Project Report: Techniques to Improve Crystallization Equipment
VerifiedAdded on 2023/06/15
|6
|583
|347
Report
AI Summary
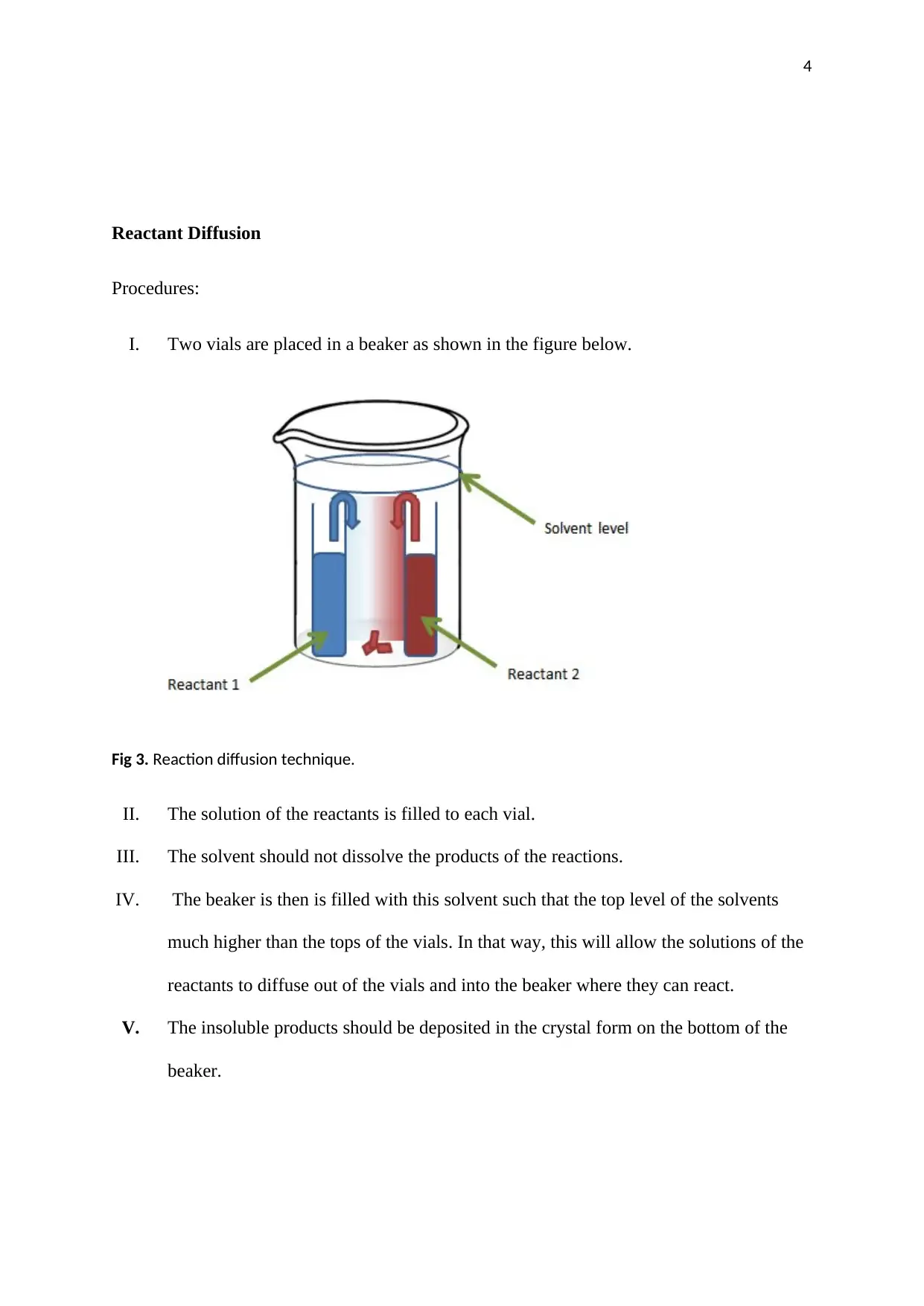
This report details methods to improve crystallization equipment for better crystal formation. It discusses slow cooling techniques using a Dewar flask and water bath setup, emphasizing controlled solvent evaporation. The report also covers reactant diffusion procedures, where solutions are allowed to diffuse and react, forming crystals. Specific steps for each method are outlined, providing a comprehensive overview of equipment enhancement for optimized crystallization processes. The document includes diagrams and references to support the explained methodologies.
1 out of 6






![[object Object]](/_next/static/media/star-bottom.7253800d.svg)