Chemistry in Forensics: Techniques and Instruments for Substance Identification
VerifiedAdded on 2023/06/10
|7
|1607
|389
AI Summary
This article discusses the application of chemistry in forensic toxicology and the use of various instruments and methodologies in identifying unknown substances found at crime scenes. It covers the properties of compounds, fundamental chemical properties, spectroscopy, atomic absorption spectroscopy, chromatography, and high performance liquid chromatography. The article also explains the use of thin layer chromatography in analyzing dyes and inks.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Name
Instructor
Course
Date
Chemistry in Forensics
Scientific Terminology
Forensic chemistry defines the application of chemistry and its related subfields in forensic
toxicology, more specifically in the legal setting context. A forensic chemist has the ability to
help in the identification of unknown substances that are found at the scene of a crime. There is
an avalanche of methodologies and instruments that are applied in the identification of unknown
substances by forensic scientist among them high liquid performance chromatography, thin layer
chromatography, atomic absorption chromatography, gas chromatography-mass spectrometry as
well as Fourier transform infrared spectroscopy (AL-Saedi & Dina 23).
Properties of Compounds
Such a range of various methods is very important owing to the destructive nature of a few of the
instruments as well as a numerous range of possible unknown substances that can be found at a
scene of crime. In most cases, forensic chemists opt for the adoption of non-destructive methods
in the final step so as to ensure that the evidence is preserved and to find out which of the
destructive methods would yield the most reliable results.
Fundamental Chemical Properties
Instructor
Course
Date
Chemistry in Forensics
Scientific Terminology
Forensic chemistry defines the application of chemistry and its related subfields in forensic
toxicology, more specifically in the legal setting context. A forensic chemist has the ability to
help in the identification of unknown substances that are found at the scene of a crime. There is
an avalanche of methodologies and instruments that are applied in the identification of unknown
substances by forensic scientist among them high liquid performance chromatography, thin layer
chromatography, atomic absorption chromatography, gas chromatography-mass spectrometry as
well as Fourier transform infrared spectroscopy (AL-Saedi & Dina 23).
Properties of Compounds
Such a range of various methods is very important owing to the destructive nature of a few of the
instruments as well as a numerous range of possible unknown substances that can be found at a
scene of crime. In most cases, forensic chemists opt for the adoption of non-destructive methods
in the final step so as to ensure that the evidence is preserved and to find out which of the
destructive methods would yield the most reliable results.
Fundamental Chemical Properties
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Numerous instruments are used in the identification of unknown substances by forensic
chemists. Still, various methods are usable in the evaluating of the identity of the same substance
and it is thus upon the examiner to examine b which methods when applied would yield the most
reliable results (Meyer et al 45). Among the factors that might be taken into consideration by
forensic chemists during conducting g of an examination include the length of time a certain
instrument requires to fully examine a substance as well as the destructive nature of that
instrument. This leaves preference to the non-destructive methods first in the preservation of the
evidence that may be used for further analysis of the very substance. However, non-destructive
methods are also applied to reduce the possibilities increasing the chances that the correct
methods will be adopted the first time when a destructive method is applied.
Spectroscopy
Atomic absorption spectroscopy and Fourier transform infrared spectroscopy are the two main
techniques of standalone spectroscope that are used by forensic chemists. Fourier transform
infrared spectroscopy is a non-destructive technique that makes use of infrared light in the
identification of a substance. The need of substance being prepared prior to the analysis has been
eliminated through the use of attenuated total reflectance technique of sampling. Attenuated total
reflectance Fourier transform infrared spectroscopy has been made simpler and quicker through
the combination of zero preparation and non-destructiveness as the first step in the analysis of
unknown substances.
Fourier transform infrared spectroscopy instruments are often loaded with databases that have
the capability of looking for known spectra that has similar properties as the unknown spectra.
This aids in the positive identification of the unknown substance. Fourier transform infrared
chemists. Still, various methods are usable in the evaluating of the identity of the same substance
and it is thus upon the examiner to examine b which methods when applied would yield the most
reliable results (Meyer et al 45). Among the factors that might be taken into consideration by
forensic chemists during conducting g of an examination include the length of time a certain
instrument requires to fully examine a substance as well as the destructive nature of that
instrument. This leaves preference to the non-destructive methods first in the preservation of the
evidence that may be used for further analysis of the very substance. However, non-destructive
methods are also applied to reduce the possibilities increasing the chances that the correct
methods will be adopted the first time when a destructive method is applied.
Spectroscopy
Atomic absorption spectroscopy and Fourier transform infrared spectroscopy are the two main
techniques of standalone spectroscope that are used by forensic chemists. Fourier transform
infrared spectroscopy is a non-destructive technique that makes use of infrared light in the
identification of a substance. The need of substance being prepared prior to the analysis has been
eliminated through the use of attenuated total reflectance technique of sampling. Attenuated total
reflectance Fourier transform infrared spectroscopy has been made simpler and quicker through
the combination of zero preparation and non-destructiveness as the first step in the analysis of
unknown substances.
Fourier transform infrared spectroscopy instruments are often loaded with databases that have
the capability of looking for known spectra that has similar properties as the unknown spectra.
This aids in the positive identification of the unknown substance. Fourier transform infrared
spectroscopy analysis of mixtures of unknown substances, even though not impossible, is
accompanied with scientific challenges owing to the cumulative nature of the response that is
generated. During the analysis of an unknown substance that is suspected to be composed of a
mixture of more than a substance, the resulting spectra will present a combination of each of the
individual spectra of every component.
In as much as common mixtures have known and identifiable spectra on the file, novel mixtures
are likely to present a challenge in solving them hence rendering Fourier transform infrared
spectroscopy an unacceptable technique of identification. Nevertheless, the instrument is usable
in the identification of the common chemical structures that can be found which then offers the
forensics chemists the opportunity to establish the best methods usable in the analysis using other
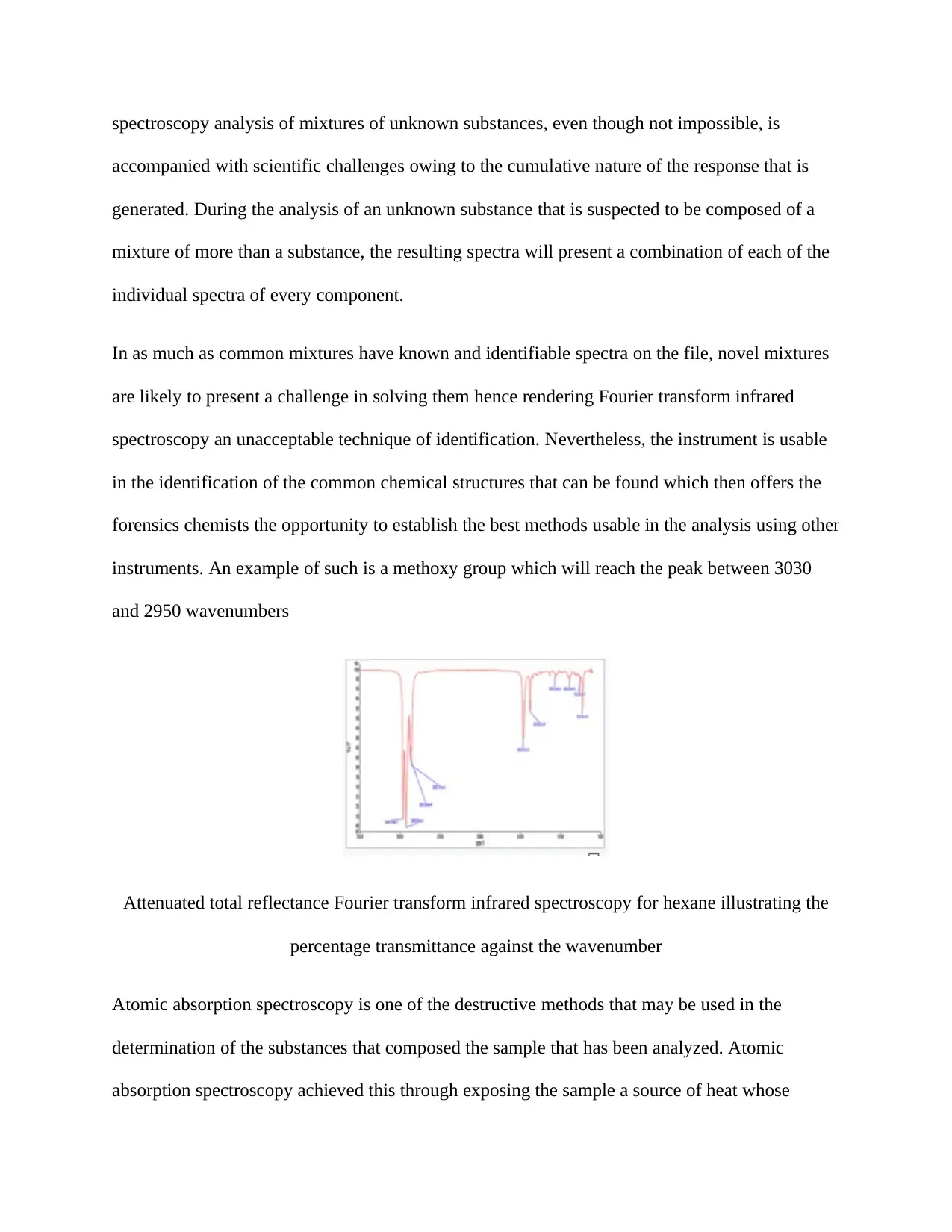
instruments. An example of such is a methoxy group which will reach the peak between 3030
and 2950 wavenumbers
Attenuated total reflectance Fourier transform infrared spectroscopy for hexane illustrating the
percentage transmittance against the wavenumber
Atomic absorption spectroscopy is one of the destructive methods that may be used in the
determination of the substances that composed the sample that has been analyzed. Atomic
absorption spectroscopy achieved this through exposing the sample a source of heat whose
accompanied with scientific challenges owing to the cumulative nature of the response that is
generated. During the analysis of an unknown substance that is suspected to be composed of a
mixture of more than a substance, the resulting spectra will present a combination of each of the
individual spectra of every component.
In as much as common mixtures have known and identifiable spectra on the file, novel mixtures
are likely to present a challenge in solving them hence rendering Fourier transform infrared
spectroscopy an unacceptable technique of identification. Nevertheless, the instrument is usable
in the identification of the common chemical structures that can be found which then offers the
forensics chemists the opportunity to establish the best methods usable in the analysis using other
instruments. An example of such is a methoxy group which will reach the peak between 3030
and 2950 wavenumbers
Attenuated total reflectance Fourier transform infrared spectroscopy for hexane illustrating the
percentage transmittance against the wavenumber
Atomic absorption spectroscopy is one of the destructive methods that may be used in the
determination of the substances that composed the sample that has been analyzed. Atomic
absorption spectroscopy achieved this through exposing the sample a source of heat whose

temperature is extremely high (Allen &Thelma 78). The high temperature breaks down the
different atomic bonds of the substance resulting into free atoms. This step is then followed by a
radiation done in the form of light which is passed through the sample making the free atoms to
shift to a higher energy state.
Each of the elements can then be tested with the aid of a corresponding wavelength of light
which makes the atoms of the elements to shift to a higher energy state during the process of
analysis. Owing to this reason and going by the destructive nature of this technique, Atomic
absorption spectroscopy is mostly used as just a confirmatory technique following preliminary
tests which have demonstrated the presence of a certain elements in an analyzed sample.
The amount of light that is absorbed during this testing process is directly proportional to the
concentration of the element that is found in the sample when comparison is made against a
blank sample. Atomic absorption spectroscopy is mostly important in such case as those of
suspected poisoning by heavy metals including lead, mercury, arsenic and cadmium (Pacold et al
112). The concentration of the identified substance in the analyzed sample may be used as a
demonstration as whether the cause of death can be attributed to heavy metals.
Chromatography
Spectroscopy techniques are applicable in cases where the sample under analysis is pure or
otherwise a very common mixture. In cases where an unknown mixture is under analysis, it has
to be broken down into the various individual proportions and parts. Chromatography techniques
are applicable in the breaking apart of the mixtures resulting into the constituents thus enabling
each of the parts to undergo a separate analysis.
different atomic bonds of the substance resulting into free atoms. This step is then followed by a
radiation done in the form of light which is passed through the sample making the free atoms to
shift to a higher energy state.
Each of the elements can then be tested with the aid of a corresponding wavelength of light
which makes the atoms of the elements to shift to a higher energy state during the process of
analysis. Owing to this reason and going by the destructive nature of this technique, Atomic
absorption spectroscopy is mostly used as just a confirmatory technique following preliminary
tests which have demonstrated the presence of a certain elements in an analyzed sample.
The amount of light that is absorbed during this testing process is directly proportional to the
concentration of the element that is found in the sample when comparison is made against a
blank sample. Atomic absorption spectroscopy is mostly important in such case as those of
suspected poisoning by heavy metals including lead, mercury, arsenic and cadmium (Pacold et al
112). The concentration of the identified substance in the analyzed sample may be used as a
demonstration as whether the cause of death can be attributed to heavy metals.
Chromatography
Spectroscopy techniques are applicable in cases where the sample under analysis is pure or
otherwise a very common mixture. In cases where an unknown mixture is under analysis, it has
to be broken down into the various individual proportions and parts. Chromatography techniques
are applicable in the breaking apart of the mixtures resulting into the constituents thus enabling
each of the parts to undergo a separate analysis.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

High performance liquid chromatography is applicable in the extraction of each of the
components from a mixture that has been dissolved in a solution. High performance liquid
chromatography is applicable in non-volatile mixture that may be used the gas chromatography
technique. This is often applicable in drug analysis in which the pharmaceutical tends to be a
combination of drugs as the components would disintegrate or elute at various times enabling
verification of the individual components.
The elutes derived from the column of High performance liquid chromatography are then taken
through different detectors that generate a peak on a graph that is relative to its concentration
while eluting off the column. Ultraviolet-visible spectrometer is the most commonly used types
of detectors since the common item of interest that under test with High performance liquid
chromatography, pharmaceuticals, have an ultra-violet absorbance.
Thin layer chromatography as a fast alternative that is usable for more chromatography methods
that are more complex. Thin layer chromatography is usable in the analysis of dyes and inks
through the extraction of each of the components (Rundberg & Robert). This can be applied in
the examination of the fibres or notes that are left at the scene as each of the products of a
company is significantly different and such differences can be noticed using Thin layer
chromatography.
For thin layer chromatography to be effective, it is required that the substances that are to be
analyzed remain soluble in any solution in order to maintain the components to the analysis
plate, and this tends to be the only limiting factor of the technique (Zavarin & Mavrik 85). Such
a solution is known as the mobile phase. A comparison is then made between the unknown
against the known standards through observing the distance travelled by the each of the
components from a mixture that has been dissolved in a solution. High performance liquid
chromatography is applicable in non-volatile mixture that may be used the gas chromatography
technique. This is often applicable in drug analysis in which the pharmaceutical tends to be a
combination of drugs as the components would disintegrate or elute at various times enabling
verification of the individual components.
The elutes derived from the column of High performance liquid chromatography are then taken
through different detectors that generate a peak on a graph that is relative to its concentration
while eluting off the column. Ultraviolet-visible spectrometer is the most commonly used types
of detectors since the common item of interest that under test with High performance liquid
chromatography, pharmaceuticals, have an ultra-violet absorbance.
Thin layer chromatography as a fast alternative that is usable for more chromatography methods
that are more complex. Thin layer chromatography is usable in the analysis of dyes and inks
through the extraction of each of the components (Rundberg & Robert). This can be applied in
the examination of the fibres or notes that are left at the scene as each of the products of a
company is significantly different and such differences can be noticed using Thin layer
chromatography.
For thin layer chromatography to be effective, it is required that the substances that are to be
analyzed remain soluble in any solution in order to maintain the components to the analysis
plate, and this tends to be the only limiting factor of the technique (Zavarin & Mavrik 85). Such
a solution is known as the mobile phase. A comparison is then made between the unknown
against the known standards through observing the distance travelled by the each of the

components. A comparison between this distance and the starting point constitute the retention
factor, Rf of each individual component. Should each Rf value be similar to a known sample, the
identity of the unknown sample is established (Orozco & Diana).
factor, Rf of each individual component. Should each Rf value be similar to a known sample, the
identity of the unknown sample is established (Orozco & Diana).

Works Cited
AL-Saedi & Dina B. Abbas. "Chemistry in Forensics and Crime Solving." Journal of Global
Pharma Technology (2018)
Meyer, Buffy M., et al. "Louisiana coastal marsh environments and MC252 oil biomarker
chemistry." Oil Spill Environmental Forensics Case Studies. 2018. 737-756
Rundberg & Robert S. Nuclear Forensics and Radiochemistry: Chemistry. No. LA-UR-17-
30555. Los Alamos National Lab.(LANL), Los Alamos, NM (United States), 2017
Allen, Thelma A. "Digital Forensics." (2018): https://link.springer.com/book/10.1007%2F978-3-
319-08470-1
Zavarin, Mavrik. 2017 LLNL Nuclear Forensics Summer Internship Program. No. LLNL-TR-
743114. Lawrence Livermore National Lab.(LLNL), Livermore, CA (United States), 2017
Allen, Thelma A. "Digital Forensics." (2018)
Pacold, J. I., et al. "Development of small particle speciation for nuclear forensics by soft X-ray
scanning transmission spectromicroscopy." Analyst 143.6 (2018): 1349-1357
Orozco & Diana. "An Evaluation of eScience Lab Kits for Online Learning." Themis: Research
Journal of Justice Studies and Forensic Science 5.1 (2017): 8
AL-Saedi & Dina B. Abbas. "Chemistry in Forensics and Crime Solving." Journal of Global
Pharma Technology (2018)
Meyer, Buffy M., et al. "Louisiana coastal marsh environments and MC252 oil biomarker
chemistry." Oil Spill Environmental Forensics Case Studies. 2018. 737-756
Rundberg & Robert S. Nuclear Forensics and Radiochemistry: Chemistry. No. LA-UR-17-
30555. Los Alamos National Lab.(LANL), Los Alamos, NM (United States), 2017
Allen, Thelma A. "Digital Forensics." (2018): https://link.springer.com/book/10.1007%2F978-3-
319-08470-1
Zavarin, Mavrik. 2017 LLNL Nuclear Forensics Summer Internship Program. No. LLNL-TR-
743114. Lawrence Livermore National Lab.(LLNL), Livermore, CA (United States), 2017
Allen, Thelma A. "Digital Forensics." (2018)
Pacold, J. I., et al. "Development of small particle speciation for nuclear forensics by soft X-ray
scanning transmission spectromicroscopy." Analyst 143.6 (2018): 1349-1357
Orozco & Diana. "An Evaluation of eScience Lab Kits for Online Learning." Themis: Research
Journal of Justice Studies and Forensic Science 5.1 (2017): 8
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
