Role of Cyclin F in G2 Cell Cycle Checkpoints and DNA Damage Response
VerifiedAdded on 2023/06/10
|4
|1139
|58
AI Summary
This article discusses the role of Cyclin F in regulating G2 cell cycle checkpoints and DNA damage response. It explains how Cyclin F suppresses B-Myb to maintain G2 cell cycle checkpoint following DNA damage. The article also highlights the importance of proper maintenance of cell cycle checkpoints to prevent unscheduled mitotic progression with damaged genome.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: CELL CYCLE
The cell cycle is defined as a series of events in which cellular components of cells are
doubled and then perfectly segregated into two daughter cells1. In eukaryotes, DNA synthesis
occurs in S-phase followed by segregation of chromosome in mitosis (M-phase). Two gap
phase separates mitosis from the S-phase of the cell cycle and this is known as G1 and G2
phase. During this gap phase, the cells obtain the desired bio-mass, integrate the growth
signals and prepare for the chromosome segregation2. The main pillar of the cell cycle
progression through the G phase of the cell cycle occurs via cyclin dependent kinases
(CDKs). This are serine-threonine kinase that phosphorylates key protein substrates in order
to promote DNA synthesis and progression into the mitotic phase3. Cell cycle check points
are used to block the process of cell division in order repair any damage DNA during the
ongoing cell-division process. Such that the faulty DNA is not transmitted to the daughter
cells. Apart from DNA damage response, cell cycle checkpoints also play an important role
in cell size control and effective monitoring of the process of DNA replication. Moreover,
there are separate mitotic spindle check points in the cell cycle which ascertains whether the
chromosome has aligned over the mitotic plate in order to perform uniform segregation4.
However, it is not clear regarding how this cell cycle checkpoint maintenance phase is
regulated during the inter-mediate phase of cell-cycle. At the same time, it is important to
understand how this phase of check-point is regulated because improper maintenance of the
cell-cycle check-points can result in unscheduled mitotic progression with damaged genome5.
This review mainly highlights over the G2 cell cycle check points, Cyclin F. This is because,
in order to explain an emerging factor behind the control and co-ordination of the cell-cycle
check-points the importance of the role of cyclin-F was highlighted6. Cyclin-F is CDK-
activating cyclin from ubiquitin ligase protein family. Cyclin F plays an important role in the
DNA damage check-point regulation when cells are radiated via ionizing radiation (IR). The
main check-point control of cyclin F is in the G2 phase of the cell cycle via the suppression
The cell cycle is defined as a series of events in which cellular components of cells are
doubled and then perfectly segregated into two daughter cells1. In eukaryotes, DNA synthesis
occurs in S-phase followed by segregation of chromosome in mitosis (M-phase). Two gap
phase separates mitosis from the S-phase of the cell cycle and this is known as G1 and G2
phase. During this gap phase, the cells obtain the desired bio-mass, integrate the growth
signals and prepare for the chromosome segregation2. The main pillar of the cell cycle
progression through the G phase of the cell cycle occurs via cyclin dependent kinases
(CDKs). This are serine-threonine kinase that phosphorylates key protein substrates in order
to promote DNA synthesis and progression into the mitotic phase3. Cell cycle check points
are used to block the process of cell division in order repair any damage DNA during the
ongoing cell-division process. Such that the faulty DNA is not transmitted to the daughter
cells. Apart from DNA damage response, cell cycle checkpoints also play an important role
in cell size control and effective monitoring of the process of DNA replication. Moreover,
there are separate mitotic spindle check points in the cell cycle which ascertains whether the
chromosome has aligned over the mitotic plate in order to perform uniform segregation4.
However, it is not clear regarding how this cell cycle checkpoint maintenance phase is
regulated during the inter-mediate phase of cell-cycle. At the same time, it is important to
understand how this phase of check-point is regulated because improper maintenance of the
cell-cycle check-points can result in unscheduled mitotic progression with damaged genome5.
This review mainly highlights over the G2 cell cycle check points, Cyclin F. This is because,
in order to explain an emerging factor behind the control and co-ordination of the cell-cycle
check-points the importance of the role of cyclin-F was highlighted6. Cyclin-F is CDK-
activating cyclin from ubiquitin ligase protein family. Cyclin F plays an important role in the
DNA damage check-point regulation when cells are radiated via ionizing radiation (IR). The
main check-point control of cyclin F is in the G2 phase of the cell cycle via the suppression
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1
CELL CYCLE
of the B-Myb-driven transcriptional programme which mediates the accumulation of mitosis-
promoting factor.
Cyclin F inhibits the action of B-Myb via causing cyclin A-mediated phosphorylation of B-
Myb. Phosphorylation of B-Myb via cyclin A mediated kinase inactives B-Myb which in turn
suppress the accumulation of mitosis promoting factor and thereby preventing cell cycle
progression. The detailed analysis of the role Cyclin F as a cell cycle check point regular was
conducted via the use of different experiments7.
The first experiment that was performed include ubiquitome library screen for the analysis of
the G2 check-point regulators. The IR with custom-made small interfering RNA (si-RNA)
targeted 559 genes involved in ubiquitylation pathway in human osteosarcoma cell line which
lacks p53 expression. This ubiquitome library screen of the cells which overcome IR-
induced G2 checkpoint and progressed to mitosis identified cyclin F (CCNF) as a principal
regulator of G2 checkpoint 7.
The second experiment was performed in order to study cyclin F and its effect on IR
induced G2 check points. G2 checkpoints consist of multiple phases which are initially
dependent on ATM and CHK1 checkpoints so the experiment was performed in order to
detect whether this preliminary checkpoints operates under the presence of cyclin F. Cyclin
F-depleted cells are passed through the nozzle of flow cytometry during the initial time-
points after IR. The analysis revealed low mitotic index during the early time point of 2 to 4
hours after IR thus proving that the IR induced G2 checkpoints are activated in cyclin-F
deficient cells. However, analysis at time-point of 6 hours after IR radiation showed that
cyclin F depleted cells overcame G2 arrest and thereby indicating that cyclin F promotes
proper maintenance of G2 cell cycle check points rather than activating the check points. In
cyclin F depleted cells showed non-homologous DNA joining in order to overcome the DNA
CELL CYCLE
of the B-Myb-driven transcriptional programme which mediates the accumulation of mitosis-
promoting factor.
Cyclin F inhibits the action of B-Myb via causing cyclin A-mediated phosphorylation of B-
Myb. Phosphorylation of B-Myb via cyclin A mediated kinase inactives B-Myb which in turn
suppress the accumulation of mitosis promoting factor and thereby preventing cell cycle
progression. The detailed analysis of the role Cyclin F as a cell cycle check point regular was
conducted via the use of different experiments7.
The first experiment that was performed include ubiquitome library screen for the analysis of
the G2 check-point regulators. The IR with custom-made small interfering RNA (si-RNA)
targeted 559 genes involved in ubiquitylation pathway in human osteosarcoma cell line which
lacks p53 expression. This ubiquitome library screen of the cells which overcome IR-
induced G2 checkpoint and progressed to mitosis identified cyclin F (CCNF) as a principal
regulator of G2 checkpoint 7.
The second experiment was performed in order to study cyclin F and its effect on IR
induced G2 check points. G2 checkpoints consist of multiple phases which are initially
dependent on ATM and CHK1 checkpoints so the experiment was performed in order to
detect whether this preliminary checkpoints operates under the presence of cyclin F. Cyclin
F-depleted cells are passed through the nozzle of flow cytometry during the initial time-
points after IR. The analysis revealed low mitotic index during the early time point of 2 to 4
hours after IR thus proving that the IR induced G2 checkpoints are activated in cyclin-F
deficient cells. However, analysis at time-point of 6 hours after IR radiation showed that
cyclin F depleted cells overcame G2 arrest and thereby indicating that cyclin F promotes
proper maintenance of G2 cell cycle check points rather than activating the check points. In
cyclin F depleted cells showed non-homologous DNA joining in order to overcome the DNA

2
CELL CYCLE
repair and the same has been revealed via staining the cells with ƳH2AX (an established
DNA damage marker). Thus overall, it showed that the cyclin F depleted cells can progress
through the cell cycle checkpoints but with damaged DNA. The reason behind this was
further elucidated via Western blotting which revealed that cyclin F promotes the
maintenance of checkpoints only after the checkpoint activating kinase-mediated phase has
been declined 7.
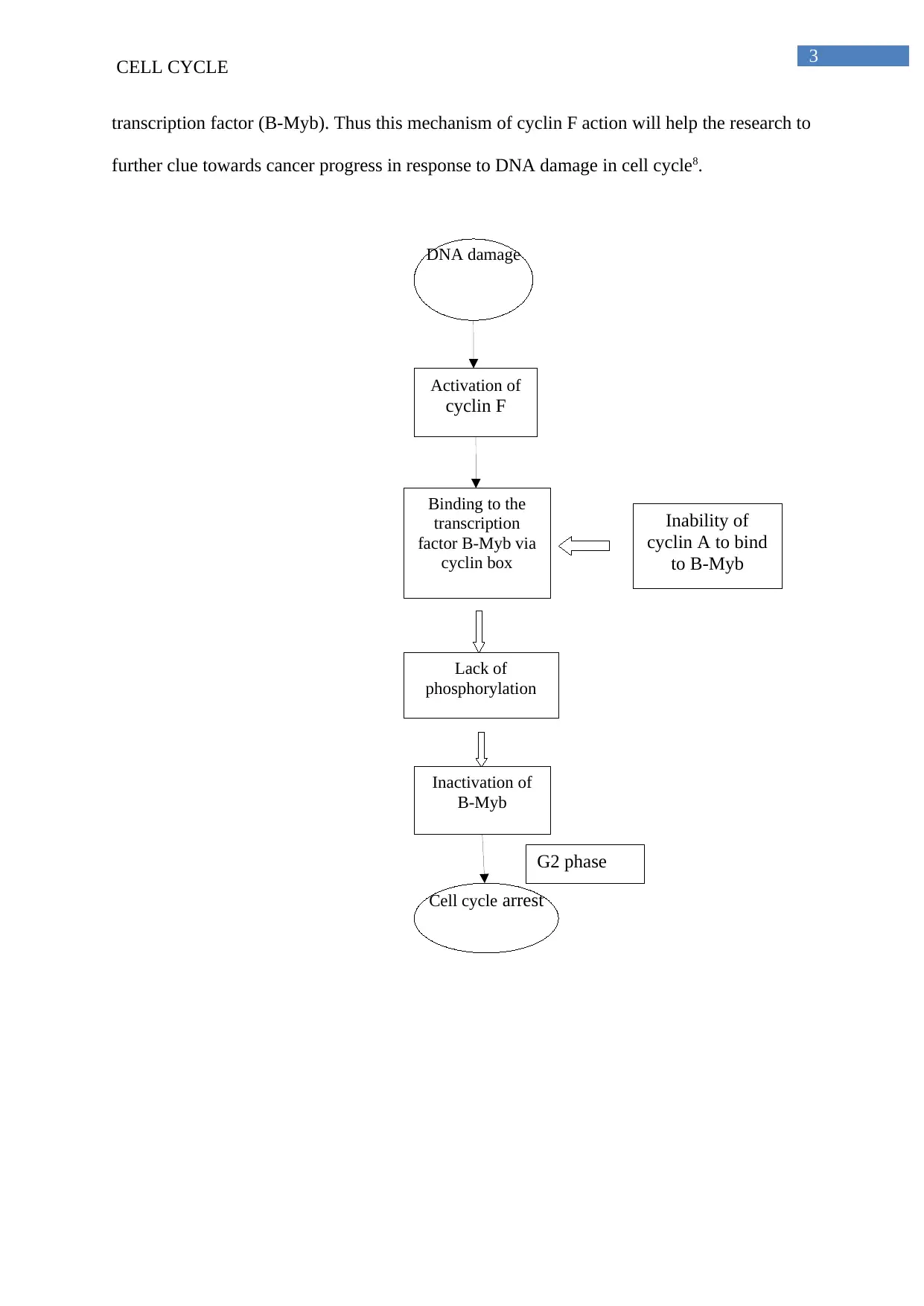
The third experiment revealed that cyclin F regulates the transcriptional activity of B-
Myb and thereby acting as mitotic index inhibitor. The inactivation of B-Myb occurs under
the action of cyclin F occurs via restriction of phosphosohorylation on B-Myb via cyclin A.
The forth experiment showed that cyclin F does not directly bounds to B-Myb but regulates
its activity via cyclin box domains. This cyclin box domain of cyclin F interacts with B-Myb
with its hydrophobic patch motif. Interaction with the cyclin box domain of cyclin Fof
hydrophobic patch motif of B-Myb prevents the phosphorylation of B-Myb via cyclin A.
Lack of phosphorylation by cyclin A prevents the activation of B-Myb and thereby
preventing the progression through cell cycle 7.
The summary of all the experiments showed that cyclin F suppresses B-Myb in order
to maintain G2 cell cycle checkpoint following the DNA damage. This DNA damage cell
cycle control occurs much before the p53 mediated DNA damage check-points. The above
study is extremely significant in the process of studying the fate of checkpoint dysfunction in
human disease development. Depending on the chronicity of the cell cycle defect, the
checkpoint dysfunction can result in several outcomes ranging from sudden cell death or cell
reprogramming which in turn may give rise to cancer. So proper study of the process of
cyclin F towards regulation G2 cell cycle check-points in response to the DNA damage
provide a detailed over-view regarding how cyclin F blocks cells in G2 check points upon
DNA damage along with the CDK phosphprylation and dephosphorylation of the
CELL CYCLE
repair and the same has been revealed via staining the cells with ƳH2AX (an established
DNA damage marker). Thus overall, it showed that the cyclin F depleted cells can progress
through the cell cycle checkpoints but with damaged DNA. The reason behind this was
further elucidated via Western blotting which revealed that cyclin F promotes the
maintenance of checkpoints only after the checkpoint activating kinase-mediated phase has
been declined 7.
The third experiment revealed that cyclin F regulates the transcriptional activity of B-
Myb and thereby acting as mitotic index inhibitor. The inactivation of B-Myb occurs under
the action of cyclin F occurs via restriction of phosphosohorylation on B-Myb via cyclin A.
The forth experiment showed that cyclin F does not directly bounds to B-Myb but regulates
its activity via cyclin box domains. This cyclin box domain of cyclin F interacts with B-Myb
with its hydrophobic patch motif. Interaction with the cyclin box domain of cyclin Fof
hydrophobic patch motif of B-Myb prevents the phosphorylation of B-Myb via cyclin A.
Lack of phosphorylation by cyclin A prevents the activation of B-Myb and thereby
preventing the progression through cell cycle 7.
The summary of all the experiments showed that cyclin F suppresses B-Myb in order
to maintain G2 cell cycle checkpoint following the DNA damage. This DNA damage cell
cycle control occurs much before the p53 mediated DNA damage check-points. The above
study is extremely significant in the process of studying the fate of checkpoint dysfunction in
human disease development. Depending on the chronicity of the cell cycle defect, the
checkpoint dysfunction can result in several outcomes ranging from sudden cell death or cell
reprogramming which in turn may give rise to cancer. So proper study of the process of
cyclin F towards regulation G2 cell cycle check-points in response to the DNA damage
provide a detailed over-view regarding how cyclin F blocks cells in G2 check points upon
DNA damage along with the CDK phosphprylation and dephosphorylation of the
3
DNA damage
Activation of
cyclin F
Binding to the
transcription
factor B-Myb via
cyclin box
Inability of
cyclin A to bind
to B-Myb
Lack of
phosphorylation
Inactivation of
B-Myb
Cell cycle arrest
G2 phase
CELL CYCLE
transcription factor (B-Myb). Thus this mechanism of cyclin F action will help the research to
further clue towards cancer progress in response to DNA damage in cell cycle8.
DNA damage
Activation of
cyclin F
Binding to the
transcription
factor B-Myb via
cyclin box
Inability of
cyclin A to bind
to B-Myb
Lack of
phosphorylation
Inactivation of
B-Myb
Cell cycle arrest
G2 phase
CELL CYCLE
transcription factor (B-Myb). Thus this mechanism of cyclin F action will help the research to
further clue towards cancer progress in response to DNA damage in cell cycle8.
1 out of 4
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
