Exercise Training Modalities and Glycemic Control in Type 2 Diabetes
VerifiedAdded on 2022/12/20
|9
|8548
|1
Report
AI Summary
This report presents a systematic review and network meta-analysis of 14 randomized controlled trials, encompassing 915 participants, to assess the impact of aerobic exercise training (AET), resistance training (RT), and combined training (CT) on glycemic control and blood lipids in individuals with type 2 diabetes mellitus. The study found that AET was more effective than RT in improving HbA1c levels and fasting glucose. Furthermore, CT demonstrated a more significant reduction in HbA1c compared to AET, and a greater reduction in HbA1c, fasting glucose, and triacylglycerols compared to RT. The findings suggest that CT might be the most efficacious exercise modality for improving glycemic control and blood lipids, although the clinical relevance is limited by the quality of included studies. The meta-analysis employed both pairwise and Bayesian network meta-analyses to synthesize the available evidence, highlighting the importance of supervised training in exercise interventions for type 2 diabetes management.

META-ANALYSIS
Impact of different training modalities on glycaemic control
and blood lipids in patients with type 2 diabetes: a systematic
review and network meta-analysis
Lukas Schwingshackl&Benjamin Missbach&Sofia Dias&
Jürgen König&Georg Hoffmann
Received: 13 March 2014 / Accepted: 2 June 2014 / Published online: 5 July 2014
# Springer-Verlag Berlin Heidelberg 2014
Abstract
Aims/hypothesis This study aimed to systematically review
randomised controlled trials comparing the effects of aerobic
exercise training (AET),resistance training (RT) and com-
bined training (CT) on glycaemic control and blood lipids in
patients with type 2 diabetes mellitus.
Methods Searches were performed in MEDLINE, EMBASE
and the Cochrane Library.Inclusion criteria were:type 2
diabetes mellitus,adult,supervised training and a minimum
intervention period of 8 weeks. Pooled effects were calculated
by fixed/random effect pairwise and Bayesian fixed/random
effects network meta-analyses.
Results A totalof 14 trials enrolling 915 participants were
included.AET was more effective than RT in improving
HbA 1 c l e v e l s( m e a nd i f f e r e n c e[MD] −0.2 0%
[−2.2 mmol/mol];95% CI −0.32,−0.08;p = 0.0007,
1 0 t r i a l s / 5 1 5 p a r t i c i p a n t s )a n d f a s t i n g g l u c o s e
(MD −0.9 mmol/l; 95% CI −1.71, −0.09; p=0.03, 8 trials/245
participants). Compared with AET, CT resulted in a significantly
more pronounced reduction in HbA1c (MD −0.17%
[−1.87 mmol/mol]; 95% CI −0.31, −0.03; p=0.02, 9 trials/493
participants).Compared with RT,the MD of the change in
HbA1c (MD −0.62%,[−6.82 mmol/mol];95% CI −0.95,
−0.30;p=0.0002,5 trials/362 participants],fasting glucose
(MD −1.99 mmol/l; 95% CI −3.07, −0.90; p=0.0003, 3 tria
99 participants) and triacylglycerols (MD −0.28 mmol/l; 95
CI −0.46, −0.10; p=0.003, 4 trials/213 participants) were
favour of CT.The exclusion of trials with a high risk of bias
yielded only non-significant results.
Conclusions/interpretation The present data suggest that
might be the most efficacious exercise modality to improv
glycaemic control and blood lipids. Interpretation with resp
to clinical relevance is limited by the low quality of the stu
included and the limited information on the clinically impo
tant outcomes or adverse effects of exercise.
Keywords Aerobic exercise. Combined training. Network
meta-analysis. Resistance training. Systematic review
Abbreviations
AET Aerobic exercise training
BW Body weight
CT Combined training
DBP Diastolic blood pressure
FG Fasting glucose
MD Mean difference
RT Resistance training
SBP Systolic blood pressure
TC Total cholesterol
TG Triacylglycerols
Introduction
Increased physical activity and improved nutritional habits
the form of hypocaloric diets (of varying macronutrient co
positions) are of particular importance to decelerate the m
ifestations oftype 2 diabetes [1–3].The ADA and the
Electronic supplementary materialThe online version of this article
(doi:10.1007/s00125-014-3303-z) contains peer-reviewed butunedited
supplementary material, which is available to authorised users.
L. Schwingshackl (*):B. Missbach:J. König:G. Hoffmann
Department of Nutritional Sciences, Faculty of Life Sciences,
University of Vienna, Althanstraße 14 (UZAII), 1090 Vienna,
Austria
e-mail: lukas.schwingshackl@univie.ac.at
S. Dias
School of Social and Community Medicine, University of Bristol,
Bristol, UK
Diabetologia (2014) 57:1789–1797
DOI 10.1007/s00125-014-3303-z
Impact of different training modalities on glycaemic control
and blood lipids in patients with type 2 diabetes: a systematic
review and network meta-analysis
Lukas Schwingshackl&Benjamin Missbach&Sofia Dias&
Jürgen König&Georg Hoffmann
Received: 13 March 2014 / Accepted: 2 June 2014 / Published online: 5 July 2014
# Springer-Verlag Berlin Heidelberg 2014
Abstract
Aims/hypothesis This study aimed to systematically review
randomised controlled trials comparing the effects of aerobic
exercise training (AET),resistance training (RT) and com-
bined training (CT) on glycaemic control and blood lipids in
patients with type 2 diabetes mellitus.
Methods Searches were performed in MEDLINE, EMBASE
and the Cochrane Library.Inclusion criteria were:type 2
diabetes mellitus,adult,supervised training and a minimum
intervention period of 8 weeks. Pooled effects were calculated
by fixed/random effect pairwise and Bayesian fixed/random
effects network meta-analyses.
Results A totalof 14 trials enrolling 915 participants were
included.AET was more effective than RT in improving
HbA 1 c l e v e l s( m e a nd i f f e r e n c e[MD] −0.2 0%
[−2.2 mmol/mol];95% CI −0.32,−0.08;p = 0.0007,
1 0 t r i a l s / 5 1 5 p a r t i c i p a n t s )a n d f a s t i n g g l u c o s e
(MD −0.9 mmol/l; 95% CI −1.71, −0.09; p=0.03, 8 trials/245
participants). Compared with AET, CT resulted in a significantly
more pronounced reduction in HbA1c (MD −0.17%
[−1.87 mmol/mol]; 95% CI −0.31, −0.03; p=0.02, 9 trials/493
participants).Compared with RT,the MD of the change in
HbA1c (MD −0.62%,[−6.82 mmol/mol];95% CI −0.95,
−0.30;p=0.0002,5 trials/362 participants],fasting glucose
(MD −1.99 mmol/l; 95% CI −3.07, −0.90; p=0.0003, 3 tria
99 participants) and triacylglycerols (MD −0.28 mmol/l; 95
CI −0.46, −0.10; p=0.003, 4 trials/213 participants) were
favour of CT.The exclusion of trials with a high risk of bias
yielded only non-significant results.
Conclusions/interpretation The present data suggest that
might be the most efficacious exercise modality to improv
glycaemic control and blood lipids. Interpretation with resp
to clinical relevance is limited by the low quality of the stu
included and the limited information on the clinically impo
tant outcomes or adverse effects of exercise.
Keywords Aerobic exercise. Combined training. Network
meta-analysis. Resistance training. Systematic review
Abbreviations
AET Aerobic exercise training
BW Body weight
CT Combined training
DBP Diastolic blood pressure
FG Fasting glucose
MD Mean difference
RT Resistance training
SBP Systolic blood pressure
TC Total cholesterol
TG Triacylglycerols
Introduction
Increased physical activity and improved nutritional habits
the form of hypocaloric diets (of varying macronutrient co
positions) are of particular importance to decelerate the m
ifestations oftype 2 diabetes [1–3].The ADA and the
Electronic supplementary materialThe online version of this article
(doi:10.1007/s00125-014-3303-z) contains peer-reviewed butunedited
supplementary material, which is available to authorised users.
L. Schwingshackl (*):B. Missbach:J. König:G. Hoffmann
Department of Nutritional Sciences, Faculty of Life Sciences,
University of Vienna, Althanstraße 14 (UZAII), 1090 Vienna,
Austria
e-mail: lukas.schwingshackl@univie.ac.at
S. Dias
School of Social and Community Medicine, University of Bristol,
Bristol, UK
Diabetologia (2014) 57:1789–1797
DOI 10.1007/s00125-014-3303-z
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

American College ofSports Medicine have stated thata
combination of resistance training (RT) and aerobic exercise
training (AET)of at least150 min ofmoderate-intensity
exercise perweek may be more effective in improving
glycaemic control than focusing solely on one single training
modality (evidence category B) [4].
The isolated effects ofeitherRT or AET, or a com-
bination ofboth (combined training [CT]),on anthropo-
metric,cardiacand metabolicrisk factorshave been
meta-analysed by Snowling and Hopkins [5]as wellas
by Chudyk and Petrella [6].Both studiesreported that
the reduction in HbA1c and fasting glucose (FG)levels,
systolic blood pressure(SBP), waist circumference,
HDL and triacylglycerols(TG) was morepronounced
following AET and CT compared with RT.In addition,
HbA1c- and blood pressure-lowering effects ofRT were
shown.However,all these systematic reviewsincluded
trialsin which training modalitieswere compared with
the data from a sedentary controlgroup [7,8].
To date, no systematicreview has compared the
directand indirecteffects ofthese three differenttrain-
ing modalitieson the outcomesof glycaemiccontrol
and blood lipidsin patientswith type 2 diabetes.A
recentpairwise meta-analysiscomparing RT (allsuper-
vised) with AET (notall supervised) exercise in patients
with type 2 diabetes concluded thatalthough differences
in some outcomevariablesreached statisticalsignifi-
cance,there was no evidence thatthey were ofclinical
relevance[9]. In a recently published network meta-
analysis,we were able to demonstrate thatCT is ranked
as the mostlikely effective exercise modelin the treat-
mentof overweightand obesity [10].
The aim of the present study was to assess the efficacy of
AET, RT and CT on glycaemic control,blood pressure and
blood lipids in patients with type 2 diabetes mellitus in a
systematic review including a pairwise and network meta-
analysis of randomised trials.The importance of supervised
training has been demonstrated by Umpierre etal, who
showed that structured training compared with training advice
only significantly improved glycaemic control in patients with
type 2 diabetes [11]. Based upon these findings, only trials that
conducted an exercise intervention which was guided by
supervised training were enrolled in the presentsystematic
review and meta-analyses.
Methods
The review was registered in PROSPERO International
Prospective Register of Systematic Reviews (www.crd.york.
ac.uk/prospero/index.asp,identifierCRD42014007502).
However,no study protocolwas published before the
initiation of the meta-analysis.
Literature search
Queries of the literature were performed using the electro
databases MEDLINE (until 2 May 2014), EMBASE (until 2
May 2014) and the Cochrane Central Register of Controlle
Trials (until 2 May 2014) with no restrictions. The following
keywords were used: (‘strength’ OR ‘resistance’ OR ‘aerob
OR ‘endurance’ OR ‘combined training’ OR ‘progressive’
OR ‘walking’OR ‘intervaltraining’OR ‘weightlifting’)
AND (‘training’ OR ‘exercise’ OR ‘physical activity’) AND
(‘diabetes’OR ‘glycemic’ OR ‘glycaemia’ OR ‘glycaemic’
OR ‘glycemia’OR ‘HbA1c’ OR ‘A 1c’ OR ‘glycated’OR
‘glycosylated’ OR ‘glucose’ OR ‘lipids’ OR ‘body weight’
OR ‘blood pressure’) AND (‘randomized controlled trial’ OR
‘randomized’ OR ‘clinical trials as topic’ OR ‘placebo’ OR
‘randomly’ OR ‘trial’) NOT (‘animals’ NOT ‘humans’).
Moreover,the reference lists from the retrieved articles,
systematic reviews and meta-analyses were checked to se
for further relevant studies. This systematic review was pl
conducted and reported in adherence with standards of qu
for reporting meta-analyses [12]. The entire literature sea
conducted independently by two authors (L. Schwingshack
B. Missbach), with disagreements resolved by consensus.
detailed search strategy for MEDLINE is given in the electr
supplementary material (ESM Methods).
Eligibility criteria
Studies were included in the meta-analysis if they met all
the following criteria: (1) a randomised controlled design;
a minimum intervention period of 8 weeks; (3) patients wi
type 2 diabetes; (4) patients’ age ≥19 years; (5) a compar
of either AET vs RT and/or CT vs AET and/or CT vs RT; (6)
an assessmentof at leastone ofthe following outcome
markers:HbA1c, blood glucose,body weight(BW), blood
pressure or blood lipids (totalcholesterol[TC],LDL, HDL
and TG);(7) the reporting of changes from baseline value
scores with SDs (or data suitable to calculate these variab
SE and 95% CI); if the SDs of the changes from baseline va
scores were not available, post-intervention values were im
puted, according to the Cochrane Handbook [13]; (8) train
that was conducted under direct (guided by a physiothera
in training classes, hospital gyms, etc.) or partial supervisi
and was not home-based; and (9) the exclusion of studies
a dietary co-intervention that was not applied in all the int
vention groups. All abstracts and full texts were independe
assessed for eligibility by two authors (L. Schwingshackl a
B. Missbach).
Risk of bias assessment
Full copies of the studies were independently assessed by
authors ( L .Schwingshackl and B. Missbach) f o r
1790 Diabetologia (2014) 57:1789–1797
combination of resistance training (RT) and aerobic exercise
training (AET)of at least150 min ofmoderate-intensity
exercise perweek may be more effective in improving
glycaemic control than focusing solely on one single training
modality (evidence category B) [4].
The isolated effects ofeitherRT or AET, or a com-
bination ofboth (combined training [CT]),on anthropo-
metric,cardiacand metabolicrisk factorshave been
meta-analysed by Snowling and Hopkins [5]as wellas
by Chudyk and Petrella [6].Both studiesreported that
the reduction in HbA1c and fasting glucose (FG)levels,
systolic blood pressure(SBP), waist circumference,
HDL and triacylglycerols(TG) was morepronounced
following AET and CT compared with RT.In addition,
HbA1c- and blood pressure-lowering effects ofRT were
shown.However,all these systematic reviewsincluded
trialsin which training modalitieswere compared with
the data from a sedentary controlgroup [7,8].
To date, no systematicreview has compared the
directand indirecteffects ofthese three differenttrain-
ing modalitieson the outcomesof glycaemiccontrol
and blood lipidsin patientswith type 2 diabetes.A
recentpairwise meta-analysiscomparing RT (allsuper-
vised) with AET (notall supervised) exercise in patients
with type 2 diabetes concluded thatalthough differences
in some outcomevariablesreached statisticalsignifi-
cance,there was no evidence thatthey were ofclinical
relevance[9]. In a recently published network meta-
analysis,we were able to demonstrate thatCT is ranked
as the mostlikely effective exercise modelin the treat-
mentof overweightand obesity [10].
The aim of the present study was to assess the efficacy of
AET, RT and CT on glycaemic control,blood pressure and
blood lipids in patients with type 2 diabetes mellitus in a
systematic review including a pairwise and network meta-
analysis of randomised trials.The importance of supervised
training has been demonstrated by Umpierre etal, who
showed that structured training compared with training advice
only significantly improved glycaemic control in patients with
type 2 diabetes [11]. Based upon these findings, only trials that
conducted an exercise intervention which was guided by
supervised training were enrolled in the presentsystematic
review and meta-analyses.
Methods
The review was registered in PROSPERO International
Prospective Register of Systematic Reviews (www.crd.york.
ac.uk/prospero/index.asp,identifierCRD42014007502).
However,no study protocolwas published before the
initiation of the meta-analysis.
Literature search
Queries of the literature were performed using the electro
databases MEDLINE (until 2 May 2014), EMBASE (until 2
May 2014) and the Cochrane Central Register of Controlle
Trials (until 2 May 2014) with no restrictions. The following
keywords were used: (‘strength’ OR ‘resistance’ OR ‘aerob
OR ‘endurance’ OR ‘combined training’ OR ‘progressive’
OR ‘walking’OR ‘intervaltraining’OR ‘weightlifting’)
AND (‘training’ OR ‘exercise’ OR ‘physical activity’) AND
(‘diabetes’OR ‘glycemic’ OR ‘glycaemia’ OR ‘glycaemic’
OR ‘glycemia’OR ‘HbA1c’ OR ‘A 1c’ OR ‘glycated’OR
‘glycosylated’ OR ‘glucose’ OR ‘lipids’ OR ‘body weight’
OR ‘blood pressure’) AND (‘randomized controlled trial’ OR
‘randomized’ OR ‘clinical trials as topic’ OR ‘placebo’ OR
‘randomly’ OR ‘trial’) NOT (‘animals’ NOT ‘humans’).
Moreover,the reference lists from the retrieved articles,
systematic reviews and meta-analyses were checked to se
for further relevant studies. This systematic review was pl
conducted and reported in adherence with standards of qu
for reporting meta-analyses [12]. The entire literature sea
conducted independently by two authors (L. Schwingshack
B. Missbach), with disagreements resolved by consensus.
detailed search strategy for MEDLINE is given in the electr
supplementary material (ESM Methods).
Eligibility criteria
Studies were included in the meta-analysis if they met all
the following criteria: (1) a randomised controlled design;
a minimum intervention period of 8 weeks; (3) patients wi
type 2 diabetes; (4) patients’ age ≥19 years; (5) a compar
of either AET vs RT and/or CT vs AET and/or CT vs RT; (6)
an assessmentof at leastone ofthe following outcome
markers:HbA1c, blood glucose,body weight(BW), blood
pressure or blood lipids (totalcholesterol[TC],LDL, HDL
and TG);(7) the reporting of changes from baseline value
scores with SDs (or data suitable to calculate these variab
SE and 95% CI); if the SDs of the changes from baseline va
scores were not available, post-intervention values were im
puted, according to the Cochrane Handbook [13]; (8) train
that was conducted under direct (guided by a physiothera
in training classes, hospital gyms, etc.) or partial supervisi
and was not home-based; and (9) the exclusion of studies
a dietary co-intervention that was not applied in all the int
vention groups. All abstracts and full texts were independe
assessed for eligibility by two authors (L. Schwingshackl a
B. Missbach).
Risk of bias assessment
Full copies of the studies were independently assessed by
authors ( L .Schwingshackl and B. Missbach) f o r
1790 Diabetologia (2014) 57:1789–1797

methodologicalquality using the risk of bias assessmenttool
from the Cochrane Collaboration [13,14].The following
sources of bias were detected: selection bias (random sequence
generation and allocation concealment), detection bias (blinding
of outcome assessment), blinding of participants and personnel
(performance bias), attrition bias (incomplete outcome data) and
reporting bias (selective reporting) (ESM Fig. 1).
Data extraction and statistical analysis
The following data were extracted from each study:the first
author’s lastname,publication year,study duration,partici-
pant’s sex,age and BMI,sample size,duration of diabetes,
HbA1c at baseline, drug treatment, change of treatment during
the trial, treatment effects, intervention type, dose, intensity and
frequency, and differences in the means of two time points or
post-intervention mean values with corresponding SDs.For
each outcome measure of interest, pairwise and network random
effects meta-analyses were performed in order to determine the
pooled relative effect of each intervention relative to every other
intervention in terms of the mean differences (MDs) between
the changes from baseline value scores (or post-intervention
values) of the differentinterventions.To process the data for
the meta-analysis,we imputed the data for the changes from
baseline means and their SDs. When the SDs for the changes
from baseline values were notavailable [15–20],the post-
intervention values with the corresponding SDs were imputed,
according the guidelines of the Cochrane Handbook [13].
Data were pooled if outcomes were reported by atleast
three studies.Heterogeneity between trial results was tested
with a Cochran’s Q test. A value for I2 of >50% was consid-
ered to representsubstantialheterogeneity [21].When sub-
stantial heterogeneity was present, the random effects model
was used to estimate MDs with 95% CIs. Forest plots were
generated to illustrate the study-specific effect sizes along with
a 95% CI. To determine the presence of publication bias, the
symmetry ofthe funnelplots in which mean MDs were
plotted againsttheircorresponding SEs were assessed.
Additionally,Begg’s and Egger’s regression tests were per-
formed to detect small study effects [22, 23].
Separate pairwise meta-analyses were first used to compare
all the interventions. Network meta-analysis was then used to
synthesise allthe available evidence [24].Network meta-
analysis methods are extensions ofthe standard pairwise
meta-analysis model that enable a simultaneous comparison
of multiple interventions while preserving the internal
randomisation of individual trials.They have the advantage
of adequately accounting for the correlation in relative effect
estimates from three-arm trials as well as providing a single
coherentsummary of allthe evidence.Random effects net-
work meta-analysis models were used when substantial het-
erogeneity was found in any of the pairwise comparisons for
thatoutcome.Otherwise,the choice between fixed and
random effects was made by comparing the deviance info
mation criteria for each model [24, 25]. The model with th
lowest deviance information criterion was chosen (differen
>3 are considered meaningful). Pooled effect sizes from th
network meta-analyses are presented as posterior median
95% credible intervals (i.e. the Bayesian equivalent of CIs)
the appropriate units, along with the estimated between-s
heterogeneity.
For pairwise meta-analyses,data were analysed using
Review Manager5.1 software,provided by the Cochrane
Collaboration (http://ims.Cochrane.org/revman).Network
meta-analyses were conducted using Markov chain Monte
Carlo simulation implemented with the open-source softwa
WinBUGS, version 1.4.3 [26]. The WinBUGS code used is
freely available online [24,27] (program ‘TSD2-5aRE_
Normal_id.odc’ or ‘TSD2-5aFE_Normal_id.odc’).
Minimally informative normalpriors were used forall
treatmenteffectvariables and a uniform prior (0,150) was
used forthe between-study SD (heterogeneity)variable.
Sensitivity to this prior was assessed, but there was no me
ingful change in the relative effects or overall conclusions.
Three Markov chain Monte Carlo chains were used to
assess convergence using Brooks–Gelman–Rubin plots and
inspection of the trace plots [28]. Convergence was achiev
after 20,000 iterations for all outcomes. Posterior summar
were then obtained from a further simulation of 50,000 ite
tions in each of the three chains (giving 150,000 in total),
resulting in a small Monte Carlo error.
The potential for inconsistency was assessed by inspect
of the available evidence.In case of possible inconsistency,
Bayesian p values for the difference between direct and in
rectevidence were calculated,and directand indirectesti-
mates were compared [29, 30].
Results
Overall, a total of 14 trials (16 reports) extracted from 9,47
articles met the eligibility requirements and were included
the presentsystematic review and meta-analysis [15–20,
31–40].One study was excluded since it was not described
as randomised [41], and two trials provided no information
whether the AET was supervised [42, 43]. The detailed ste
of the article selection process for the meta-analysis are d
scribed as a flow diagram in ESM Fig.2. The studies were
published between 2003 and 2013 and had enrolled a tota
915 participants.The study duration ranged between 2 and
12 months;the patients’mean age was between 49 and
62.5 years,and theirBMI between 27.1 and 43.8 kg/m2.
Fourteen trials met the objectives for meta-analysis: 10 co
pared RT vs AET, 9 compared CT vs AET, and 5 compared
CT vs RT (ESM Fig. 3). The generaland specific study
Diabetologia (2014) 57:1789–1797 1791
from the Cochrane Collaboration [13,14].The following
sources of bias were detected: selection bias (random sequence
generation and allocation concealment), detection bias (blinding
of outcome assessment), blinding of participants and personnel
(performance bias), attrition bias (incomplete outcome data) and
reporting bias (selective reporting) (ESM Fig. 1).
Data extraction and statistical analysis
The following data were extracted from each study:the first
author’s lastname,publication year,study duration,partici-
pant’s sex,age and BMI,sample size,duration of diabetes,
HbA1c at baseline, drug treatment, change of treatment during
the trial, treatment effects, intervention type, dose, intensity and
frequency, and differences in the means of two time points or
post-intervention mean values with corresponding SDs.For
each outcome measure of interest, pairwise and network random
effects meta-analyses were performed in order to determine the
pooled relative effect of each intervention relative to every other
intervention in terms of the mean differences (MDs) between
the changes from baseline value scores (or post-intervention
values) of the differentinterventions.To process the data for
the meta-analysis,we imputed the data for the changes from
baseline means and their SDs. When the SDs for the changes
from baseline values were notavailable [15–20],the post-
intervention values with the corresponding SDs were imputed,
according the guidelines of the Cochrane Handbook [13].
Data were pooled if outcomes were reported by atleast
three studies.Heterogeneity between trial results was tested
with a Cochran’s Q test. A value for I2 of >50% was consid-
ered to representsubstantialheterogeneity [21].When sub-
stantial heterogeneity was present, the random effects model
was used to estimate MDs with 95% CIs. Forest plots were
generated to illustrate the study-specific effect sizes along with
a 95% CI. To determine the presence of publication bias, the
symmetry ofthe funnelplots in which mean MDs were
plotted againsttheircorresponding SEs were assessed.
Additionally,Begg’s and Egger’s regression tests were per-
formed to detect small study effects [22, 23].
Separate pairwise meta-analyses were first used to compare
all the interventions. Network meta-analysis was then used to
synthesise allthe available evidence [24].Network meta-
analysis methods are extensions ofthe standard pairwise
meta-analysis model that enable a simultaneous comparison
of multiple interventions while preserving the internal
randomisation of individual trials.They have the advantage
of adequately accounting for the correlation in relative effect
estimates from three-arm trials as well as providing a single
coherentsummary of allthe evidence.Random effects net-
work meta-analysis models were used when substantial het-
erogeneity was found in any of the pairwise comparisons for
thatoutcome.Otherwise,the choice between fixed and
random effects was made by comparing the deviance info
mation criteria for each model [24, 25]. The model with th
lowest deviance information criterion was chosen (differen
>3 are considered meaningful). Pooled effect sizes from th
network meta-analyses are presented as posterior median
95% credible intervals (i.e. the Bayesian equivalent of CIs)
the appropriate units, along with the estimated between-s
heterogeneity.
For pairwise meta-analyses,data were analysed using
Review Manager5.1 software,provided by the Cochrane
Collaboration (http://ims.Cochrane.org/revman).Network
meta-analyses were conducted using Markov chain Monte
Carlo simulation implemented with the open-source softwa
WinBUGS, version 1.4.3 [26]. The WinBUGS code used is
freely available online [24,27] (program ‘TSD2-5aRE_
Normal_id.odc’ or ‘TSD2-5aFE_Normal_id.odc’).
Minimally informative normalpriors were used forall
treatmenteffectvariables and a uniform prior (0,150) was
used forthe between-study SD (heterogeneity)variable.
Sensitivity to this prior was assessed, but there was no me
ingful change in the relative effects or overall conclusions.
Three Markov chain Monte Carlo chains were used to
assess convergence using Brooks–Gelman–Rubin plots and
inspection of the trace plots [28]. Convergence was achiev
after 20,000 iterations for all outcomes. Posterior summar
were then obtained from a further simulation of 50,000 ite
tions in each of the three chains (giving 150,000 in total),
resulting in a small Monte Carlo error.
The potential for inconsistency was assessed by inspect
of the available evidence.In case of possible inconsistency,
Bayesian p values for the difference between direct and in
rectevidence were calculated,and directand indirectesti-
mates were compared [29, 30].
Results
Overall, a total of 14 trials (16 reports) extracted from 9,47
articles met the eligibility requirements and were included
the presentsystematic review and meta-analysis [15–20,
31–40].One study was excluded since it was not described
as randomised [41], and two trials provided no information
whether the AET was supervised [42, 43]. The detailed ste
of the article selection process for the meta-analysis are d
scribed as a flow diagram in ESM Fig.2. The studies were
published between 2003 and 2013 and had enrolled a tota
915 participants.The study duration ranged between 2 and
12 months;the patients’mean age was between 49 and
62.5 years,and theirBMI between 27.1 and 43.8 kg/m2.
Fourteen trials met the objectives for meta-analysis: 10 co
pared RT vs AET, 9 compared CT vs AET, and 5 compared
CT vs RT (ESM Fig. 3). The generaland specific study
Diabetologia (2014) 57:1789–1797 1791
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
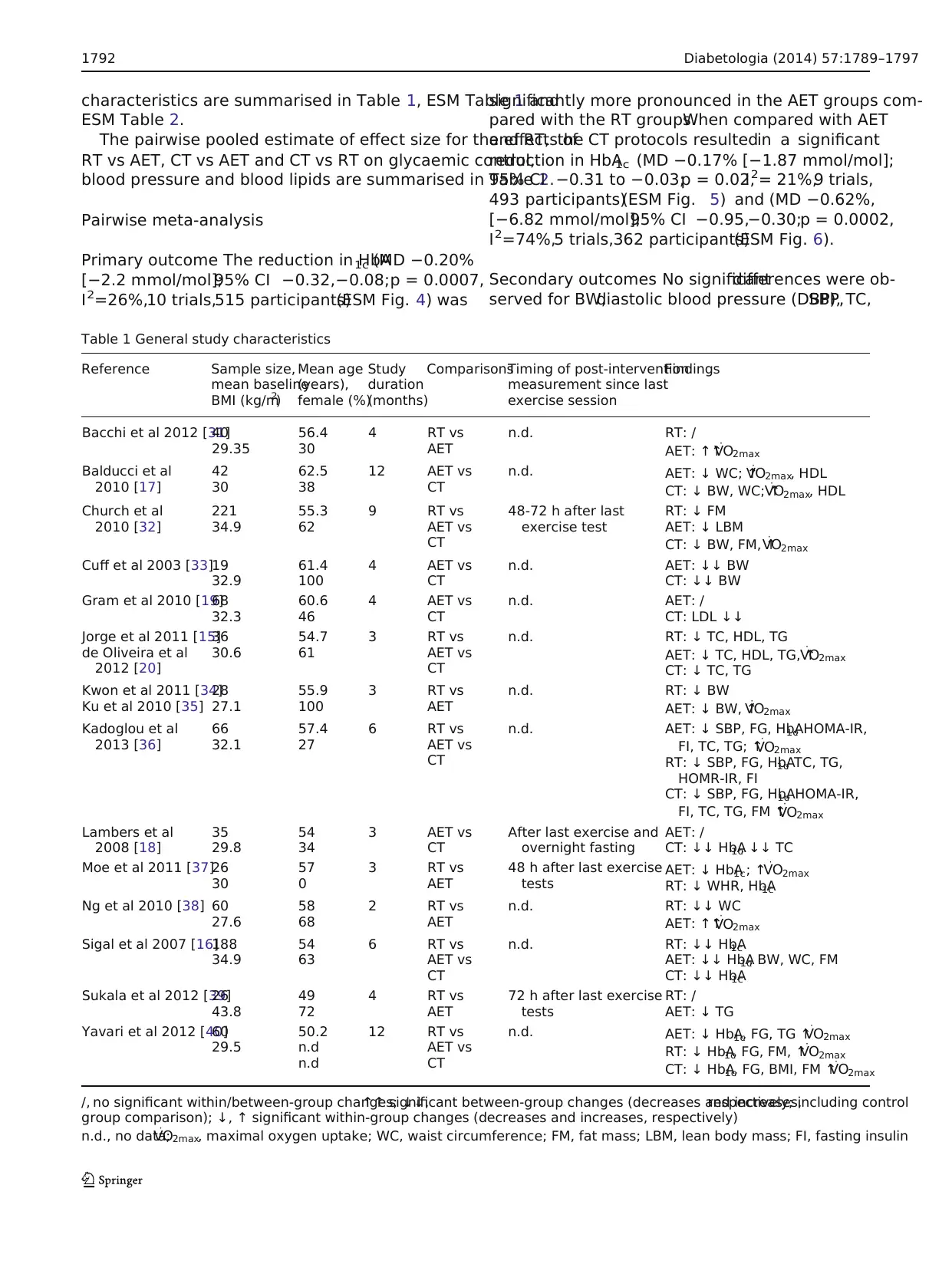
characteristics are summarised in Table 1, ESM Table 1 and
ESM Table 2.
The pairwise pooled estimate of effect size for the effects of
RT vs AET, CT vs AET and CT vs RT on glycaemic control,
blood pressure and blood lipids are summarised in Table 2.
Pairwise meta-analysis
Primary outcome The reduction in HbA1c (MD −0.20%
[−2.2 mmol/mol];95% CI −0.32,−0.08;p = 0.0007,
I 2=26%,10 trials,515 participants)(ESM Fig. 4) was
significantly more pronounced in the AET groups com-
pared with the RT groups.When compared with AET
and RT, the CT protocols resultedin a significant
reduction in HbA1c (MD −0.17% [−1.87 mmol/mol];
95% CI −0.31 to −0.03;p = 0.02,I 2= 21%,9 trials,
493 participants)(ESM Fig. 5) and (MD −0.62%,
[−6.82 mmol/mol];95% CI −0.95,−0.30;p = 0.0002,
I 2=74%,5 trials,362 participants)(ESM Fig. 6).
Secondary outcomes No significantdifferences were ob-
served for BW,diastolic blood pressure (DBP),SBP,TC,
Table 1 General study characteristics
Reference Sample size,
mean baseline
BMI (kg/m2)
Mean age
(years),
female (%)
Study
duration
(months)
ComparisonsTiming of post-intervention
measurement since last
exercise session
Findings
Bacchi et al 2012 [31]40
29.35
56.4
30
4 RT vs
AET
n.d. RT: /
AET: ↑↑V
⋅O2max
Balducci et al
2010 [17]
42
30
62.5
38
12 AET vs
CT
n.d. AET: ↓ WC; ↑V
⋅O2max, HDL
CT: ↓ BW, WC; ↑V
⋅O2max, HDL
Church et al
2010 [32]
221
34.9
55.3
62
9 RT vs
AET vs
CT
48-72 h after last
exercise test
RT: ↓ FM
AET: ↓ LBM
CT: ↓ BW, FM, ↑V
⋅O2max
Cuff et al 2003 [33]19
32.9
61.4
100
4 AET vs
CT
n.d. AET: ↓↓ BW
CT: ↓↓ BW
Gram et al 2010 [19]68
32.3
60.6
46
4 AET vs
CT
n.d. AET: /
CT: LDL ↓↓
Jorge et al 2011 [15]
de Oliveira et al
2012 [20]
36
30.6
54.7
61
3 RT vs
AET vs
CT
n.d. RT: ↓ TC, HDL, TG
AET: ↓ TC, HDL, TG, ↑V
⋅O2max
CT: ↓ TC, TG
Kwon et al 2011 [34]
Ku et al 2010 [35]
28
27.1
55.9
100
3 RT vs
AET
n.d. RT: ↓ BW
AET: ↓ BW, ↑V
⋅O2max
Kadoglou et al
2013 [36]
66
32.1
57.4
27
6 RT vs
AET vs
CT
n.d. AET: ↓ SBP, FG, HbA1c, HOMA-IR,
FI, TC, TG; ↑V
⋅O2max
RT: ↓ SBP, FG, HbA1c, TC, TG,
HOMR-IR, FI
CT: ↓ SBP, FG, HbA1c, HOMA-IR,
FI, TC, TG, FM ↑V
⋅O2max
Lambers et al
2008 [18]
35
29.8
54
34
3 AET vs
CT
After last exercise and
overnight fasting
AET: /
CT: ↓↓ HbA1c, ↓↓ TC
Moe et al 2011 [37]26
30
57
0
3 RT vs
AET
48 h after last exercise
tests
AET: ↓ HbA1c; ↑V
⋅O2max
RT: ↓ WHR, HbA1c
Ng et al 2010 [38] 60
27.6
58
68
2 RT vs
AET
n.d. RT: ↓↓ WC
AET: ↑↑V
⋅O2max
Sigal et al 2007 [16]188
34.9
54
63
6 RT vs
AET vs
CT
n.d. RT: ↓↓ HbA1c
AET: ↓↓ HbA1c, BW, WC, FM
CT: ↓↓ HbA1c
Sukala et al 2012 [39]26
43.8
49
72
4 RT vs
AET
72 h after last exercise
tests
RT: /
AET: ↓ TG
Yavari et al 2012 [40]60
29.5
50.2
n.d
n.d
12 RT vs
AET vs
CT
n.d. AET: ↓ HbA1c, FG, TG ↑V
⋅O2max
RT: ↓ HbA1c, FG, FM, ↑V
⋅O2max
CT: ↓ HbA1c, FG, BMI, FM ↑V
⋅O2max
/, no significant within/between-group changes; ↓↓,↑↑ significant between-group changes (decreases and increases,respectively; including control
group comparison); ↓, ↑ significant within-group changes (decreases and increases, respectively)
n.d., no data;V
⋅O2max, maximal oxygen uptake; WC, waist circumference; FM, fat mass; LBM, lean body mass; FI, fasting insulin
1792 Diabetologia (2014) 57:1789–1797
ESM Table 2.
The pairwise pooled estimate of effect size for the effects of
RT vs AET, CT vs AET and CT vs RT on glycaemic control,
blood pressure and blood lipids are summarised in Table 2.
Pairwise meta-analysis
Primary outcome The reduction in HbA1c (MD −0.20%
[−2.2 mmol/mol];95% CI −0.32,−0.08;p = 0.0007,
I 2=26%,10 trials,515 participants)(ESM Fig. 4) was
significantly more pronounced in the AET groups com-
pared with the RT groups.When compared with AET
and RT, the CT protocols resultedin a significant
reduction in HbA1c (MD −0.17% [−1.87 mmol/mol];
95% CI −0.31 to −0.03;p = 0.02,I 2= 21%,9 trials,
493 participants)(ESM Fig. 5) and (MD −0.62%,
[−6.82 mmol/mol];95% CI −0.95,−0.30;p = 0.0002,
I 2=74%,5 trials,362 participants)(ESM Fig. 6).
Secondary outcomes No significantdifferences were ob-
served for BW,diastolic blood pressure (DBP),SBP,TC,
Table 1 General study characteristics
Reference Sample size,
mean baseline
BMI (kg/m2)
Mean age
(years),
female (%)
Study
duration
(months)
ComparisonsTiming of post-intervention
measurement since last
exercise session
Findings
Bacchi et al 2012 [31]40
29.35
56.4
30
4 RT vs
AET
n.d. RT: /
AET: ↑↑V
⋅O2max
Balducci et al
2010 [17]
42
30
62.5
38
12 AET vs
CT
n.d. AET: ↓ WC; ↑V
⋅O2max, HDL
CT: ↓ BW, WC; ↑V
⋅O2max, HDL
Church et al
2010 [32]
221
34.9
55.3
62
9 RT vs
AET vs
CT
48-72 h after last
exercise test
RT: ↓ FM
AET: ↓ LBM
CT: ↓ BW, FM, ↑V
⋅O2max
Cuff et al 2003 [33]19
32.9
61.4
100
4 AET vs
CT
n.d. AET: ↓↓ BW
CT: ↓↓ BW
Gram et al 2010 [19]68
32.3
60.6
46
4 AET vs
CT
n.d. AET: /
CT: LDL ↓↓
Jorge et al 2011 [15]
de Oliveira et al
2012 [20]
36
30.6
54.7
61
3 RT vs
AET vs
CT
n.d. RT: ↓ TC, HDL, TG
AET: ↓ TC, HDL, TG, ↑V
⋅O2max
CT: ↓ TC, TG
Kwon et al 2011 [34]
Ku et al 2010 [35]
28
27.1
55.9
100
3 RT vs
AET
n.d. RT: ↓ BW
AET: ↓ BW, ↑V
⋅O2max
Kadoglou et al
2013 [36]
66
32.1
57.4
27
6 RT vs
AET vs
CT
n.d. AET: ↓ SBP, FG, HbA1c, HOMA-IR,
FI, TC, TG; ↑V
⋅O2max
RT: ↓ SBP, FG, HbA1c, TC, TG,
HOMR-IR, FI
CT: ↓ SBP, FG, HbA1c, HOMA-IR,
FI, TC, TG, FM ↑V
⋅O2max
Lambers et al
2008 [18]
35
29.8
54
34
3 AET vs
CT
After last exercise and
overnight fasting
AET: /
CT: ↓↓ HbA1c, ↓↓ TC
Moe et al 2011 [37]26
30
57
0
3 RT vs
AET
48 h after last exercise
tests
AET: ↓ HbA1c; ↑V
⋅O2max
RT: ↓ WHR, HbA1c
Ng et al 2010 [38] 60
27.6
58
68
2 RT vs
AET
n.d. RT: ↓↓ WC
AET: ↑↑V
⋅O2max
Sigal et al 2007 [16]188
34.9
54
63
6 RT vs
AET vs
CT
n.d. RT: ↓↓ HbA1c
AET: ↓↓ HbA1c, BW, WC, FM
CT: ↓↓ HbA1c
Sukala et al 2012 [39]26
43.8
49
72
4 RT vs
AET
72 h after last exercise
tests
RT: /
AET: ↓ TG
Yavari et al 2012 [40]60
29.5
50.2
n.d
n.d
12 RT vs
AET vs
CT
n.d. AET: ↓ HbA1c, FG, TG ↑V
⋅O2max
RT: ↓ HbA1c, FG, FM, ↑V
⋅O2max
CT: ↓ HbA1c, FG, BMI, FM ↑V
⋅O2max
/, no significant within/between-group changes; ↓↓,↑↑ significant between-group changes (decreases and increases,respectively; including control
group comparison); ↓, ↑ significant within-group changes (decreases and increases, respectively)
n.d., no data;V
⋅O2max, maximal oxygen uptake; WC, waist circumference; FM, fat mass; LBM, lean body mass; FI, fasting insulin
1792 Diabetologia (2014) 57:1789–1797
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
LDL, HDL and TG between AET and RT.However,AET
resulted in a significant reduction in FG (MD −0.90 mmol/l;
95% CI −1.71, −0.09; p=0.03, I2=72%, 8 trials, 245 partici-
pants) compared with RT (ESM Fig. 7). Compared with RT,
CT resulted in a more pronounced decrease in FG (MD
−1.99 mmol/l; 95% CI −3.07, −0.90; p=0.0003, I2=61%, 3
trials, 99 participants) (ESM Fig. 8), TG (MD −0.28 mmol/l;
95% CI −0.46, −0.10; p=0.003, I2=0%, 4 trials, 213 partici-
pants) (ESM Fig.9) and SBP (MD −4.42 mmHg;95% CI
−8.62,−0.21;p=0.04,I 2=41%,4 trials,206 participants)
(ESM Fig. 10).
Network meta-analysis
ESM Fig. 3 shows the network of the included trials.The
pooled estimates of effectsize for the comparison of AET
vs RT vs CT using both directand indirectevidence on
glycaemiccontroland cardiovascularrisk outcomesare
summarised in ESM Table3 (a fixed effectnetwork
meta-analysisfor BW and TG, since I2≤50%).For each
outcome,a common between-study heterogeneity variable
was assumed to reflectthe variability between studies of
all the interventions(ESM Table 3). The ranking
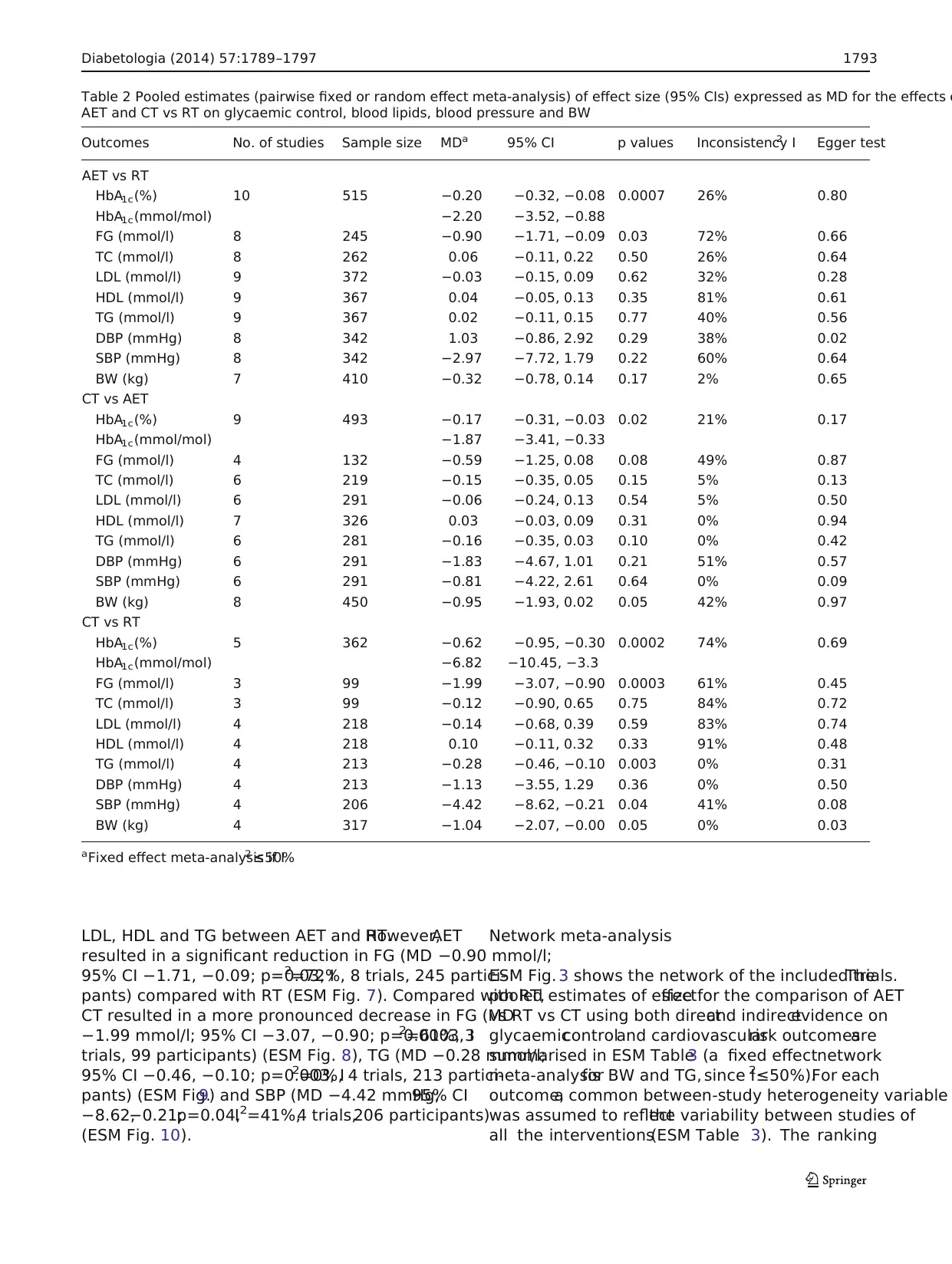
Table 2 Pooled estimates (pairwise fixed or random effect meta-analysis) of effect size (95% CIs) expressed as MD for the effects o
AET and CT vs RT on glycaemic control, blood lipids, blood pressure and BW
Outcomes No. of studies Sample size MDa 95% CI p values Inconsistency I2 Egger test
AET vs RT
HbA1c(%) 10 515 −0.20 −0.32, −0.08 0.0007 26% 0.80
HbA1c(mmol/mol) −2.20 −3.52, −0.88
FG (mmol/l) 8 245 −0.90 −1.71, −0.09 0.03 72% 0.66
TC (mmol/l) 8 262 0.06 −0.11, 0.22 0.50 26% 0.64
LDL (mmol/l) 9 372 −0.03 −0.15, 0.09 0.62 32% 0.28
HDL (mmol/l) 9 367 0.04 −0.05, 0.13 0.35 81% 0.61
TG (mmol/l) 9 367 0.02 −0.11, 0.15 0.77 40% 0.56
DBP (mmHg) 8 342 1.03 −0.86, 2.92 0.29 38% 0.02
SBP (mmHg) 8 342 −2.97 −7.72, 1.79 0.22 60% 0.64
BW (kg) 7 410 −0.32 −0.78, 0.14 0.17 2% 0.65
CT vs AET
HbA1c(%) 9 493 −0.17 −0.31, −0.03 0.02 21% 0.17
HbA1c(mmol/mol) −1.87 −3.41, −0.33
FG (mmol/l) 4 132 −0.59 −1.25, 0.08 0.08 49% 0.87
TC (mmol/l) 6 219 −0.15 −0.35, 0.05 0.15 5% 0.13
LDL (mmol/l) 6 291 −0.06 −0.24, 0.13 0.54 5% 0.50
HDL (mmol/l) 7 326 0.03 −0.03, 0.09 0.31 0% 0.94
TG (mmol/l) 6 281 −0.16 −0.35, 0.03 0.10 0% 0.42
DBP (mmHg) 6 291 −1.83 −4.67, 1.01 0.21 51% 0.57
SBP (mmHg) 6 291 −0.81 −4.22, 2.61 0.64 0% 0.09
BW (kg) 8 450 −0.95 −1.93, 0.02 0.05 42% 0.97
CT vs RT
HbA1c(%) 5 362 −0.62 −0.95, −0.30 0.0002 74% 0.69
HbA1c(mmol/mol) −6.82 −10.45, −3.3
FG (mmol/l) 3 99 −1.99 −3.07, −0.90 0.0003 61% 0.45
TC (mmol/l) 3 99 −0.12 −0.90, 0.65 0.75 84% 0.72
LDL (mmol/l) 4 218 −0.14 −0.68, 0.39 0.59 83% 0.74
HDL (mmol/l) 4 218 0.10 −0.11, 0.32 0.33 91% 0.48
TG (mmol/l) 4 213 −0.28 −0.46, −0.10 0.003 0% 0.31
DBP (mmHg) 4 213 −1.13 −3.55, 1.29 0.36 0% 0.50
SBP (mmHg) 4 206 −4.42 −8.62, −0.21 0.04 41% 0.08
BW (kg) 4 317 −1.04 −2.07, −0.00 0.05 0% 0.03
aFixed effect meta-analysis if I2 ≤50%
Diabetologia (2014) 57:1789–1797 1793
resulted in a significant reduction in FG (MD −0.90 mmol/l;
95% CI −1.71, −0.09; p=0.03, I2=72%, 8 trials, 245 partici-
pants) compared with RT (ESM Fig. 7). Compared with RT,
CT resulted in a more pronounced decrease in FG (MD
−1.99 mmol/l; 95% CI −3.07, −0.90; p=0.0003, I2=61%, 3
trials, 99 participants) (ESM Fig. 8), TG (MD −0.28 mmol/l;
95% CI −0.46, −0.10; p=0.003, I2=0%, 4 trials, 213 partici-
pants) (ESM Fig.9) and SBP (MD −4.42 mmHg;95% CI
−8.62,−0.21;p=0.04,I 2=41%,4 trials,206 participants)
(ESM Fig. 10).
Network meta-analysis
ESM Fig. 3 shows the network of the included trials.The
pooled estimates of effectsize for the comparison of AET
vs RT vs CT using both directand indirectevidence on
glycaemiccontroland cardiovascularrisk outcomesare
summarised in ESM Table3 (a fixed effectnetwork
meta-analysisfor BW and TG, since I2≤50%).For each
outcome,a common between-study heterogeneity variable
was assumed to reflectthe variability between studies of
all the interventions(ESM Table 3). The ranking
Table 2 Pooled estimates (pairwise fixed or random effect meta-analysis) of effect size (95% CIs) expressed as MD for the effects o
AET and CT vs RT on glycaemic control, blood lipids, blood pressure and BW
Outcomes No. of studies Sample size MDa 95% CI p values Inconsistency I2 Egger test
AET vs RT
HbA1c(%) 10 515 −0.20 −0.32, −0.08 0.0007 26% 0.80
HbA1c(mmol/mol) −2.20 −3.52, −0.88
FG (mmol/l) 8 245 −0.90 −1.71, −0.09 0.03 72% 0.66
TC (mmol/l) 8 262 0.06 −0.11, 0.22 0.50 26% 0.64
LDL (mmol/l) 9 372 −0.03 −0.15, 0.09 0.62 32% 0.28
HDL (mmol/l) 9 367 0.04 −0.05, 0.13 0.35 81% 0.61
TG (mmol/l) 9 367 0.02 −0.11, 0.15 0.77 40% 0.56
DBP (mmHg) 8 342 1.03 −0.86, 2.92 0.29 38% 0.02
SBP (mmHg) 8 342 −2.97 −7.72, 1.79 0.22 60% 0.64
BW (kg) 7 410 −0.32 −0.78, 0.14 0.17 2% 0.65
CT vs AET
HbA1c(%) 9 493 −0.17 −0.31, −0.03 0.02 21% 0.17
HbA1c(mmol/mol) −1.87 −3.41, −0.33
FG (mmol/l) 4 132 −0.59 −1.25, 0.08 0.08 49% 0.87
TC (mmol/l) 6 219 −0.15 −0.35, 0.05 0.15 5% 0.13
LDL (mmol/l) 6 291 −0.06 −0.24, 0.13 0.54 5% 0.50
HDL (mmol/l) 7 326 0.03 −0.03, 0.09 0.31 0% 0.94
TG (mmol/l) 6 281 −0.16 −0.35, 0.03 0.10 0% 0.42
DBP (mmHg) 6 291 −1.83 −4.67, 1.01 0.21 51% 0.57
SBP (mmHg) 6 291 −0.81 −4.22, 2.61 0.64 0% 0.09
BW (kg) 8 450 −0.95 −1.93, 0.02 0.05 42% 0.97
CT vs RT
HbA1c(%) 5 362 −0.62 −0.95, −0.30 0.0002 74% 0.69
HbA1c(mmol/mol) −6.82 −10.45, −3.3
FG (mmol/l) 3 99 −1.99 −3.07, −0.90 0.0003 61% 0.45
TC (mmol/l) 3 99 −0.12 −0.90, 0.65 0.75 84% 0.72
LDL (mmol/l) 4 218 −0.14 −0.68, 0.39 0.59 83% 0.74
HDL (mmol/l) 4 218 0.10 −0.11, 0.32 0.33 91% 0.48
TG (mmol/l) 4 213 −0.28 −0.46, −0.10 0.003 0% 0.31
DBP (mmHg) 4 213 −1.13 −3.55, 1.29 0.36 0% 0.50
SBP (mmHg) 4 206 −4.42 −8.62, −0.21 0.04 41% 0.08
BW (kg) 4 317 −1.04 −2.07, −0.00 0.05 0% 0.03
aFixed effect meta-analysis if I2 ≤50%
Diabetologia (2014) 57:1789–1797 1793
probabilitiesof AET, RT and CT for each outcome are
presented in ESM Table 4.
Both AET and CT were significantly more effective in
reducing HbA1cwhen compared with RT. As shown in ESM
Table 4,CT turned outto be the mosteffective exercise
intervention with respect to reducing HbA1c, FG, TC, LDL,
TG, DBP, SBP and BW, and increasing HDL. CT resulted in a
high (>75%) probability of being bestfor mostoutcomes.
There is greater uncertainty regarding which treatment is the
best for LDL- and TC, although again CT yielded the highest
probability of being best.
No evidence of inconsistency was found with Bayesian
p values for the difference between directand indirectevi-
dence all greater than 0.90.
Risk of bias
The dropout rates ranged from 0% to 31%, with five studies
reporting dropoutrates <10% (ESM Table 1).Seven trials
reported random sequence generation [16,31,32,36–39],
and only five trials reported allocation concealment [16, 31,
32,37,38].None ofthe studies reported the blinding of
volunteers towards the mode of intervention (ESM Fig.1).
Eight trials performed intention-to-treat analysis [15–17, 32,
35, 37–39], and six trials appear to have had adequate blinding
of the outcome assessment [16, 17, 31, 32, 37, 38]. High risk
of bias was defined as fewer than four out of a maximum yield
of six low risk of bias items using the risk of bias assessment
toolfrom the Cochrane Collaboration (ESM Fig.1).Seven
high risk of bias trials (nine reports) were identified [15, 19,
20, 33–36, 39, 40], and sensitivity analyses were performe
for studies with a high vs low risk of bias.
Subgroup analysis/sensitivity analysis
Subgroup analyseswere performed comparing short-
term (<6 months)vs long-term (≥6months)trials
(ESM Figs 11–13),obese (BMI ≥30 kg/m2) vs non-obese
(BMI <30 kg/m2) participants (ESM Figs 14–16) and sample
size (≥50 vs <50) (ESM Fig.17–19).Overall,pooling the
long-term trials resulted in significantly greater reductions
HbA1c compared with short-term trials for allcomparison
groups.Furthermore,including only obese patients resulted
in significant reductions in HbA1c. A smaller vs bigger sample
size showed non-significant differences for HbA1cwhen com-
paring AET vs RT.In contrast,comparisons for CT yielded
significantly higher reductions in trials with a bigger samp
size when compared with either AET or RT. Subgroup anal
sis comparing different measurement time points for HbA1c
provided no additionalinformation (ESM Figs 20–22).
Sensitivity analyses excluding trials with a high risk of bias
changed the summary estimates and became statistically
significant (Table 3).
Publication bias
Begg’s and Egger’s regression tests provided no evidence
substantial publication bias. Funnel plots were generated
if specific outcome measures were provided by atleastten
different trials. The plot with respect to change in effect si
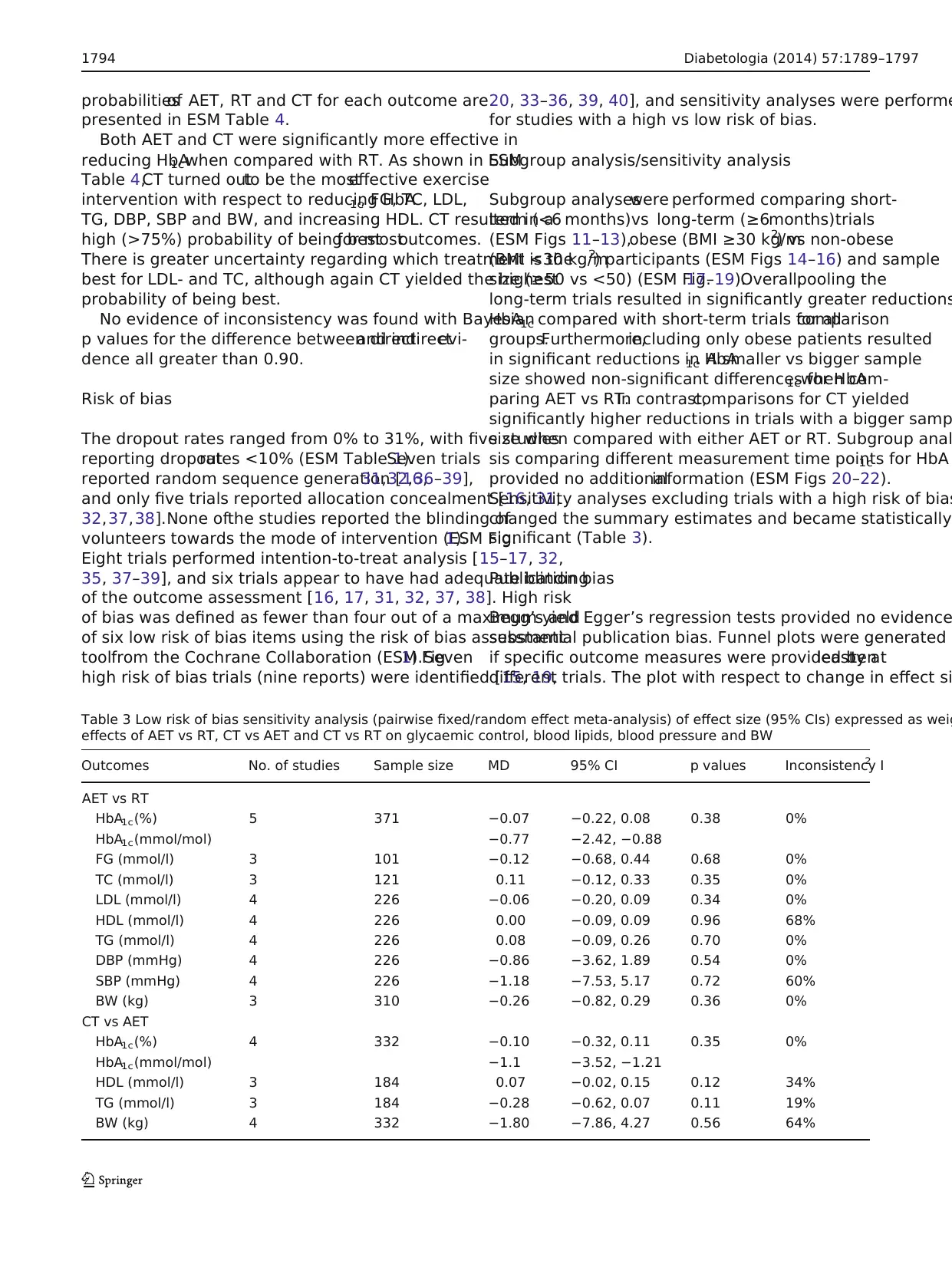
Table 3 Low risk of bias sensitivity analysis (pairwise fixed/random effect meta-analysis) of effect size (95% CIs) expressed as weig
effects of AET vs RT, CT vs AET and CT vs RT on glycaemic control, blood lipids, blood pressure and BW
Outcomes No. of studies Sample size MD 95% CI p values Inconsistency I2
AET vs RT
HbA1c(%) 5 371 −0.07 −0.22, 0.08 0.38 0%
HbA1c(mmol/mol) −0.77 −2.42, −0.88
FG (mmol/l) 3 101 −0.12 −0.68, 0.44 0.68 0%
TC (mmol/l) 3 121 0.11 −0.12, 0.33 0.35 0%
LDL (mmol/l) 4 226 −0.06 −0.20, 0.09 0.34 0%
HDL (mmol/l) 4 226 0.00 −0.09, 0.09 0.96 68%
TG (mmol/l) 4 226 0.08 −0.09, 0.26 0.70 0%
DBP (mmHg) 4 226 −0.86 −3.62, 1.89 0.54 0%
SBP (mmHg) 4 226 −1.18 −7.53, 5.17 0.72 60%
BW (kg) 3 310 −0.26 −0.82, 0.29 0.36 0%
CT vs AET
HbA1c(%) 4 332 −0.10 −0.32, 0.11 0.35 0%
HbA1c(mmol/mol) −1.1 −3.52, −1.21
HDL (mmol/l) 3 184 0.07 −0.02, 0.15 0.12 34%
TG (mmol/l) 3 184 −0.28 −0.62, 0.07 0.11 19%
BW (kg) 4 332 −1.80 −7.86, 4.27 0.56 64%
1794 Diabetologia (2014) 57:1789–1797
presented in ESM Table 4.
Both AET and CT were significantly more effective in
reducing HbA1cwhen compared with RT. As shown in ESM
Table 4,CT turned outto be the mosteffective exercise
intervention with respect to reducing HbA1c, FG, TC, LDL,
TG, DBP, SBP and BW, and increasing HDL. CT resulted in a
high (>75%) probability of being bestfor mostoutcomes.
There is greater uncertainty regarding which treatment is the
best for LDL- and TC, although again CT yielded the highest
probability of being best.
No evidence of inconsistency was found with Bayesian
p values for the difference between directand indirectevi-
dence all greater than 0.90.
Risk of bias
The dropout rates ranged from 0% to 31%, with five studies
reporting dropoutrates <10% (ESM Table 1).Seven trials
reported random sequence generation [16,31,32,36–39],
and only five trials reported allocation concealment [16, 31,
32,37,38].None ofthe studies reported the blinding of
volunteers towards the mode of intervention (ESM Fig.1).
Eight trials performed intention-to-treat analysis [15–17, 32,
35, 37–39], and six trials appear to have had adequate blinding
of the outcome assessment [16, 17, 31, 32, 37, 38]. High risk
of bias was defined as fewer than four out of a maximum yield
of six low risk of bias items using the risk of bias assessment
toolfrom the Cochrane Collaboration (ESM Fig.1).Seven
high risk of bias trials (nine reports) were identified [15, 19,
20, 33–36, 39, 40], and sensitivity analyses were performe
for studies with a high vs low risk of bias.
Subgroup analysis/sensitivity analysis
Subgroup analyseswere performed comparing short-
term (<6 months)vs long-term (≥6months)trials
(ESM Figs 11–13),obese (BMI ≥30 kg/m2) vs non-obese
(BMI <30 kg/m2) participants (ESM Figs 14–16) and sample
size (≥50 vs <50) (ESM Fig.17–19).Overall,pooling the
long-term trials resulted in significantly greater reductions
HbA1c compared with short-term trials for allcomparison
groups.Furthermore,including only obese patients resulted
in significant reductions in HbA1c. A smaller vs bigger sample
size showed non-significant differences for HbA1cwhen com-
paring AET vs RT.In contrast,comparisons for CT yielded
significantly higher reductions in trials with a bigger samp
size when compared with either AET or RT. Subgroup anal
sis comparing different measurement time points for HbA1c
provided no additionalinformation (ESM Figs 20–22).
Sensitivity analyses excluding trials with a high risk of bias
changed the summary estimates and became statistically
significant (Table 3).
Publication bias
Begg’s and Egger’s regression tests provided no evidence
substantial publication bias. Funnel plots were generated
if specific outcome measures were provided by atleastten
different trials. The plot with respect to change in effect si
Table 3 Low risk of bias sensitivity analysis (pairwise fixed/random effect meta-analysis) of effect size (95% CIs) expressed as weig
effects of AET vs RT, CT vs AET and CT vs RT on glycaemic control, blood lipids, blood pressure and BW
Outcomes No. of studies Sample size MD 95% CI p values Inconsistency I2
AET vs RT
HbA1c(%) 5 371 −0.07 −0.22, 0.08 0.38 0%
HbA1c(mmol/mol) −0.77 −2.42, −0.88
FG (mmol/l) 3 101 −0.12 −0.68, 0.44 0.68 0%
TC (mmol/l) 3 121 0.11 −0.12, 0.33 0.35 0%
LDL (mmol/l) 4 226 −0.06 −0.20, 0.09 0.34 0%
HDL (mmol/l) 4 226 0.00 −0.09, 0.09 0.96 68%
TG (mmol/l) 4 226 0.08 −0.09, 0.26 0.70 0%
DBP (mmHg) 4 226 −0.86 −3.62, 1.89 0.54 0%
SBP (mmHg) 4 226 −1.18 −7.53, 5.17 0.72 60%
BW (kg) 3 310 −0.26 −0.82, 0.29 0.36 0%
CT vs AET
HbA1c(%) 4 332 −0.10 −0.32, 0.11 0.35 0%
HbA1c(mmol/mol) −1.1 −3.52, −1.21
HDL (mmol/l) 3 184 0.07 −0.02, 0.15 0.12 34%
TG (mmol/l) 3 184 −0.28 −0.62, 0.07 0.11 19%
BW (kg) 4 332 −1.80 −7.86, 4.27 0.56 64%
1794 Diabetologia (2014) 57:1789–1797
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

for HbA1c in responseto AET vs RT indicateslittle
asymmetry.Thus,publication bias cannotbe completely
excluded as a factoraffecting the results ofthe present
meta-analysis (ESM Fig.23).
Discussion
According to our literature search,this is the firstnetwork
meta-analysis comparing the pooled effects of AET, RT and
CT on glycaemic control, blood pressure and blood lipids in
patients with type 2 diabetes. The results of the present meta-
analyses showed that,in patients with established diabetes,
AET might be more effective in reducing HbA1cand FG when
compared with RT. CT was more powerful in reducing HbA1c
compared with AET, and more effective in reducing HbA1c,
FG and TG when compared with RT. However, these results
could notbe confirmed when only low risk of bias studies
were included.Pooling both direct and indirect evidence on
AET, RT and CT via network meta-analysis demonstrated that
CT was the most efficacious exercise intervention regarding
its impact on HbA1c, FG, HDL, TG, DBP and BW (with the
respective probabilities ofbeing ranked bestfollowing
Bayesian network meta-analysis of 94%,94%,78%,99%,
84% and 97%).
HbA1cis not unanimously regarded to be a valid predictor
of cardiovascular disease, thereby limiting the relevance of the
present findings with respect to their clinical implications. The
interpretation of the present data is further restricted by the fact
that none of the studies evaluated the impact of their interven-
tions on clinical outcomes. Data from epidemiological studies
suggestthatgreaterphysicalactivity is associated with a
reduced risk of all-cause mortality, mortality from cardiovas-
cular disease and risk of type 2 diabetes [44–47].
A recent meta-analysis comparing RT with AET concluded
that there is no evidence that RT differs from AET in its impact
on cardiovascular risk factors and safety [9].However,for
some risk factors,the ranking probabilities of the Bayesian
network meta-analysis suggest that AET was the second best
exercise modality. However, these probabilities should not be
overinterpreted, particularly since they are not very large (not
close to 80–90%). A dose–response meta-regression analysis
by Umpierre et al [48] summarised the effects of CT, AET and
RT on glycaemic control in patients with type 2 diabetes and
concluded thatthe reduction in HbA1c was associated with
exercise frequency in supervised AET,and with the weekly
volume of RT in supervised CT. Regarding the optimal dose,
the authors speculated that there should be a minimal amount
of AET (33 min per session) to elicitthe effects of high-
volume RT in CT [11].
The results ofthis meta-analysis are in line with data
published by Chudyk et al [6] comparing CT,AET and RT
with controlgroups.The authors concluded thatRT is not
significantly related to changes in HbA1clevels or to changes
in SBP in patients with type 2 diabetes if it is not combined
with other forms of exercise. Moreover, splitting AET and R
sessions between different days might have additional ben
for glycaemic control [49]. This indicates that more elabor
training programmes might be of relevance.
According to a mechanistic model linking the combinati
of AET and RT with the improvement in glycaemic control
type 2 diabetes,RT enhances insulin sensitivity [50] via an
increase in glucose transporter(GLUT)-4 contentand an
amplification of insulin signalling in muscle [51]. Similarly,
AET increased GLUT-4 expression in the adipose tissue an
skeletal muscle of patients with type 2 diabetes; however,
benefit of this adaption appears to be dependent on optim
beta cell function [52].
One strength of this systematic review is the application
a network meta-analysis.Directand indirectevidence was
used, taking into account the fact that AET, RT and CT wer
compared simultaneously in some studies. However, the m
tiple use of data from three-arm trials will lead to an overe
timation of the corresponding data and should be avoided
relevance of the presentdata is further emphasised by the
smallestimated between-studies heterogeneity variables a
well as by the fact that there wasno evidence of
inconsistencies.
On the otherhand,this systematic review has several
limitations that should be taken into account when interpr
its findings. There is evidence that supervised exercise is m
effective than unsupervised training [11],butin practice it
seems unlikely that most patients would have access to su
vised exercise regimens of this intensity.It is possible that
either AET,RT or CT may be easier to perform effectively
withoutsupervision,thus affecting the externalvalidity of
these results since only studies with supervised training w
included.
Although the network meta-analysis included all individ-
uals for each outcome, the sample size of volunteers migh
considered low when compared with drug trials. Several p
tentialrisk of bias characteristics were identified in the 14
included trials (7 trials described random sequence genera
5 trials performed allocation concealment, 8 trials perform
intention-to-treat analysis, and 6 trials had adequate blind
of outcome assessment).Taken together,more than 50% of
the included trials were judged as being at high risk of bia
Therefore, the results of the present meta-analyses should
interpreted in a conservative manner.
There were some heterogeneities in study design espec
with respect to the population characteristics (e.g. duratio
type 2 diabetes, study length, BMI, age and ratio of male t
female participants).Subgroup analyses showed thatlong-
term trials as well as trials including obese participants wi
type 2 diabetes resulted in more pronounced beneficial eff
Diabetologia (2014) 57:1789–1797 1795
asymmetry.Thus,publication bias cannotbe completely
excluded as a factoraffecting the results ofthe present
meta-analysis (ESM Fig.23).
Discussion
According to our literature search,this is the firstnetwork
meta-analysis comparing the pooled effects of AET, RT and
CT on glycaemic control, blood pressure and blood lipids in
patients with type 2 diabetes. The results of the present meta-
analyses showed that,in patients with established diabetes,
AET might be more effective in reducing HbA1cand FG when
compared with RT. CT was more powerful in reducing HbA1c
compared with AET, and more effective in reducing HbA1c,
FG and TG when compared with RT. However, these results
could notbe confirmed when only low risk of bias studies
were included.Pooling both direct and indirect evidence on
AET, RT and CT via network meta-analysis demonstrated that
CT was the most efficacious exercise intervention regarding
its impact on HbA1c, FG, HDL, TG, DBP and BW (with the
respective probabilities ofbeing ranked bestfollowing
Bayesian network meta-analysis of 94%,94%,78%,99%,
84% and 97%).
HbA1cis not unanimously regarded to be a valid predictor
of cardiovascular disease, thereby limiting the relevance of the
present findings with respect to their clinical implications. The
interpretation of the present data is further restricted by the fact
that none of the studies evaluated the impact of their interven-
tions on clinical outcomes. Data from epidemiological studies
suggestthatgreaterphysicalactivity is associated with a
reduced risk of all-cause mortality, mortality from cardiovas-
cular disease and risk of type 2 diabetes [44–47].
A recent meta-analysis comparing RT with AET concluded
that there is no evidence that RT differs from AET in its impact
on cardiovascular risk factors and safety [9].However,for
some risk factors,the ranking probabilities of the Bayesian
network meta-analysis suggest that AET was the second best
exercise modality. However, these probabilities should not be
overinterpreted, particularly since they are not very large (not
close to 80–90%). A dose–response meta-regression analysis
by Umpierre et al [48] summarised the effects of CT, AET and
RT on glycaemic control in patients with type 2 diabetes and
concluded thatthe reduction in HbA1c was associated with
exercise frequency in supervised AET,and with the weekly
volume of RT in supervised CT. Regarding the optimal dose,
the authors speculated that there should be a minimal amount
of AET (33 min per session) to elicitthe effects of high-
volume RT in CT [11].
The results ofthis meta-analysis are in line with data
published by Chudyk et al [6] comparing CT,AET and RT
with controlgroups.The authors concluded thatRT is not
significantly related to changes in HbA1clevels or to changes
in SBP in patients with type 2 diabetes if it is not combined
with other forms of exercise. Moreover, splitting AET and R
sessions between different days might have additional ben
for glycaemic control [49]. This indicates that more elabor
training programmes might be of relevance.
According to a mechanistic model linking the combinati
of AET and RT with the improvement in glycaemic control
type 2 diabetes,RT enhances insulin sensitivity [50] via an
increase in glucose transporter(GLUT)-4 contentand an
amplification of insulin signalling in muscle [51]. Similarly,
AET increased GLUT-4 expression in the adipose tissue an
skeletal muscle of patients with type 2 diabetes; however,
benefit of this adaption appears to be dependent on optim
beta cell function [52].
One strength of this systematic review is the application
a network meta-analysis.Directand indirectevidence was
used, taking into account the fact that AET, RT and CT wer
compared simultaneously in some studies. However, the m
tiple use of data from three-arm trials will lead to an overe
timation of the corresponding data and should be avoided
relevance of the presentdata is further emphasised by the
smallestimated between-studies heterogeneity variables a
well as by the fact that there wasno evidence of
inconsistencies.
On the otherhand,this systematic review has several
limitations that should be taken into account when interpr
its findings. There is evidence that supervised exercise is m
effective than unsupervised training [11],butin practice it
seems unlikely that most patients would have access to su
vised exercise regimens of this intensity.It is possible that
either AET,RT or CT may be easier to perform effectively
withoutsupervision,thus affecting the externalvalidity of
these results since only studies with supervised training w
included.
Although the network meta-analysis included all individ-
uals for each outcome, the sample size of volunteers migh
considered low when compared with drug trials. Several p
tentialrisk of bias characteristics were identified in the 14
included trials (7 trials described random sequence genera
5 trials performed allocation concealment, 8 trials perform
intention-to-treat analysis, and 6 trials had adequate blind
of outcome assessment).Taken together,more than 50% of
the included trials were judged as being at high risk of bia
Therefore, the results of the present meta-analyses should
interpreted in a conservative manner.
There were some heterogeneities in study design espec
with respect to the population characteristics (e.g. duratio
type 2 diabetes, study length, BMI, age and ratio of male t
female participants).Subgroup analyses showed thatlong-
term trials as well as trials including obese participants wi
type 2 diabetes resulted in more pronounced beneficial eff
Diabetologia (2014) 57:1789–1797 1795
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

on HbA1c, which mightbe due to carrying forward HbA1c
values under conditions of high dropout rates.However,no
significant differences could be observed following a compar-
ison of the dropout rates between short- and long-term studies
in the presentnetwork meta-analysis.Anotherconfounder
mightbe the variations in the volume of exercise (min per
week) prescribed. One study reported exercise duration (min
per session) in the CT group to be twice as high as with their
respective RT and/orAET counterparts [16].However,a
sensitivity analysis excluding this trialwas able to confirm
the results of the primary analysis.
With respect to the potential side effects (ESM Table 1),
eighttrials in the presentmeta-analysis reported adverse
events such as hypoglycaemia,back pain,shoulderpain,
musculoskeletal injury,tendonitis and other musculoskeletal
discomforts following exercise,with no significantdiffer-
ences between the intervention groups.However,it remains
possible that the number of adverse events will increase with
the duration and intensity of exercise.
This systematic review and meta-analysis focused on
randomised controlled trials comparing AET,RT and CT.
Compared with AET orRT, CT interventions resulted in
significantly more pronounced improvements in variables
related to glycaemic control. With respect to single types of
exercise intervention,AET was more effective in reducing
HbA1cand FG when compared with RT. However, the inter-
pretation of these findings with respect to their clinical rele-
vance is limited by the overall low to moderate quality of the
studies included, the lack of information on clinically impor-
tantoutcomes,and the limited information on the adverse
effects of exercise.
Funding This research received no specific grantfrom any funding
agency in the public, commercial or not-for-profit sectors.
Duality ofinterest The authors declare thatthere is no duality of
interest associated with this manuscript.
Contribution statementLS and GH conceived this study, LS, BM and
SD analysed the data,and LS,BM, JK and GH contributed to the
collection of data.All authors critically reviewed various drafts of the
manuscript, and all authors approved the final version. GH is responsible
for the integrity of the work as a whole.
References
1. Tuomilehto J, Lindstrom J, Eriksson JG et al (2001) Prevention of
type 2 diabetes mellitus by changes in lifestyle among subjects with
impaired glucose tolerance. N Engl J Med 344:1343–1350
2. Ajala O, English P, Pinkney J (2013) Systematic review and meta-
analysis of different dietary approaches to the management of type 2
diabetes. Am J Clin Nutr 97:505–516
3. Schwingshackl L, Hoffmann G (2014) Comparison of the long-term
effects of high-fatv. low-fatdietconsumption on cardiometabolic
risk factors in subjects with abnormal glucose metabolism: a syste
atic review and meta-analysis. Br J Nutr 111:2047–2058
4. Colberg SR,Sigal RJ, FernhallB et al (2010)Exercise and
type 2 diabetes:the American College of Sports Medicine and
the American DiabetesAssociation:joint position statement.
Diabetes Care 33:e147–e167
5. Snowling NJ,Hopkins WG (2006) Effects of differentmodes of
exercise training on glucose control and risk factors for complicati
in type 2 diabetic patients: a meta-analysis. Diabetes Care 29:251
2527
6. Chudyk A, Petrella RJ (2011) Effects of exercise on cardiovascular
risk factors in type 2 diabetes:a meta-analysis.Diabetes Care 34:
1228–1237
7. Strasser B, Siebert U, Schobersberger W (2010) Resistance trainin
in the treatment of the metabolic syndrome: a systematic review
meta-analysis of the effect of resistance training on metabolic clus
tering in patients with abnormal glucose metabolism. Sports Med
397–415
8. Kelley GA,Kelley KS (2000) Progressive resistance exercise and
resting blood pressure:a meta-analysis of randomized controlled
trials. Hypertension 35:838–843
9. Yang Z,ScottCA, Mao C,Tang J,Farmer AJ (2013) Resistance
exercise versus aerobic exercise for type 2 diabetes:a systematic
review and meta-analysis. Sports Med 44:487–499
10.Schwingshackl L, Dias S, Strasser B, Hoffmann G (2013) Impact of
different training modalities on anthropometric and metabolic cha
acteristics in overweight/obese subjects:a systematic review and
network meta-analysis. PLoS One 8:e82853
11.Umpierre D, Ribeiro PA, Kramer CK et al (2011) Physical activity
advice only orstructured exercise training and association with
HbA1c levels in type 2 diabetes:a systematic review and meta-
analysis. JAMA 305:1790–1799
12.MoherD, LiberatiA, TetzlaffJ, Altman DG (2009)Preferred
reporting items forsystematic reviews and meta-analyses:the
PRISMA statement. PLoS Med 6:e1000097
13.Higgins JP, Green S (eds) (2011) Cochrane handbook for systemat
reviews on interventions 5.1.0 [updated March 2011] The Cochran
Collaboration. Available from www.cochrane-handbook.org
14.Higgins JP,Altman DG,Gotzsche PC etal (2011) The Cochrane
Collaboration’s tool for assessing risk of bias in randomised trials.
BMJ 343:d5928
15.Jorge ML, de Oliveira VN, Resende NM et al (2011) The effects of
aerobic,resistance,and combined exercise on metabolic control,
inflammatory markers, adipocytokines, and muscle insulin signalin
in patients with type 2 diabetes mellitus. Metabolism 60:1244–125
16.SigalRJ, Kenny GP,Boule NG etal (2007)Effects ofaerobic
training,resistance training,or both on glycemic controlin type 2
diabetes – a randomized trial. Ann Intern Med 147:357–369
17.Balducci S, Zanuso S, Nicolucci A et al (2010) Anti-inflammatory
effectof exercise training in subjects with type 2 diabetes and the
metabolic syndrome isdependenton exercise modalitiesand
independentof weightloss. Nutr Metab Cardiovasc Dis20:
608–617
18.Lambers S, van Laethem C, van Acker K, Calders P (2008) Influenc
of combined exercise training on indices of obesity,diabetes and
cardiovascular risk in type 2 diabetes patients. Clin Rehabil 22:483
492
19.Gram B,Christensen R,Christiansen C,Gram J (2010) Effects of
nordic walking and exercise in type 2 diabetes mellitus: a random
controlled trial. Clin J Sport Med 20:355–361
20.de Oliveira VN,Bessa A,Jorge ML etal (2012) The effectof
differenttraining programs on antioxidantstatus,oxidative stress,
and metabolic control in type 2 diabetes. Appl Physiol Nutr Metab
37:334–344
21.Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring
inconsistency in meta-analyses. BMJ 327:557–560
1796 Diabetologia (2014) 57:1789–1797
values under conditions of high dropout rates.However,no
significant differences could be observed following a compar-
ison of the dropout rates between short- and long-term studies
in the presentnetwork meta-analysis.Anotherconfounder
mightbe the variations in the volume of exercise (min per
week) prescribed. One study reported exercise duration (min
per session) in the CT group to be twice as high as with their
respective RT and/orAET counterparts [16].However,a
sensitivity analysis excluding this trialwas able to confirm
the results of the primary analysis.
With respect to the potential side effects (ESM Table 1),
eighttrials in the presentmeta-analysis reported adverse
events such as hypoglycaemia,back pain,shoulderpain,
musculoskeletal injury,tendonitis and other musculoskeletal
discomforts following exercise,with no significantdiffer-
ences between the intervention groups.However,it remains
possible that the number of adverse events will increase with
the duration and intensity of exercise.
This systematic review and meta-analysis focused on
randomised controlled trials comparing AET,RT and CT.
Compared with AET orRT, CT interventions resulted in
significantly more pronounced improvements in variables
related to glycaemic control. With respect to single types of
exercise intervention,AET was more effective in reducing
HbA1cand FG when compared with RT. However, the inter-
pretation of these findings with respect to their clinical rele-
vance is limited by the overall low to moderate quality of the
studies included, the lack of information on clinically impor-
tantoutcomes,and the limited information on the adverse
effects of exercise.
Funding This research received no specific grantfrom any funding
agency in the public, commercial or not-for-profit sectors.
Duality ofinterest The authors declare thatthere is no duality of
interest associated with this manuscript.
Contribution statementLS and GH conceived this study, LS, BM and
SD analysed the data,and LS,BM, JK and GH contributed to the
collection of data.All authors critically reviewed various drafts of the
manuscript, and all authors approved the final version. GH is responsible
for the integrity of the work as a whole.
References
1. Tuomilehto J, Lindstrom J, Eriksson JG et al (2001) Prevention of
type 2 diabetes mellitus by changes in lifestyle among subjects with
impaired glucose tolerance. N Engl J Med 344:1343–1350
2. Ajala O, English P, Pinkney J (2013) Systematic review and meta-
analysis of different dietary approaches to the management of type 2
diabetes. Am J Clin Nutr 97:505–516
3. Schwingshackl L, Hoffmann G (2014) Comparison of the long-term
effects of high-fatv. low-fatdietconsumption on cardiometabolic
risk factors in subjects with abnormal glucose metabolism: a syste
atic review and meta-analysis. Br J Nutr 111:2047–2058
4. Colberg SR,Sigal RJ, FernhallB et al (2010)Exercise and
type 2 diabetes:the American College of Sports Medicine and
the American DiabetesAssociation:joint position statement.
Diabetes Care 33:e147–e167
5. Snowling NJ,Hopkins WG (2006) Effects of differentmodes of
exercise training on glucose control and risk factors for complicati
in type 2 diabetic patients: a meta-analysis. Diabetes Care 29:251
2527
6. Chudyk A, Petrella RJ (2011) Effects of exercise on cardiovascular
risk factors in type 2 diabetes:a meta-analysis.Diabetes Care 34:
1228–1237
7. Strasser B, Siebert U, Schobersberger W (2010) Resistance trainin
in the treatment of the metabolic syndrome: a systematic review
meta-analysis of the effect of resistance training on metabolic clus
tering in patients with abnormal glucose metabolism. Sports Med
397–415
8. Kelley GA,Kelley KS (2000) Progressive resistance exercise and
resting blood pressure:a meta-analysis of randomized controlled
trials. Hypertension 35:838–843
9. Yang Z,ScottCA, Mao C,Tang J,Farmer AJ (2013) Resistance
exercise versus aerobic exercise for type 2 diabetes:a systematic
review and meta-analysis. Sports Med 44:487–499
10.Schwingshackl L, Dias S, Strasser B, Hoffmann G (2013) Impact of
different training modalities on anthropometric and metabolic cha
acteristics in overweight/obese subjects:a systematic review and
network meta-analysis. PLoS One 8:e82853
11.Umpierre D, Ribeiro PA, Kramer CK et al (2011) Physical activity
advice only orstructured exercise training and association with
HbA1c levels in type 2 diabetes:a systematic review and meta-
analysis. JAMA 305:1790–1799
12.MoherD, LiberatiA, TetzlaffJ, Altman DG (2009)Preferred
reporting items forsystematic reviews and meta-analyses:the
PRISMA statement. PLoS Med 6:e1000097
13.Higgins JP, Green S (eds) (2011) Cochrane handbook for systemat
reviews on interventions 5.1.0 [updated March 2011] The Cochran
Collaboration. Available from www.cochrane-handbook.org
14.Higgins JP,Altman DG,Gotzsche PC etal (2011) The Cochrane
Collaboration’s tool for assessing risk of bias in randomised trials.
BMJ 343:d5928
15.Jorge ML, de Oliveira VN, Resende NM et al (2011) The effects of
aerobic,resistance,and combined exercise on metabolic control,
inflammatory markers, adipocytokines, and muscle insulin signalin
in patients with type 2 diabetes mellitus. Metabolism 60:1244–125
16.SigalRJ, Kenny GP,Boule NG etal (2007)Effects ofaerobic
training,resistance training,or both on glycemic controlin type 2
diabetes – a randomized trial. Ann Intern Med 147:357–369
17.Balducci S, Zanuso S, Nicolucci A et al (2010) Anti-inflammatory
effectof exercise training in subjects with type 2 diabetes and the
metabolic syndrome isdependenton exercise modalitiesand
independentof weightloss. Nutr Metab Cardiovasc Dis20:
608–617
18.Lambers S, van Laethem C, van Acker K, Calders P (2008) Influenc
of combined exercise training on indices of obesity,diabetes and
cardiovascular risk in type 2 diabetes patients. Clin Rehabil 22:483
492
19.Gram B,Christensen R,Christiansen C,Gram J (2010) Effects of
nordic walking and exercise in type 2 diabetes mellitus: a random
controlled trial. Clin J Sport Med 20:355–361
20.de Oliveira VN,Bessa A,Jorge ML etal (2012) The effectof
differenttraining programs on antioxidantstatus,oxidative stress,
and metabolic control in type 2 diabetes. Appl Physiol Nutr Metab
37:334–344
21.Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring
inconsistency in meta-analyses. BMJ 327:557–560
1796 Diabetologia (2014) 57:1789–1797

22.Begg CB, Mazumdar M (1994) Operating characteristics of a rank
correlation test for publication bias. Biometrics 50:1088–1101
23.Egger M, Davey Smith G, Schneider M,Minder C (1997) Bias in
meta-analysis detected by a simple, graphical test. BMJ 315:629–634
24.Dias S, Sutton AJ, Ades AE, Welton NJ (2013) Evidence synthesis
for decision making 2: a generalized linear modeling framework for
pairwise and network meta-analysis of randomized controlled trials.
Med Decis Making 33:607–617
25.SpiegelhalterDJ, BestNG, Carlin BP,van derLinde A (2002)
Bayesian measures of modelcomplexity and fit.J R StatSoc (B)
64:583–616
26.Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS - a
Bayesian modelling framework: concepts, structure, and extensibili-
ty. Stat Comput 10:325–337
27.Dias S, Welton NJ, Sutton AJ, Ades AE (2011) NICE DSU Technical
Support Document 2: A Generalised Linear Modelling Framework
for Pairwise and Network Meta-Analysis of Randomised Controlled
Trials. Available from http://www.nicedsu.org.uk. Accessed 05 Sept
2013
28.Brooks SP,Gelman A (1998) Alternative methods for monitoring
convergence of iterative simulations.J ComputGraph Stat7:434–
455
29.Dias S, Welton NJ, Caldwell DM, Ades AE (2010) Checking con-
sistency in mixed treatment comparison meta-analysis. Stat Med 29:
932–944
30.Dias S,Welton NJ,Sutton AJ et al(2013) Evidence synthesis for
decision making 4: inconsistency in networks of evidence based on
randomized controlled trials. Med Decis Making 33:641–656
31.BacchiE, NegriC, Zanolin ME etal (2012) Metabolic effects of
aerobic training and resistance training in type 2 diabetic subjects: a
randomized controlled trial (the RAED2 study).Diabetes Care 35:
676–682
32.Church TS, Blair SN, Cocreham S et al (2010) Effects of aerobic and
resistance training on hemoglobin A1c levels in patients with type 2
diabetes: a randomized controlled trial. JAMA 304:2253–2262
33.Cuff DJ, Meneilly GS,Martin A etal (2003) Effective exercise
modality to reduce insulin resistance in women with type 2 diabetes.
Diabetes Care 26:2977–2982
34.Kwon HR, Min KW, Ahn HJ et al (2011) Effects of aerobic exercise
vs resistance training on endothelial function in women with type 2
diabetes mellitus. Diabetes Metab J 35:364–373
35.Ku YH, Han KA,Ahn H et al(2010) Resistance exercise did not
alterintramuscularadiposetissuebut reduced retinol-binding
protein-4 concentration in individualswith type 2 diabetes
mellitus.J Int Med Res 38:782–791
36.Kadoglou NP,Fotiadis G,Kapelouzou A etal (2013) The differ-
entialanti-inflammatory effectsof exercise modalitiesand their
association with early carotid atherosclerosis progression in patients
with type 2 diabetes. Diabet Med 30:e41–e50
37.Moe B, Augestad LB, Åsvold BO, Flanders WD (2011) Effects of
aerobic versus resistance training on glycaemic control in men with
type 2 diabetes. Eur J Sport Sci 11:365–374
38.Ng C, Goh S, Malhotra R, Ostbye T, Tai S (2010) Minimal difference
between aerobic and progressive resistance exercise on metabolic
profile and fitness in older adults with diabetes mellitus: a random
trial. J Physiother 56:163–170
39.Sukala WR,Page R,Rowlands DS etal (2012)South Pacific
Islanders resist type 2 diabetes: comparison of aerobic and resista
training. Eur J Appl Physiol 112:317–325
40.YavariA, Najafipoor F,Aliasgharzadeh A etal (2012) Effectof
aerobic exercise,resistance training orcombined training on
glycaemic controland cardiovascular risk factors in patients with
type 2 diabetes. Biol Sport 29:135–143
41.Marcus RL, Smith S, Morrell G et al (2008) Comparison of combine
aerobic and high-force eccentric resistance exercise with aerobic
exercise only for people with type 2 diabetes mellitus.Phys Ther
88:1345–1354
42.Cauza E,Hanusch-Enserer U,Strasser B etal (2005) The relative
benefits of endurance and strength training on the metabolic facto
and muscle function ofpeople with type 2 diabetes mellitus.
Arch Phys Med Rehabil86:1527–1533
43.Shenoy S, Arora E, Jaspal S (2009) Effects of progressive resistanc
training and aerobic exercise on type 2 diabetics in Indian populat
Int J Diabetes Metab 17:27–30
44.Grontved A, Pan A, Mekary RA et al (2014) Muscle-strengthening
and conditioning activities and risk of type 2 diabetes: a prospecti
study in two cohorts of US women. PLoS Med 11:e1001587
45.Grontved A, Rimm EB, Willett WC, Andersen LB, Hu FB (2012) A
prospective study of weighttraining and risk of type 2 diabetes
mellitus in men. Arch Intern Med 172:1306–1312
46.Li J, SiegristJ (2012)Physicalactivity and risk ofcardiovas-
cular disease–ameta-analysisof prospectivecohortstudies.
Int J Environ Res PublHealth 9:391–407
47.Kokkinos P,Myers J, Nylen E et al (2009)Exercise capacity
and all-causemortality in African American and Caucasian
men with type 2 diabetes.Diabetes Care 32:623–628
48.Umpierre D, Ribeiro PA, Schaan BD, Ribeiro JP (2013) Volume of
supervised exercise training impacts glycaemic controlin patients
with type 2 diabetes: a systematic review with meta-regression an
sis. Diabetologia 56:242–251
49.Oliveira C,Simoes M,Carvalho J,Ribeiro J (2012)Combined
exercise for people with type 2 diabetes mellitus: a systematic rev
Diabetes Res Clin Pract 98:187–198
50.Misra A, Alappan NK, Vikram NK et al (2008) Effect of supervised
progressive resistance-exercise training protocol on insulin sensiti
ity,glycemia,lipids,and body composition in Asian Indians with
type 2 diabetes. Diabetes Care 31:1282–1287
51.Tabata I,SuzukiY, Fukunaga T etal (1985) (1999) Resistance
training affects GLUT-4 contentin skeletalmuscle ofhumans
after19 days ofhead-down bed rest.J Appl Physiol86:909–
914
52.Hussey SE,McGee SL,Garnham A et al (2011) Exercise training
increases adiposetissueGLUT4 expression in patientswith
type 2 diabetes.Diabetes Obes Metab 13:959–962
Diabetologia (2014) 57:1789–1797 1797
correlation test for publication bias. Biometrics 50:1088–1101
23.Egger M, Davey Smith G, Schneider M,Minder C (1997) Bias in
meta-analysis detected by a simple, graphical test. BMJ 315:629–634
24.Dias S, Sutton AJ, Ades AE, Welton NJ (2013) Evidence synthesis
for decision making 2: a generalized linear modeling framework for
pairwise and network meta-analysis of randomized controlled trials.
Med Decis Making 33:607–617
25.SpiegelhalterDJ, BestNG, Carlin BP,van derLinde A (2002)
Bayesian measures of modelcomplexity and fit.J R StatSoc (B)
64:583–616
26.Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS - a
Bayesian modelling framework: concepts, structure, and extensibili-
ty. Stat Comput 10:325–337
27.Dias S, Welton NJ, Sutton AJ, Ades AE (2011) NICE DSU Technical
Support Document 2: A Generalised Linear Modelling Framework
for Pairwise and Network Meta-Analysis of Randomised Controlled
Trials. Available from http://www.nicedsu.org.uk. Accessed 05 Sept
2013
28.Brooks SP,Gelman A (1998) Alternative methods for monitoring
convergence of iterative simulations.J ComputGraph Stat7:434–
455
29.Dias S, Welton NJ, Caldwell DM, Ades AE (2010) Checking con-
sistency in mixed treatment comparison meta-analysis. Stat Med 29:
932–944
30.Dias S,Welton NJ,Sutton AJ et al(2013) Evidence synthesis for
decision making 4: inconsistency in networks of evidence based on
randomized controlled trials. Med Decis Making 33:641–656
31.BacchiE, NegriC, Zanolin ME etal (2012) Metabolic effects of
aerobic training and resistance training in type 2 diabetic subjects: a
randomized controlled trial (the RAED2 study).Diabetes Care 35:
676–682
32.Church TS, Blair SN, Cocreham S et al (2010) Effects of aerobic and
resistance training on hemoglobin A1c levels in patients with type 2
diabetes: a randomized controlled trial. JAMA 304:2253–2262
33.Cuff DJ, Meneilly GS,Martin A etal (2003) Effective exercise
modality to reduce insulin resistance in women with type 2 diabetes.
Diabetes Care 26:2977–2982
34.Kwon HR, Min KW, Ahn HJ et al (2011) Effects of aerobic exercise
vs resistance training on endothelial function in women with type 2
diabetes mellitus. Diabetes Metab J 35:364–373
35.Ku YH, Han KA,Ahn H et al(2010) Resistance exercise did not
alterintramuscularadiposetissuebut reduced retinol-binding
protein-4 concentration in individualswith type 2 diabetes
mellitus.J Int Med Res 38:782–791
36.Kadoglou NP,Fotiadis G,Kapelouzou A etal (2013) The differ-
entialanti-inflammatory effectsof exercise modalitiesand their
association with early carotid atherosclerosis progression in patients
with type 2 diabetes. Diabet Med 30:e41–e50
37.Moe B, Augestad LB, Åsvold BO, Flanders WD (2011) Effects of
aerobic versus resistance training on glycaemic control in men with
type 2 diabetes. Eur J Sport Sci 11:365–374
38.Ng C, Goh S, Malhotra R, Ostbye T, Tai S (2010) Minimal difference
between aerobic and progressive resistance exercise on metabolic
profile and fitness in older adults with diabetes mellitus: a random
trial. J Physiother 56:163–170
39.Sukala WR,Page R,Rowlands DS etal (2012)South Pacific
Islanders resist type 2 diabetes: comparison of aerobic and resista
training. Eur J Appl Physiol 112:317–325
40.YavariA, Najafipoor F,Aliasgharzadeh A etal (2012) Effectof
aerobic exercise,resistance training orcombined training on
glycaemic controland cardiovascular risk factors in patients with
type 2 diabetes. Biol Sport 29:135–143
41.Marcus RL, Smith S, Morrell G et al (2008) Comparison of combine
aerobic and high-force eccentric resistance exercise with aerobic
exercise only for people with type 2 diabetes mellitus.Phys Ther
88:1345–1354
42.Cauza E,Hanusch-Enserer U,Strasser B etal (2005) The relative
benefits of endurance and strength training on the metabolic facto
and muscle function ofpeople with type 2 diabetes mellitus.
Arch Phys Med Rehabil86:1527–1533
43.Shenoy S, Arora E, Jaspal S (2009) Effects of progressive resistanc
training and aerobic exercise on type 2 diabetics in Indian populat
Int J Diabetes Metab 17:27–30
44.Grontved A, Pan A, Mekary RA et al (2014) Muscle-strengthening
and conditioning activities and risk of type 2 diabetes: a prospecti
study in two cohorts of US women. PLoS Med 11:e1001587
45.Grontved A, Rimm EB, Willett WC, Andersen LB, Hu FB (2012) A
prospective study of weighttraining and risk of type 2 diabetes
mellitus in men. Arch Intern Med 172:1306–1312
46.Li J, SiegristJ (2012)Physicalactivity and risk ofcardiovas-
cular disease–ameta-analysisof prospectivecohortstudies.
Int J Environ Res PublHealth 9:391–407
47.Kokkinos P,Myers J, Nylen E et al (2009)Exercise capacity
and all-causemortality in African American and Caucasian
men with type 2 diabetes.Diabetes Care 32:623–628
48.Umpierre D, Ribeiro PA, Schaan BD, Ribeiro JP (2013) Volume of
supervised exercise training impacts glycaemic controlin patients
with type 2 diabetes: a systematic review with meta-regression an
sis. Diabetologia 56:242–251
49.Oliveira C,Simoes M,Carvalho J,Ribeiro J (2012)Combined
exercise for people with type 2 diabetes mellitus: a systematic rev
Diabetes Res Clin Pract 98:187–198
50.Misra A, Alappan NK, Vikram NK et al (2008) Effect of supervised
progressive resistance-exercise training protocol on insulin sensiti
ity,glycemia,lipids,and body composition in Asian Indians with
type 2 diabetes. Diabetes Care 31:1282–1287
51.Tabata I,SuzukiY, Fukunaga T etal (1985) (1999) Resistance
training affects GLUT-4 contentin skeletalmuscle ofhumans
after19 days ofhead-down bed rest.J Appl Physiol86:909–
914
52.Hussey SE,McGee SL,Garnham A et al (2011) Exercise training
increases adiposetissueGLUT4 expression in patientswith
type 2 diabetes.Diabetes Obes Metab 13:959–962
Diabetologia (2014) 57:1789–1797 1797
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.