Kinetics of Hydrogen Peroxide Decomposition
VerifiedAdded on 2021/04/21
|9
|1406
|339
AI Summary
This assignment explores the kinetics of hydrogen peroxide decomposition, focusing on the role of manganese dioxide as a catalyst. It delves into the effect of temperature on reaction rate and estimates the activation energy using experimental data. The solved assignment provides a thorough analysis of the results, including error estimation and comparison with literature values.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: PHYSICAL CHEMISTRY LAB REPORT
PHYSICAL CHEMISTRY LAB REPORT
[Author Name(s), First M. Last, Omit Titles and Degrees]
[Institutional Affiliation(s)]
PHYSICAL CHEMISTRY LAB REPORT
[Author Name(s), First M. Last, Omit Titles and Degrees]
[Institutional Affiliation(s)]
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

2
PHYSICAL CHEMISTRY LAB REPORT
Abstract
The kinetics of the decomposition of hydrogen peroxide using manganese dioxide as the catalyst
was studied at 25⁰C and 34⁰C. The findings from the experiment showed that the rate of
decomposition of hydrogen peroxide increases with an increase in the amount of manganese
dioxide and a rise in the temperature.
Introduction
The decomposition of hydrogen peroxide at room temperature proceeds at a very slow rate to
yield water and oxygen gas. Under such conditions, there rate of collision between the molecules
of hydrogen peroxide is very slow as they have insufficient energy that is to be used in the
activation of the reaction (Pixel, 2017). Still, the commercial hydrogen peroxide solutions among
them 6% solution that is sold by beautician supply stores and the 3% hydrogen peroxide solution
available in the drug stores are normally treated with stabilizers, which are at times referred to as
negative catalysts (Brent, 2015). These stabilizers are important in raising the activation energy
for reactions involving hydrogen peroxide. In so doing, any reaction is thus further inhibited
from occurring.
A catalyst is a chemical substance that alters the rate of a chemical reaction and itself not
consumed during the reaction. A catalyst works by lowering the activation energy that is
required to initiate and maintain the reaction (Kadish, 2012). An example is where two hydrogen
peroxide molecules react with each other forming two molecules of water and a molecule of
oxygen gas as shown in the reaction below
2H2O 2H2O + O2
PHYSICAL CHEMISTRY LAB REPORT
Abstract
The kinetics of the decomposition of hydrogen peroxide using manganese dioxide as the catalyst
was studied at 25⁰C and 34⁰C. The findings from the experiment showed that the rate of
decomposition of hydrogen peroxide increases with an increase in the amount of manganese
dioxide and a rise in the temperature.
Introduction
The decomposition of hydrogen peroxide at room temperature proceeds at a very slow rate to
yield water and oxygen gas. Under such conditions, there rate of collision between the molecules
of hydrogen peroxide is very slow as they have insufficient energy that is to be used in the
activation of the reaction (Pixel, 2017). Still, the commercial hydrogen peroxide solutions among
them 6% solution that is sold by beautician supply stores and the 3% hydrogen peroxide solution
available in the drug stores are normally treated with stabilizers, which are at times referred to as
negative catalysts (Brent, 2015). These stabilizers are important in raising the activation energy
for reactions involving hydrogen peroxide. In so doing, any reaction is thus further inhibited
from occurring.
A catalyst is a chemical substance that alters the rate of a chemical reaction and itself not
consumed during the reaction. A catalyst works by lowering the activation energy that is
required to initiate and maintain the reaction (Kadish, 2012). An example is where two hydrogen
peroxide molecules react with each other forming two molecules of water and a molecule of
oxygen gas as shown in the reaction below
2H2O 2H2O + O2

3
PHYSICAL CHEMISTRY LAB REPORT
A catalyst has an immediate effect on a solution of hydrogen peroxide. The solution is observed
to bubble due to the production of oxygen gas.
Experimental procedure
1. Mix 150 ml of distilled water, 50 ml of borate buffer solution and 10 ml of hydrogen
peroxide solution in 250 ml conical flask
2. Place the flask in a 298K thermostat and allow it to reach temperature equilibrium
3. Add 5 ml of 0.002M KMnO4 mix well and start the stopwatch. Keep the stopwatch
running throughout the experiment
4. Remove a 10.0 ml sample and deliver into a conical flask containing 5-10 ml of dilute
H2SO4 after 3 minutes. Record the accurate time, t when the pipette is half-empty. NOTE:
When the sample is removed from the conical flask, the reaction is till occurring in the
pipette. It must be quenched before analyzing. The catalyst works in alkaline solution
(the borate buffer solution has a pH of about 9), so the reaction can be stopped by making
the solution acidic (Siegrist, 2011).
5. Titrate the sample with 0.002 M KMnO4 until a faint pink coloration is obtained. Record
the volume, (Vt)
6. Take samples after every 5 minutes for 50 minutes, or until the titre volume is around 1-2
ml, whichever comes first
7. Repeat the entire experiment at 308 K except take samples every 3 minutes until the titre
volume is around 1-2 ml
Results
PHYSICAL CHEMISTRY LAB REPORT
A catalyst has an immediate effect on a solution of hydrogen peroxide. The solution is observed
to bubble due to the production of oxygen gas.
Experimental procedure
1. Mix 150 ml of distilled water, 50 ml of borate buffer solution and 10 ml of hydrogen
peroxide solution in 250 ml conical flask
2. Place the flask in a 298K thermostat and allow it to reach temperature equilibrium
3. Add 5 ml of 0.002M KMnO4 mix well and start the stopwatch. Keep the stopwatch
running throughout the experiment
4. Remove a 10.0 ml sample and deliver into a conical flask containing 5-10 ml of dilute
H2SO4 after 3 minutes. Record the accurate time, t when the pipette is half-empty. NOTE:
When the sample is removed from the conical flask, the reaction is till occurring in the
pipette. It must be quenched before analyzing. The catalyst works in alkaline solution
(the borate buffer solution has a pH of about 9), so the reaction can be stopped by making
the solution acidic (Siegrist, 2011).
5. Titrate the sample with 0.002 M KMnO4 until a faint pink coloration is obtained. Record
the volume, (Vt)
6. Take samples after every 5 minutes for 50 minutes, or until the titre volume is around 1-2
ml, whichever comes first
7. Repeat the entire experiment at 308 K except take samples every 3 minutes until the titre
volume is around 1-2 ml
Results
4
PHYSICAL CHEMISTRY LAB REPORT
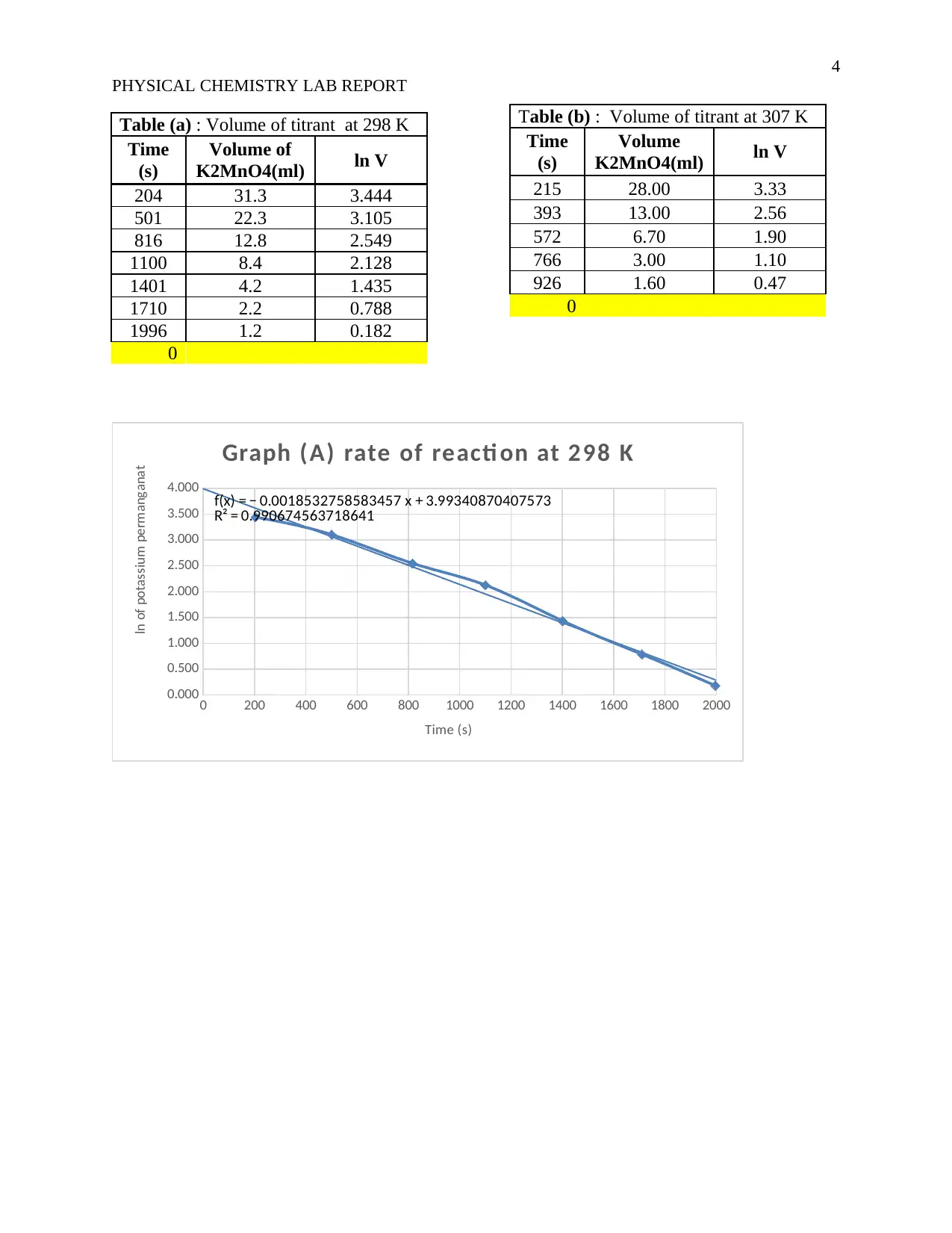
Table (a) : Volume of titrant at 298 K
Time
(s)
Volume of
K2MnO4(ml) ln V
204 31.3 3.444
501 22.3 3.105
816 12.8 2.549
1100 8.4 2.128
1401 4.2 1.435
1710 2.2 0.788
1996 1.2 0.182
0
0 200 400 600 800 1000 1200 1400 1600 1800 2000
0.000
0.500
1.000
1.500
2.000
2.500
3.000
3.500
4.000 f(x) = − 0.0018532758583457 x + 3.99340870407573
R² = 0.990674563718641
Graph (A) rate of reacti on at 298 K
Time (s)
ln of potassium permanganat
Table (b) : Volume of titrant at 307 K
Time
(s)
Volume
K2MnO4(ml) ln V
215 28.00 3.33
393 13.00 2.56
572 6.70 1.90
766 3.00 1.10
926 1.60 0.47
0
PHYSICAL CHEMISTRY LAB REPORT
Table (a) : Volume of titrant at 298 K
Time
(s)
Volume of
K2MnO4(ml) ln V
204 31.3 3.444
501 22.3 3.105
816 12.8 2.549
1100 8.4 2.128
1401 4.2 1.435
1710 2.2 0.788
1996 1.2 0.182
0
0 200 400 600 800 1000 1200 1400 1600 1800 2000
0.000
0.500
1.000
1.500
2.000
2.500
3.000
3.500
4.000 f(x) = − 0.0018532758583457 x + 3.99340870407573
R² = 0.990674563718641
Graph (A) rate of reacti on at 298 K
Time (s)
ln of potassium permanganat
Table (b) : Volume of titrant at 307 K
Time
(s)
Volume
K2MnO4(ml) ln V
215 28.00 3.33
393 13.00 2.56
572 6.70 1.90
766 3.00 1.10
926 1.60 0.47
0
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
5
PHYSICAL CHEMISTRY LAB REPORT
0 100 200 300 400 500 600 700 800 900 1000
0.00
0.50
1.00
1.50
2.00
2.50
3.00
3.50
4.00
4.50
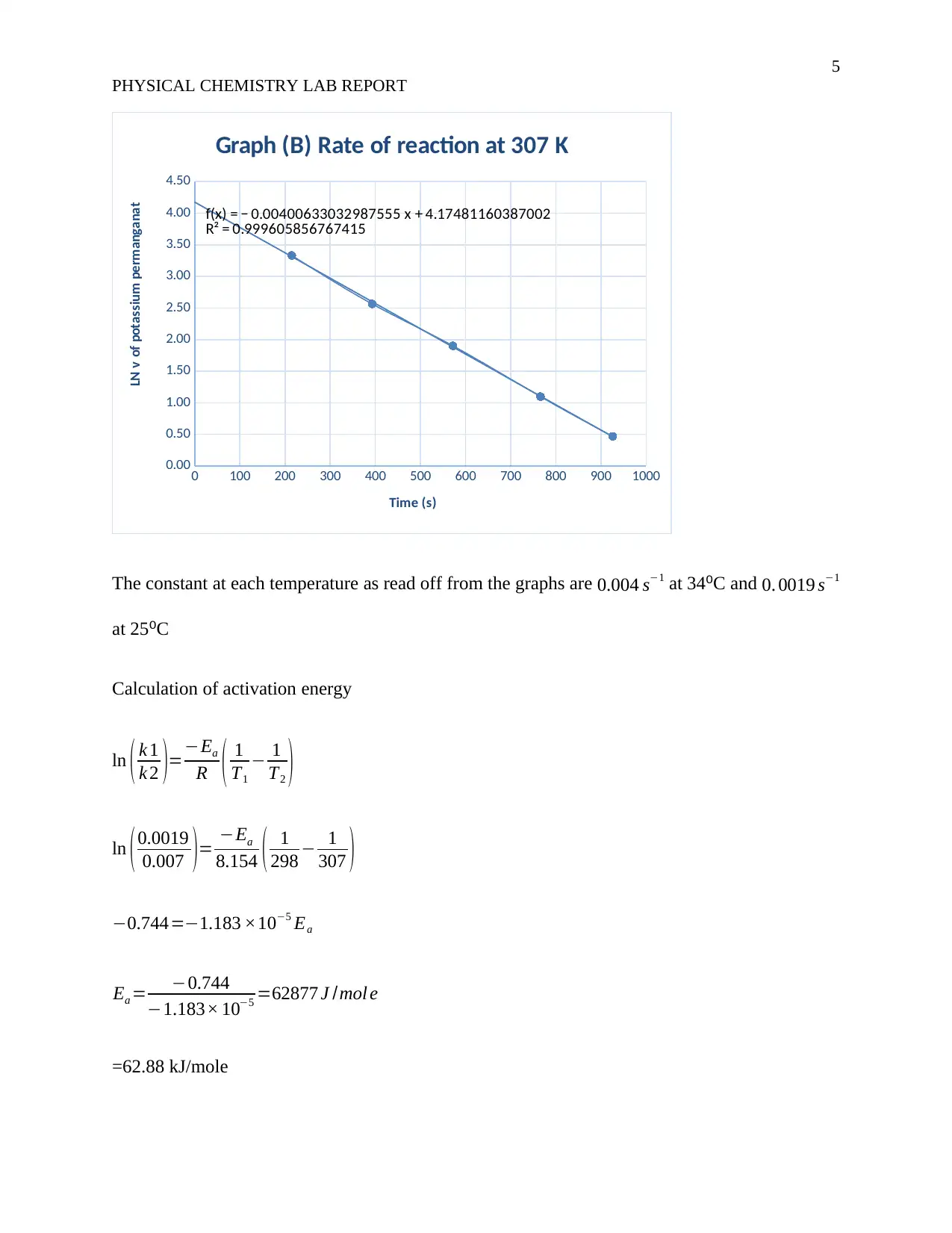
f(x) = − 0.00400633032987555 x + 4.17481160387002
R² = 0.999605856767415
Graph (B) Rate of reaction at 307 K
Time (s)
LN v of potassium permanganat
The constant at each temperature as read off from the graphs are 0.004 s−1 at 34⁰C and 0. 0019 s−1
at 25⁰C
Calculation of activation energy
ln ( k 1
k 2 )=−Ea
R ( 1
T1
− 1
T2 )
ln (0.0019
0.007 )= −Ea
8.154 ( 1
298 − 1
307 )
−0.744=−1.183 ×10−5 Ea
Ea = −0.744
−1.183× 10−5 =62877 J /mol e
=62.88 kJ/mole
PHYSICAL CHEMISTRY LAB REPORT
0 100 200 300 400 500 600 700 800 900 1000
0.00
0.50
1.00
1.50
2.00
2.50
3.00
3.50
4.00
4.50
f(x) = − 0.00400633032987555 x + 4.17481160387002
R² = 0.999605856767415
Graph (B) Rate of reaction at 307 K
Time (s)
LN v of potassium permanganat
The constant at each temperature as read off from the graphs are 0.004 s−1 at 34⁰C and 0. 0019 s−1
at 25⁰C
Calculation of activation energy
ln ( k 1
k 2 )=−Ea
R ( 1
T1
− 1
T2 )
ln (0.0019
0.007 )= −Ea
8.154 ( 1
298 − 1
307 )
−0.744=−1.183 ×10−5 Ea
Ea = −0.744
−1.183× 10−5 =62877 J /mol e
=62.88 kJ/mole

6
PHYSICAL CHEMISTRY LAB REPORT
By drawing the graphs and using the Arrhenius equation, the pre-exponential factor for the
reaction can be estimated. The Arrhenius equation is
k = A e−E a/ RT where k is the rate constant, A the pre-exponential factor which correlates with the
quantity of correctly oriented collisions, Ea is the activation energy , R the universal gas constant
which is usually 8.314472 J/mol.K and T the temperature of the reactants in K
By taking the natural log, A can be solved as shown below
ln k =ln A e
Ea
RT
¿ ln A− Ea
RT
By graphing the multiple values of ln k against the corresponding temperature reciprocals, ln A
would be estimated as the y-intercept thereafter ln A can be expontiated.
For graph A;
k = A e−E a/ RT =0.0019=A e
−62.88
8.314472 × 298
A=3.9934
For graph B;
k = A e−E a/ RT
0. 007=A e
−62.88
8.314472 × 307
PHYSICAL CHEMISTRY LAB REPORT
By drawing the graphs and using the Arrhenius equation, the pre-exponential factor for the
reaction can be estimated. The Arrhenius equation is
k = A e−E a/ RT where k is the rate constant, A the pre-exponential factor which correlates with the
quantity of correctly oriented collisions, Ea is the activation energy , R the universal gas constant
which is usually 8.314472 J/mol.K and T the temperature of the reactants in K
By taking the natural log, A can be solved as shown below
ln k =ln A e
Ea
RT
¿ ln A− Ea
RT
By graphing the multiple values of ln k against the corresponding temperature reciprocals, ln A
would be estimated as the y-intercept thereafter ln A can be expontiated.
For graph A;
k = A e−E a/ RT =0.0019=A e
−62.88
8.314472 × 298
A=3.9934
For graph B;
k = A e−E a/ RT
0. 007=A e
−62.88
8.314472 × 307

7
PHYSICAL CHEMISTRY LAB REPORT
A=4.1748
Discussion
A first order reaction is a factor of the concentration of one of the reactants and the rate law is
r = dA
dt =k ⌈ A ⌉ where k is the constant of the first order. In as much as there could be other
reactants present in a first order reaction, each of the other reacts will be assumed to be order
zero as the concentration of such reactants would not be having an effect on the rate of the
reaction (Thompson, 2012). In this regard, the rate law relating to an elementary reaction which
is of a first order with respect to a reactant A would be given by
r =−dA
dt =k ⌈ A ⌉
From the obtained results of the experiment, it is deducible that decomposition of hydrogen
peroxide is a first order reaction.
When the temperature of the reactants is increased, the rate of reaction is observed to increase.
An increase in temperature increases the rate of collision of the molecules of hydrogen peroxide.
This leads to a faster rate of collision and thereby faster production of the products of the
reaction. In this regard, the molecules of hydrogen peroxide gain more kinetic energy that make
the molecules move rapidly and thus collide with each other at high rates.
The experiment was largely free from errors with most of the values of rate constant kept
relatively constant. The biggest deviation was experienced was an uncertainty of 3.85 in te initial
volume of the titrant. The deviation can be attributed to the time differences where one part of
PHYSICAL CHEMISTRY LAB REPORT
A=4.1748
Discussion
A first order reaction is a factor of the concentration of one of the reactants and the rate law is
r = dA
dt =k ⌈ A ⌉ where k is the constant of the first order. In as much as there could be other
reactants present in a first order reaction, each of the other reacts will be assumed to be order
zero as the concentration of such reactants would not be having an effect on the rate of the
reaction (Thompson, 2012). In this regard, the rate law relating to an elementary reaction which
is of a first order with respect to a reactant A would be given by
r =−dA
dt =k ⌈ A ⌉
From the obtained results of the experiment, it is deducible that decomposition of hydrogen
peroxide is a first order reaction.
When the temperature of the reactants is increased, the rate of reaction is observed to increase.
An increase in temperature increases the rate of collision of the molecules of hydrogen peroxide.
This leads to a faster rate of collision and thereby faster production of the products of the
reaction. In this regard, the molecules of hydrogen peroxide gain more kinetic energy that make
the molecules move rapidly and thus collide with each other at high rates.
The experiment was largely free from errors with most of the values of rate constant kept
relatively constant. The biggest deviation was experienced was an uncertainty of 3.85 in te initial
volume of the titrant. The deviation can be attributed to the time differences where one part of
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
8
PHYSICAL CHEMISTRY LAB REPORT
the experiment was done in the morning hours while the other was carried out in the afternoon
hours (Baxter, 2017).
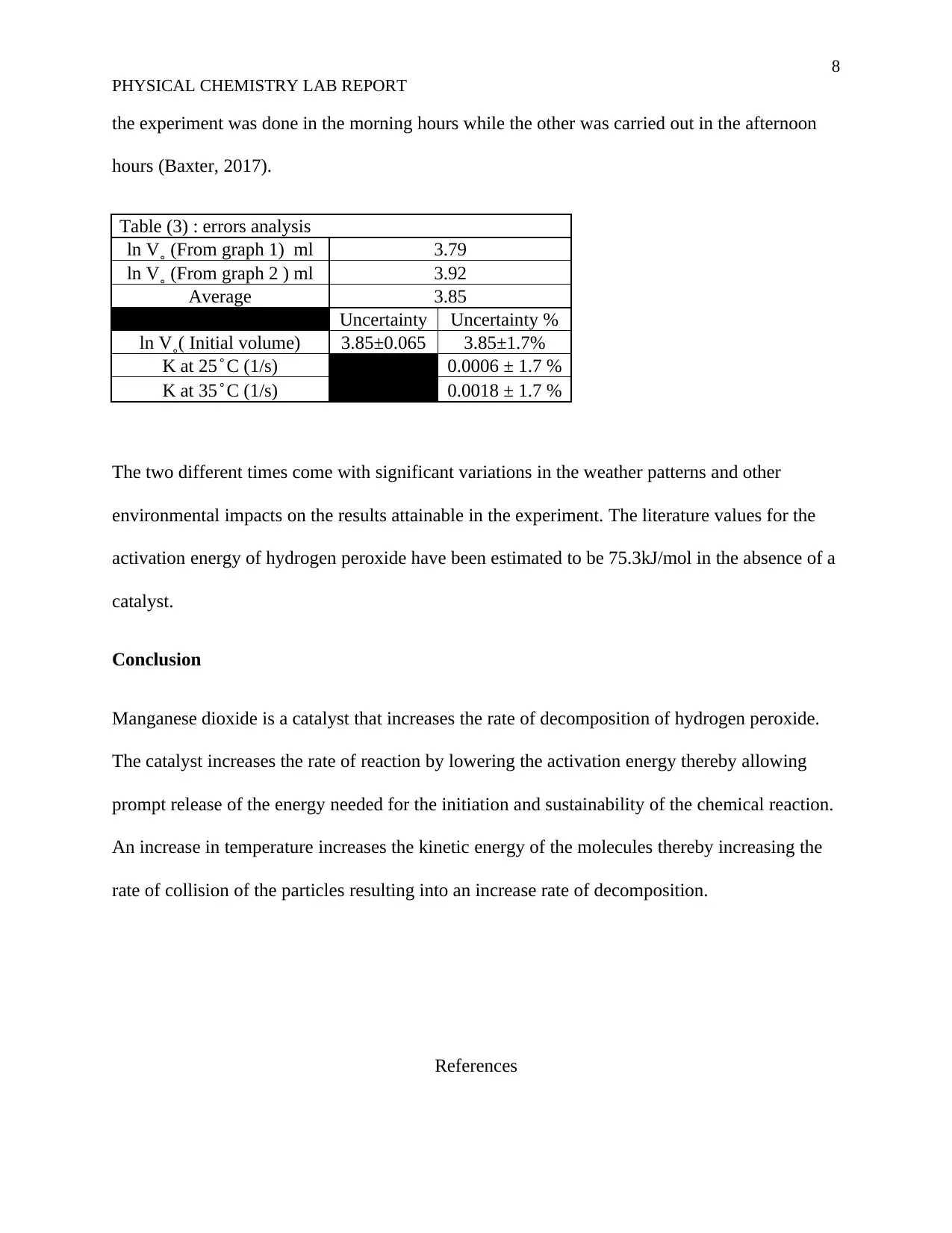
Table (3) : errors analysis
ln V˳ (From graph 1) ml 3.79
ln V˳ (From graph 2 ) ml 3.92
Average 3.85
Uncertainty Uncertainty %
ln V˳( Initial volume) 3.85±0.065 3.85±1.7%
K at 25 ̊ C (1/s) 0.0006 ± 1.7 %
K at 35 ̊ C (1/s) 0.0018 ± 1.7 %
The two different times come with significant variations in the weather patterns and other
environmental impacts on the results attainable in the experiment. The literature values for the
activation energy of hydrogen peroxide have been estimated to be 75.3kJ/mol in the absence of a
catalyst.
Conclusion
Manganese dioxide is a catalyst that increases the rate of decomposition of hydrogen peroxide.
The catalyst increases the rate of reaction by lowering the activation energy thereby allowing
prompt release of the energy needed for the initiation and sustainability of the chemical reaction.
An increase in temperature increases the kinetic energy of the molecules thereby increasing the
rate of collision of the particles resulting into an increase rate of decomposition.
References
PHYSICAL CHEMISTRY LAB REPORT
the experiment was done in the morning hours while the other was carried out in the afternoon
hours (Baxter, 2017).
Table (3) : errors analysis
ln V˳ (From graph 1) ml 3.79
ln V˳ (From graph 2 ) ml 3.92
Average 3.85
Uncertainty Uncertainty %
ln V˳( Initial volume) 3.85±0.065 3.85±1.7%
K at 25 ̊ C (1/s) 0.0006 ± 1.7 %
K at 35 ̊ C (1/s) 0.0018 ± 1.7 %
The two different times come with significant variations in the weather patterns and other
environmental impacts on the results attainable in the experiment. The literature values for the
activation energy of hydrogen peroxide have been estimated to be 75.3kJ/mol in the absence of a
catalyst.
Conclusion
Manganese dioxide is a catalyst that increases the rate of decomposition of hydrogen peroxide.
The catalyst increases the rate of reaction by lowering the activation energy thereby allowing
prompt release of the energy needed for the initiation and sustainability of the chemical reaction.
An increase in temperature increases the kinetic energy of the molecules thereby increasing the
rate of collision of the particles resulting into an increase rate of decomposition.
References

9
PHYSICAL CHEMISTRY LAB REPORT
Baxter, T. (2017). AQA KS3 Science Student Book, Part 2. New York: HarperCollins Publishers
Limited.
Brent, R. (2015). The Golden Book of Chemistry Experiment. Oxford: CreateSpace Independent
Publishing Platform.
Kadish, K. (2012). The Porphyrin Handbook: Phthalocyanines: Properties and Material. New
York: Elsevier.
Pixel. (2017). Conference Proceedings. New Perspectives in Science Education: 6th Edition.
Kansas: libreriauniversitaria.it Edizioni.
Siegrist, R. L. (2011). n Situ Chemical Oxidation for Groundwater Remediation. London:
Springer Science & Business Media.
Thompson, R. B. (2012). Illustrated Guide to Home Chemistry Experiments: All Lab, No
Lecture. London: O'Reilly Media, Inc.".
PHYSICAL CHEMISTRY LAB REPORT
Baxter, T. (2017). AQA KS3 Science Student Book, Part 2. New York: HarperCollins Publishers
Limited.
Brent, R. (2015). The Golden Book of Chemistry Experiment. Oxford: CreateSpace Independent
Publishing Platform.
Kadish, K. (2012). The Porphyrin Handbook: Phthalocyanines: Properties and Material. New
York: Elsevier.
Pixel. (2017). Conference Proceedings. New Perspectives in Science Education: 6th Edition.
Kansas: libreriauniversitaria.it Edizioni.
Siegrist, R. L. (2011). n Situ Chemical Oxidation for Groundwater Remediation. London:
Springer Science & Business Media.
Thompson, R. B. (2012). Illustrated Guide to Home Chemistry Experiments: All Lab, No
Lecture. London: O'Reilly Media, Inc.".
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.