Gasification of Char: CO2 and H2O Mixture Effects on Residual Carbon
VerifiedAdded on 2023/06/11
|28
|5362
|280
Report
AI Summary
This report investigates the effects of carbon dioxide and water mixtures on the intensified gasification of residual carbon in partially gasified char, a critical aspect of clean coal technology. The study details the gasification process, including coal pyrolysis, and outlines the experimental setup involving fixed, fluidized, and entrained beds. It aims to evaluate the impact of water and carbon dioxide on carbon residue (coke and ash) by analyzing parameter changes during the gasification process. The methodology involves examining the effects of CO2 and CO2+H2O mixtures on the gasification of char, with a focus on temperature variations and ash composition. The report references existing literature on gasification kinetics and reaction models, highlighting the challenges of simultaneous char-steam and char-CO2 reactions. The ultimate goal is to optimize gasification conditions for cleaner coal utilization, and Desklib provides access to similar reports and study resources for students.

Dissertation Project
Report
Clean coal technology: Effects of CO2 and H2O mixture on
intensified gasification of residual carbon in partially
gasified char
By
Susmitha Dugiveedi Lakshmipathy
5573294
Supervisor: Dr. Guangqing Zhang
Report
Clean coal technology: Effects of CO2 and H2O mixture on
intensified gasification of residual carbon in partially
gasified char
By
Susmitha Dugiveedi Lakshmipathy
5573294
Supervisor: Dr. Guangqing Zhang
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Progress report
Contents
Abstract:........................................................................................................................3
1.Introduction...............................................................................................................4
1.1 Gasification:........................................................................................................4
1.2 Coal Pyrolysis process:.......................................................................................7
2. Aim, Objectives and Scope of the Experiment:.....................................................9
2.1 The aim of the experiment:................................................................................9
2.2 Experiment objectives........................................................................................9
2.3 The scope of the experiment:.............................................................................9
3. Literature Review:.................................................................................................10
4. A Detailed description of the materials, procedure and factors involved with
the process...................................................................................................................14
4.1 Materials:...........................................................................................................14
4.2 Process of Description :....................................................................................15
4.3 The Factors that are to be evaluated from the experiment:.........................16
5. Experimental setup:...............................................................................................17
A. Effects of CO2 and H2O Mixture on Intensified Gasification of Residual
Carbon in Partially Gasified Char (CO2 CO)................................................17
B. Effects of CO2 and H2O Mixture on Intensified Gasification of Residual
Carbon in Partially Gasified Char (CO2+H20):..................................................18
6. Methodology...........................................................................................................19
6.1 Effects of CO2 Mixture on Intensified Gasification of Residual Carbon in
Partially Gasified Char (CO2 CO) – procedure:............................................19
6.2 Effects of CO2 and H2O Mixture on Intensified Gasification of Residual
Carbon in Partially Gasified Char (CO2 + H2O) – procedure:.........................20
5573294 Page 2
Contents
Abstract:........................................................................................................................3
1.Introduction...............................................................................................................4
1.1 Gasification:........................................................................................................4
1.2 Coal Pyrolysis process:.......................................................................................7
2. Aim, Objectives and Scope of the Experiment:.....................................................9
2.1 The aim of the experiment:................................................................................9
2.2 Experiment objectives........................................................................................9
2.3 The scope of the experiment:.............................................................................9
3. Literature Review:.................................................................................................10
4. A Detailed description of the materials, procedure and factors involved with
the process...................................................................................................................14
4.1 Materials:...........................................................................................................14
4.2 Process of Description :....................................................................................15
4.3 The Factors that are to be evaluated from the experiment:.........................16
5. Experimental setup:...............................................................................................17
A. Effects of CO2 and H2O Mixture on Intensified Gasification of Residual
Carbon in Partially Gasified Char (CO2 CO)................................................17
B. Effects of CO2 and H2O Mixture on Intensified Gasification of Residual
Carbon in Partially Gasified Char (CO2+H20):..................................................18
6. Methodology...........................................................................................................19
6.1 Effects of CO2 Mixture on Intensified Gasification of Residual Carbon in
Partially Gasified Char (CO2 CO) – procedure:............................................19
6.2 Effects of CO2 and H2O Mixture on Intensified Gasification of Residual
Carbon in Partially Gasified Char (CO2 + H2O) – procedure:.........................20
5573294 Page 2

Progress report
7. Results:....................................................................................................................22
8.Work plan:...............................................................................................................25
9.Conclusion................................................................................................................26
10.References..............................................................................................................27
Abstract:
To resolve the problem of the carbon impurities is a major issue for consumption of
the coal for various activities.so we need to purify the coal through the process of
gasification. To know the experimental procedure of the coal gasification process by
using a mixture of water and carbon dioxide using the conventional Chinese coal.
This can also give a brief description of the effects of these mixtures on an intensified
carbon residue in a partially gasified char. This composition of the produced products
from the process depends on the changes of certain factors like temperature, pressure
etc., The results we obtain from this process can be used to evaluate the effects of the
parameters like temperature and pressure on the carbon residue in a partially gasified
char. This Can help to obtain pure coke from the process of gasification at high
temperatures with the composition of ash.
5573294 Page 3
7. Results:....................................................................................................................22
8.Work plan:...............................................................................................................25
9.Conclusion................................................................................................................26
10.References..............................................................................................................27
Abstract:
To resolve the problem of the carbon impurities is a major issue for consumption of
the coal for various activities.so we need to purify the coal through the process of
gasification. To know the experimental procedure of the coal gasification process by
using a mixture of water and carbon dioxide using the conventional Chinese coal.
This can also give a brief description of the effects of these mixtures on an intensified
carbon residue in a partially gasified char. This composition of the produced products
from the process depends on the changes of certain factors like temperature, pressure
etc., The results we obtain from this process can be used to evaluate the effects of the
parameters like temperature and pressure on the carbon residue in a partially gasified
char. This Can help to obtain pure coke from the process of gasification at high
temperatures with the composition of ash.
5573294 Page 3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Progress report
1.Introduction
Coal can be considered as one of the important fuel and can be used in the various
sectors in the day-to-day activities. Coal is considered as the carbon substitute and
there is a need to obtain pure coal for several processes. This can be called as the
Clean Coal Technology and This process can be done through gasification, which is
prorated into 2 distinct Processes like Pyrolysis of coal and gasification of Char (Zhou
et al. 2006, p. 13).
1.1 Gasification:
The process of Gasification involves evaporation of water and therefore Water vapor
and CO2can be considered as the important components in the process of gasification
and to obtain a pure coke from the coal. The clean coal technology is able to involve
gasification of coal with Syngas (N, S Sox, and NOx). To produce various
products, Carbon can react with water and carbon.
CO + H2O CO2 + H2O Storage
In this process, while heating the coal, Char is being produced and Later on, during
the process of the Gasification-The residue of the char can be produced. This Residue
of Char is a combination of Ash and Coke (Malekshahian & Hill 2011, p. 22).
5573294 Page 4
O2
Cok
Coke + Ash
Char Residue
1.Introduction
Coal can be considered as one of the important fuel and can be used in the various
sectors in the day-to-day activities. Coal is considered as the carbon substitute and
there is a need to obtain pure coal for several processes. This can be called as the
Clean Coal Technology and This process can be done through gasification, which is
prorated into 2 distinct Processes like Pyrolysis of coal and gasification of Char (Zhou
et al. 2006, p. 13).
1.1 Gasification:
The process of Gasification involves evaporation of water and therefore Water vapor
and CO2can be considered as the important components in the process of gasification
and to obtain a pure coke from the coal. The clean coal technology is able to involve
gasification of coal with Syngas (N, S Sox, and NOx). To produce various
products, Carbon can react with water and carbon.
CO + H2O CO2 + H2O Storage
In this process, while heating the coal, Char is being produced and Later on, during
the process of the Gasification-The residue of the char can be produced. This Residue
of Char is a combination of Ash and Coke (Malekshahian & Hill 2011, p. 22).
5573294 Page 4
O2
Cok
Coke + Ash
Char Residue
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
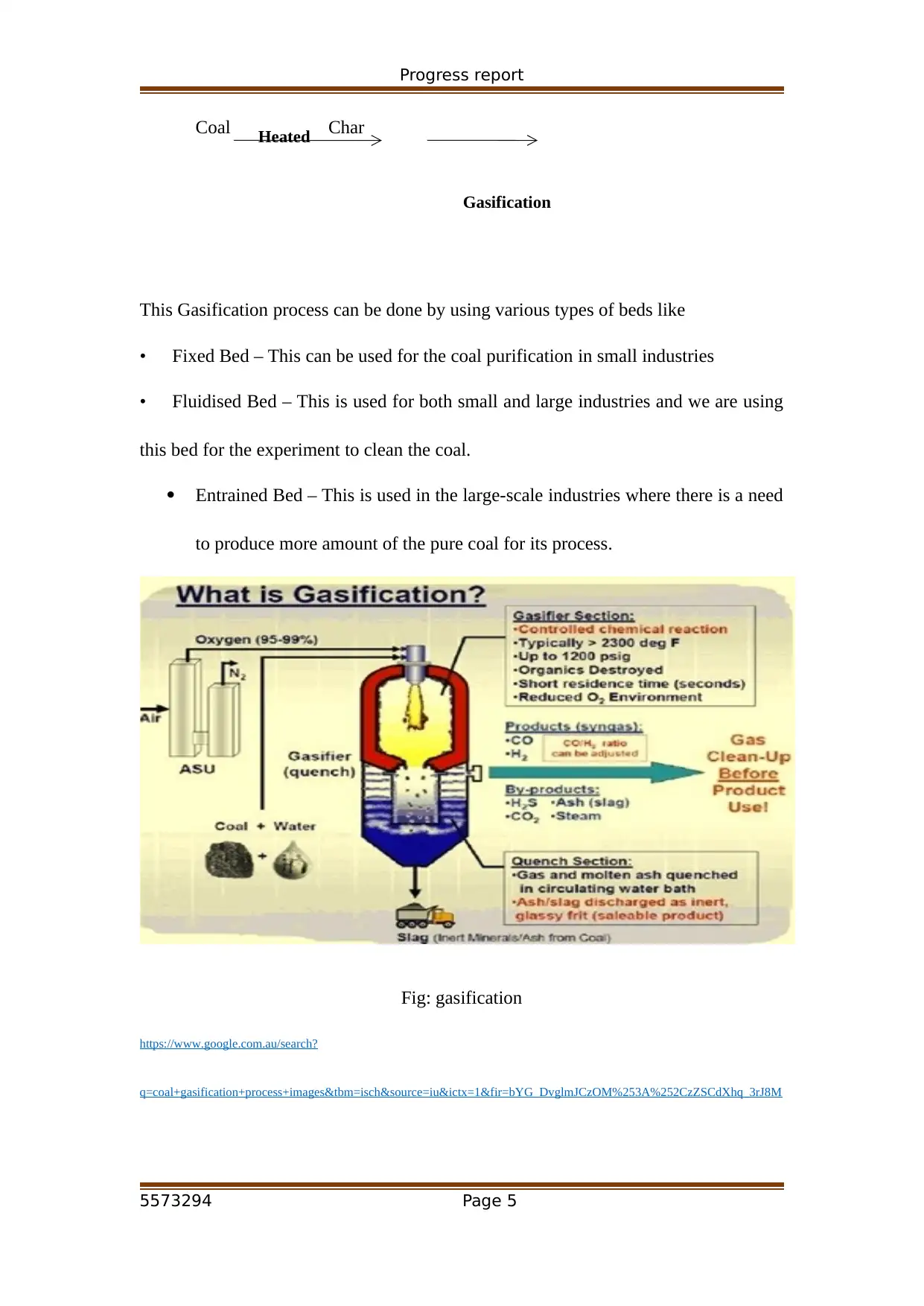
Progress report
Coal Char
This Gasification process can be done by using various types of beds like
• Fixed Bed – This can be used for the coal purification in small industries
• Fluidised Bed – This is used for both small and large industries and we are using
this bed for the experiment to clean the coal.
Entrained Bed – This is used in the large-scale industries where there is a need
to produce more amount of the pure coal for its process.
Fig: gasification
https://www.google.com.au/search?
q=coal+gasification+process+images&tbm=isch&source=iu&ictx=1&fir=bYG_DvglmJCzOM%253A%252CzZSCdXhq_3rJ8M
5573294 Page 5
Heated
Gasification
Coal Char
This Gasification process can be done by using various types of beds like
• Fixed Bed – This can be used for the coal purification in small industries
• Fluidised Bed – This is used for both small and large industries and we are using
this bed for the experiment to clean the coal.
Entrained Bed – This is used in the large-scale industries where there is a need
to produce more amount of the pure coal for its process.
Fig: gasification
https://www.google.com.au/search?
q=coal+gasification+process+images&tbm=isch&source=iu&ictx=1&fir=bYG_DvglmJCzOM%253A%252CzZSCdXhq_3rJ8M
5573294 Page 5
Heated
Gasification

Progress report
%252C_&usg=__kLtM9q5g5rysjcFiwYC7PSuWETw%3D&sa=X&ved=0ahUKEwjR-
cPd35_bAhUHU7wKHYQ4AQ4Q9QEIKzAA#imgrc=Qf-Na6yvv7bejM:
Moreover, the coal can be obtained from several levels of the process and from this
there will be an effect on the process of Coal Gasification. There are few parameters,
which have an ability to change during the process of Gasification like pressure,
temperature, Char, and content of Ash. All of these parameters are capable to rely
upon the quantity of the water and the carbon dioxide present and used during the
process of coal gasification. Amid of the gasification process, the coal can be passed
through different beds like fixed, fluid bed, etc., Hydrogen gas reacts with CO during
the existence of the oxygen, to produce the water and carbon dioxide. Char produced
during this process is another form of the coal and can be capable of reacting with the
water for the production of Hydrogen gas and Carbon Monoxide and with the Carbon
dioxide to produce Carbon monoxide (Jeong, Park, & Hwang 2014, p. 31).
Thus, the combination of the water and the carbon dioxide are capable of affecting the
process of coal Gasification as well as the carbon residue. During this project, several
materials are examined. Shenmu, Tejing, and the Chinese coal are used to evaluate the
carbon dioxide and water effects in the process of Coal Gasification. From this, we
can add the composition of the two coals in the report. Along with this, we can also
highlight the effects of the water and the carbon dioxide during the process of coal
Gasification (Hou et al. 2014, p. 45). The Carbon will be in the form of Residue. The
changes in the temperature can affect the consequences of the produced ash in several
5573294 Page 6
%252C_&usg=__kLtM9q5g5rysjcFiwYC7PSuWETw%3D&sa=X&ved=0ahUKEwjR-
cPd35_bAhUHU7wKHYQ4AQ4Q9QEIKzAA#imgrc=Qf-Na6yvv7bejM:
Moreover, the coal can be obtained from several levels of the process and from this
there will be an effect on the process of Coal Gasification. There are few parameters,
which have an ability to change during the process of Gasification like pressure,
temperature, Char, and content of Ash. All of these parameters are capable to rely
upon the quantity of the water and the carbon dioxide present and used during the
process of coal gasification. Amid of the gasification process, the coal can be passed
through different beds like fixed, fluid bed, etc., Hydrogen gas reacts with CO during
the existence of the oxygen, to produce the water and carbon dioxide. Char produced
during this process is another form of the coal and can be capable of reacting with the
water for the production of Hydrogen gas and Carbon Monoxide and with the Carbon
dioxide to produce Carbon monoxide (Jeong, Park, & Hwang 2014, p. 31).
Thus, the combination of the water and the carbon dioxide are capable of affecting the
process of coal Gasification as well as the carbon residue. During this project, several
materials are examined. Shenmu, Tejing, and the Chinese coal are used to evaluate the
carbon dioxide and water effects in the process of Coal Gasification. From this, we
can add the composition of the two coals in the report. Along with this, we can also
highlight the effects of the water and the carbon dioxide during the process of coal
Gasification (Hou et al. 2014, p. 45). The Carbon will be in the form of Residue. The
changes in the temperature can affect the consequences of the produced ash in several
5573294 Page 6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Progress report
circumstances. From this project and the report, we can evaluate the several types of
ash products, which are produced from the different coals.
1.2 Coal Pyrolysis process:
Pyrolysis is a technological process, which became popular by grabbing the attention
of the scientists/researchers as this is an in-between process in the coal
purification/conversion of the coal. In this process, we need to consider some
parameters like size of the coal particle, Temperature, Reactor type, pressure, etc., to
decide the carbon conversion rate and its distribution throughout the process. In detail,
we can say as:
25-300*c –Dry H20
Where there is a need to remove the gas from the surface.
300-550*c- Physical RXNs
Semi-coke
C---C---C
H --- C---C---C---C
H C O
Fig: Semicoke (uneven distribution of carbon components by a mixture of other
gases)
550-1050*c- semi-coke to coke
--C---C---C---C--
--C---C---C---C--
--C---C---C---C--
5573294 Page 7
circumstances. From this project and the report, we can evaluate the several types of
ash products, which are produced from the different coals.
1.2 Coal Pyrolysis process:
Pyrolysis is a technological process, which became popular by grabbing the attention
of the scientists/researchers as this is an in-between process in the coal
purification/conversion of the coal. In this process, we need to consider some
parameters like size of the coal particle, Temperature, Reactor type, pressure, etc., to
decide the carbon conversion rate and its distribution throughout the process. In detail,
we can say as:
25-300*c –Dry H20
Where there is a need to remove the gas from the surface.
300-550*c- Physical RXNs
Semi-coke
C---C---C
H --- C---C---C---C
H C O
Fig: Semicoke (uneven distribution of carbon components by a mixture of other
gases)
550-1050*c- semi-coke to coke
--C---C---C---C--
--C---C---C---C--
--C---C---C---C--
5573294 Page 7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
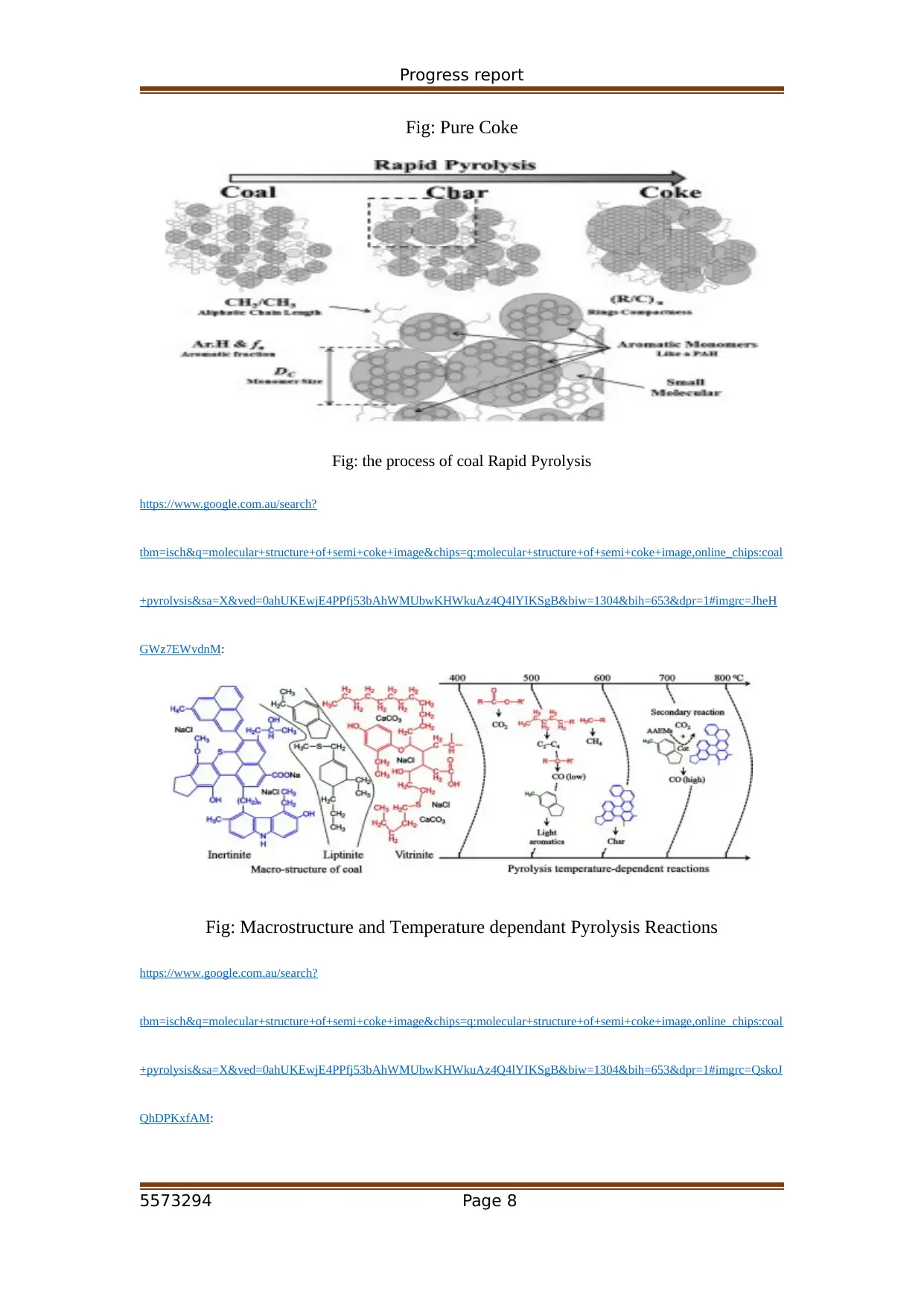
Progress report
Fig: Pure Coke
Fig: the process of coal Rapid Pyrolysis
https://www.google.com.au/search?
tbm=isch&q=molecular+structure+of+semi+coke+image&chips=q:molecular+structure+of+semi+coke+image,online_chips:coal
+pyrolysis&sa=X&ved=0ahUKEwjE4PPfj53bAhWMUbwKHWkuAz4Q4lYIKSgB&biw=1304&bih=653&dpr=1#imgrc=JheH
GWz7EWvdnM:
Fig: Macrostructure and Temperature dependant Pyrolysis Reactions
https://www.google.com.au/search?
tbm=isch&q=molecular+structure+of+semi+coke+image&chips=q:molecular+structure+of+semi+coke+image,online_chips:coal
+pyrolysis&sa=X&ved=0ahUKEwjE4PPfj53bAhWMUbwKHWkuAz4Q4lYIKSgB&biw=1304&bih=653&dpr=1#imgrc=QskoJ
QhDPKxfAM:
5573294 Page 8
Fig: Pure Coke
Fig: the process of coal Rapid Pyrolysis
https://www.google.com.au/search?
tbm=isch&q=molecular+structure+of+semi+coke+image&chips=q:molecular+structure+of+semi+coke+image,online_chips:coal
+pyrolysis&sa=X&ved=0ahUKEwjE4PPfj53bAhWMUbwKHWkuAz4Q4lYIKSgB&biw=1304&bih=653&dpr=1#imgrc=JheH
GWz7EWvdnM:
Fig: Macrostructure and Temperature dependant Pyrolysis Reactions
https://www.google.com.au/search?
tbm=isch&q=molecular+structure+of+semi+coke+image&chips=q:molecular+structure+of+semi+coke+image,online_chips:coal
+pyrolysis&sa=X&ved=0ahUKEwjE4PPfj53bAhWMUbwKHWkuAz4Q4lYIKSgB&biw=1304&bih=653&dpr=1#imgrc=QskoJ
QhDPKxfAM:
5573294 Page 8

Progress report
2. Aim, Objectives, and Scope of the Experiment:
2.1 The aim of the experiment:
To evaluate the effects of the water and carbon dioxide on the carbon residue (Coke +
ash) in the process of the coal gasification by analyzing the changes in various
parameters required for the experiment.
2.2 Experiment objectives
The experiment will evaluate the effect of the water and the carbon dioxide on
the residue of the carbon by the analysis of the changes in the parameters used
for this process.
To evaluate the effect temperature changes during the process of the coal
gasification.
To get a detailed analysis of the composition of the ash in 2 types of the coal
used in the experiment. To investigate the amount of ash produced when
different amounts of carbon dioxide and water are used
The experiment gives a chance to evaluate the amount of the ash that is
produced from the different amounts of the water and carbon dioxide used in
the gasification process.
2.3 The scope of the experiment:
The experiment/ project mainly aims at the effects that are achieved during the coal
gasification process. The changes in the temperature play a key role in the coal
gasification process. Carbon dioxide and water play an important role in coal process
and temperature increase affects the functionality of these components. Apart from
this, The experiment also helps us to evaluate the production of several products from
5573294 Page 9
2. Aim, Objectives, and Scope of the Experiment:
2.1 The aim of the experiment:
To evaluate the effects of the water and carbon dioxide on the carbon residue (Coke +
ash) in the process of the coal gasification by analyzing the changes in various
parameters required for the experiment.
2.2 Experiment objectives
The experiment will evaluate the effect of the water and the carbon dioxide on
the residue of the carbon by the analysis of the changes in the parameters used
for this process.
To evaluate the effect temperature changes during the process of the coal
gasification.
To get a detailed analysis of the composition of the ash in 2 types of the coal
used in the experiment. To investigate the amount of ash produced when
different amounts of carbon dioxide and water are used
The experiment gives a chance to evaluate the amount of the ash that is
produced from the different amounts of the water and carbon dioxide used in
the gasification process.
2.3 The scope of the experiment:
The experiment/ project mainly aims at the effects that are achieved during the coal
gasification process. The changes in the temperature play a key role in the coal
gasification process. Carbon dioxide and water play an important role in coal process
and temperature increase affects the functionality of these components. Apart from
this, The experiment also helps us to evaluate the production of several products from
5573294 Page 9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Progress report
the process during the functionality of the experiment. The main important parameters
and the change in the conditions can affect the amount of the products produced from
this the process of gasification. These products include Char and ash (Deng, Luo,
Zhang & Wang 2012). The process of the gasification is well affected by the increase
in temperature and this will also evaluate the way how the products in this process are
affected by the continuous changes in the temperature within the gasification process.
The project will also evaluate the effect of the increase in heat while producing
different products from the gasification process, The change in the heat can affect the
rate of reactivity of the reactants present I the process as well as coal (Bai et al. 2014).
3. Literature Review:
Gasification is a very fundamental technology when it comes to the uncontaminated
and energy-saving use of coal, coke, petroleum as well as other fuels in solid form.
The technology has been in the large-scale industry for a long time owing to its high
efficiency, almost zero carbon dioxide pollution as well as high productivity in the
industries in which it is being applied. Research and studies through theoretical and
experimental analyses of the processes of gasification have picked on gasification rate
as an important factor that is used in controlling gasification behaviors. Such
conclusions based on the findings were mainly arrived at based on the relatively slow
kinetics of char-steam reactions and char-CO2 reactions under the different
gasification conditions. This thus makes it important to steer off a proper
5573294 Page 10
the process during the functionality of the experiment. The main important parameters
and the change in the conditions can affect the amount of the products produced from
this the process of gasification. These products include Char and ash (Deng, Luo,
Zhang & Wang 2012). The process of the gasification is well affected by the increase
in temperature and this will also evaluate the way how the products in this process are
affected by the continuous changes in the temperature within the gasification process.
The project will also evaluate the effect of the increase in heat while producing
different products from the gasification process, The change in the heat can affect the
rate of reactivity of the reactants present I the process as well as coal (Bai et al. 2014).
3. Literature Review:
Gasification is a very fundamental technology when it comes to the uncontaminated
and energy-saving use of coal, coke, petroleum as well as other fuels in solid form.
The technology has been in the large-scale industry for a long time owing to its high
efficiency, almost zero carbon dioxide pollution as well as high productivity in the
industries in which it is being applied. Research and studies through theoretical and
experimental analyses of the processes of gasification have picked on gasification rate
as an important factor that is used in controlling gasification behaviors. Such
conclusions based on the findings were mainly arrived at based on the relatively slow
kinetics of char-steam reactions and char-CO2 reactions under the different
gasification conditions. This thus makes it important to steer off a proper
5573294 Page 10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Progress report
comprehension of the kinetics of gasification of char-steam as well as that of char-
CO2 (Overend, 2012, p.820).
Numerous reaction models have been developed by various scholars of char-CO2 and
char-steam in that order in order to estimate the rates of their reactions. Still, there
have also been elaborate and extensive studies made on char-CO2 gasification and
char steam gasification kinetics. The main disadvantage with the reaction models that
have been developed in the previous studies by researchers is their inability to be
applied in systems in which there are simultaneous reactions of char-steam and char-
CO2 having a sole gasifying agent (Puigjaner, 2011, p.315). Under real gasification
conditions, a reaction is carried out between the chars and with the mixtures of
gasifying agent and CO2.
A research was thus carried out by the scholars to investigate the reactivities of
gasification of metallurgical coke in a steam and CO2 mixture unfortunately it
became quite a challenge to perform an analysis on the intrinsic chair-steam-CO2
reaction since the sizes of the particles that were used in the sample were quite large
ranging between 3 mm and 6 mm. This led to the impact of internal diffusion. Other
researcher has also done studies on the mechanisms of gasification of coal char
exposed to conditions of a mixture of CO2 and steam and made proposals on
empirical approaches that could be used to elaborate the impacts of the degree of
change of carbon on the rate of gasification (Shelton, 2012, p.177).
5573294 Page 11
comprehension of the kinetics of gasification of char-steam as well as that of char-
CO2 (Overend, 2012, p.820).
Numerous reaction models have been developed by various scholars of char-CO2 and
char-steam in that order in order to estimate the rates of their reactions. Still, there
have also been elaborate and extensive studies made on char-CO2 gasification and
char steam gasification kinetics. The main disadvantage with the reaction models that
have been developed in the previous studies by researchers is their inability to be
applied in systems in which there are simultaneous reactions of char-steam and char-
CO2 having a sole gasifying agent (Puigjaner, 2011, p.315). Under real gasification
conditions, a reaction is carried out between the chars and with the mixtures of
gasifying agent and CO2.
A research was thus carried out by the scholars to investigate the reactivities of
gasification of metallurgical coke in a steam and CO2 mixture unfortunately it
became quite a challenge to perform an analysis on the intrinsic chair-steam-CO2
reaction since the sizes of the particles that were used in the sample were quite large
ranging between 3 mm and 6 mm. This led to the impact of internal diffusion. Other
researcher has also done studies on the mechanisms of gasification of coal char
exposed to conditions of a mixture of CO2 and steam and made proposals on
empirical approaches that could be used to elaborate the impacts of the degree of
change of carbon on the rate of gasification (Shelton, 2012, p.177).
5573294 Page 11

Progress report
A recent study was developed by some researchers recently to come out to examine
the relations in char-CO2-steam reaction with the aid of Langmuir-Hinshelwood
model. This model is pegged on the philosophy of resorption and absorption. It was
established for the research that char-CO2-steam reaction mainly occurs at the
common active sites. The findings also illustrated that hot vapor and CO2 were in a
competition for the active sites of the chars. However, the same research was done by
some other researchers who proposed an opposite view over the findings (Puigjaner,
2011, p.247). These researchers, in the perception, argued that the char-CO2 reaction
and the char-CO2 reaction went on a separate and different active site and thus the
entire rate of gasification should be determined by adding the gasification rate of the
char-CO2 reaction and the rate of char-steam reaction.
Still, these researchers identified that the mechanism of reaction of lignite gasification
was in line with the different reactive site mechanism of reaction under relatively
reduced pressures of gasification, but was greater than the mechanism of a reaction at
the common reactive site in case the pressures of gasification was increased (Shelton,
2012, p.258). On the other hand, another scholar found out that the entire rate of
gasification is not equivalent to the some of the char-CO2 reaction and the rate of
char-steam reaction rates and that there was no proof of competition for the active site
between the two gasifying agents. A Langmuir-Hinshelwood model that had two
parameters with no dimension was then proposed by the researchers to examine the
rate of char-steam and char-CO2 gasification and thus could further be used in the
estimation of the interactions more accurately in comparison to other models (Cooper,
5573294 Page 12
A recent study was developed by some researchers recently to come out to examine
the relations in char-CO2-steam reaction with the aid of Langmuir-Hinshelwood
model. This model is pegged on the philosophy of resorption and absorption. It was
established for the research that char-CO2-steam reaction mainly occurs at the
common active sites. The findings also illustrated that hot vapor and CO2 were in a
competition for the active sites of the chars. However, the same research was done by
some other researchers who proposed an opposite view over the findings (Puigjaner,
2011, p.247). These researchers, in the perception, argued that the char-CO2 reaction
and the char-CO2 reaction went on a separate and different active site and thus the
entire rate of gasification should be determined by adding the gasification rate of the
char-CO2 reaction and the rate of char-steam reaction.
Still, these researchers identified that the mechanism of reaction of lignite gasification
was in line with the different reactive site mechanism of reaction under relatively
reduced pressures of gasification, but was greater than the mechanism of a reaction at
the common reactive site in case the pressures of gasification was increased (Shelton,
2012, p.258). On the other hand, another scholar found out that the entire rate of
gasification is not equivalent to the some of the char-CO2 reaction and the rate of
char-steam reaction rates and that there was no proof of competition for the active site
between the two gasifying agents. A Langmuir-Hinshelwood model that had two
parameters with no dimension was then proposed by the researchers to examine the
rate of char-steam and char-CO2 gasification and thus could further be used in the
estimation of the interactions more accurately in comparison to other models (Cooper,
5573294 Page 12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 28
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


