Development of a Dipstick Platelet Function Test
VerifiedAdded on 2023/06/18
|13
|4361
|315
AI Summary
This report discusses the functioning of platelets, the different pathways of platelets, thrombus formation process, and the Haemostasis process. It also covers the chronological order of platelet activation and the process of blockage formed by thrombus formation.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

DEVELOPMENT OF A DIP-
STICK PLATELET
FUNCTION TEST
STICK PLATELET
FUNCTION TEST
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

TABLE OF CONTENTS
INTRODUCTION...........................................................................................................................3
LITERATURE REVIEW................................................................................................................3
Defining platelets.........................................................................................................................3
Functions performed by platelets.................................................................................................3
Platelet pathways.........................................................................................................................4
Formation of thrombus................................................................................................................5
Haemostasis process....................................................................................................................5
Pathological process for thrombus formation..............................................................................6
Process of blockage formed by thrombus formation...................................................................6
Chronological order of platelets activations and things responsible for the platelet activation
with associated response..............................................................................................................7
Changes in platelets and reason behind it....................................................................................8
Measuring platelet functions........................................................................................................8
Platelet functions related with physiological...............................................................................9
CONCLUSION................................................................................................................................9
REFERENCES..............................................................................................................................11
INTRODUCTION...........................................................................................................................3
LITERATURE REVIEW................................................................................................................3
Defining platelets.........................................................................................................................3
Functions performed by platelets.................................................................................................3
Platelet pathways.........................................................................................................................4
Formation of thrombus................................................................................................................5
Haemostasis process....................................................................................................................5
Pathological process for thrombus formation..............................................................................6
Process of blockage formed by thrombus formation...................................................................6
Chronological order of platelets activations and things responsible for the platelet activation
with associated response..............................................................................................................7
Changes in platelets and reason behind it....................................................................................8
Measuring platelet functions........................................................................................................8
Platelet functions related with physiological...............................................................................9
CONCLUSION................................................................................................................................9
REFERENCES..............................................................................................................................11

INTRODUCTION
Platelets are tiny blood cells that help the body form clots, essential to stop the bleeding
(Nam and et al., 2019). Platelets are known as thrombocytes. Thrombocytes are the component
that reacts during the bleeding and is present within the blood vessels during some injury. The
tests on platelet functions are done to evaluate the functional defects and help determine the
proteins present on the surface of the platelets, along with the change that happens when platelets
are activated. This report deals with the functioning of platelets and the different pathways of
platelets. There is a detailed discussion about the thrombus formation process as well as the
Haemostasis process. The platelets are activated in a particular chronological order and will
change in shape in the process.
LITERATURE REVIEW
Defining platelets
Platelets are small disc-shaped cells that are found in the blood and spleen of a human
body. These are pieces made from extensive cells within bone marrow known as
megakaryocytes. Platelets are one of the three types of blood cells. Platelets are small and cells
without a nucleus. They are also known as thrombocytes and are very small as well as colorless
fragments of cells. These are a part of blood and help form clots that help to prevent bleeding.
There are other studies that further help in elaborating the definition of platelets Koupenova et
al., (2018) that they are produced in the bone marrow of an individual, a sponge-like tissue
present inside the bones. Moreover, Etulain (2018) added that platelets are essential for surviving
surgeries like an organ transplant, fighting cancer, and dealing with some chronic diseases.
However, The rise in the number of individual platelet counts will reduce the risk of any
dangerous or fatal bleeding. The average count of platelets is ranged from between 150000 to
450000 platelets per microliter of blood. This count is increased when some problems lead to
causing many platelets in the bone marrow. In some conditions, it is due to infection or primary
and secondary thrombocytosis. Clinical diagnosis involves blood tests that will measure the
substances involved in the process of clotting.
Platelets are tiny blood cells that help the body form clots, essential to stop the bleeding
(Nam and et al., 2019). Platelets are known as thrombocytes. Thrombocytes are the component
that reacts during the bleeding and is present within the blood vessels during some injury. The
tests on platelet functions are done to evaluate the functional defects and help determine the
proteins present on the surface of the platelets, along with the change that happens when platelets
are activated. This report deals with the functioning of platelets and the different pathways of
platelets. There is a detailed discussion about the thrombus formation process as well as the
Haemostasis process. The platelets are activated in a particular chronological order and will
change in shape in the process.
LITERATURE REVIEW
Defining platelets
Platelets are small disc-shaped cells that are found in the blood and spleen of a human
body. These are pieces made from extensive cells within bone marrow known as
megakaryocytes. Platelets are one of the three types of blood cells. Platelets are small and cells
without a nucleus. They are also known as thrombocytes and are very small as well as colorless
fragments of cells. These are a part of blood and help form clots that help to prevent bleeding.
There are other studies that further help in elaborating the definition of platelets Koupenova et
al., (2018) that they are produced in the bone marrow of an individual, a sponge-like tissue
present inside the bones. Moreover, Etulain (2018) added that platelets are essential for surviving
surgeries like an organ transplant, fighting cancer, and dealing with some chronic diseases.
However, The rise in the number of individual platelet counts will reduce the risk of any
dangerous or fatal bleeding. The average count of platelets is ranged from between 150000 to
450000 platelets per microliter of blood. This count is increased when some problems lead to
causing many platelets in the bone marrow. In some conditions, it is due to infection or primary
and secondary thrombocytosis. Clinical diagnosis involves blood tests that will measure the
substances involved in the process of clotting.

Functions performed by platelets
Maiocchi and et al. (2018) stated that platelets play an essential role in the blood vessel,
such as fixing the damage caused due to some injury. They exist in circulation for 5 to 7 days,
mainly working as regulators of hemostasis and thrombosis. When the count of platelets from the
blood vessel is altered as either increased or decreased by high rates will create a lot of problems.
When this count is low, the individual's body will be unable to form clots, and spontaneous
bleeding can lead to conditions as severe as death. A high count of platelets can cause diseases
such as stroke, heart attack as clots are formed within the blood vessels. Checking the number of
platelets present in the blood will help diagnose certain diseases or any health conditions related
to them.
On the other hand, platelets become activated within the blood during any vascular injury, which
results in adhesion to the unprotected extracellular matrix. The term used for the process by
which platelets from the clot is adhesion. In the case of some pathological conditions, platelets
play a vital part in the formation of occlusive thrombus formation, which helps form a target on
prevention from platelet adhesion to the vascular wall. Colberg and et al. (2020) added that it
even helps regulate the growth of the tumor and the extravasation in the blood vessels. These are
some of the primary functions performed by platelets, representing the standard functions and
generating more versatility in blood circulation.
Platelet pathways
Trugilho and et al. (2017) mentioned that some interactive pathways help outline the
process of platelet activation concerning some specific aspects of the process. One course will
depict the interaction of the adhesion receptors of the platelets. Aslan, (2021) the primary
function of platelets is related to the initiation of the coagulation cascades. The adhesion of the
platelets to the extracellular matrix is the beginning step for the process of hemostasis. It is done
under the condition of high shear, which will form a bridge between the exposed collagen and
the platelet glycoprotein, a complex receptor on the platelet membrane. Lien and et al. (2017)
explained that the exposed collagen binds with the platelet glycoprotein and its receptors.
Platelets are activated during this process and change their shape to release the granules'
contents. The bonded fibrinogen cross-links the platelets, which contributes to the stabilization of
the thrombus. The activation of the platelets is stimulated with the help of platelet secretion and
Maiocchi and et al. (2018) stated that platelets play an essential role in the blood vessel,
such as fixing the damage caused due to some injury. They exist in circulation for 5 to 7 days,
mainly working as regulators of hemostasis and thrombosis. When the count of platelets from the
blood vessel is altered as either increased or decreased by high rates will create a lot of problems.
When this count is low, the individual's body will be unable to form clots, and spontaneous
bleeding can lead to conditions as severe as death. A high count of platelets can cause diseases
such as stroke, heart attack as clots are formed within the blood vessels. Checking the number of
platelets present in the blood will help diagnose certain diseases or any health conditions related
to them.
On the other hand, platelets become activated within the blood during any vascular injury, which
results in adhesion to the unprotected extracellular matrix. The term used for the process by
which platelets from the clot is adhesion. In the case of some pathological conditions, platelets
play a vital part in the formation of occlusive thrombus formation, which helps form a target on
prevention from platelet adhesion to the vascular wall. Colberg and et al. (2020) added that it
even helps regulate the growth of the tumor and the extravasation in the blood vessels. These are
some of the primary functions performed by platelets, representing the standard functions and
generating more versatility in blood circulation.
Platelet pathways
Trugilho and et al. (2017) mentioned that some interactive pathways help outline the
process of platelet activation concerning some specific aspects of the process. One course will
depict the interaction of the adhesion receptors of the platelets. Aslan, (2021) the primary
function of platelets is related to the initiation of the coagulation cascades. The adhesion of the
platelets to the extracellular matrix is the beginning step for the process of hemostasis. It is done
under the condition of high shear, which will form a bridge between the exposed collagen and
the platelet glycoprotein, a complex receptor on the platelet membrane. Lien and et al. (2017)
explained that the exposed collagen binds with the platelet glycoprotein and its receptors.
Platelets are activated during this process and change their shape to release the granules'
contents. The bonded fibrinogen cross-links the platelets, which contributes to the stabilization of
the thrombus. The activation of the platelets is stimulated with the help of platelet secretion and
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

the local pro-thrombotic factors. Multiple pathways lead to the activation of the platelets. PI3-
kinase., PLC, and PKC are the main pathways that are involved in the process of platelet
activation.
PI3- Kinase (Phosphoinositide 3- kinases)
It is a central enzyme acting as a signaling pathway and helps in mediating the cellular
response and other growth factors. It is essential in modulating the platelet functions that is to
form blood clots.
PKC (Protein Kinase C)
This enzyme plays an essential part in signal transduction cascades. It helps in regulating
the platelet granule secretion in its activation and aggregation and spreading activity. It helps in
controlling the function of other proteins from the hydroxyl groups.
Formation of thrombus
Qiao and et al. (2018) explained thrombosis occurs when the clots are formed in the
blood, which blocks the veins and arteries. The thrombus formation occurs when the platelets
bind with collagen, which is exposed at the site of vascular injury. This binding will lead to the
activation of the platelets, which leads to the platelet membranes gaining the ability to provide
catalytic support for the biochemical reactions. This results in the formation of thrombin. During
the time of some vascular injury, immediate local cellular cells respond. Platelets will migrate to
the area of the damage, which includes several cellular factors and some mediators. On the same
note, Iba and Levy (2018) continued and said these mediators are responsible for promoting the
formation of clots. Thrombus is more likely to occur in immobile individuals and have a genetic
predisposition to the blood clotting process. A thrombus is formed after any damage occurs in
the artery or vein or even in the surrounding tissue. Thrombus formation is a dynamic process in
which the platelets adhere to the thrombus while the others are separated. The number of
platelets in the circulation impacts the formation of the clot.
Hemostasis process
As mentioned by Asada et al. (2020), hemostasis leads to developing cessation of the
bleeding coming from the blood vessels. The hemostasis mechanism is categorized into four
parts: constriction of the blood vessel, formation of the temporary platelet plug, activation of the
kinase., PLC, and PKC are the main pathways that are involved in the process of platelet
activation.
PI3- Kinase (Phosphoinositide 3- kinases)
It is a central enzyme acting as a signaling pathway and helps in mediating the cellular
response and other growth factors. It is essential in modulating the platelet functions that is to
form blood clots.
PKC (Protein Kinase C)
This enzyme plays an essential part in signal transduction cascades. It helps in regulating
the platelet granule secretion in its activation and aggregation and spreading activity. It helps in
controlling the function of other proteins from the hydroxyl groups.
Formation of thrombus
Qiao and et al. (2018) explained thrombosis occurs when the clots are formed in the
blood, which blocks the veins and arteries. The thrombus formation occurs when the platelets
bind with collagen, which is exposed at the site of vascular injury. This binding will lead to the
activation of the platelets, which leads to the platelet membranes gaining the ability to provide
catalytic support for the biochemical reactions. This results in the formation of thrombin. During
the time of some vascular injury, immediate local cellular cells respond. Platelets will migrate to
the area of the damage, which includes several cellular factors and some mediators. On the same
note, Iba and Levy (2018) continued and said these mediators are responsible for promoting the
formation of clots. Thrombus is more likely to occur in immobile individuals and have a genetic
predisposition to the blood clotting process. A thrombus is formed after any damage occurs in
the artery or vein or even in the surrounding tissue. Thrombus formation is a dynamic process in
which the platelets adhere to the thrombus while the others are separated. The number of
platelets in the circulation impacts the formation of the clot.
Hemostasis process
As mentioned by Asada et al. (2020), hemostasis leads to developing cessation of the
bleeding coming from the blood vessels. The hemostasis mechanism is categorized into four
parts: constriction of the blood vessel, formation of the temporary platelet plug, activation of the

coagulation cascade, and the formation of the final clot. Dietrich-Muszalska and Wachowicz
(2017) explained that one of the primary purposes behind the hemostasis process is to facilitate
the series of enzyme actions activations which leads to the formation of the clots with the help of
platelets as well as fibrin polymer. This process involves multiple interlinked steps and
culminates in the construction of the plug, which closes the site of injury of the blood vessel,
which controls the bleeding. The blob is sealed on the injured area and is maintained and
prevented from bleeding when the tissue regression process occurs. When the injury begins to
heal, the plug remodels at a slow pace and starts dissolving with restoring the normal tissue at the
site of damage.
On the other hand, Derbalah and et al. (2021) mentioned that hemostasis is a natural process that
stops having any blood loss during an injury, consisting of three steps of vasoconstriction,
formation of a platelet plug, and coagulation. The different steps involved in hemostasis are
followed by vasoconstriction, which forms the platelet plug. It involves three stages in the
platelet plug formation, beginning with platelet adherence, activation, and lastly, aggregation.
Pathological process for thrombus formation
A thrombus can block the flow of blood flowing through the vein or an artery. While
Maas and Renné (2018) when these thrombi detach from the wall of the vessel, they lodges in
the vital organs of the human body and can be life-threatening. The body's coagulation system
depends on forming a balance between the natural coagulation and anticoagulant factors and in
the coagulation with the fibrinolytic system. Shou and et al. (2017) stated that the pathological
thrombus is produced when there is an imbalance in the blood coagulation system, leading to
many serious health conditions, including heart attacks, cardioembolic stroke, and so on. The
pathological process occurs at the site of attachment, such as insertions of joint capsules,
ligaments, and so on, which generally deals with inflammation and infection. Transport of flow
of platelets and the coagulation proteins is required in the thrombus formation. This thrombus
formation results in having an imbalance within the blood coagulation system. This leads to
various health conditions such as heart attacks and cardiac stroke. The outcomes received from
thrombus formation are propagation, dissolution, and re- canalization.
(2017) explained that one of the primary purposes behind the hemostasis process is to facilitate
the series of enzyme actions activations which leads to the formation of the clots with the help of
platelets as well as fibrin polymer. This process involves multiple interlinked steps and
culminates in the construction of the plug, which closes the site of injury of the blood vessel,
which controls the bleeding. The blob is sealed on the injured area and is maintained and
prevented from bleeding when the tissue regression process occurs. When the injury begins to
heal, the plug remodels at a slow pace and starts dissolving with restoring the normal tissue at the
site of damage.
On the other hand, Derbalah and et al. (2021) mentioned that hemostasis is a natural process that
stops having any blood loss during an injury, consisting of three steps of vasoconstriction,
formation of a platelet plug, and coagulation. The different steps involved in hemostasis are
followed by vasoconstriction, which forms the platelet plug. It involves three stages in the
platelet plug formation, beginning with platelet adherence, activation, and lastly, aggregation.
Pathological process for thrombus formation
A thrombus can block the flow of blood flowing through the vein or an artery. While
Maas and Renné (2018) when these thrombi detach from the wall of the vessel, they lodges in
the vital organs of the human body and can be life-threatening. The body's coagulation system
depends on forming a balance between the natural coagulation and anticoagulant factors and in
the coagulation with the fibrinolytic system. Shou and et al. (2017) stated that the pathological
thrombus is produced when there is an imbalance in the blood coagulation system, leading to
many serious health conditions, including heart attacks, cardioembolic stroke, and so on. The
pathological process occurs at the site of attachment, such as insertions of joint capsules,
ligaments, and so on, which generally deals with inflammation and infection. Transport of flow
of platelets and the coagulation proteins is required in the thrombus formation. This thrombus
formation results in having an imbalance within the blood coagulation system. This leads to
various health conditions such as heart attacks and cardiac stroke. The outcomes received from
thrombus formation are propagation, dissolution, and re- canalization.

Process of blockage formed by thrombus formation
Scharf (2018) said that thrombus forms a plug that helps reduce and prevent bleeding.
However, Lim, Lloyd, and Bhattacharyya, (2017) contradicted that formation of a thrombus can
lead to causing severe health problems as it interrupts the functioning of blood vessels. The
blood clot section, which breaks free from the thrombus, then circulates within the bloodstream.
This is known as an embolus. This embolus is dangerous and can be fatal, especially when it
reaches the heart, lungs, or brain. Thrombus formation can cause the blockage of blood flow in
veins and arteries. In a similar context, Ahmed and et al., (2020) said that there are different
complications that depend on the location of the formed thrombus. The consequences of
thrombosis can be life-threatening in some cases, such as leading to stroke or a heart attack. A
thrombus is a blood clot that is present in the circulatory system and attaches to the site where it
is formed and is stuck there, which hinders the flow of blood. It is most likely to occur in people
who are more immobile and have a predisposition to blood clotting.
Chronological order of platelets activations and things responsible for the platelet activation with
associated response
Platelet activation is the primary process that involves protective hemostasis and
pathological thrombosis. This is done by activating multiple pathways that include binding of
several agonists. The chronological order of platelet activation is the adhesion of the platelets to
the adjacent platelets, spreading and aggregation of the platelets, and lastly, the activation of
platelets by the formation of thrombus clot. Activation of the platelets is in a different order that
follows a particular chronological order. The inhibitory signals play an essential part in providing
prevention from activation in healthy vasculature. The activation process begins with the
adhesion of the platelets, which expose the components from the extracellular matrix present in
the blood vessel wall. The adhesion process includes a von wille- brand factor (vWF) which is
helpful in binding with the platelet glycoprotein (Wang, et et al., 2017). This process is
significant for the initiation step for haemostasis. This binding process of vWF with the
glycoprotein triggers the signals of pathways present within the platelets, leading to their
activation. It involves the stimulation of phospholipase, which results in the activation of protein
kinase C. Platelet and collagen interacts, which slows the movements of the platelets in the
blood. This is because of the formation of rope, which allows the platelets to bind directly which
Scharf (2018) said that thrombus forms a plug that helps reduce and prevent bleeding.
However, Lim, Lloyd, and Bhattacharyya, (2017) contradicted that formation of a thrombus can
lead to causing severe health problems as it interrupts the functioning of blood vessels. The
blood clot section, which breaks free from the thrombus, then circulates within the bloodstream.
This is known as an embolus. This embolus is dangerous and can be fatal, especially when it
reaches the heart, lungs, or brain. Thrombus formation can cause the blockage of blood flow in
veins and arteries. In a similar context, Ahmed and et al., (2020) said that there are different
complications that depend on the location of the formed thrombus. The consequences of
thrombosis can be life-threatening in some cases, such as leading to stroke or a heart attack. A
thrombus is a blood clot that is present in the circulatory system and attaches to the site where it
is formed and is stuck there, which hinders the flow of blood. It is most likely to occur in people
who are more immobile and have a predisposition to blood clotting.
Chronological order of platelets activations and things responsible for the platelet activation with
associated response
Platelet activation is the primary process that involves protective hemostasis and
pathological thrombosis. This is done by activating multiple pathways that include binding of
several agonists. The chronological order of platelet activation is the adhesion of the platelets to
the adjacent platelets, spreading and aggregation of the platelets, and lastly, the activation of
platelets by the formation of thrombus clot. Activation of the platelets is in a different order that
follows a particular chronological order. The inhibitory signals play an essential part in providing
prevention from activation in healthy vasculature. The activation process begins with the
adhesion of the platelets, which expose the components from the extracellular matrix present in
the blood vessel wall. The adhesion process includes a von wille- brand factor (vWF) which is
helpful in binding with the platelet glycoprotein (Wang, et et al., 2017). This process is
significant for the initiation step for haemostasis. This binding process of vWF with the
glycoprotein triggers the signals of pathways present within the platelets, leading to their
activation. It involves the stimulation of phospholipase, which results in the activation of protein
kinase C. Platelet and collagen interacts, which slows the movements of the platelets in the
blood. This is because of the formation of rope, which allows the platelets to bind directly which
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

is exposed to collagen with the help of α2β1 and GPVI receptors present on the platelets' surface.
Collagen stimulates glycoprotein VI (GPVI), which activates tyrosine kinase, which finally
results in the activation of platelets. The following order is to spread and aggregate the platelets
with the help of autocatalytic signaling. This is followed by activation of platelets and formation
of thrombus. Thrombin is a serine protease which is an effective platelet agonist. It is essential
for cleavage of fibrinogen in the fibrin, which strengthens the platelet plug. Platelets consist of
specific G protein-coupled receptors (GPCR) on their surface. These are also known as protease-
activated receptors. Thrombin will cleave these receptors, which will activate G proteins
inducing the signaling pathways, which leads to platelet activation. After any vascular injury,
vWF binds with the uncovered collagen present in the damaged wall of the blood vessel along
with the platelets with the help of GPIb- IX- V. The formation of the tether is between the
platelets and the blood vessel wall (Cowman and et al., 2017). It allows the α2β1 and GPVI to
bind directly with the collagen, promoting the activation of platelets and adhesion. Thrombin
activates the platelets with the help of protease-activated receptors (PAR) on the surface of
platelets through GPCR. The PAR- 1 works as a mediator for the platelet activation process
when the thrombin concentration is low. On the other hand, PAR- 4 is when this concentration is
higher during platelet activation. The activation of platelets begins when the adhesion occurs,
which is triggered by collagen-binding with the receptors of α2β1 and GPVI on the platelet.
Changes in platelets and reason behind it
The activation of platelets will stimulate the rapid reorganization, which transforms the
platelets' shapes from being biconcave disk to spread cells completely. In this process, the
platelets extend from the filopodia and generate lamellipodia, which rapidly increases the platelet
surface area, due to the exposure of various agonists, the shape of the platelet changes with the
dismantling and the reorganization of the cytoskeleton. The form of inactive platelets is biconvex
discoid, while activated platelets have projection on the cell membrane covering the surface
(Hanke, et al., 2018). After the activation, platelets undergo several changes such as activation of
integrin receptors, synthesis of TxA2 (Thromboxane A2), and changes in the shape. Activation
of β3 receptors on the platelets is in cross link with the fibrinogen and vWF, leading to platelet
aggregation. The capability of the platelets to aggregate at the sites shows that they are helpful in
stopping blood loss. In the formation of thrombus, the activation of platelets after the rupture of
Collagen stimulates glycoprotein VI (GPVI), which activates tyrosine kinase, which finally
results in the activation of platelets. The following order is to spread and aggregate the platelets
with the help of autocatalytic signaling. This is followed by activation of platelets and formation
of thrombus. Thrombin is a serine protease which is an effective platelet agonist. It is essential
for cleavage of fibrinogen in the fibrin, which strengthens the platelet plug. Platelets consist of
specific G protein-coupled receptors (GPCR) on their surface. These are also known as protease-
activated receptors. Thrombin will cleave these receptors, which will activate G proteins
inducing the signaling pathways, which leads to platelet activation. After any vascular injury,
vWF binds with the uncovered collagen present in the damaged wall of the blood vessel along
with the platelets with the help of GPIb- IX- V. The formation of the tether is between the
platelets and the blood vessel wall (Cowman and et al., 2017). It allows the α2β1 and GPVI to
bind directly with the collagen, promoting the activation of platelets and adhesion. Thrombin
activates the platelets with the help of protease-activated receptors (PAR) on the surface of
platelets through GPCR. The PAR- 1 works as a mediator for the platelet activation process
when the thrombin concentration is low. On the other hand, PAR- 4 is when this concentration is
higher during platelet activation. The activation of platelets begins when the adhesion occurs,
which is triggered by collagen-binding with the receptors of α2β1 and GPVI on the platelet.
Changes in platelets and reason behind it
The activation of platelets will stimulate the rapid reorganization, which transforms the
platelets' shapes from being biconcave disk to spread cells completely. In this process, the
platelets extend from the filopodia and generate lamellipodia, which rapidly increases the platelet
surface area, due to the exposure of various agonists, the shape of the platelet changes with the
dismantling and the reorganization of the cytoskeleton. The form of inactive platelets is biconvex
discoid, while activated platelets have projection on the cell membrane covering the surface
(Hanke, et al., 2018). After the activation, platelets undergo several changes such as activation of
integrin receptors, synthesis of TxA2 (Thromboxane A2), and changes in the shape. Activation
of β3 receptors on the platelets is in cross link with the fibrinogen and vWF, leading to platelet
aggregation. The capability of the platelets to aggregate at the sites shows that they are helpful in
stopping blood loss. In the formation of thrombus, the activation of platelets after the rupture of
atherosclerotic plaque will expose the thrombogenic components, which can lead to severe
complications such as heart stroke.
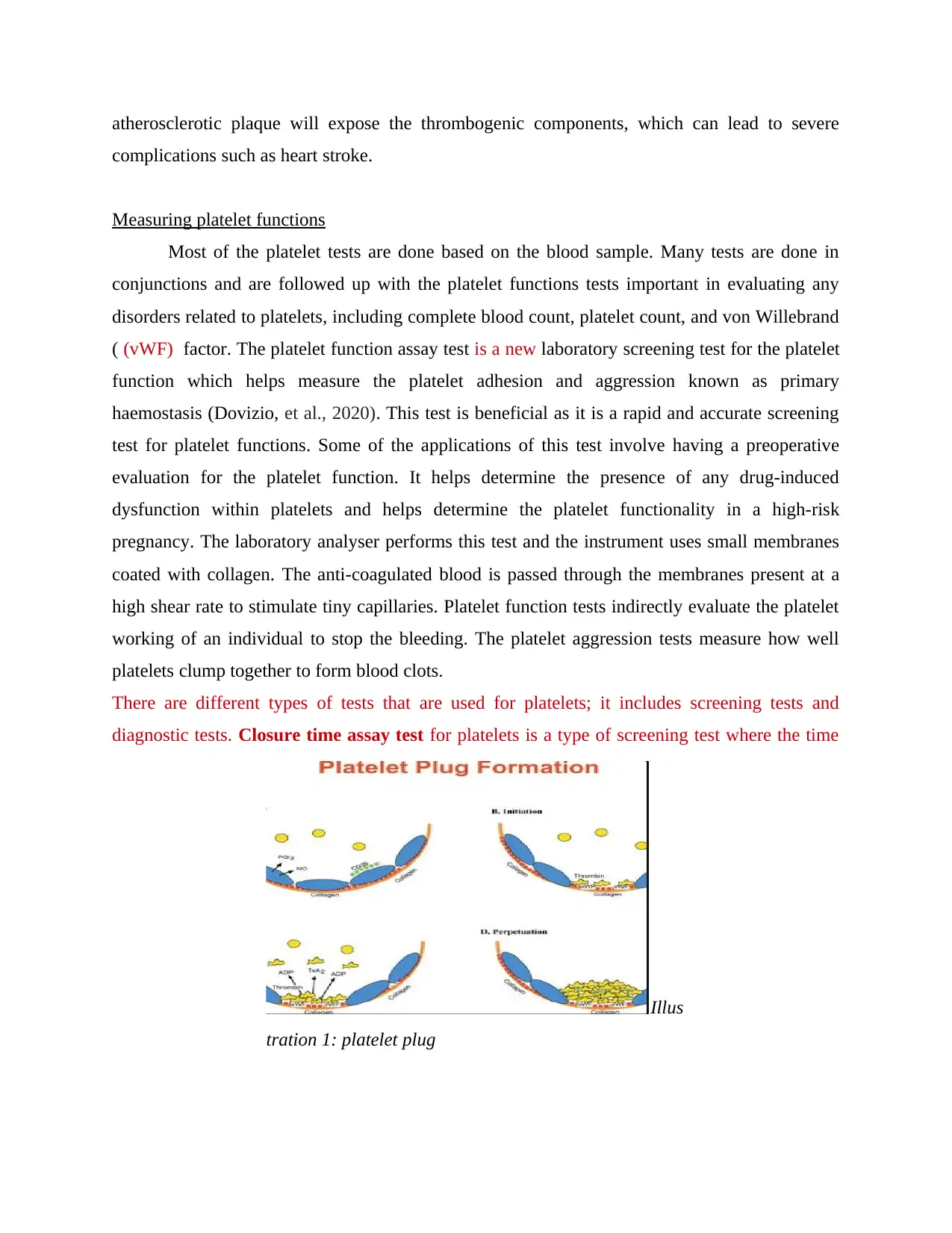
Measuring platelet functions
Most of the platelet tests are done based on the blood sample. Many tests are done in
conjunctions and are followed up with the platelet functions tests important in evaluating any
disorders related to platelets, including complete blood count, platelet count, and von Willebrand
( (vWF) factor. The platelet function assay test is a new laboratory screening test for the platelet
function which helps measure the platelet adhesion and aggression known as primary
haemostasis (Dovizio, et al., 2020). This test is beneficial as it is a rapid and accurate screening
test for platelet functions. Some of the applications of this test involve having a preoperative
evaluation for the platelet function. It helps determine the presence of any drug-induced
dysfunction within platelets and helps determine the platelet functionality in a high-risk
pregnancy. The laboratory analyser performs this test and the instrument uses small membranes
coated with collagen. The anti-coagulated blood is passed through the membranes present at a
high shear rate to stimulate tiny capillaries. Platelet function tests indirectly evaluate the platelet
working of an individual to stop the bleeding. The platelet aggression tests measure how well
platelets clump together to form blood clots.
There are different types of tests that are used for platelets; it includes screening tests and
diagnostic tests. Closure time assay test for platelets is a type of screening test where the time
Illus
tration 1: platelet plug
complications such as heart stroke.
Measuring platelet functions
Most of the platelet tests are done based on the blood sample. Many tests are done in
conjunctions and are followed up with the platelet functions tests important in evaluating any
disorders related to platelets, including complete blood count, platelet count, and von Willebrand
( (vWF) factor. The platelet function assay test is a new laboratory screening test for the platelet
function which helps measure the platelet adhesion and aggression known as primary
haemostasis (Dovizio, et al., 2020). This test is beneficial as it is a rapid and accurate screening
test for platelet functions. Some of the applications of this test involve having a preoperative
evaluation for the platelet function. It helps determine the presence of any drug-induced
dysfunction within platelets and helps determine the platelet functionality in a high-risk
pregnancy. The laboratory analyser performs this test and the instrument uses small membranes
coated with collagen. The anti-coagulated blood is passed through the membranes present at a
high shear rate to stimulate tiny capillaries. Platelet function tests indirectly evaluate the platelet
working of an individual to stop the bleeding. The platelet aggression tests measure how well
platelets clump together to form blood clots.
There are different types of tests that are used for platelets; it includes screening tests and
diagnostic tests. Closure time assay test for platelets is a type of screening test where the time
Illus
tration 1: platelet plug

taken by the platelets present within a blood sample plug into a small hole in a tiny tube after
they are exposed to different activating substances. This is known as closure time and indicates
lower functions of platelets; however, these tests cannot identify the leading cause. Platelet
aggregometry is the test used in a different clinical settingss and helps monitor the response
towards the anti-platelet therapies. It diagnoses the inherited bleeding disorders within an
individual. Thromboelastometry is a viscoelastic method used for testing hemostasis in the
whole blood.
Flow cytometry is a platelet function test that helps in monitoring the complete panel for the
activation of platelets and their interaction with other cells. It includes the use of lasers and
identifying the proteins present on the surface of platelets. It also tells about the changes
happening during the time of platelet activation.
Bleeding time is a clinical laboratory test that is performed to evaluate the functions of platelets.
It measures the ability of platelets to from a clot. It involves the creation of incision and timing
the cessation of bleeding.
Platelet functions related to physiological
The primary function of platelet is hemostasis, thrombosis, and healing the wound with a
complex activation process. This leads to integrin activation and the formation at the site of
injury and other physiological roles for the platelet, including immunity and communication.
Platelets secrete many factors which are involved in coagulation and wound healing. During
coagulation, platelets will release several factors that will increase the local platelet aggregation
and mediate inflammation. It promotes blood coagulation by increasing the thrombin and fibrin
(Guo and Rondina, 2019). The physiological effects of low platelet count can result in dangerous
internal bleeding; however, it is very rare. Platelet physiology is small anucleate cell fragments
that circulate in the blood, playing an essential part in managing vascular integrity and regulating
hemostasis. They are involved in the fundamental biological process for chronic inflammation,
which is associated with disease pathology.
they are exposed to different activating substances. This is known as closure time and indicates
lower functions of platelets; however, these tests cannot identify the leading cause. Platelet
aggregometry is the test used in a different clinical settingss and helps monitor the response
towards the anti-platelet therapies. It diagnoses the inherited bleeding disorders within an
individual. Thromboelastometry is a viscoelastic method used for testing hemostasis in the
whole blood.
Flow cytometry is a platelet function test that helps in monitoring the complete panel for the
activation of platelets and their interaction with other cells. It includes the use of lasers and
identifying the proteins present on the surface of platelets. It also tells about the changes
happening during the time of platelet activation.
Bleeding time is a clinical laboratory test that is performed to evaluate the functions of platelets.
It measures the ability of platelets to from a clot. It involves the creation of incision and timing
the cessation of bleeding.
Platelet functions related to physiological
The primary function of platelet is hemostasis, thrombosis, and healing the wound with a
complex activation process. This leads to integrin activation and the formation at the site of
injury and other physiological roles for the platelet, including immunity and communication.
Platelets secrete many factors which are involved in coagulation and wound healing. During
coagulation, platelets will release several factors that will increase the local platelet aggregation
and mediate inflammation. It promotes blood coagulation by increasing the thrombin and fibrin
(Guo and Rondina, 2019). The physiological effects of low platelet count can result in dangerous
internal bleeding; however, it is very rare. Platelet physiology is small anucleate cell fragments
that circulate in the blood, playing an essential part in managing vascular integrity and regulating
hemostasis. They are involved in the fundamental biological process for chronic inflammation,
which is associated with disease pathology.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

CONCLUSION
Platelets circulate with the help of vasculature in an inactive state. When some vascular injury in
the endothelium or alterations to the normal blood flow, platelets will rapidly decelerate and roll
on the endothelium to firmly adhere to the vascular walls. Platelets play an essential role in the
normal hemostatic process by maintaining vascular patency. Platelets are far more complex and
are equipped with elaborated intracellular machinery. They can interact with almost all the
immune cells. Understanding the platelets' activation pathways results in generating new
possibilities in monitoring the diseases and driving responses for its treatment. Moreover, it can
be seen that the shape of the platelets changes after the process of its activation due to various
reason. There are different ways and tests which can be used to measure the platelet functions,
such as platelet dip-stick function tests, which will measure the behavior of platelets while
forming blood clots.
Illustration 2: Haemostasis
Platelets circulate with the help of vasculature in an inactive state. When some vascular injury in
the endothelium or alterations to the normal blood flow, platelets will rapidly decelerate and roll
on the endothelium to firmly adhere to the vascular walls. Platelets play an essential role in the
normal hemostatic process by maintaining vascular patency. Platelets are far more complex and
are equipped with elaborated intracellular machinery. They can interact with almost all the
immune cells. Understanding the platelets' activation pathways results in generating new
possibilities in monitoring the diseases and driving responses for its treatment. Moreover, it can
be seen that the shape of the platelets changes after the process of its activation due to various
reason. There are different ways and tests which can be used to measure the platelet functions,
such as platelet dip-stick function tests, which will measure the behavior of platelets while
forming blood clots.
Illustration 2: Haemostasis

REFERENCES
Books and Journals
Ahmed, and et.al., 2020. Pharmacological blockade of glycoprotein VI promotes thrombus
disaggregation in the absence of thrombin. Arteriosclerosis, Thrombosis, and Vascular
Biology, 40(9), pp.2127-2142.
Asada, and et.al., 2020. Pathophysiology of atherothrombosis: Mechanisms of thrombus
formation on disrupted atherosclerotic plaques. Pathology international, 70(6), pp.309-
322.
Aslan, J.E., 2021. Platelet proteomes, pathways, and phenotypes as informants of vascular
wellness and disease. Arteriosclerosis, thrombosis, and vascular biology, 41(3), pp.999-
1011.
Colberg, and et.al., 2020. Structure and function of the ubiquitin‐proteasome system in
platelets. Journal of Thrombosis and Haemostasis, 18(4), pp.771-780.
Cowman, and et.al., 2017. Platelet behaviour on von Willebrand factor changes in pregnancy:
consequences of haemodilution and intrinsic changes in platelet function. Scientific
reports, 7(1), pp.1-7.
Derbalah, and et.al., 2021. The Influence of Haemostatic System Maturation on the Dose–
Response Relationship of Unfractionated Heparin. Clinical Pharmacokinetics, 60(4),
pp.491-499.
Dietrich-Muszalska, A. and Wachowicz, B., 2017. Platelet haemostatic function in psychiatric
disorders: Effects of antidepressants and antipsychotic drugs. The World Journal of
Biological Psychiatry, 18(8), pp.564-574.
Dovizio, and et.al., 2020. Multifaceted functions of platelets in cancer: From tumorigenesis to
liquid biopsy tool and drug delivery system. International Journal of Molecular
Sciences, 21(24), p.9585.
Etulain, J., 2018. Platelets in wound healing and regenerative medicine. Platelets, 29(6), pp.556-
568.
Guo, L. and Rondina, M.T., 2019. The era of thromboinflammation: platelets are dynamic
sensors and effector cells during infectious diseases. Frontiers in immunology, 10,
p.2204.
Hanke, and et.al., 2018. Dynamics of force generation by spreading platelets. Soft
Matter, 14(31), pp.6571-6581.
Iba, T. and Levy, J.H., 2018. Inflammation and thrombosis: roles of neutrophils, platelets and
endothelial cells and their interactions in thrombus formation during sepsis. Journal of
Thrombosis and Haemostasis, 16(2), pp.231-241.
Koupenova, and et.al., 2018. Circulating platelets as mediators of immunity, inflammation, and
thrombosis. Circulation research, 122(2), pp.337-351.
Lien, and et.al., 2017. Licochalcone A prevents platelet activation and thrombus formation
through the inhibition of PLCγ2-PKC, Akt, and MAPK pathways. International journal
of molecular sciences, 18(7), p.1500.
Lim, W.Y., Lloyd, G. and Bhattacharyya, S., 2017. Mechanical and surgical bioprosthetic valve
thrombosis. Heart, 103(24), pp.1934-1941.
Maas, C. and Renné, T., 2018. Coagulation factor XII in thrombosis and inflammation. Blood,
The Journal of the American Society of Hematology, 131(17), pp.1903-1909.
Books and Journals
Ahmed, and et.al., 2020. Pharmacological blockade of glycoprotein VI promotes thrombus
disaggregation in the absence of thrombin. Arteriosclerosis, Thrombosis, and Vascular
Biology, 40(9), pp.2127-2142.
Asada, and et.al., 2020. Pathophysiology of atherothrombosis: Mechanisms of thrombus
formation on disrupted atherosclerotic plaques. Pathology international, 70(6), pp.309-
322.
Aslan, J.E., 2021. Platelet proteomes, pathways, and phenotypes as informants of vascular
wellness and disease. Arteriosclerosis, thrombosis, and vascular biology, 41(3), pp.999-
1011.
Colberg, and et.al., 2020. Structure and function of the ubiquitin‐proteasome system in
platelets. Journal of Thrombosis and Haemostasis, 18(4), pp.771-780.
Cowman, and et.al., 2017. Platelet behaviour on von Willebrand factor changes in pregnancy:
consequences of haemodilution and intrinsic changes in platelet function. Scientific
reports, 7(1), pp.1-7.
Derbalah, and et.al., 2021. The Influence of Haemostatic System Maturation on the Dose–
Response Relationship of Unfractionated Heparin. Clinical Pharmacokinetics, 60(4),
pp.491-499.
Dietrich-Muszalska, A. and Wachowicz, B., 2017. Platelet haemostatic function in psychiatric
disorders: Effects of antidepressants and antipsychotic drugs. The World Journal of
Biological Psychiatry, 18(8), pp.564-574.
Dovizio, and et.al., 2020. Multifaceted functions of platelets in cancer: From tumorigenesis to
liquid biopsy tool and drug delivery system. International Journal of Molecular
Sciences, 21(24), p.9585.
Etulain, J., 2018. Platelets in wound healing and regenerative medicine. Platelets, 29(6), pp.556-
568.
Guo, L. and Rondina, M.T., 2019. The era of thromboinflammation: platelets are dynamic
sensors and effector cells during infectious diseases. Frontiers in immunology, 10,
p.2204.
Hanke, and et.al., 2018. Dynamics of force generation by spreading platelets. Soft
Matter, 14(31), pp.6571-6581.
Iba, T. and Levy, J.H., 2018. Inflammation and thrombosis: roles of neutrophils, platelets and
endothelial cells and their interactions in thrombus formation during sepsis. Journal of
Thrombosis and Haemostasis, 16(2), pp.231-241.
Koupenova, and et.al., 2018. Circulating platelets as mediators of immunity, inflammation, and
thrombosis. Circulation research, 122(2), pp.337-351.
Lien, and et.al., 2017. Licochalcone A prevents platelet activation and thrombus formation
through the inhibition of PLCγ2-PKC, Akt, and MAPK pathways. International journal
of molecular sciences, 18(7), p.1500.
Lim, W.Y., Lloyd, G. and Bhattacharyya, S., 2017. Mechanical and surgical bioprosthetic valve
thrombosis. Heart, 103(24), pp.1934-1941.
Maas, C. and Renné, T., 2018. Coagulation factor XII in thrombosis and inflammation. Blood,
The Journal of the American Society of Hematology, 131(17), pp.1903-1909.

Maiocchi, and et.al., 2018, March. Thromboinflammatory Functions of Platelets in Ischemia–
Reperfusion Injury and Its Dysregulation in Diabetes. In Seminars in thrombosis and
hemostasis (Vol. 44, No. 02, pp. 102-113). Thieme Medical Publishers.
Nam, and et.al., 2019. Value of computed tomography radiomic features for differentiation of
periprosthetic mass in patients with suspected prosthetic valve obstruction. Circulation:
Cardiovascular Imaging, 12(11), p.e009496.
Qiao and et.al., 2018. Regulation of platelet activation and thrombus formation by reactive
oxygen species. Redox biology, 14, pp.126-130.
Scharf, R.E., 2018. Platelet signaling in primary haemostasis and arterial thrombus formation:
Part 1. Hämostaseologie, 38(04), pp.203-210.
Shou and et.al., 2017. Multifunctional biomedical imaging in physiological and pathological
conditions using a NIR‐II Probe. Advanced functional materials, 27(23), p.1700995.
Trugilho, and et.al., 2017. Platelet proteome reveals novel pathways of platelet activation and
platelet-mediated immunoregulation in dengue. PLoS pathogens, 13(5), p.e1006385.
Wang, and et.al., 2017. Platelet mitochondrial dysfunction and the correlation with human
diseases. Biochemical Society Transactions, 45(6), pp.1213-1223.
Online
Hemostasis, 2021. [Online]. Available through:
<https://www.lecturio.com/concepts/hemostasis/>
Platelet plug formation, 2021. [Online]. Available through:
<https://www.researchgate.net/figure/Steps-in-platelet-plug-formation-A-Prior-to-
vascular-injury-platelets-are-maintained_fig2_8888918
Reperfusion Injury and Its Dysregulation in Diabetes. In Seminars in thrombosis and
hemostasis (Vol. 44, No. 02, pp. 102-113). Thieme Medical Publishers.
Nam, and et.al., 2019. Value of computed tomography radiomic features for differentiation of
periprosthetic mass in patients with suspected prosthetic valve obstruction. Circulation:
Cardiovascular Imaging, 12(11), p.e009496.
Qiao and et.al., 2018. Regulation of platelet activation and thrombus formation by reactive
oxygen species. Redox biology, 14, pp.126-130.
Scharf, R.E., 2018. Platelet signaling in primary haemostasis and arterial thrombus formation:
Part 1. Hämostaseologie, 38(04), pp.203-210.
Shou and et.al., 2017. Multifunctional biomedical imaging in physiological and pathological
conditions using a NIR‐II Probe. Advanced functional materials, 27(23), p.1700995.
Trugilho, and et.al., 2017. Platelet proteome reveals novel pathways of platelet activation and
platelet-mediated immunoregulation in dengue. PLoS pathogens, 13(5), p.e1006385.
Wang, and et.al., 2017. Platelet mitochondrial dysfunction and the correlation with human
diseases. Biochemical Society Transactions, 45(6), pp.1213-1223.
Online
Hemostasis, 2021. [Online]. Available through:
<https://www.lecturio.com/concepts/hemostasis/>
Platelet plug formation, 2021. [Online]. Available through:
<https://www.researchgate.net/figure/Steps-in-platelet-plug-formation-A-Prior-to-
vascular-injury-platelets-are-maintained_fig2_8888918
1 out of 13
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.





