Comparison of Known and Unknown Halophilic Archaeans
VerifiedAdded on 2023/01/09
|6
|2242
|30
AI Summary
This experiment investigates the characteristics of two known species of halophilic Archaeans and an unknown species discovered in Dead Moose Lake in Canada. The study compares the morphological, genetic, and phenotypic characteristics of the three microorganisms to classify the unknown halophilic archaea.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Comparison of Known Halophilic Archaeans Characteristics with Unknown Halophilic Archaeans.
Aldhafiri, Danah a
a Department of Biological Sciences, Brock University, St. Catharines, Ontario. Canada, L2S 3A1
Journal of Biological Sciences 01 (2018) 001–001
Abstract
This experiment investigated two known species of halophilic Archaeans and an unknown species that
was discovered in Dead moose lake in Canada. The known species were Haloferax volcanii and
Halobacterium salinarum. The purpose of the experiment was to compare the morphological, genetic
and phenotypic characteristics of the three microorganisms with the view to classify the unknown
halophilic archaea. Different cultures were made and the organisms put in them under different
conditions in order to study the said characteristics. The ability of the halophiles to withstand extreme
conditions was put to test with variations in temperatures, pH and concentration of the culture being
made in order to identify the optimal conditions for growth in the different species. The results revealed
that the three organisms shared some characteristics and also had some unique features which suggested
that they belonged to different genres. The unknown sample, in particular, was quite distinct in a
majority of its properties, suggesting that this might be a new species. This study evaluates the optimal
conditions of temperature, salt concentration, and pH required for the three species and comparisons of
the evolutionary relationships between the three species made using phenotypic analysis by the use of
dendograms. From the results obtained, the unknown species should be classified as a member of the
genus Haloferax.
Keywords: Dihydrolase; Galactosidase; Halophilic; Hydrolysis; Morphology.
1. Introduction
The domain of archaea was first put in place in 1977 by a group of scientists who had spent time
studying the rRNA (ribosomal ribonucleic acid) genes (Oren, 2006). They were classified into their
own kingdom, separated from bacteria, as they lacked the properties and features of other bacterial
cells in normal habitats (Oren, 2006). Halophilic archaea are a group of microorganisms that belongs
to the phylum Euryarchaeota (Gibtan et al., 2017). They were initially categorized as halobacteria
but they are now referred to archaea (Gibtan et al., 2017). There are ten haloarchaeal genera that are
classified in both order halobacteriales and halofercales (Noha, et al., 2012). About 50 species have
been documented to exist in halobacteriales genera (Noha, et al., 2012). These microorganisms have
the ability to withstand very saline conditions hence the name halo which means salt (Stan-Lotter &
Fendrihan, 2015). They also exist in rocks that have existed on the surface of the earth for many
years (Stan-Lotter & Fendrihan, 2015). Also known as haloarchaea, the bacteria thrive in salty
conditions having the ability to conduct its biological processes without being adversely affected
(Stan-Lotter & Fendrihan, 2015). Scientists have continued to conduct the research and discover new
sequences of rRNA genes that suggest the existence of a new species of haloarchaea (Stan-Lotter &
Fendrihan, 2015). Haloarchaea are able to withstand very extreme conditions of very high
temperatures, super saline conditions and in some cases ultraviolet radiations (Oren, 2011). For this
reason, halophilic Archaeans are considered to be extremophiles (Oren, 2011). To ensure the
Aldhafiri, Danah a
a Department of Biological Sciences, Brock University, St. Catharines, Ontario. Canada, L2S 3A1
Journal of Biological Sciences 01 (2018) 001–001
Abstract
This experiment investigated two known species of halophilic Archaeans and an unknown species that
was discovered in Dead moose lake in Canada. The known species were Haloferax volcanii and
Halobacterium salinarum. The purpose of the experiment was to compare the morphological, genetic
and phenotypic characteristics of the three microorganisms with the view to classify the unknown
halophilic archaea. Different cultures were made and the organisms put in them under different
conditions in order to study the said characteristics. The ability of the halophiles to withstand extreme
conditions was put to test with variations in temperatures, pH and concentration of the culture being
made in order to identify the optimal conditions for growth in the different species. The results revealed
that the three organisms shared some characteristics and also had some unique features which suggested
that they belonged to different genres. The unknown sample, in particular, was quite distinct in a
majority of its properties, suggesting that this might be a new species. This study evaluates the optimal
conditions of temperature, salt concentration, and pH required for the three species and comparisons of
the evolutionary relationships between the three species made using phenotypic analysis by the use of
dendograms. From the results obtained, the unknown species should be classified as a member of the
genus Haloferax.
Keywords: Dihydrolase; Galactosidase; Halophilic; Hydrolysis; Morphology.
1. Introduction
The domain of archaea was first put in place in 1977 by a group of scientists who had spent time
studying the rRNA (ribosomal ribonucleic acid) genes (Oren, 2006). They were classified into their
own kingdom, separated from bacteria, as they lacked the properties and features of other bacterial
cells in normal habitats (Oren, 2006). Halophilic archaea are a group of microorganisms that belongs
to the phylum Euryarchaeota (Gibtan et al., 2017). They were initially categorized as halobacteria
but they are now referred to archaea (Gibtan et al., 2017). There are ten haloarchaeal genera that are
classified in both order halobacteriales and halofercales (Noha, et al., 2012). About 50 species have
been documented to exist in halobacteriales genera (Noha, et al., 2012). These microorganisms have
the ability to withstand very saline conditions hence the name halo which means salt (Stan-Lotter &
Fendrihan, 2015). They also exist in rocks that have existed on the surface of the earth for many
years (Stan-Lotter & Fendrihan, 2015). Also known as haloarchaea, the bacteria thrive in salty
conditions having the ability to conduct its biological processes without being adversely affected
(Stan-Lotter & Fendrihan, 2015). Scientists have continued to conduct the research and discover new
sequences of rRNA genes that suggest the existence of a new species of haloarchaea (Stan-Lotter &
Fendrihan, 2015). Haloarchaea are able to withstand very extreme conditions of very high
temperatures, super saline conditions and in some cases ultraviolet radiations (Oren, 2011). For this
reason, halophilic Archaeans are considered to be extremophiles (Oren, 2011). To ensure the
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

survival of halophiles in the saline conditions, their cells are configured to have charged amino acids
on the cell membranes which are essential in the retention of water molecules within the cell (Stan-
Lotter & Fendrihan, 2015). Being unable to produce spores which are a domicile form of most
bacteria when the environment becomes oligotrophic, some forms haloarchaea form cysts and other
resting states that are still the subject of study by various scholars (Oren, 2006). These organisms
have demonstrated resilience to ensure that their survival is guaranteed including resistance to
desiccation (Najjari, et al., 2015). Due to their ability to survive extreme conditions, halophilic
archaea have some very distinct features associated with eukaryotic organisms (Oren, 2006).
Presence of multiple RNA polymerases and leaderless transcripts make them very unique from other
types of bacteria at the level of molecules (Oren, 2006). Some of the most studied genera of
halophiles include Halobacterium, Haloferax, and Haloarcula (Oren, 2011). Scholars have little
information about many other genres that exist in terms of their structures and patterns. Given the
wide range of diversity in halobacteria, scientists have conducted many studies to determine the
significance of the different habitat factors and how they affect the growth of the halophiles (Gibtan
et al., 2017). Understanding that these organisms exist in special environments, a study of their
physiological and other important features is imperative to critically evaluate their survival. An in-
depth look at the genetic features is also a key to notice any genetic disparities between the known
halophiles and the new isolates that continue to be discovered every day. This study investigated two
species of halophiles and made comparisons of their various morphological features, growth
characteristics, nutrition patterns and phylogenetic features. The unknown isolate under study was
discovered in Deadmoose lake in Canada.
2. Materials and methods
Isolation and culture conditions
20 μL of liquid culture was inoculated into 5 mL of growth media. Cultures were then incubated for
a period of 14 days after which absorbance was measured at 600 nm. NaCl concentration was
increased from 0 to 6.0 M. The pH was then increased from 3-9. Cultures were then grown at
different temperatures from 5-60 °C.
Physiological and biochemical tests
Physiological tests are done to determine which factors affect the growth of halophiles.
Physiological tests are carried out by altering the temperature, pH and the concentration of the
culture keeping the level of nutrients constant (Bowers and Wiegel, 2011). Biochemical tests are
conducted to determine the end products of enzymatic and metabolic reactions. Reagents that change
colour when the targeted enzyme is present are used for this particular part of the experiment. Few
biochemical tests were conducted such as ß-Galactosidase Activity, acid production from
carbohydrates, Arginine Dihydrolase activity, starch hydrolysis.
Phylogenetic analysis
A phylogenetic tree is constructed to establish the relationship between the two known species and
the unknown species by considering their individual characteristics.
3. Results and Discussion
Colony Morphology
2
on the cell membranes which are essential in the retention of water molecules within the cell (Stan-
Lotter & Fendrihan, 2015). Being unable to produce spores which are a domicile form of most
bacteria when the environment becomes oligotrophic, some forms haloarchaea form cysts and other
resting states that are still the subject of study by various scholars (Oren, 2006). These organisms
have demonstrated resilience to ensure that their survival is guaranteed including resistance to
desiccation (Najjari, et al., 2015). Due to their ability to survive extreme conditions, halophilic
archaea have some very distinct features associated with eukaryotic organisms (Oren, 2006).
Presence of multiple RNA polymerases and leaderless transcripts make them very unique from other
types of bacteria at the level of molecules (Oren, 2006). Some of the most studied genera of
halophiles include Halobacterium, Haloferax, and Haloarcula (Oren, 2011). Scholars have little
information about many other genres that exist in terms of their structures and patterns. Given the
wide range of diversity in halobacteria, scientists have conducted many studies to determine the
significance of the different habitat factors and how they affect the growth of the halophiles (Gibtan
et al., 2017). Understanding that these organisms exist in special environments, a study of their
physiological and other important features is imperative to critically evaluate their survival. An in-
depth look at the genetic features is also a key to notice any genetic disparities between the known
halophiles and the new isolates that continue to be discovered every day. This study investigated two
species of halophiles and made comparisons of their various morphological features, growth
characteristics, nutrition patterns and phylogenetic features. The unknown isolate under study was
discovered in Deadmoose lake in Canada.
2. Materials and methods
Isolation and culture conditions
20 μL of liquid culture was inoculated into 5 mL of growth media. Cultures were then incubated for
a period of 14 days after which absorbance was measured at 600 nm. NaCl concentration was
increased from 0 to 6.0 M. The pH was then increased from 3-9. Cultures were then grown at
different temperatures from 5-60 °C.
Physiological and biochemical tests
Physiological tests are done to determine which factors affect the growth of halophiles.
Physiological tests are carried out by altering the temperature, pH and the concentration of the
culture keeping the level of nutrients constant (Bowers and Wiegel, 2011). Biochemical tests are
conducted to determine the end products of enzymatic and metabolic reactions. Reagents that change
colour when the targeted enzyme is present are used for this particular part of the experiment. Few
biochemical tests were conducted such as ß-Galactosidase Activity, acid production from
carbohydrates, Arginine Dihydrolase activity, starch hydrolysis.
Phylogenetic analysis
A phylogenetic tree is constructed to establish the relationship between the two known species and
the unknown species by considering their individual characteristics.
3. Results and Discussion
Colony Morphology
2
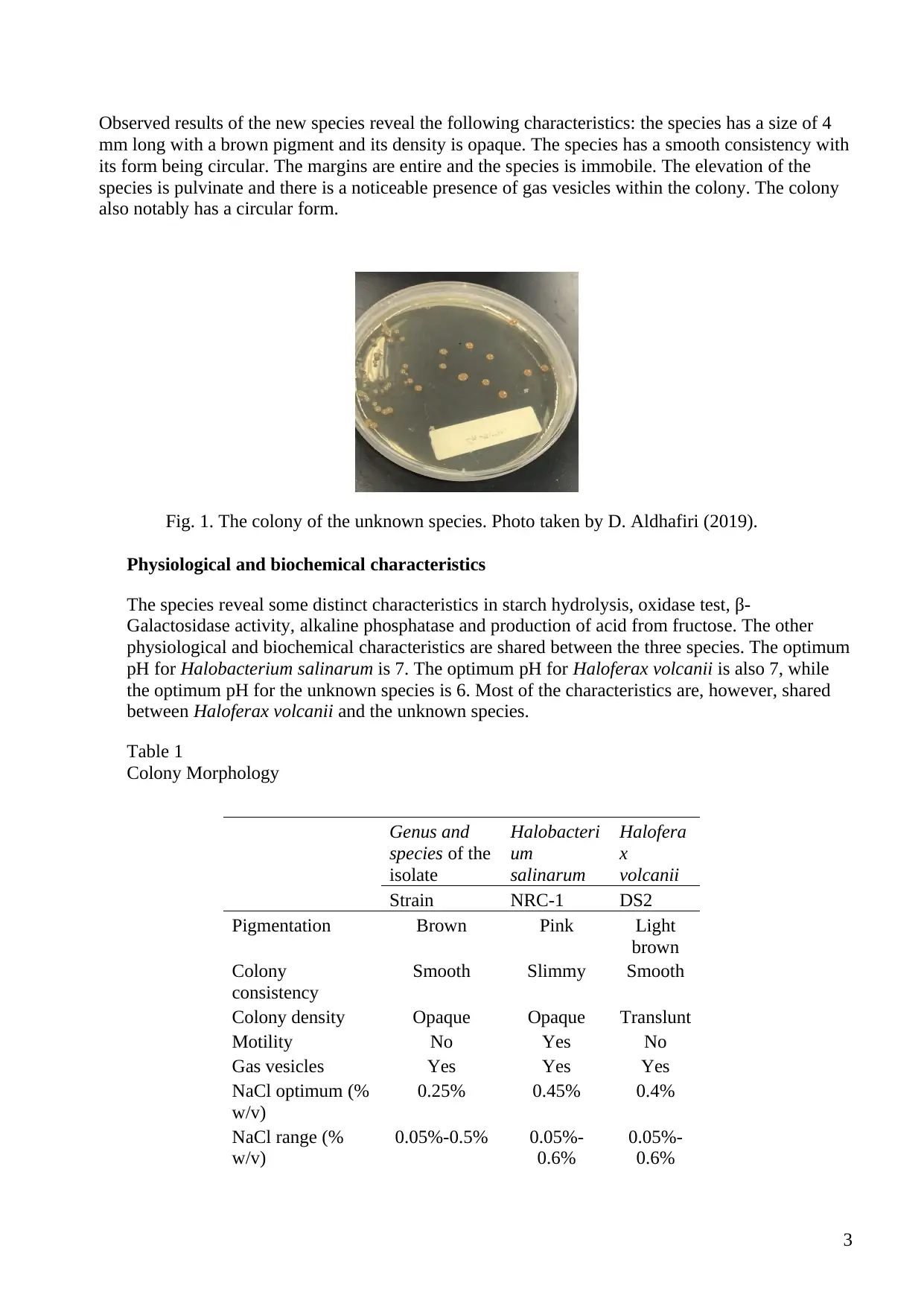
Observed results of the new species reveal the following characteristics: the species has a size of 4
mm long with a brown pigment and its density is opaque. The species has a smooth consistency with
its form being circular. The margins are entire and the species is immobile. The elevation of the
species is pulvinate and there is a noticeable presence of gas vesicles within the colony. The colony
also notably has a circular form.
Fig. 1. The colony of the unknown species. Photo taken by D. Aldhafiri (2019).
Physiological and biochemical characteristics
The species reveal some distinct characteristics in starch hydrolysis, oxidase test, β-
Galactosidase activity, alkaline phosphatase and production of acid from fructose. The other
physiological and biochemical characteristics are shared between the three species. The optimum
pH for Halobacterium salinarum is 7. The optimum pH for Haloferax volcanii is also 7, while
the optimum pH for the unknown species is 6. Most of the characteristics are, however, shared
between Haloferax volcanii and the unknown species.
Table 1
Colony Morphology
Genus and
species of the
isolate
Halobacteri
um
salinarum
Halofera
x
volcanii
Strain NRC-1 DS2
Pigmentation Brown Pink Light
brown
Colony
consistency
Smooth Slimmy Smooth
Colony density Opaque Opaque Translunt
Motility No Yes No
Gas vesicles Yes Yes Yes
NaCl optimum (%
w/v)
0.25% 0.45% 0.4%
NaCl range (%
w/v)
0.05%-0.5% 0.05%-
0.6%
0.05%-
0.6%
3
mm long with a brown pigment and its density is opaque. The species has a smooth consistency with
its form being circular. The margins are entire and the species is immobile. The elevation of the
species is pulvinate and there is a noticeable presence of gas vesicles within the colony. The colony
also notably has a circular form.
Fig. 1. The colony of the unknown species. Photo taken by D. Aldhafiri (2019).
Physiological and biochemical characteristics
The species reveal some distinct characteristics in starch hydrolysis, oxidase test, β-
Galactosidase activity, alkaline phosphatase and production of acid from fructose. The other
physiological and biochemical characteristics are shared between the three species. The optimum
pH for Halobacterium salinarum is 7. The optimum pH for Haloferax volcanii is also 7, while
the optimum pH for the unknown species is 6. Most of the characteristics are, however, shared
between Haloferax volcanii and the unknown species.
Table 1
Colony Morphology
Genus and
species of the
isolate
Halobacteri
um
salinarum
Halofera
x
volcanii
Strain NRC-1 DS2
Pigmentation Brown Pink Light
brown
Colony
consistency
Smooth Slimmy Smooth
Colony density Opaque Opaque Translunt
Motility No Yes No
Gas vesicles Yes Yes Yes
NaCl optimum (%
w/v)
0.25% 0.45% 0.4%
NaCl range (%
w/v)
0.05%-0.5% 0.05%-
0.6%
0.05%-
0.6%
3
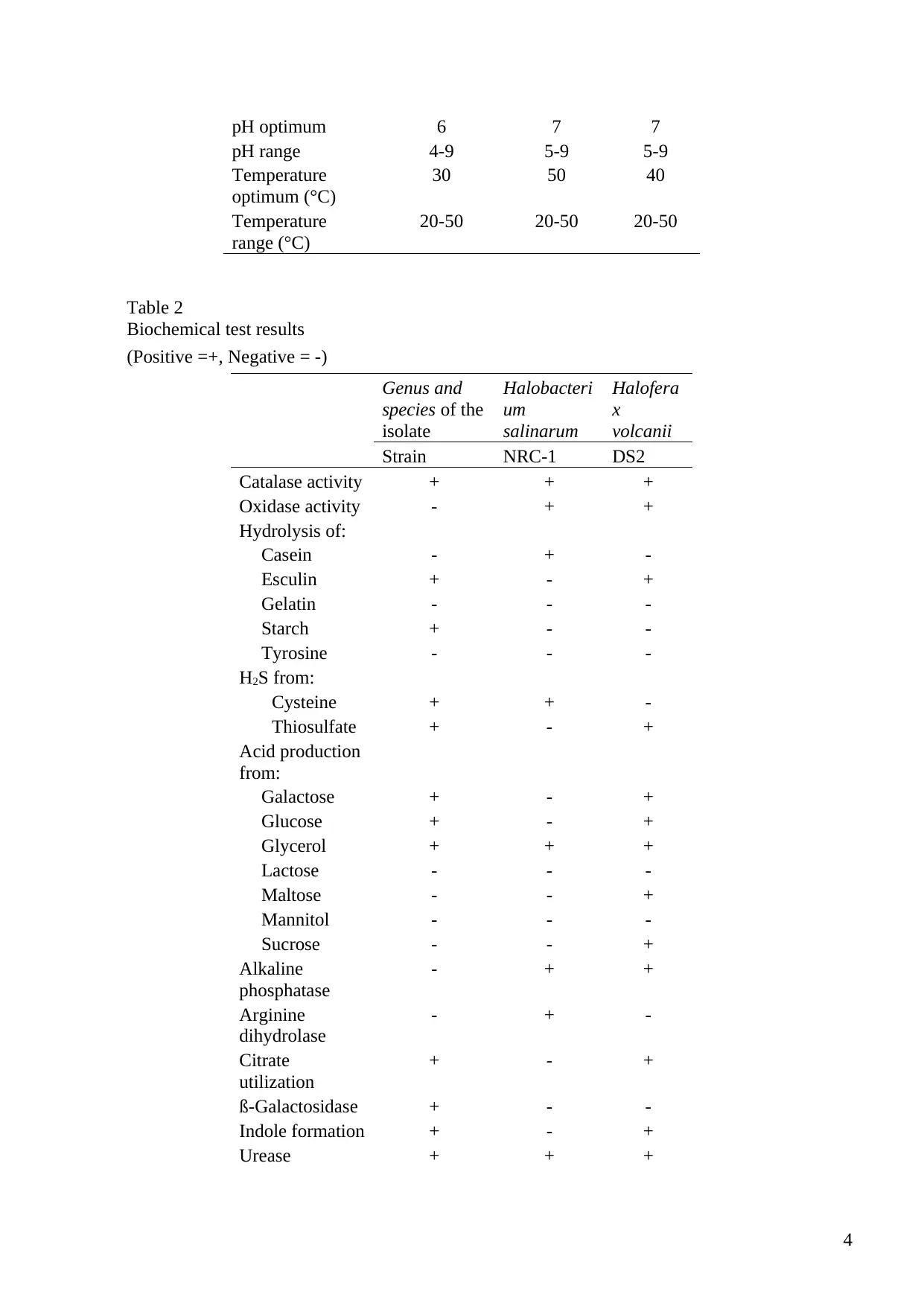
pH optimum 6 7 7
pH range 4-9 5-9 5-9
Temperature
optimum (°C)
30 50 40
Temperature
range (°C)
20-50 20-50 20-50
Table 2
Biochemical test results
(Positive =+, Negative = -)
Genus and
species of the
isolate
Halobacteri
um
salinarum
Halofera
x
volcanii
Strain NRC-1 DS2
Catalase activity + + +
Oxidase activity - + +
Hydrolysis of:
Casein - + -
Esculin + - +
Gelatin - - -
Starch + - -
Tyrosine - - -
H2S from:
Cysteine + + -
Thiosulfate + - +
Acid production
from:
Galactose + - +
Glucose + - +
Glycerol + + +
Lactose - - -
Maltose - - +
Mannitol - - -
Sucrose - - +
Alkaline
phosphatase
- + +
Arginine
dihydrolase
- + -
Citrate
utilization
+ - +
ß-Galactosidase + - -
Indole formation + - +
Urease + + +
4
pH range 4-9 5-9 5-9
Temperature
optimum (°C)
30 50 40
Temperature
range (°C)
20-50 20-50 20-50
Table 2
Biochemical test results
(Positive =+, Negative = -)
Genus and
species of the
isolate
Halobacteri
um
salinarum
Halofera
x
volcanii
Strain NRC-1 DS2
Catalase activity + + +
Oxidase activity - + +
Hydrolysis of:
Casein - + -
Esculin + - +
Gelatin - - -
Starch + - -
Tyrosine - - -
H2S from:
Cysteine + + -
Thiosulfate + - +
Acid production
from:
Galactose + - +
Glucose + - +
Glycerol + + +
Lactose - - -
Maltose - - +
Mannitol - - -
Sucrose - - +
Alkaline
phosphatase
- + +
Arginine
dihydrolase
- + -
Citrate
utilization
+ - +
ß-Galactosidase + - -
Indole formation + - +
Urease + + +
4
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
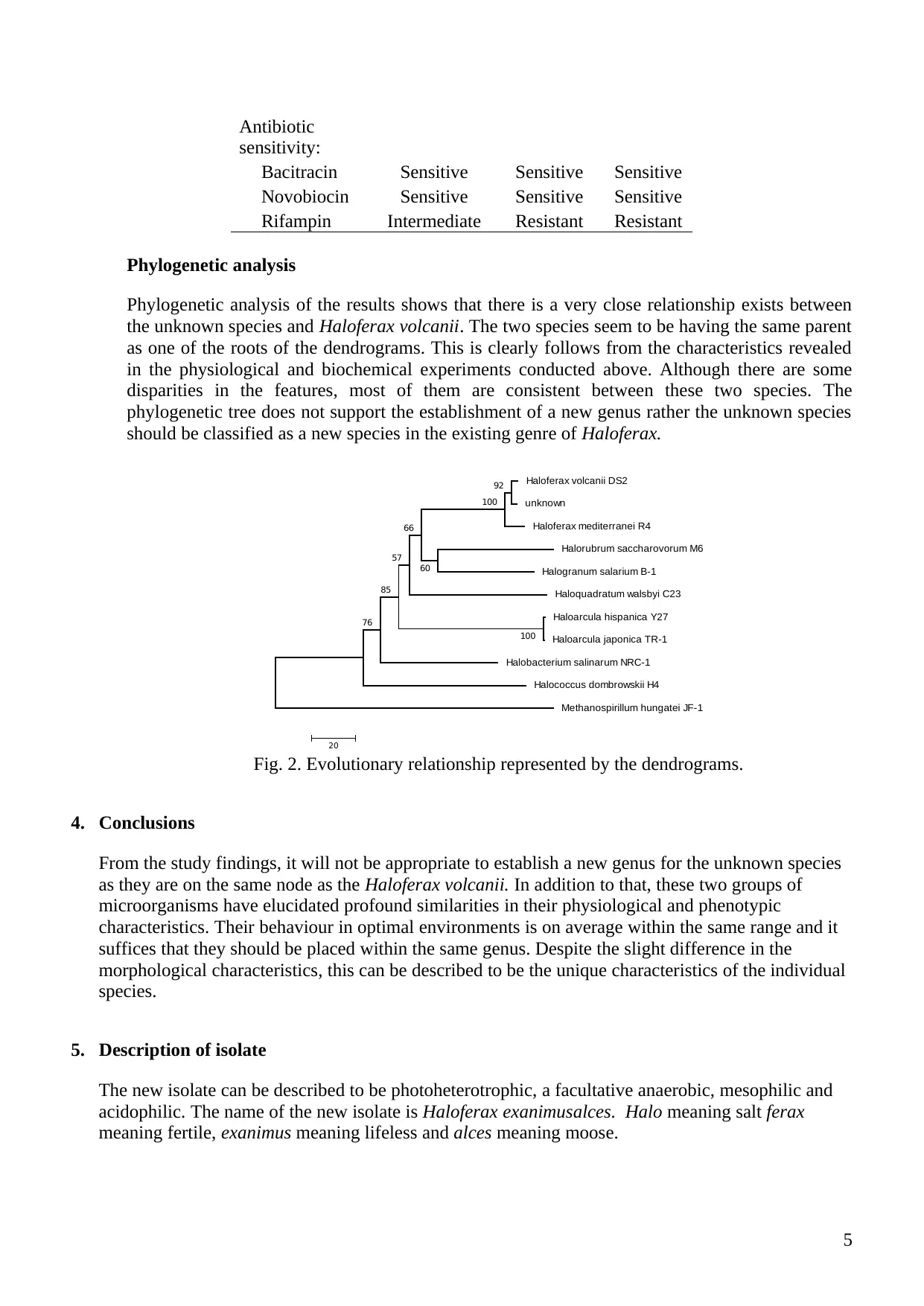
Antibiotic
sensitivity:
Bacitracin Sensitive Sensitive Sensitive
Novobiocin Sensitive Sensitive Sensitive
Rifampin Intermediate Resistant Resistant
Phylogenetic analysis
Phylogenetic analysis of the results shows that there is a very close relationship exists between
the unknown species and Haloferax volcanii. The two species seem to be having the same parent
as one of the roots of the dendrograms. This is clearly follows from the characteristics revealed
in the physiological and biochemical experiments conducted above. Although there are some
disparities in the features, most of them are consistent between these two species. The
phylogenetic tree does not support the establishment of a new genus rather the unknown species
should be classified as a new species in the existing genre of Haloferax.
Haloferax volcanii DS2
unknown
Haloferax mediterranei R4
Halorubrum saccharovorum M6
Halogranum salarium B-1
Haloquadratum walsbyi C23
Haloarcula hispanica Y27
Haloarcula japonica TR-1
Halobacterium salinarum NRC-1
Halococcus dombrowskii H4
Methanospirillum hungatei JF-1
100
92
100
76
60
66
57
85
20
Fig. 2. Evolutionary relationship represented by the dendrograms.
4. Conclusions
From the study findings, it will not be appropriate to establish a new genus for the unknown species
as they are on the same node as the Haloferax volcanii. In addition to that, these two groups of
microorganisms have elucidated profound similarities in their physiological and phenotypic
characteristics. Their behaviour in optimal environments is on average within the same range and it
suffices that they should be placed within the same genus. Despite the slight difference in the
morphological characteristics, this can be described to be the unique characteristics of the individual
species.
5. Description of isolate
The new isolate can be described to be photoheterotrophic, a facultative anaerobic, mesophilic and
acidophilic. The name of the new isolate is Haloferax exanimusalces. Halo meaning salt ferax
meaning fertile, exanimus meaning lifeless and alces meaning moose.
5
sensitivity:
Bacitracin Sensitive Sensitive Sensitive
Novobiocin Sensitive Sensitive Sensitive
Rifampin Intermediate Resistant Resistant
Phylogenetic analysis
Phylogenetic analysis of the results shows that there is a very close relationship exists between
the unknown species and Haloferax volcanii. The two species seem to be having the same parent
as one of the roots of the dendrograms. This is clearly follows from the characteristics revealed
in the physiological and biochemical experiments conducted above. Although there are some
disparities in the features, most of them are consistent between these two species. The
phylogenetic tree does not support the establishment of a new genus rather the unknown species
should be classified as a new species in the existing genre of Haloferax.
Haloferax volcanii DS2
unknown
Haloferax mediterranei R4
Halorubrum saccharovorum M6
Halogranum salarium B-1
Haloquadratum walsbyi C23
Haloarcula hispanica Y27
Haloarcula japonica TR-1
Halobacterium salinarum NRC-1
Halococcus dombrowskii H4
Methanospirillum hungatei JF-1
100
92
100
76
60
66
57
85
20
Fig. 2. Evolutionary relationship represented by the dendrograms.
4. Conclusions
From the study findings, it will not be appropriate to establish a new genus for the unknown species
as they are on the same node as the Haloferax volcanii. In addition to that, these two groups of
microorganisms have elucidated profound similarities in their physiological and phenotypic
characteristics. Their behaviour in optimal environments is on average within the same range and it
suffices that they should be placed within the same genus. Despite the slight difference in the
morphological characteristics, this can be described to be the unique characteristics of the individual
species.
5. Description of isolate
The new isolate can be described to be photoheterotrophic, a facultative anaerobic, mesophilic and
acidophilic. The name of the new isolate is Haloferax exanimusalces. Halo meaning salt ferax
meaning fertile, exanimus meaning lifeless and alces meaning moose.
5

6. Acknowledgments
Acknowledgements are made to Brock University and my teacher assistants for the instrumental
help in the documenting of this journal article.
7. References
Bowers, K. and Wiegel, J. (2011) Temperature and pH optima of extremely halophilic archaea: a
mini-review. Extremophiles 15: 119–128
Brooker, R. J., Widmaier, E. P., Graham, L. E., & Stiling, P. D. (2017). Biology (4th ed.). New
York, NY: McGraw-Hill Education.
Gibtan, A., Park, K., Woo, M., Shin, J., Lee, D., Sohn, J., Song, M., Roh, S., Lee, S., & Lee, H.
(2017). Diversity of Extremely Halophilic Archaeal and Bacterial Communities from Commercial
Salts. Frontiers in Microbiology, 8. doi: 10.3389/fmicb.2017.00799
Najjari, A., Elshahed, M., Cherif, A., & Youssef, N. (2015). Patterns and Determinants of
Halophilic Archaea (Class Halobacteria) Diversity in Tunisian Endorheic Salt Lakes and Sebkhet
Systems. Applied and Environmental Microbiology, 81(13), 4432-4441. doi: 10.1128/aem.01097-15
Noha, Y., Kristen, A., & Mostafa, E. (2012). Phylogenetic Diversities and Community Structure
of Members of the Extremely Halophilic Archaea (Order Halobacteriales) in Multiple Saline Sediment
Habitats. Applied and Environmental Microbiology, 1332-1344.
Oren, A. (2006) The Order Halobacteriales 3: 113-164 In: Dworkin, M., Falkow, S., Rosenberg,
E., Schleifer, K., Stackebrandt, E. (eds) The Prokaryotes. Springer, New York.
Oren, A. (2011) Diversity of Halophiles. In: Horikoshi K. (ed) Extremophiles Handbook.
Springer, Tokyo.
Stan-Lotter, H., & Fendrihan, S. (2015). Halophilic Archaea: Life with Desiccation, Radiation
and Oligotrophy over Geological Times. Life, 5(3), 1487-1496. doi: 10.3390/life5031487
6
Acknowledgements are made to Brock University and my teacher assistants for the instrumental
help in the documenting of this journal article.
7. References
Bowers, K. and Wiegel, J. (2011) Temperature and pH optima of extremely halophilic archaea: a
mini-review. Extremophiles 15: 119–128
Brooker, R. J., Widmaier, E. P., Graham, L. E., & Stiling, P. D. (2017). Biology (4th ed.). New
York, NY: McGraw-Hill Education.
Gibtan, A., Park, K., Woo, M., Shin, J., Lee, D., Sohn, J., Song, M., Roh, S., Lee, S., & Lee, H.
(2017). Diversity of Extremely Halophilic Archaeal and Bacterial Communities from Commercial
Salts. Frontiers in Microbiology, 8. doi: 10.3389/fmicb.2017.00799
Najjari, A., Elshahed, M., Cherif, A., & Youssef, N. (2015). Patterns and Determinants of
Halophilic Archaea (Class Halobacteria) Diversity in Tunisian Endorheic Salt Lakes and Sebkhet
Systems. Applied and Environmental Microbiology, 81(13), 4432-4441. doi: 10.1128/aem.01097-15
Noha, Y., Kristen, A., & Mostafa, E. (2012). Phylogenetic Diversities and Community Structure
of Members of the Extremely Halophilic Archaea (Order Halobacteriales) in Multiple Saline Sediment
Habitats. Applied and Environmental Microbiology, 1332-1344.
Oren, A. (2006) The Order Halobacteriales 3: 113-164 In: Dworkin, M., Falkow, S., Rosenberg,
E., Schleifer, K., Stackebrandt, E. (eds) The Prokaryotes. Springer, New York.
Oren, A. (2011) Diversity of Halophiles. In: Horikoshi K. (ed) Extremophiles Handbook.
Springer, Tokyo.
Stan-Lotter, H., & Fendrihan, S. (2015). Halophilic Archaea: Life with Desiccation, Radiation
and Oligotrophy over Geological Times. Life, 5(3), 1487-1496. doi: 10.3390/life5031487
6
1 out of 6
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
