid
3. DL-Lactic acid
Its formula is: C3 H 6 O3 and CH3 CHOHCOOH. C represents carbon, H represents hydrogen, O
represents oxygen. Structure of lactic acid:
Structure of valine: Valine is one of the essential amino acids. It is present in proteins. In
the absence of valine, cells cannot form. Generally, all the amino acids have similar structure
where an amino group is attached with a hydrogen, a carboxyl group and a side chain group
which is denoted by R. Chemical formula of valine is C5H11NO2. It is a branched chain amino
14
Illustration 2: 3D structure of lactic acid
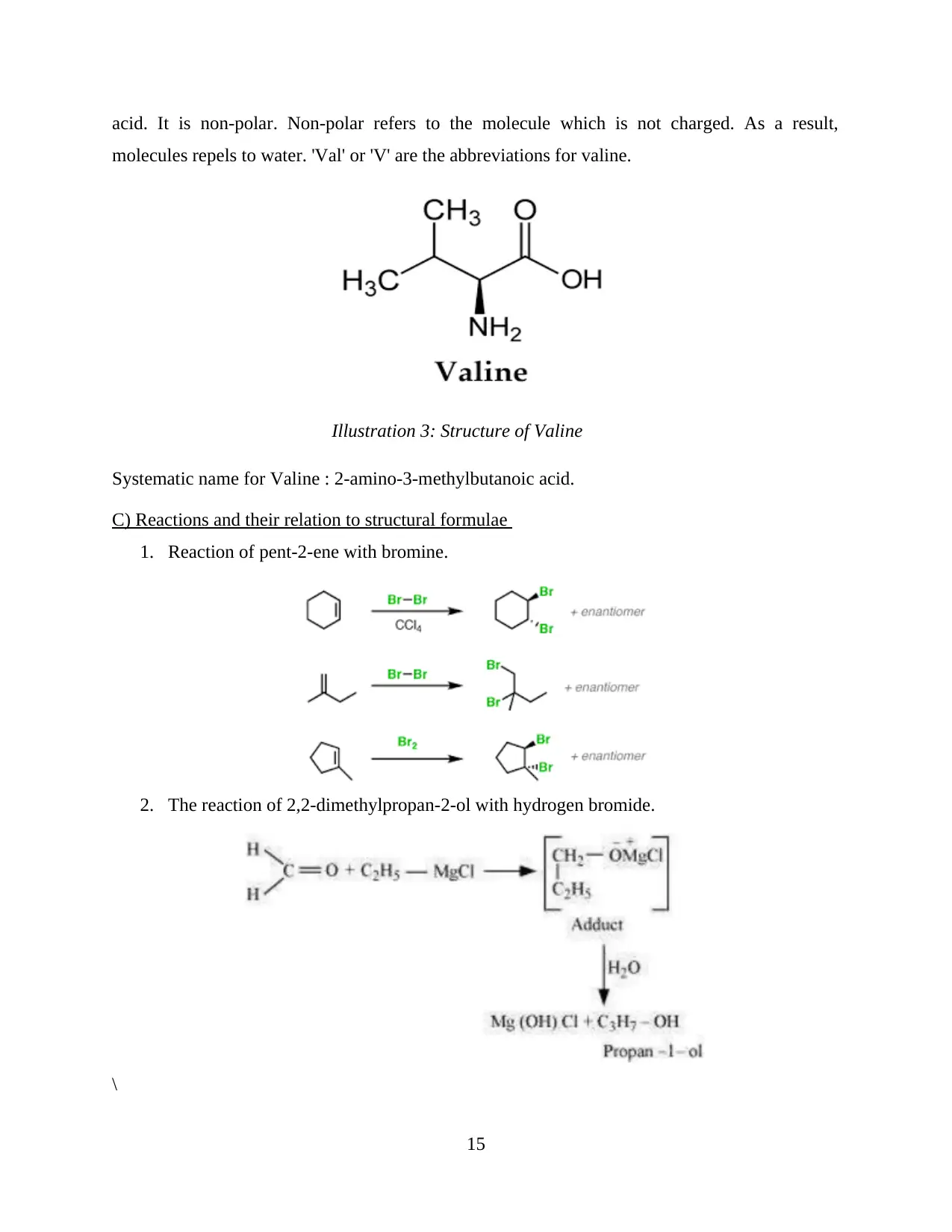
acid. It is non-polar. Non-polar refers to the molecule which is not charged. As a result,
molecules repels to water. 'Val' or 'V' are the abbreviations for valine.
Systematic name for Valine : 2-amino-3-methylbutanoic acid.
C) Reactions and their relation to structural formulae
1. Reaction of pent-2-ene with bromine.
2. The reaction of 2,2-dimethylpropan-2-ol with hydrogen bromide.
\
15
Illustration 3: Structure of Valine
molecules repels to water. 'Val' or 'V' are the abbreviations for valine.
Systematic name for Valine : 2-amino-3-methylbutanoic acid.
C) Reactions and their relation to structural formulae
1. Reaction of pent-2-ene with bromine.
2. The reaction of 2,2-dimethylpropan-2-ol with hydrogen bromide.
\
15
Illustration 3: Structure of Valine
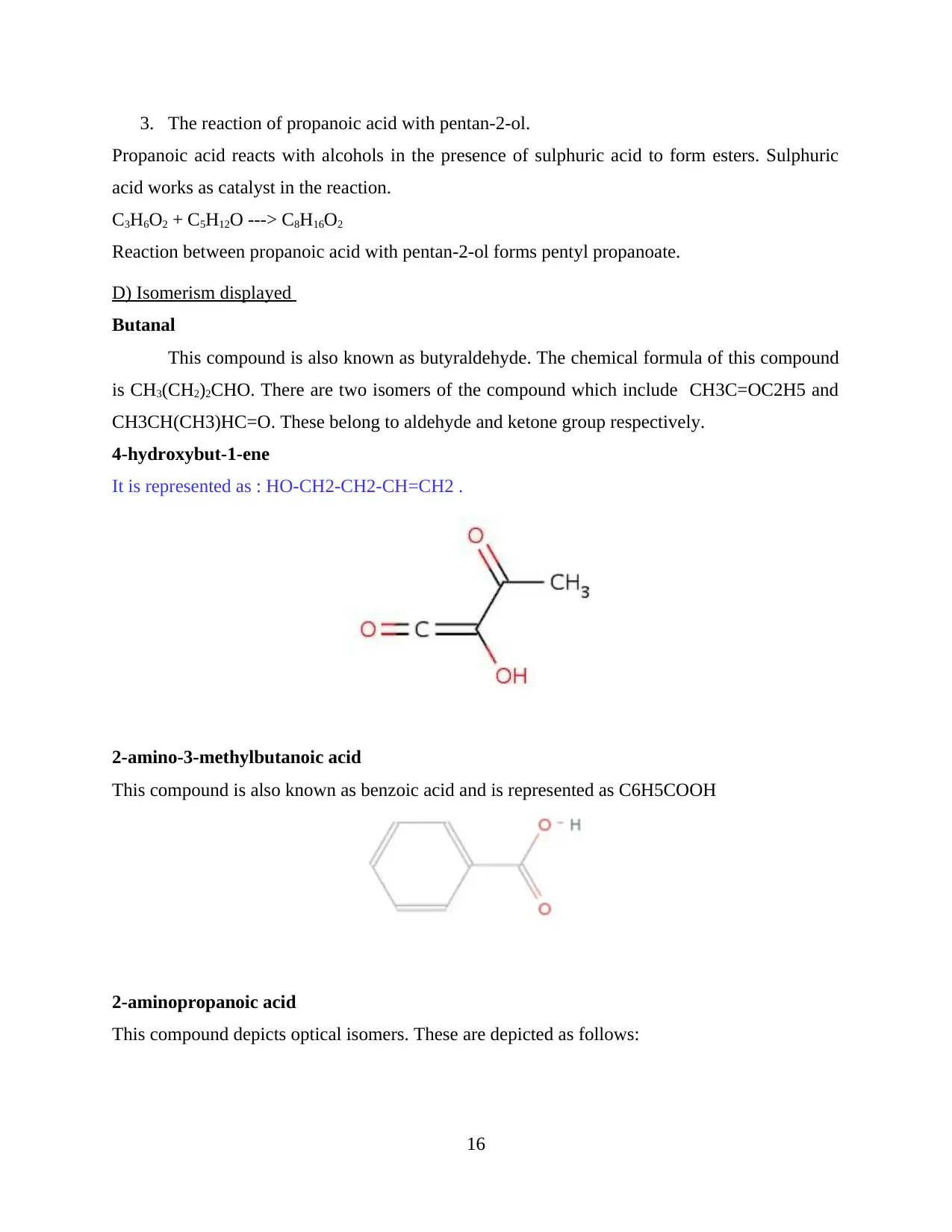
3. The reaction of propanoic acid with pentan-2-ol.
Propanoic acid reacts with alcohols in the presence of sulphuric acid to form esters. Sulphuric
acid works as catalyst in the reaction.
C3H6O2 + C5H12O ---> C8H16O2
Reaction between propanoic acid with pentan-2-ol forms pentyl propanoate.
D) Isomerism displayed
Butanal
This compound is also known as butyraldehyde. The chemical formula of this compound
is CH3(CH2)2CHO. There are two isomers of the compound which include CH3C=OC2H5 and
CH3CH(CH3)HC=O. These belong to aldehyde and ketone group respectively.
4-hydroxybut-1-ene
It is represented as : HO-CH2-CH2-CH=CH2 .
2-amino-3-methylbutanoic acid
This compound is also known as benzoic acid and is represented as C6H5COOH
2-aminopropanoic acid
This compound depicts optical isomers. These are depicted as follows:
16
Propanoic acid reacts with alcohols in the presence of sulphuric acid to form esters. Sulphuric
acid works as catalyst in the reaction.
C3H6O2 + C5H12O ---> C8H16O2
Reaction between propanoic acid with pentan-2-ol forms pentyl propanoate.
D) Isomerism displayed
Butanal
This compound is also known as butyraldehyde. The chemical formula of this compound
is CH3(CH2)2CHO. There are two isomers of the compound which include CH3C=OC2H5 and
CH3CH(CH3)HC=O. These belong to aldehyde and ketone group respectively.
4-hydroxybut-1-ene
It is represented as : HO-CH2-CH2-CH=CH2 .
2-amino-3-methylbutanoic acid
This compound is also known as benzoic acid and is represented as C6H5COOH
2-aminopropanoic acid
This compound depicts optical isomers. These are depicted as follows:
16
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
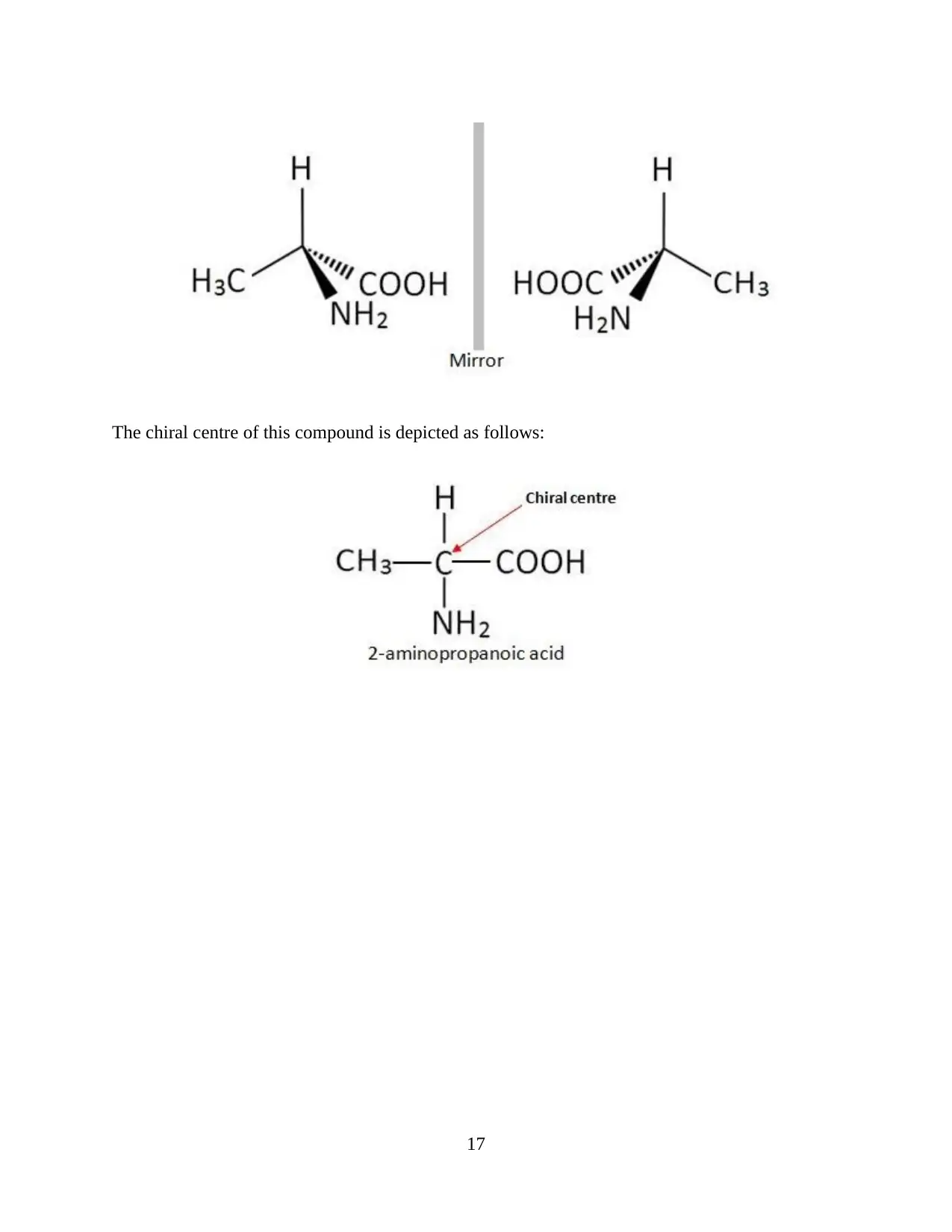
The chiral centre of this compound is depicted as follows:
17
17
1 out of 20
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.


