In-depth Review: Solanezumab Trial for Mild Dementia in Alzheimer’s
VerifiedAdded on 2023/04/04
|10
|2649
|93
AI Summary
This critical review analyzes the research article "Trial of solanezumab for mild dementia due to Alzheimer’s disease" by Lawrence S. Honig et al., which investigated the efficacy and safety of solanezumab, a monoclonal antibody, in treating mild Alzheimer's disease. The review summarizes the study's design, including its double-blind, placebo-controlled phase 3 trial involving 2129 patients, and discusses the methods used for safety and efficacy assessments. The analysis points out limitations such as the lack of statistically significant results in the primary outcome, the absence of discussion on pharmacokinetics and pharmacodynamics, and potential biases due to the large sample size. The review concludes that solanezumab at a dose of 400 mg did not significantly slow cognitive decline in patients with mild Alzheimer's and highlights the need for further research and dose adjustments. The document is contributed by a student and available on Desklib, a platform offering study tools and resources for students.

CRITICAL REVIEW ON TRIAL OF SOLANEZUMAB IN MILD
DEMENTIA DUE TO ALZHEIMER’S DISEASE
DEMENTIA DUE TO ALZHEIMER’S DISEASE
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

INTRODUCTION
The research article titled “Trial of solanezumab for mild dementia due
to Alzheimer’s disease” was done by Lawrence S. Honig, Bruno Vellas,
Michael Woodward, Merce Boada et al. 2129 patients were enrolled for
this study. The article was published in the journal article “THE NEW
ENGLAND JOURNAL OF MEDICINE” in January 25, 2018.
Alzheimer’s disease is a common neurodegenerative disorder with no
effective disease-modifying treatments (Crespi et al., 2015).
Neurofibrillary tangles and amyloid-beta (Aβ) plaques are essential
characteristics for Alzheimer’s disease. Solanezumab is a humanized
monoclonal antibody tested by Eli Lilly, which is a neuroprotecter for
patients suffer with Alzheimer’s disease. Solanezumab binds amyloid-
beta peptides that aggregate and form plaques in the brain. It was
designed to increase the clearance of amyloid-beta peptides from brain
and it leads to the toxic effects in the synapses and leads to the
deposition of amyloid (Honig et al., 2018).
This debilitating illness, which affects quality of life, productivity, and
the economy negatively, affects more than 55 million people globally.
The prevalence of Alzheimer's disease is predicted to rise because of the
growing number of elderly people (Chen, 2018). Although the exact
causes of AD are yet unknown, ageing, environmental, genetic, and
lifestyle variables are all linked to its development. It is believed that the
pathophysiology of AD is associated with the accumulation of beta-
amyloid protein peptides and neurofibrillary tangles (NFT), both of
which cause damage to the neurons (Guo et al., 2020).
The research article titled “Trial of solanezumab for mild dementia due
to Alzheimer’s disease” was done by Lawrence S. Honig, Bruno Vellas,
Michael Woodward, Merce Boada et al. 2129 patients were enrolled for
this study. The article was published in the journal article “THE NEW
ENGLAND JOURNAL OF MEDICINE” in January 25, 2018.
Alzheimer’s disease is a common neurodegenerative disorder with no
effective disease-modifying treatments (Crespi et al., 2015).
Neurofibrillary tangles and amyloid-beta (Aβ) plaques are essential
characteristics for Alzheimer’s disease. Solanezumab is a humanized
monoclonal antibody tested by Eli Lilly, which is a neuroprotecter for
patients suffer with Alzheimer’s disease. Solanezumab binds amyloid-
beta peptides that aggregate and form plaques in the brain. It was
designed to increase the clearance of amyloid-beta peptides from brain
and it leads to the toxic effects in the synapses and leads to the
deposition of amyloid (Honig et al., 2018).
This debilitating illness, which affects quality of life, productivity, and
the economy negatively, affects more than 55 million people globally.
The prevalence of Alzheimer's disease is predicted to rise because of the
growing number of elderly people (Chen, 2018). Although the exact
causes of AD are yet unknown, ageing, environmental, genetic, and
lifestyle variables are all linked to its development. It is believed that the
pathophysiology of AD is associated with the accumulation of beta-
amyloid protein peptides and neurofibrillary tangles (NFT), both of
which cause damage to the neurons (Guo et al., 2020).

SUMMARY:
This research article was written by Lawrence S. Honig et al 2018 to
study the trial of solanezumab for mild dementia due to Alzheimer’s
disease. This study is to discuss the safety and efficacy of the
monoclonal antibody, solanezumab.
The research article's abstract provides an explanation of the study's
outcomes, research methodology, results, and conclusion. This article
did not adhere to the APA style manual's formatting guidelines. The
word limit for an abstract is 250 words, yet this article does not adhere to
the format because it is 342 words long. Furthermore, the writers
neglected to include negative outcomes in the result section. The authors
made an effort to provide all of the study's specifics in the abstract so
that readers would understand it completely..
The phase 3 trials double-blind, placebo-controlled phase is the only set
of results presented in this article. Sample size, trial design, safety
assessment, outcome measure, and adverse events are discussed in the
methods section. The study's age group and diagnostic criteria are easily
understood by the readers. Routine physical and neurologic examination,
clinical laboratory assessment, and collection of adverse event data are
the main safety assessments used for this study.
The primary and secondary efficacies were measured by using different
score scale methods (Delrieu et al., 2022). Only patients with outcome
measurements at and after baseline were included in this analysis. The
experimental procedure and method for data analysis, as well as the
methodological approach employed in the study, have all been
thoroughly discussed.
The statistical significance assessment for the secondary cognitive and
functional measures in this study was not provided because the primary
This research article was written by Lawrence S. Honig et al 2018 to
study the trial of solanezumab for mild dementia due to Alzheimer’s
disease. This study is to discuss the safety and efficacy of the
monoclonal antibody, solanezumab.
The research article's abstract provides an explanation of the study's
outcomes, research methodology, results, and conclusion. This article
did not adhere to the APA style manual's formatting guidelines. The
word limit for an abstract is 250 words, yet this article does not adhere to
the format because it is 342 words long. Furthermore, the writers
neglected to include negative outcomes in the result section. The authors
made an effort to provide all of the study's specifics in the abstract so
that readers would understand it completely..
The phase 3 trials double-blind, placebo-controlled phase is the only set
of results presented in this article. Sample size, trial design, safety
assessment, outcome measure, and adverse events are discussed in the
methods section. The study's age group and diagnostic criteria are easily
understood by the readers. Routine physical and neurologic examination,
clinical laboratory assessment, and collection of adverse event data are
the main safety assessments used for this study.
The primary and secondary efficacies were measured by using different
score scale methods (Delrieu et al., 2022). Only patients with outcome
measurements at and after baseline were included in this analysis. The
experimental procedure and method for data analysis, as well as the
methodological approach employed in the study, have all been
thoroughly discussed.
The statistical significance assessment for the secondary cognitive and
functional measures in this study was not provided because the primary
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

outcome did not meet expectations. The previous studies raised
questions about cardiac side effects, but in this trial, there is no evidence
for the study.
The authors explain why solanezumab is less effective. The dose of
solanezumab will be adjusted because the current dose of 400 mg was
insufficient to produce a meaningful effect. A dose adjustment of
solanezumab should be considered for patients because 400 mg was
insufficient to produce a clinical effect.
In the EXPEDITION and EXPEDITION 2 trials, solanezumab did not
significantly slow the decline in cognition and function. In contrast,
patients with moderate Alzheimer's disease who got solanezumab
treatment experienced less cognitive and functional impairment than
those who received a placebo. Only people with early-stage Alzheimer's
disease who had biomarker evidence of beta-amyloid deposition were
enrolled in the EXPEDITION 3 trial (as determined by a PET scan or
amyloid-beta1-42 readings in CSF). According to the findings from the
two phase 3 trials that were completed, there is no decline in cognitive
function in those who have been diagnosed with mild to moderate
Alzheimer's disease. Two groups; one receiving solanezumab and the
other a placebo were taken into account in this study.
STUDY DESIGN AND ASSESSMENT
This clinical trial was designed to test whether the monoclonal antibody,
solanezumab, will slow the cognitive decline of Alzheimer’s disease as
compared with a placebo in patients with mild AD. It was a double-
blind, placebo-controlled, phase 3 trial. Male and female patients
between the ages of 55 and 90 were enrolled in this trial. The study
protocol, funded by Eli Lilly and Company, Indianapolis, Indiana, USA
46285, was electronically signed and approved by Lilly on April 29,
questions about cardiac side effects, but in this trial, there is no evidence
for the study.
The authors explain why solanezumab is less effective. The dose of
solanezumab will be adjusted because the current dose of 400 mg was
insufficient to produce a meaningful effect. A dose adjustment of
solanezumab should be considered for patients because 400 mg was
insufficient to produce a clinical effect.
In the EXPEDITION and EXPEDITION 2 trials, solanezumab did not
significantly slow the decline in cognition and function. In contrast,
patients with moderate Alzheimer's disease who got solanezumab
treatment experienced less cognitive and functional impairment than
those who received a placebo. Only people with early-stage Alzheimer's
disease who had biomarker evidence of beta-amyloid deposition were
enrolled in the EXPEDITION 3 trial (as determined by a PET scan or
amyloid-beta1-42 readings in CSF). According to the findings from the
two phase 3 trials that were completed, there is no decline in cognitive
function in those who have been diagnosed with mild to moderate
Alzheimer's disease. Two groups; one receiving solanezumab and the
other a placebo were taken into account in this study.
STUDY DESIGN AND ASSESSMENT
This clinical trial was designed to test whether the monoclonal antibody,
solanezumab, will slow the cognitive decline of Alzheimer’s disease as
compared with a placebo in patients with mild AD. It was a double-
blind, placebo-controlled, phase 3 trial. Male and female patients
between the ages of 55 and 90 were enrolled in this trial. The study
protocol, funded by Eli Lilly and Company, Indianapolis, Indiana, USA
46285, was electronically signed and approved by Lilly on April 29,
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2013. Before the screening period, the consent will be explained and
given to the patient and caregiver for signing. The ERB will review the
protocol. For the patient's eligibility, blood and urine tests, an MRI, and
a florbetapir PET scan will be performed. Patients who meet the
eligibility requirements will be enrolled and randomized to receive
solanezumab 400 mg once every four weeks or a placebo once every
four weeks. Participation in an open-label extension study may be
available to patients who complete this study. Each patient will receive
400 mg of solanezumab once every four weeks during the open-label
extension study (Honig et al., 2018).
RESULT
Tables and figures are used to describe the study's conclusion. In the
study, 2129 individuals were randomly assigned, with 86.5% of patients
receiving solanezumab and 84.7% receiving a placebo completing the
trial. Results from the primary outcome were examined as changes over
time relative to the baseline. Fisher's exact test or Pearson's chi-square
test were applied to categorical data, while analysis of variance with
independent components for treatment and site was applied to
continuous data.
A mixed-model repeated analysis and the change from baseline in the
ADAS-co14 score were used to evaluate the primary outcome. The
baseline ADAS-cog14 score, the site, the visit, the trial group, and the
trial group by visit interaction, the baseline use of acetylcholinesterase,
and the baseline age are all included in this model. ADCS-iADL,
MMSE, and FAQ analyze the secondary result. Only the baseline CDR-
SB analysis was done. Between the solanezumab group and the placebo
group, the safety analysis was compared.
given to the patient and caregiver for signing. The ERB will review the
protocol. For the patient's eligibility, blood and urine tests, an MRI, and
a florbetapir PET scan will be performed. Patients who meet the
eligibility requirements will be enrolled and randomized to receive
solanezumab 400 mg once every four weeks or a placebo once every
four weeks. Participation in an open-label extension study may be
available to patients who complete this study. Each patient will receive
400 mg of solanezumab once every four weeks during the open-label
extension study (Honig et al., 2018).
RESULT
Tables and figures are used to describe the study's conclusion. In the
study, 2129 individuals were randomly assigned, with 86.5% of patients
receiving solanezumab and 84.7% receiving a placebo completing the
trial. Results from the primary outcome were examined as changes over
time relative to the baseline. Fisher's exact test or Pearson's chi-square
test were applied to categorical data, while analysis of variance with
independent components for treatment and site was applied to
continuous data.
A mixed-model repeated analysis and the change from baseline in the
ADAS-co14 score were used to evaluate the primary outcome. The
baseline ADAS-cog14 score, the site, the visit, the trial group, and the
trial group by visit interaction, the baseline use of acetylcholinesterase,
and the baseline age are all included in this model. ADCS-iADL,
MMSE, and FAQ analyze the secondary result. Only the baseline CDR-
SB analysis was done. Between the solanezumab group and the placebo
group, the safety analysis was compared.
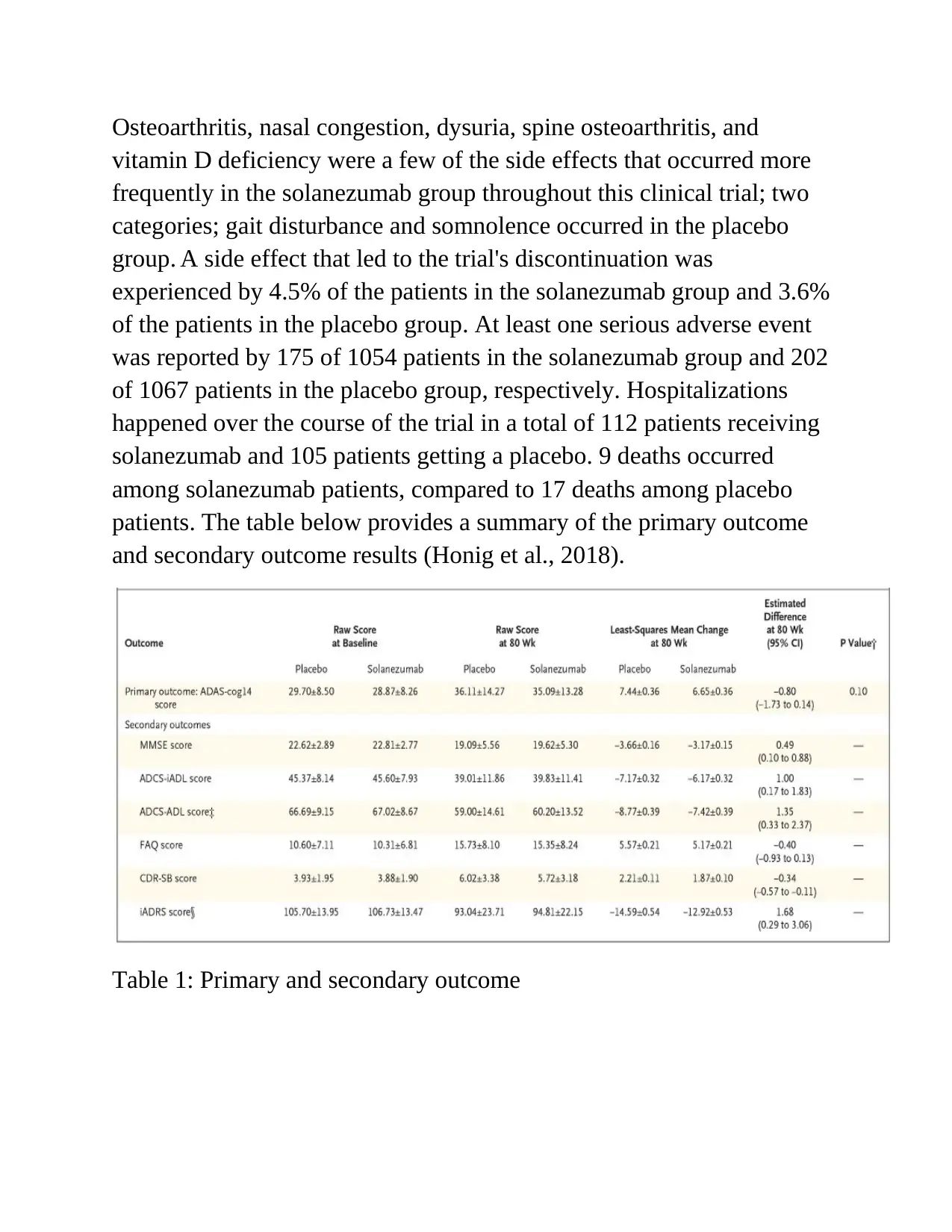
Osteoarthritis, nasal congestion, dysuria, spine osteoarthritis, and
vitamin D deficiency were a few of the side effects that occurred more
frequently in the solanezumab group throughout this clinical trial; two
categories; gait disturbance and somnolence occurred in the placebo
group. A side effect that led to the trial's discontinuation was
experienced by 4.5% of the patients in the solanezumab group and 3.6%
of the patients in the placebo group. At least one serious adverse event
was reported by 175 of 1054 patients in the solanezumab group and 202
of 1067 patients in the placebo group, respectively. Hospitalizations
happened over the course of the trial in a total of 112 patients receiving
solanezumab and 105 patients getting a placebo. 9 deaths occurred
among solanezumab patients, compared to 17 deaths among placebo
patients. The table below provides a summary of the primary outcome
and secondary outcome results (Honig et al., 2018).
Table 1: Primary and secondary outcome
vitamin D deficiency were a few of the side effects that occurred more
frequently in the solanezumab group throughout this clinical trial; two
categories; gait disturbance and somnolence occurred in the placebo
group. A side effect that led to the trial's discontinuation was
experienced by 4.5% of the patients in the solanezumab group and 3.6%
of the patients in the placebo group. At least one serious adverse event
was reported by 175 of 1054 patients in the solanezumab group and 202
of 1067 patients in the placebo group, respectively. Hospitalizations
happened over the course of the trial in a total of 112 patients receiving
solanezumab and 105 patients getting a placebo. 9 deaths occurred
among solanezumab patients, compared to 17 deaths among placebo
patients. The table below provides a summary of the primary outcome
and secondary outcome results (Honig et al., 2018).
Table 1: Primary and secondary outcome
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

CRITIQUE
The paper goes into great detail about the effectiveness of solanezumab.
Clinical results have not improved as a result of the anti-amyloid
medicines (Hardy et al., 2014). In the past two years, the trials haven't
achieved their main goals. 26 deaths and serious side effects occurred
throughout this experiment (Honig et al., 2018). The information is
given in such a concise and clear way that the trial's full course is
defined.
Both advantages and disadvantages can be found in this research paper.
The study was finished, which was good for this article. Lack of
scientific support results in many trials being discontinued. Solanezumab
failed in this instance, although the trial was successful. The trial's goals,
which included seeing if solanezumab was safe or effective enough to
treat mild dementia are thoroughly described by the authors.
The research article has some limitations, like how cognitive
performance wasn't looked at in this trial. Solanezumab failed to achieve
the primary aim of the EXPEDITION 3 clinical trial. According to the
study's findings, the size of the treatment differences was negligible, and
no new safety signals were found.
Comparatively speaking, solanezumab is less effective than a placebo. It
was unable to show a significant benefit. The pharmacokinetics and
pharmacodynamics aspects were not discussed in this study (Beshir et
al., 2022).
LIMITATIONS
Over 2000 participants were included in the study's sample. In this
article, patients are divided into categories according to gender, although
only female patients are depicted in the patient characteristics. This
The paper goes into great detail about the effectiveness of solanezumab.
Clinical results have not improved as a result of the anti-amyloid
medicines (Hardy et al., 2014). In the past two years, the trials haven't
achieved their main goals. 26 deaths and serious side effects occurred
throughout this experiment (Honig et al., 2018). The information is
given in such a concise and clear way that the trial's full course is
defined.
Both advantages and disadvantages can be found in this research paper.
The study was finished, which was good for this article. Lack of
scientific support results in many trials being discontinued. Solanezumab
failed in this instance, although the trial was successful. The trial's goals,
which included seeing if solanezumab was safe or effective enough to
treat mild dementia are thoroughly described by the authors.
The research article has some limitations, like how cognitive
performance wasn't looked at in this trial. Solanezumab failed to achieve
the primary aim of the EXPEDITION 3 clinical trial. According to the
study's findings, the size of the treatment differences was negligible, and
no new safety signals were found.
Comparatively speaking, solanezumab is less effective than a placebo. It
was unable to show a significant benefit. The pharmacokinetics and
pharmacodynamics aspects were not discussed in this study (Beshir et
al., 2022).
LIMITATIONS
Over 2000 participants were included in the study's sample. In this
article, patients are divided into categories according to gender, although
only female patients are depicted in the patient characteristics. This
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

article states that phase 3 research will be rejected since it cannot show a
drug is both safe and effective. More explanation than data facts are
presented in this article. An outcome that is biased due to a large sample
size. The drug solanezumab failed due to a lack of data demonstrating its
commercial viability.
CONCLUSION
According to Lawrence S. Honig et al. (2018)'s research journal paper
"Trial of solanezumab for mild dementia due to Alzheimer's disease,"
solanezumab 400 mg may not have been enough to achieve the desired
effects because it did not slow cognitive decline. The solanezumab
group and the placebo group did not vary significantly in terms of major
adverse events. Mixed-model repeated measures were employed by the
authors to analyze the data. The experiment revealed that solanezumab
was ineffective for Alzheimer's disease.
REFERENCE
1. Beshir, S. A., Aadithsoorya, A. M., Parveen, A., Goh, S. S. L., Hussain, N., & Menon, V.
B. (2022). Aducanumab Therapy to Treat Alzheimer’s Disease: A Narrative Review.
International Journal of Alzheimer’s Disease, 2022, 1–10.
https://doi.org/10.1155/2022/9343514
2. Chen, Y.-G. (2018). Research Progress in the Pathogenesis of Alzheimer’s Disease.
Chinese Medical Journal, 131(13), 1618–1624. https://doi.org/10.4103/0366-
6999.235112
3. Crespi, G. A. N., Hermans, S. J., Parker, M. W., & Miles, L. A. (2015). Molecular basis
for mid-region amyloid-β capture by leading Alzheimer’s disease immunotherapies.
Scientific Reports, 5(1). https://doi.org/10.1038/srep09649
drug is both safe and effective. More explanation than data facts are
presented in this article. An outcome that is biased due to a large sample
size. The drug solanezumab failed due to a lack of data demonstrating its
commercial viability.
CONCLUSION
According to Lawrence S. Honig et al. (2018)'s research journal paper
"Trial of solanezumab for mild dementia due to Alzheimer's disease,"
solanezumab 400 mg may not have been enough to achieve the desired
effects because it did not slow cognitive decline. The solanezumab
group and the placebo group did not vary significantly in terms of major
adverse events. Mixed-model repeated measures were employed by the
authors to analyze the data. The experiment revealed that solanezumab
was ineffective for Alzheimer's disease.
REFERENCE
1. Beshir, S. A., Aadithsoorya, A. M., Parveen, A., Goh, S. S. L., Hussain, N., & Menon, V.
B. (2022). Aducanumab Therapy to Treat Alzheimer’s Disease: A Narrative Review.
International Journal of Alzheimer’s Disease, 2022, 1–10.
https://doi.org/10.1155/2022/9343514
2. Chen, Y.-G. (2018). Research Progress in the Pathogenesis of Alzheimer’s Disease.
Chinese Medical Journal, 131(13), 1618–1624. https://doi.org/10.4103/0366-
6999.235112
3. Crespi, G. A. N., Hermans, S. J., Parker, M. W., & Miles, L. A. (2015). Molecular basis
for mid-region amyloid-β capture by leading Alzheimer’s disease immunotherapies.
Scientific Reports, 5(1). https://doi.org/10.1038/srep09649

4. Delrieu, J., Bateman, R. J., Touchon, J., Sabbagh, M., & Cummings, J. (2022). The
Future of AD Clinical Trials with the Advent of Anti-Amyloid Therapies: An CTAD
Task Force Report. The Journal of Prevention of Alzheimer’s Disease.
https://doi.org/10.14283/jpad.2022.48
5. Gold, M. (2017). Phase II clinical trials of anti–amyloid β antibodies: When is enough,
enough? Alzheimer’s & Dementia: Translational Research & Clinical Interventions,
3(3), 402–409. https://doi.org/10.1016/j.trci.2017.04.005
6. Guo, T., Zhang, D., Zeng, Y., Huang, T. Y., Xu, H., & Zhao, Y. (2020). Molecular and
cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Molecular
Neurodegeneration, 15(1). https://doi.org/10.1186/s13024-020-00391-7
7. Hardy, J., Bogdanovic, N., Winblad, B., Portelius, E., Andreasen, N., Cedazo-Minguez,
A., & Zetterberg, H. (2014). Pathways to Alzheimer’s disease. Journal of Internal
Medicine, 275(3), 296–303. https://doi.org/10.1111/joim.12192
8. Honig, L. S., Vellas, B., Woodward, M., Boada, M., Bullock, R., Borrie, M., Hager, K.,
Andreasen, N., Scarpini, E., Liu-Seifert, H., Case, M., Dean, R. A., Hake, A., Sundell, K.,
Poole Hoffmann, V., Carlson, C., Khanna, R., Mintun, M., DeMattos, R., & Selzler, K. J.
(2018). Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. New
England Journal of Medicine, 378(4), 321–330. https://doi.org/10.1056/nejmoa1705971
9. Karran, E., & Hardy, J. (2014). Antiamyloid Therapy for Alzheimer’s Disease — Are We
on the Right Road? New England Journal of Medicine, 370(4), 377–378.
https://doi.org/10.1056/nejme1313943
10. Liu-Seifert, H., Andersen, S., Case, M., Sparks, J., Holdridge, K. C., Wessels, A. M.,
Hendrix, S., Aisen, P., & Siemers, E. (2017). Statistical properties of continuous
Future of AD Clinical Trials with the Advent of Anti-Amyloid Therapies: An CTAD
Task Force Report. The Journal of Prevention of Alzheimer’s Disease.
https://doi.org/10.14283/jpad.2022.48
5. Gold, M. (2017). Phase II clinical trials of anti–amyloid β antibodies: When is enough,
enough? Alzheimer’s & Dementia: Translational Research & Clinical Interventions,
3(3), 402–409. https://doi.org/10.1016/j.trci.2017.04.005
6. Guo, T., Zhang, D., Zeng, Y., Huang, T. Y., Xu, H., & Zhao, Y. (2020). Molecular and
cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Molecular
Neurodegeneration, 15(1). https://doi.org/10.1186/s13024-020-00391-7
7. Hardy, J., Bogdanovic, N., Winblad, B., Portelius, E., Andreasen, N., Cedazo-Minguez,
A., & Zetterberg, H. (2014). Pathways to Alzheimer’s disease. Journal of Internal
Medicine, 275(3), 296–303. https://doi.org/10.1111/joim.12192
8. Honig, L. S., Vellas, B., Woodward, M., Boada, M., Bullock, R., Borrie, M., Hager, K.,
Andreasen, N., Scarpini, E., Liu-Seifert, H., Case, M., Dean, R. A., Hake, A., Sundell, K.,
Poole Hoffmann, V., Carlson, C., Khanna, R., Mintun, M., DeMattos, R., & Selzler, K. J.
(2018). Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. New
England Journal of Medicine, 378(4), 321–330. https://doi.org/10.1056/nejmoa1705971
9. Karran, E., & Hardy, J. (2014). Antiamyloid Therapy for Alzheimer’s Disease — Are We
on the Right Road? New England Journal of Medicine, 370(4), 377–378.
https://doi.org/10.1056/nejme1313943
10. Liu-Seifert, H., Andersen, S., Case, M., Sparks, J., Holdridge, K. C., Wessels, A. M.,
Hendrix, S., Aisen, P., & Siemers, E. (2017). Statistical properties of continuous
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

composite scales and implications for drug development. Journal of Biopharmaceutical
Statistics, 27(6), 1104–1114. https://doi.org/10.1080/10543406.2017.1315819
11. Schwarz, A. J., Sundell, K. L., Charil, A., Case, M. G., Jaeger, R. K., Scott, D., Bracoud,
L., Oh, J., Suhy, J., Pontecorvo, M. J., Dickerson, B. C., & Siemers, E. R. (2019).
Magnetic resonance imaging measures of brain atrophy from the EXPEDITION3 trial in
mild Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical
Interventions, 5(1), 328–337. https://doi.org/10.1016/j.trci.2019.05.007
12. Siemers, E. R., Sundell, K. L., Carlson, C., Case, M., Sethuraman, G., Liu-Seifert, H.,
Dowsett, S. A., Pontecorvo, M. J., Dean, R. A., & Demattos, R. (2016). Phase 3
solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients.
Alzheimer’s & Dementia, 12(2), 110–120. https://doi.org/10.1016/j.jalz.2015.06.1893
Word count: 2077
Statistics, 27(6), 1104–1114. https://doi.org/10.1080/10543406.2017.1315819
11. Schwarz, A. J., Sundell, K. L., Charil, A., Case, M. G., Jaeger, R. K., Scott, D., Bracoud,
L., Oh, J., Suhy, J., Pontecorvo, M. J., Dickerson, B. C., & Siemers, E. R. (2019).
Magnetic resonance imaging measures of brain atrophy from the EXPEDITION3 trial in
mild Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical
Interventions, 5(1), 328–337. https://doi.org/10.1016/j.trci.2019.05.007
12. Siemers, E. R., Sundell, K. L., Carlson, C., Case, M., Sethuraman, G., Liu-Seifert, H.,
Dowsett, S. A., Pontecorvo, M. J., Dean, R. A., & Demattos, R. (2016). Phase 3
solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients.
Alzheimer’s & Dementia, 12(2), 110–120. https://doi.org/10.1016/j.jalz.2015.06.1893
Word count: 2077
1 out of 10
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





