Investigation of the complementary and alternative medicine’s benefits in cancer pain management: A comparative study between cannabis and morphine
VerifiedAdded on 2023/01/19
|51
|12010
|89
AI Summary
This research investigation aims to evaluate and explore the effect of traditional and alternative medicine such as Cannabis and Morphine in pain management of cancer patients.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: DISSERTATION
DISSERTATION ON EVIDENCE BASED NURSING
Name of the Student
Name of the University
Submission Date:
Word Count:
DISSERTATION ON EVIDENCE BASED NURSING
Name of the Student
Name of the University
Submission Date:
Word Count:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1DISSERTATION
Investigation of the complementary and alternative medicine’s benefits
in cancer pain management: A comparative study between cannabis and
morphine.
Investigation of the complementary and alternative medicine’s benefits
in cancer pain management: A comparative study between cannabis and
morphine.

2DISSERTATION
Abstract
Background: Morphine has been one of the frequently used opioids to treat serious cancer
injuries. In this regard, cancer is an acute and chronic disease sufficient to cause immense
suffering for the person, and also to reduce the willingness to live longer. Thus, morphine, a
chain opiate painkiller, is amongst the most commonly used analgesics for pain. The absence of
clinical evidence on cannabis used as medicinal purpose for the treatment of cancer pain is still
inconclusive which limited its investigation as a potential medical product because of its lack
of classification as an agent for schedule I by the Controlled Substances Act of 1970.
Aim: This research investigation aim was to evaluate and explore the effect of traditional and
alternative medicine such as Cannabis and Morphine in pain management of cancer patient.
Research question: Research questions for this investigation was “Does Morphine or Cannabis
improve the pain management among the patients living with cancer?”
Design: Rapid Evidence Assessment was used as a study design for this investigation.
Method: Electronic searches have been conducted in two databases with key words for this
investigation. Identified research publication were selected for relevancy according to the
inclusion criterion adopted or this study, evaluated for quality, and relevant data and the analysis
were conducted on the extracted data.
Ethical consideration: No primary research has been conducted for this research and only
secondary research has been conducted for this study. Therefore, no ethical consideration was
required for this study.
Abstract
Background: Morphine has been one of the frequently used opioids to treat serious cancer
injuries. In this regard, cancer is an acute and chronic disease sufficient to cause immense
suffering for the person, and also to reduce the willingness to live longer. Thus, morphine, a
chain opiate painkiller, is amongst the most commonly used analgesics for pain. The absence of
clinical evidence on cannabis used as medicinal purpose for the treatment of cancer pain is still
inconclusive which limited its investigation as a potential medical product because of its lack
of classification as an agent for schedule I by the Controlled Substances Act of 1970.
Aim: This research investigation aim was to evaluate and explore the effect of traditional and
alternative medicine such as Cannabis and Morphine in pain management of cancer patient.
Research question: Research questions for this investigation was “Does Morphine or Cannabis
improve the pain management among the patients living with cancer?”
Design: Rapid Evidence Assessment was used as a study design for this investigation.
Method: Electronic searches have been conducted in two databases with key words for this
investigation. Identified research publication were selected for relevancy according to the
inclusion criterion adopted or this study, evaluated for quality, and relevant data and the analysis
were conducted on the extracted data.
Ethical consideration: No primary research has been conducted for this research and only
secondary research has been conducted for this study. Therefore, no ethical consideration was
required for this study.

3DISSERTATION
Results: In total six recent researches journal was used to evaluate the research question
presented in this study. Three of them related to morphine and three of them are related to the
Cannabis. In the data extraction process, it has been found out that both cannabis and morphine
can be used to treat pain among the patients suffering from cancer.
Conclusion: In a nutshell, it can be stated that the both methods used for the treatment of the pain
among cancer patients can be compared. However, the significant difference among them is that
morphine has more adverse effect when used as a first line treatment. Nonetheless, further
studies are required for the standardisation and regulatory administration of morphine and
cannabis as treatment for cancer pain.
Results: In total six recent researches journal was used to evaluate the research question
presented in this study. Three of them related to morphine and three of them are related to the
Cannabis. In the data extraction process, it has been found out that both cannabis and morphine
can be used to treat pain among the patients suffering from cancer.
Conclusion: In a nutshell, it can be stated that the both methods used for the treatment of the pain
among cancer patients can be compared. However, the significant difference among them is that
morphine has more adverse effect when used as a first line treatment. Nonetheless, further
studies are required for the standardisation and regulatory administration of morphine and
cannabis as treatment for cancer pain.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

4DISSERTATION
Glossary of Acronyms
CBD :Cannabidiol
REA : Rapid Evidence Assessment
THC : Tetrahydrocannabinol
PICO : Population, Intervention, Comparison, Outcome
ECLIPSE : Expectation, Client group, Location, Impact, Professionals, Service
CIMO : Context, Intervention, Mechanism, Outcome
RCT : Randomised Controlled Trial
QOL : Quality of Life
Glossary of Acronyms
CBD :Cannabidiol
REA : Rapid Evidence Assessment
THC : Tetrahydrocannabinol
PICO : Population, Intervention, Comparison, Outcome
ECLIPSE : Expectation, Client group, Location, Impact, Professionals, Service
CIMO : Context, Intervention, Mechanism, Outcome
RCT : Randomised Controlled Trial
QOL : Quality of Life

5DISSERTATION
Table of Contents
1. Introduction..................................................................................................................................8
1.1 Rationale behind the research investigation..........................................................................8
1.2 Introduction to Cannabis for pain management in cancer.....................................................9
1.3 Introduction to Morphine for pain management in cancer..................................................10
1.4 Aim and objectives..............................................................................................................11
1.5 Study design.........................................................................................................................12
1.6 Ethical consideration...........................................................................................................13
2. Methods.....................................................................................................................................14
2.1 Research question................................................................................................................14
2.2 Search strategy.....................................................................................................................15
2.3 Rationale behind the search terms.......................................................................................17
2.4 Article Selection..................................................................................................................19
2.5 Inclusion and exclusion criteria...........................................................................................19
2.6 Data synthesis and extraction..............................................................................................20
2.7 Quality appraisal..................................................................................................................22
Table of Contents
1. Introduction..................................................................................................................................8
1.1 Rationale behind the research investigation..........................................................................8
1.2 Introduction to Cannabis for pain management in cancer.....................................................9
1.3 Introduction to Morphine for pain management in cancer..................................................10
1.4 Aim and objectives..............................................................................................................11
1.5 Study design.........................................................................................................................12
1.6 Ethical consideration...........................................................................................................13
2. Methods.....................................................................................................................................14
2.1 Research question................................................................................................................14
2.2 Search strategy.....................................................................................................................15
2.3 Rationale behind the search terms.......................................................................................17
2.4 Article Selection..................................................................................................................19
2.5 Inclusion and exclusion criteria...........................................................................................19
2.6 Data synthesis and extraction..............................................................................................20
2.7 Quality appraisal..................................................................................................................22

6DISSERTATION
3. Results........................................................................................................................................24
3.1 Summary of the included studies.........................................................................................24
3.2 Summary of the excluded studies and rationale behind exclusion......................................24
3.3 Description of the study findings.........................................................................................24
3.3.1 Bandieri et al. 2016.......................................................................................................24
3.3.2 Riley et al. 2015............................................................................................................25
3.3.3Nunes, Garcia and Sakata 2014.....................................................................................27
3.3.4Khaiser et al. 2016.........................................................................................................28
3.3.5 Abrams 2016.................................................................................................................30
3.3.6 Shaw et al. 2017............................................................................................................31
4. Discussion..................................................................................................................................33
4.1 Discussion on morphine as a pain management among cancer patients.............................33
4.2 Discussion on Cannabis as a pain management among cancer patients..............................35
5. Conclusion.................................................................................................................................38
5.1 Summary..............................................................................................................................38
5.2 Limitations...........................................................................................................................39
5.3 Recommendations................................................................................................................39
3. Results........................................................................................................................................24
3.1 Summary of the included studies.........................................................................................24
3.2 Summary of the excluded studies and rationale behind exclusion......................................24
3.3 Description of the study findings.........................................................................................24
3.3.1 Bandieri et al. 2016.......................................................................................................24
3.3.2 Riley et al. 2015............................................................................................................25
3.3.3Nunes, Garcia and Sakata 2014.....................................................................................27
3.3.4Khaiser et al. 2016.........................................................................................................28
3.3.5 Abrams 2016.................................................................................................................30
3.3.6 Shaw et al. 2017............................................................................................................31
4. Discussion..................................................................................................................................33
4.1 Discussion on morphine as a pain management among cancer patients.............................33
4.2 Discussion on Cannabis as a pain management among cancer patients..............................35
5. Conclusion.................................................................................................................................38
5.1 Summary..............................................................................................................................38
5.2 Limitations...........................................................................................................................39
5.3 Recommendations................................................................................................................39
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7DISSERTATION
6. References..................................................................................................................................41
7. Appendices................................................................................................................................47
7.1 Appendix – I: Evidence level..............................................................................................47
7.2 Appendix – II: Evidence hierarchy level.............................................................................48
7.3 Appendix – III: Evidence hierarchy level for qualitative studies........................................49
6. References..................................................................................................................................41
7. Appendices................................................................................................................................47
7.1 Appendix – I: Evidence level..............................................................................................47
7.2 Appendix – II: Evidence hierarchy level.............................................................................48
7.3 Appendix – III: Evidence hierarchy level for qualitative studies........................................49

8DISSERTATION
Chapter 1
1. Introduction
1.1 Rationale behind the research investigation
The adverse impacts of morphine or any opioid derived drugs have been studied several times
over but no sufficient attempts have been made to create alternative medications to substitute the
widely used opioid- based pain relievers even though various adverse impacts of long-term use
are present (Wiffen, Wee and Moore 2016). Morphine, though, has been one of the frequently
used opioids to treat serious cancer injuries. In this regard, cancer is an acute and chronic disease
sufficient to cause immense suffering for the person, and also to reduce the willingness to live
longer. Thus, morphine, a chain opiate painkiller, is amongst the most commonly used analgesics
for pain (Money and Garber 2018). However opioid-based treatment such as the morphine, as
described by Bandieri et al. (2015), is still very tentative and with many discretions used in
clinical exercise, even in the palliative medication field, despite its effectively and efficiently
reducing even severe suffering such as cancer-related suffering. As Wiffen et al. (2017) have
mentioned, the use of morphine can be viewed as a balance act, and many writers and clinics
have even taken into account the use of moral suffering as a compensation between pain relief
and negative impacts (Mercadante et al. 2016). Recent study uses morphine as a synonym for or
marker of imminent mortality and cancer suffering is also a benchmark for a deep existential
illness. In addition, the morphine mode of action is mediated by a receptor binding process,
particularly the CNS Opioid cells. As Mercadante et al. (2015) discusses, morphine mainly
operates on mu- opioid receptors by catalysing a sequence of responses that in turn alter the
Chapter 1
1. Introduction
1.1 Rationale behind the research investigation
The adverse impacts of morphine or any opioid derived drugs have been studied several times
over but no sufficient attempts have been made to create alternative medications to substitute the
widely used opioid- based pain relievers even though various adverse impacts of long-term use
are present (Wiffen, Wee and Moore 2016). Morphine, though, has been one of the frequently
used opioids to treat serious cancer injuries. In this regard, cancer is an acute and chronic disease
sufficient to cause immense suffering for the person, and also to reduce the willingness to live
longer. Thus, morphine, a chain opiate painkiller, is amongst the most commonly used analgesics
for pain (Money and Garber 2018). However opioid-based treatment such as the morphine, as
described by Bandieri et al. (2015), is still very tentative and with many discretions used in
clinical exercise, even in the palliative medication field, despite its effectively and efficiently
reducing even severe suffering such as cancer-related suffering. As Wiffen et al. (2017) have
mentioned, the use of morphine can be viewed as a balance act, and many writers and clinics
have even taken into account the use of moral suffering as a compensation between pain relief
and negative impacts (Mercadante et al. 2016). Recent study uses morphine as a synonym for or
marker of imminent mortality and cancer suffering is also a benchmark for a deep existential
illness. In addition, the morphine mode of action is mediated by a receptor binding process,
particularly the CNS Opioid cells. As Mercadante et al. (2015) discusses, morphine mainly
operates on mu- opioid receptors by catalysing a sequence of responses that in turn alter the

9DISSERTATION
regulation of pain response in the human body. Morphine is undoubtedly an amazing treatment
of pain and, but at the other side, it is also has a highly significant list of secondary impacts. The
danger of dependency and withdrawal is particularly strong for patients who receive morphine.
Furthermore, the administration of morphine has several other side impacts for a lengthy moment
(Abrams 2016). These adverse implications include severe nausea, constipation, dullness,
severe dry throat, headache, mood depression, sudden and uncontrollable bodily wobbles, loss of
gender or libido, localized discomfort, particularly in the abdomen, insomnia, drowsiness, and
excessive tiredness in multiple areas of the body, lack of appetites, swelling of both arm and
ultimate bodily limbs (Bandieri et al. 2016). The use of an alternative option that can be given by
marijuana can therefore be a very important answer to the upcoming opioid outbreak. The
patients can create an opiates’ tolerance which requires an increasing amount of the drug to
obtain the required effects (Abrams and Guzman 2015). In an elaborate sense, Cannabis is an
ancient natural plant compound claimed to have a variety of medicinal characteristics. One of the
countless health advantages is managing acute pain sensations Cannabis, if efficiently applied,
can surpass morphine while being secure and non-toxic. This research aims to strengthen and
evaluate current proof on this subject using the organizational form of the systematic evaluation
and to solve the query whether marijuana and morphine are leading to better clinical pain
leadership, particularly for cancer- related injuries (Garud et al. 2017).
1.2 Introduction to Cannabis for pain management in cancer
The absence of clinical evidence on cannabis used as medical remedy for the treatment of cancer
pain is still inconclusive which limited its examination as a potential medical product because of
its lack of classification as an agent for schedule I by the Controlled Substances Act of 1970
(Borgelt et al. 2013). Nevertheless, several surveys generated on the treatment of cancer
regulation of pain response in the human body. Morphine is undoubtedly an amazing treatment
of pain and, but at the other side, it is also has a highly significant list of secondary impacts. The
danger of dependency and withdrawal is particularly strong for patients who receive morphine.
Furthermore, the administration of morphine has several other side impacts for a lengthy moment
(Abrams 2016). These adverse implications include severe nausea, constipation, dullness,
severe dry throat, headache, mood depression, sudden and uncontrollable bodily wobbles, loss of
gender or libido, localized discomfort, particularly in the abdomen, insomnia, drowsiness, and
excessive tiredness in multiple areas of the body, lack of appetites, swelling of both arm and
ultimate bodily limbs (Bandieri et al. 2016). The use of an alternative option that can be given by
marijuana can therefore be a very important answer to the upcoming opioid outbreak. The
patients can create an opiates’ tolerance which requires an increasing amount of the drug to
obtain the required effects (Abrams and Guzman 2015). In an elaborate sense, Cannabis is an
ancient natural plant compound claimed to have a variety of medicinal characteristics. One of the
countless health advantages is managing acute pain sensations Cannabis, if efficiently applied,
can surpass morphine while being secure and non-toxic. This research aims to strengthen and
evaluate current proof on this subject using the organizational form of the systematic evaluation
and to solve the query whether marijuana and morphine are leading to better clinical pain
leadership, particularly for cancer- related injuries (Garud et al. 2017).
1.2 Introduction to Cannabis for pain management in cancer
The absence of clinical evidence on cannabis used as medical remedy for the treatment of cancer
pain is still inconclusive which limited its examination as a potential medical product because of
its lack of classification as an agent for schedule I by the Controlled Substances Act of 1970
(Borgelt et al. 2013). Nevertheless, several surveys generated on the treatment of cancer
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

10DISSERTATION
suffering with medical marijuana indicate that they have beneficial capacity and at least need
further research. There are no dosing rules in clinical exercise for the use of therapies based on
cannabinoid usage. The perfect dose would allow an efficient treatment of pain but does not
generate unbearable side impacts. Moreover, it is difficult for the complicated cancer patient
populations to achieve this ideal dose (Blake et al. 2017). One of those is the person to person
variation according to the outcomes of the drugs and other pharmaceuticals intervention. Since
the appropriate dose has been observed to differ from patient to patient, doctors need to know
how the right dosage is determined when a fresh patient is prescribed. Advanced patients with
disease are also expected to show complicated symptoms, which render it hard, and often take
several concomitant medicines, to precisely evaluate the side impacts of marijuana medicines
(Blake et al. 2017).However, a multitude of such studies have noted that detected side effects
appear to not be limiting the therapy and that dose titration may be controlled by pain relief in a
low dosage of 2.7 to 10.8 milligrams THC combined with 2.5–10.0 milligrams CBD. This shows
the need to establish and validate a titration protocol to enable scientists to safely and effectively
define efficient and tolerated dosages (Lynch, Cesar - Rittenberg and Hohmann 2014).
1.3 Introduction to Morphine for pain management in cancer
After the World Health organization's (WHO) instructions were published in the mid-1980s,
treatment of mild to serious cancer suffering became common every four hours of daily
administration of aqueous morphine remedy (Wiffen, Wee and Moore 2016). Morphine was
initially commercialised at around the same moment in a release tablet form to extend the dosage
period to 12 hours. Both 12 hour and 24 hour discharge tablets are accessible and should be
swallowed in their entirety (Wiffen, Wee and Moore 2016). Modified release tablets contain
covered tiny beads and, if needed, may be sprinkled with food. Most trials contrasted oral dosing
suffering with medical marijuana indicate that they have beneficial capacity and at least need
further research. There are no dosing rules in clinical exercise for the use of therapies based on
cannabinoid usage. The perfect dose would allow an efficient treatment of pain but does not
generate unbearable side impacts. Moreover, it is difficult for the complicated cancer patient
populations to achieve this ideal dose (Blake et al. 2017). One of those is the person to person
variation according to the outcomes of the drugs and other pharmaceuticals intervention. Since
the appropriate dose has been observed to differ from patient to patient, doctors need to know
how the right dosage is determined when a fresh patient is prescribed. Advanced patients with
disease are also expected to show complicated symptoms, which render it hard, and often take
several concomitant medicines, to precisely evaluate the side impacts of marijuana medicines
(Blake et al. 2017).However, a multitude of such studies have noted that detected side effects
appear to not be limiting the therapy and that dose titration may be controlled by pain relief in a
low dosage of 2.7 to 10.8 milligrams THC combined with 2.5–10.0 milligrams CBD. This shows
the need to establish and validate a titration protocol to enable scientists to safely and effectively
define efficient and tolerated dosages (Lynch, Cesar - Rittenberg and Hohmann 2014).
1.3 Introduction to Morphine for pain management in cancer
After the World Health organization's (WHO) instructions were published in the mid-1980s,
treatment of mild to serious cancer suffering became common every four hours of daily
administration of aqueous morphine remedy (Wiffen, Wee and Moore 2016). Morphine was
initially commercialised at around the same moment in a release tablet form to extend the dosage
period to 12 hours. Both 12 hour and 24 hour discharge tablets are accessible and should be
swallowed in their entirety (Wiffen, Wee and Moore 2016). Modified release tablets contain
covered tiny beads and, if needed, may be sprinkled with food. Most trials contrasted oral dosing

11DISSERTATION
of morphine or oral morphine formulations with other oral opioids on separate paths of
administration on occasion. For any specific comparative there was no collection of proof.
Evidence should have been still helpful if continuously reported results were that a patient
reported result of recognized benefits was obtained with respondents receiving excellent pain
relief ideally not worse than moderate pain. A particular focus on research that attempted to
identify tiny effective distinctions or harms between formulas, opioids or both has also hindered
the application of the proof (Wiffen, Wee and Moore 2016).
1.4 Aim and objectives
Aim and objectives are two most vital part of a research investigation and it is a very integral
part of the research topic. Therefore, the aim and objectives of a research topic should be
designed and developed in a concise and clear manner, so that it can effectively and concisely
convey the message and objectives of the research topic. Academics have suggested that the
aims and objective should clearly state the purpose of the whole project, the clear reason it is
being conducted for, outline and overall structure of the project and expected outcome of the
project (Booth, Sutton and Papaioannou 2016). Therefore, during the development of this
project’s aim and objective, the above criteria of the aims and objectives were considered. The
aim and objective of this investigation project is mentioned below:
Aim: This research investigation’s aim was evaluate and explore the effect of traditional and
alternative medicine such as Cannabis and Morphine in pain management of cancer patient.
Objectives:
Analysis and identification of current research publication on the use and efficacy of
of morphine or oral morphine formulations with other oral opioids on separate paths of
administration on occasion. For any specific comparative there was no collection of proof.
Evidence should have been still helpful if continuously reported results were that a patient
reported result of recognized benefits was obtained with respondents receiving excellent pain
relief ideally not worse than moderate pain. A particular focus on research that attempted to
identify tiny effective distinctions or harms between formulas, opioids or both has also hindered
the application of the proof (Wiffen, Wee and Moore 2016).
1.4 Aim and objectives
Aim and objectives are two most vital part of a research investigation and it is a very integral
part of the research topic. Therefore, the aim and objectives of a research topic should be
designed and developed in a concise and clear manner, so that it can effectively and concisely
convey the message and objectives of the research topic. Academics have suggested that the
aims and objective should clearly state the purpose of the whole project, the clear reason it is
being conducted for, outline and overall structure of the project and expected outcome of the
project (Booth, Sutton and Papaioannou 2016). Therefore, during the development of this
project’s aim and objective, the above criteria of the aims and objectives were considered. The
aim and objective of this investigation project is mentioned below:
Aim: This research investigation’s aim was evaluate and explore the effect of traditional and
alternative medicine such as Cannabis and Morphine in pain management of cancer patient.
Objectives:
Analysis and identification of current research publication on the use and efficacy of

12DISSERTATION
alternative and traditional method like Cannabis and Morphine in pain management.
Evaluation and exploration of current research publication on the efficacy of Cannabis as
pain management among cancer patient.
Evaluation and exploration of current research publication on the efficacy of Morphine as
pain management among cancer patient.
Report and identification of relevant data whether these pain management techniques
effectively applied in clinical settings.
1.5 Study design
REA or Rapid evidence assessment is an effective tool for analysing and evaluating existing
research among a limited time frame. It is allows the investigator of the research to provide a
critical evidence based overview on the research topic as well as to find a research gap among
the existing research publication (Haby et al. 2016). In addition to that, REA also allows the
investigator to find, appraise, gather and summarize data on a particular research topic and it can
be done on the within a short time frame like 2 – 3 months. This application and implementation
of evidence based method is particularly effective in the area of the health care practice (Haby et
al. 2016). From the above discussion, it can be stated that the REA can be used effectively to
gather, analyse, and explore high quality research publication within short frame which very
much in line with the timeframe of this investigation. Therefore, REA will be used as a study
design for this research investigation.
However, there are some limits for the Rapid Evidence Assessment study design. The assurance
of the outcomes of this study design can be lower due to the publication bias (Ganann, Ciliska
and Thomas2010). Owing to the short time frame of this study design, it is less demanding than a
alternative and traditional method like Cannabis and Morphine in pain management.
Evaluation and exploration of current research publication on the efficacy of Cannabis as
pain management among cancer patient.
Evaluation and exploration of current research publication on the efficacy of Morphine as
pain management among cancer patient.
Report and identification of relevant data whether these pain management techniques
effectively applied in clinical settings.
1.5 Study design
REA or Rapid evidence assessment is an effective tool for analysing and evaluating existing
research among a limited time frame. It is allows the investigator of the research to provide a
critical evidence based overview on the research topic as well as to find a research gap among
the existing research publication (Haby et al. 2016). In addition to that, REA also allows the
investigator to find, appraise, gather and summarize data on a particular research topic and it can
be done on the within a short time frame like 2 – 3 months. This application and implementation
of evidence based method is particularly effective in the area of the health care practice (Haby et
al. 2016). From the above discussion, it can be stated that the REA can be used effectively to
gather, analyse, and explore high quality research publication within short frame which very
much in line with the timeframe of this investigation. Therefore, REA will be used as a study
design for this research investigation.
However, there are some limits for the Rapid Evidence Assessment study design. The assurance
of the outcomes of this study design can be lower due to the publication bias (Ganann, Ciliska
and Thomas2010). Owing to the short time frame of this study design, it is less demanding than a
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

13DISSERTATION
systematic review and it omits the area of dissertation and unpublished research. However, if
strict exclusion and inclusion criteria can be implemented to the REA study design to make it
less prone to publication bias (Ganann, Ciliska and Thomas2010).
1.6 Ethical consideration
The study design of this assignment is REA and no primary research has been conducted for this
research. Only secondary research has been conducted for this study. Therefore, no ethical
consideration was exclusively required for this study.
systematic review and it omits the area of dissertation and unpublished research. However, if
strict exclusion and inclusion criteria can be implemented to the REA study design to make it
less prone to publication bias (Ganann, Ciliska and Thomas2010).
1.6 Ethical consideration
The study design of this assignment is REA and no primary research has been conducted for this
research. Only secondary research has been conducted for this study. Therefore, no ethical
consideration was exclusively required for this study.

14DISSERTATION
Chapter 2
2. Methods
2.1 Research question
In all scientific studies, it is highly important to implement a focused question model. In this
sense, the significance of a focused study issue must be recognized as ideal in simplifying and
clarifying the precise subject on which the study will be based. A focused research issue enables
scientists to advance a research study in a well guided manner and enables them explore and deal
with the precise and particular fields of interest in the subject in an ideal way (Peters et al. 2015).
Different designs are accessible for study questions. Other design includes Context, Intervention,
Mechanism, Outcome (CIMO) model (Wong et al. 2013), and the Expectation, Client group,
Location, Impact, Professionals, Service (ECLIPSE) framework (Wildridge and Bell 2002).
PICO is a well-developed medical study structure (Davies 2011) that could assist to formulate a
clearly specified and restricted query that can be used to guide the REA method. The PICO
structure has, however, been established to assist scientists respond to and adapt the layout of
this REA to health-related issues (Davies 2011).
PICO question for this research is presented in the Table 1 below.
Table 1: PICO Questions
Population : Patients living with cancer
Chapter 2
2. Methods
2.1 Research question
In all scientific studies, it is highly important to implement a focused question model. In this
sense, the significance of a focused study issue must be recognized as ideal in simplifying and
clarifying the precise subject on which the study will be based. A focused research issue enables
scientists to advance a research study in a well guided manner and enables them explore and deal
with the precise and particular fields of interest in the subject in an ideal way (Peters et al. 2015).
Different designs are accessible for study questions. Other design includes Context, Intervention,
Mechanism, Outcome (CIMO) model (Wong et al. 2013), and the Expectation, Client group,
Location, Impact, Professionals, Service (ECLIPSE) framework (Wildridge and Bell 2002).
PICO is a well-developed medical study structure (Davies 2011) that could assist to formulate a
clearly specified and restricted query that can be used to guide the REA method. The PICO
structure has, however, been established to assist scientists respond to and adapt the layout of
this REA to health-related issues (Davies 2011).
PICO question for this research is presented in the Table 1 below.
Table 1: PICO Questions
Population : Patients living with cancer

15DISSERTATION
Intervention : Cannabis
Comparison : Morphine
Outcome : Improved pain management and pain free rates
Hence, the questions chosen for this research investigation was “Does Morphine or Cannabis
improve the pain management among the patients living with cancer?”
2.2 Search strategy
This segment of the research proposition presents a brief idea of the precise steps adopted to
effectively finish the research study. A research technique is used step-by-step for the precise
operations which were conducted in the precise series to produce the required result for
completing the research. Here, the selected research technique was systemic evaluation
(Silverman 2016) to be used in this specific research analysis. In this situation, the scholarly
databases related to health care and therapeutic or clinical studies have been researched in
detailed manner. In order to define the pattern of the information findings on the subject, a
preliminary check will be made with Google's information repository. Based on the PICO query
a search will then be conducted for a comprehensive literature search the particular databases,
such as Medline and CINAHL.The search phrases that was utilized in conducting the research
study includes “morphine”, “cannabis”, “alternative pain medication”, “traditional pain
management”, “cancer pain”, “side effects”, “benefits”, “pain management” and “comparative
benefits”. A thorough and critical analysis of the studies was carried out using the inclusion and
Intervention : Cannabis
Comparison : Morphine
Outcome : Improved pain management and pain free rates
Hence, the questions chosen for this research investigation was “Does Morphine or Cannabis
improve the pain management among the patients living with cancer?”
2.2 Search strategy
This segment of the research proposition presents a brief idea of the precise steps adopted to
effectively finish the research study. A research technique is used step-by-step for the precise
operations which were conducted in the precise series to produce the required result for
completing the research. Here, the selected research technique was systemic evaluation
(Silverman 2016) to be used in this specific research analysis. In this situation, the scholarly
databases related to health care and therapeutic or clinical studies have been researched in
detailed manner. In order to define the pattern of the information findings on the subject, a
preliminary check will be made with Google's information repository. Based on the PICO query
a search will then be conducted for a comprehensive literature search the particular databases,
such as Medline and CINAHL.The search phrases that was utilized in conducting the research
study includes “morphine”, “cannabis”, “alternative pain medication”, “traditional pain
management”, “cancer pain”, “side effects”, “benefits”, “pain management” and “comparative
benefits”. A thorough and critical analysis of the studies was carried out using the inclusion and
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

16DISSERTATION
exclusion criteria. For this investigation, the research papers published after 2014, edited in
English, evaluated by peers and research on human subject chosen. All research plans and
evidence syntheses have been prioritized through publications that have consistently
strengthened accessible evidence. Scientific studies published in other languages than English
have been published before 2014, have not been peer- reviewed and have been omitted from
study clinical current studies on topics other than individuals (Flick 2018). Scientific papers
included in the analysis of evidence or systematic studies as the main format in this specific
investigation were not included in the evaluation separately but were also included as evidence in
the systemic assessment or the synthesis of evidence.
exclusion criteria. For this investigation, the research papers published after 2014, edited in
English, evaluated by peers and research on human subject chosen. All research plans and
evidence syntheses have been prioritized through publications that have consistently
strengthened accessible evidence. Scientific studies published in other languages than English
have been published before 2014, have not been peer- reviewed and have been omitted from
study clinical current studies on topics other than individuals (Flick 2018). Scientific papers
included in the analysis of evidence or systematic studies as the main format in this specific
investigation were not included in the evaluation separately but were also included as evidence in
the systemic assessment or the synthesis of evidence.
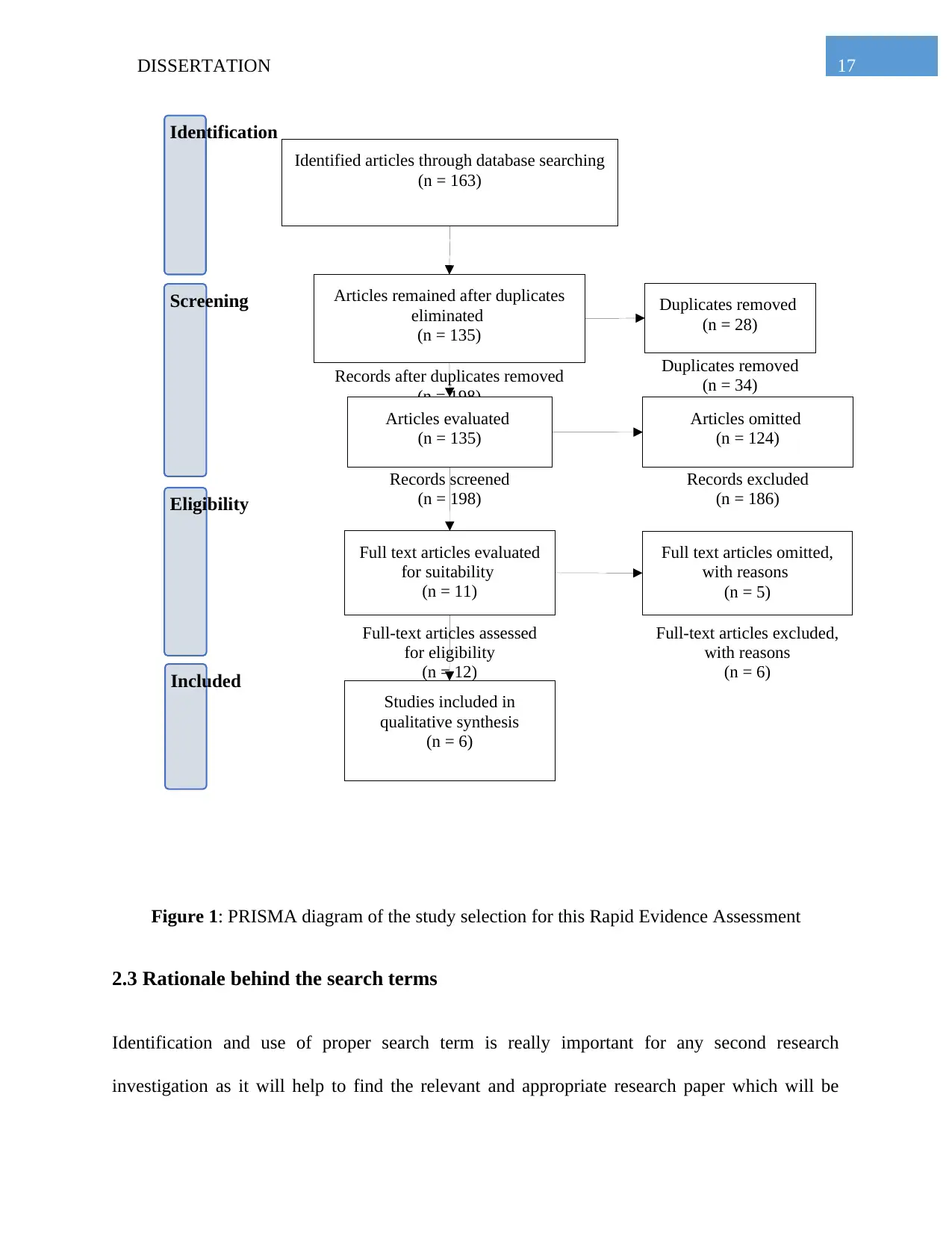
Identified articles through database searching
(n = 163)
Articles remained after duplicates
eliminated
(n = 135)
Records after duplicates removed
(n = 198)
Duplicates removed
(n = 28)
Duplicates removed
(n = 34)
Articles evaluated
(n = 135)
Records screened
(n = 198)
Articles omitted
(n = 124)
Records excluded
(n = 186)
Full text articles evaluated
for suitability
(n = 11)
Full-text articles assessed
for eligibility
(n = 12)
Full text articles omitted,
with reasons
(n = 5)
Full-text articles excluded,
with reasons
(n = 6)
Studies included in
qualitative synthesis
(n = 6)
Included
Screening
Eligibility
Identification
17DISSERTATION
Figure 1: PRISMA diagram of the study selection for this Rapid Evidence Assessment
2.3 Rationale behind the search terms
Identification and use of proper search term is really important for any second research
investigation as it will help to find the relevant and appropriate research paper which will be
(n = 163)
Articles remained after duplicates
eliminated
(n = 135)
Records after duplicates removed
(n = 198)
Duplicates removed
(n = 28)
Duplicates removed
(n = 34)
Articles evaluated
(n = 135)
Records screened
(n = 198)
Articles omitted
(n = 124)
Records excluded
(n = 186)
Full text articles evaluated
for suitability
(n = 11)
Full-text articles assessed
for eligibility
(n = 12)
Full text articles omitted,
with reasons
(n = 5)
Full-text articles excluded,
with reasons
(n = 6)
Studies included in
qualitative synthesis
(n = 6)
Included
Screening
Eligibility
Identification
17DISSERTATION
Figure 1: PRISMA diagram of the study selection for this Rapid Evidence Assessment
2.3 Rationale behind the search terms
Identification and use of proper search term is really important for any second research
investigation as it will help to find the relevant and appropriate research paper which will be

18DISSERTATION
effective for the determination and evaluation of the research question mentioned above (Smith
et al. 2011). In this research, the research term was initially derived from the PICO questions
provided above. The search term that were used are “morphine”, “cannabis”, “alternative pain
medication”, “traditional pain management”, “cancer pain”, “side effects”, “benefits”, “pain
management” and “comparative benefits”. However, all these search term are not provided in the
title of the research papers only, hence, the search throughout text of the articles was chosen in
this investigation. By this method, adequate amount of research paper were extracted which were
then screened for the selection of the proper and appropriate study. However, for the
convenience, the search terms that were used are provided in the tabular format in the Table 2
below.
Table 2: List of search terms used in this research investigation.
Search Terms used for the retrieval of the studies
Morphine
Cannabis
Alternative Pain Medication
Traditional Pain Management
Cancer Pain
Side Effects
Benefits
Pain Management
Comparative Benefits
* CINAHL and Medline databases were used for the search of the research papers.
effective for the determination and evaluation of the research question mentioned above (Smith
et al. 2011). In this research, the research term was initially derived from the PICO questions
provided above. The search term that were used are “morphine”, “cannabis”, “alternative pain
medication”, “traditional pain management”, “cancer pain”, “side effects”, “benefits”, “pain
management” and “comparative benefits”. However, all these search term are not provided in the
title of the research papers only, hence, the search throughout text of the articles was chosen in
this investigation. By this method, adequate amount of research paper were extracted which were
then screened for the selection of the proper and appropriate study. However, for the
convenience, the search terms that were used are provided in the tabular format in the Table 2
below.
Table 2: List of search terms used in this research investigation.
Search Terms used for the retrieval of the studies
Morphine
Cannabis
Alternative Pain Medication
Traditional Pain Management
Cancer Pain
Side Effects
Benefits
Pain Management
Comparative Benefits
* CINAHL and Medline databases were used for the search of the research papers.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

19DISSERTATION
2.4 Article Selection
By using the above mentioned search terms all total of 163 articles were extracted from the
search databases. Out of these 163 articles, there were some duplicates of the retrieved articles. A
total number of 28 duplicates were present and they were removed from the search results. After
the removal of the duplicates, 135 articles were left in the search results which were then
screened for the eligibility of their relevancy. Out of these 135 screened articles, 11 articles were
selected which deals with the pain management by the interventions of either morphine or
cannabis. From these 11 articles, 6 were included in the study, as they are able to shed light in
the research question of this investigation and 5 were excluded due to their irrelevancy. The
details of the included study can be found in the Table 3 below and details of the excluded study
can be found on the Table 4 below.
2.5 Inclusion and exclusion criteria
Qualitative study has been considered as gold standards with regard to the health care studies
among the academics as this type of study allow the findings of the result to be generalised
among the populations (Bettany- Saltikov et al. 2012). Hence, particular focus has been given to
this type of the studies which have reported specifically in the area of formulation of practice
while screening and selecting the studies. Several inclusion criteria were utilised for the retrieval
of the research articles used in this research articles. First of all, the studies have to be published
between the year 2014 and 2019 for updated information related to the research questions. Any
journals published outside of this area of publication range were not further screened. Another
inclusion criterion was used that the full articles should be available in the database so that
information reported in that study can be included for the justification of research question
2.4 Article Selection
By using the above mentioned search terms all total of 163 articles were extracted from the
search databases. Out of these 163 articles, there were some duplicates of the retrieved articles. A
total number of 28 duplicates were present and they were removed from the search results. After
the removal of the duplicates, 135 articles were left in the search results which were then
screened for the eligibility of their relevancy. Out of these 135 screened articles, 11 articles were
selected which deals with the pain management by the interventions of either morphine or
cannabis. From these 11 articles, 6 were included in the study, as they are able to shed light in
the research question of this investigation and 5 were excluded due to their irrelevancy. The
details of the included study can be found in the Table 3 below and details of the excluded study
can be found on the Table 4 below.
2.5 Inclusion and exclusion criteria
Qualitative study has been considered as gold standards with regard to the health care studies
among the academics as this type of study allow the findings of the result to be generalised
among the populations (Bettany- Saltikov et al. 2012). Hence, particular focus has been given to
this type of the studies which have reported specifically in the area of formulation of practice
while screening and selecting the studies. Several inclusion criteria were utilised for the retrieval
of the research articles used in this research articles. First of all, the studies have to be published
between the year 2014 and 2019 for updated information related to the research questions. Any
journals published outside of this area of publication range were not further screened. Another
inclusion criterion was used that the full articles should be available in the database so that
information reported in that study can be included for the justification of research question

20DISSERTATION
presented in the study. In addition to that, it was checked that the study should be available in the
English language for the understanding of the information portrayed in the study. During the
screening of the articles, particular focus was given in the relevancy of the information presented
in the journal with regard to the research question presented above. The studies whose
information were not relevant to the research question were excluded from this study despite
meeting the above mentioned inclusion criteria. There were 5 research articles retrieved from the
database which met these criteria. In addition to that all the articles have to be published in a
peer-reviewed journal; otherwise the study was not selected. Finally, 6 articles were selected
which have met all the criteria and relevant to the research questions. The details of the included
study can be found in the Table 3 below and details of the excluded study can be found on the
Table 4 below.
2.6 Data synthesis and extraction
In this REA, 11 articles were extracted from the database of CINAHL and Medline. Among
them, six articles were included and five articles have been excluded. These six included articles
have been synthesised for the evaluation purpose. The details of the studies can be found in the
Table 3 and Table 4. Findings from these extracted studies have been summarised in the result
section below. In addition to this, the included studies were apprised using the GRADE tool in
the below section.
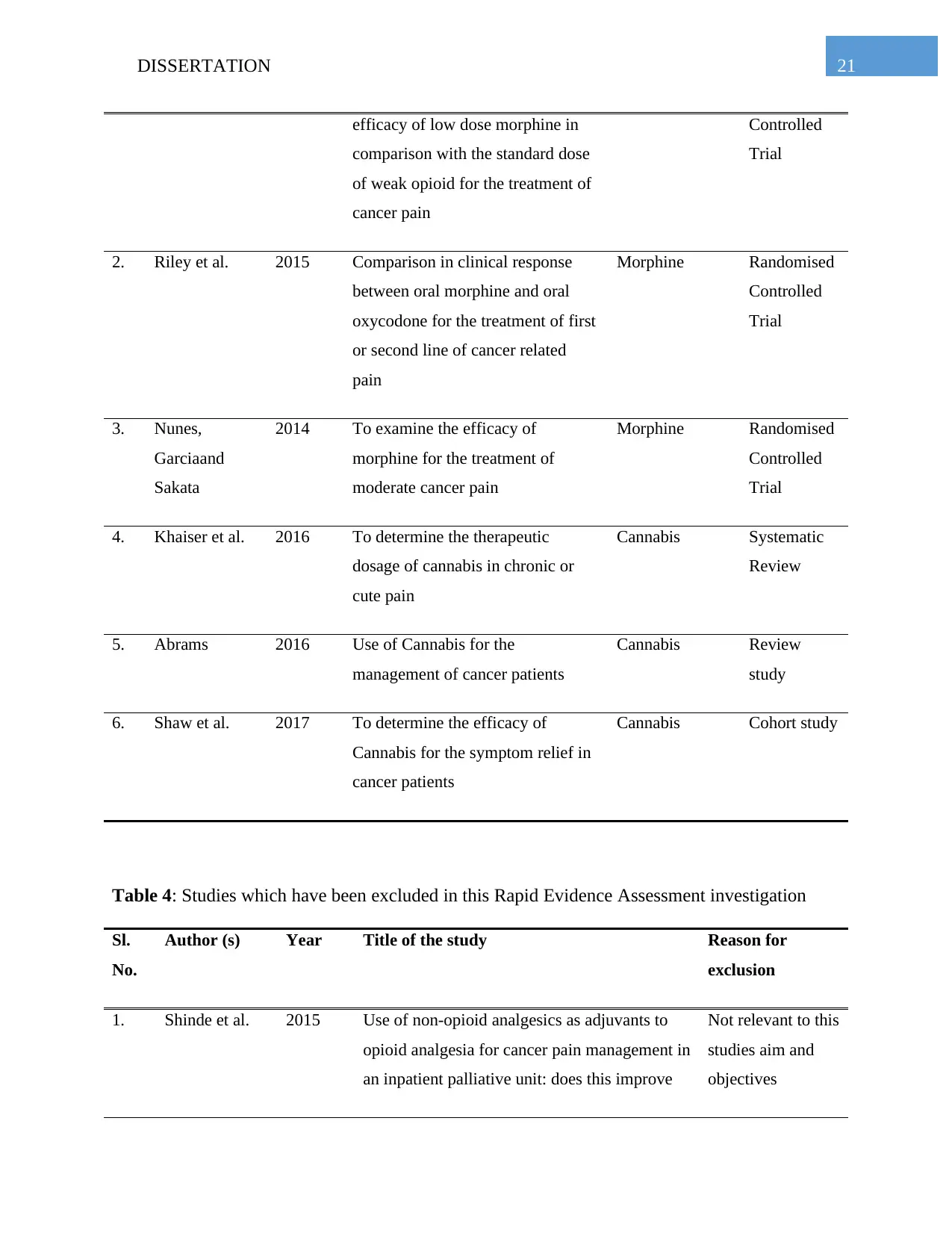
Table 3: Studies which have been included in this Rapid Evidence Assessment investigation
Sl.
No.
Author (s) Year Aim of the Study Intervention
for cancer pain
management
Study Type
1. Bandieri et al. 2016 To determine the tolerability and Morphine Randomised
presented in the study. In addition to that, it was checked that the study should be available in the
English language for the understanding of the information portrayed in the study. During the
screening of the articles, particular focus was given in the relevancy of the information presented
in the journal with regard to the research question presented above. The studies whose
information were not relevant to the research question were excluded from this study despite
meeting the above mentioned inclusion criteria. There were 5 research articles retrieved from the
database which met these criteria. In addition to that all the articles have to be published in a
peer-reviewed journal; otherwise the study was not selected. Finally, 6 articles were selected
which have met all the criteria and relevant to the research questions. The details of the included
study can be found in the Table 3 below and details of the excluded study can be found on the
Table 4 below.
2.6 Data synthesis and extraction
In this REA, 11 articles were extracted from the database of CINAHL and Medline. Among
them, six articles were included and five articles have been excluded. These six included articles
have been synthesised for the evaluation purpose. The details of the studies can be found in the
Table 3 and Table 4. Findings from these extracted studies have been summarised in the result
section below. In addition to this, the included studies were apprised using the GRADE tool in
the below section.
Table 3: Studies which have been included in this Rapid Evidence Assessment investigation
Sl.
No.
Author (s) Year Aim of the Study Intervention
for cancer pain
management
Study Type
1. Bandieri et al. 2016 To determine the tolerability and Morphine Randomised
21DISSERTATION
efficacy of low dose morphine in
comparison with the standard dose
of weak opioid for the treatment of
cancer pain
Controlled
Trial
2. Riley et al. 2015 Comparison in clinical response
between oral morphine and oral
oxycodone for the treatment of first
or second line of cancer related
pain
Morphine Randomised
Controlled
Trial
3. Nunes,
Garciaand
Sakata
2014 To examine the efficacy of
morphine for the treatment of
moderate cancer pain
Morphine Randomised
Controlled
Trial
4. Khaiser et al. 2016 To determine the therapeutic
dosage of cannabis in chronic or
cute pain
Cannabis Systematic
Review
5. Abrams 2016 Use of Cannabis for the
management of cancer patients
Cannabis Review
study
6. Shaw et al. 2017 To determine the efficacy of
Cannabis for the symptom relief in
cancer patients
Cannabis Cohort study
Table 4: Studies which have been excluded in this Rapid Evidence Assessment investigation
Sl.
No.
Author (s) Year Title of the study Reason for
exclusion
1. Shinde et al. 2015 Use of non-opioid analgesics as adjuvants to
opioid analgesia for cancer pain management in
an inpatient palliative unit: does this improve
Not relevant to this
studies aim and
objectives
efficacy of low dose morphine in
comparison with the standard dose
of weak opioid for the treatment of
cancer pain
Controlled
Trial
2. Riley et al. 2015 Comparison in clinical response
between oral morphine and oral
oxycodone for the treatment of first
or second line of cancer related
pain
Morphine Randomised
Controlled
Trial
3. Nunes,
Garciaand
Sakata
2014 To examine the efficacy of
morphine for the treatment of
moderate cancer pain
Morphine Randomised
Controlled
Trial
4. Khaiser et al. 2016 To determine the therapeutic
dosage of cannabis in chronic or
cute pain
Cannabis Systematic
Review
5. Abrams 2016 Use of Cannabis for the
management of cancer patients
Cannabis Review
study
6. Shaw et al. 2017 To determine the efficacy of
Cannabis for the symptom relief in
cancer patients
Cannabis Cohort study
Table 4: Studies which have been excluded in this Rapid Evidence Assessment investigation
Sl.
No.
Author (s) Year Title of the study Reason for
exclusion
1. Shinde et al. 2015 Use of non-opioid analgesics as adjuvants to
opioid analgesia for cancer pain management in
an inpatient palliative unit: does this improve
Not relevant to this
studies aim and
objectives
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

22DISSERTATION
pain control and reduce opioid requirements?
2. Bruce et al. 2018 Preferences for medical marijuana over
prescription medications among persons living
with chronic conditions: Alternative,
complementary, and tapering uses.
Not relevant to this
studies aim and
objectives
3. Paice 2018 Navigating cancer pain management in the
midst of the opioid epidemic
Not relevant to this
studies aim and
objectives
4. Singh
andChaturvedi
2015 Complementary and alternative medicine in
cancer pain management: a systematic review
Not relevant to this
studies aim and
objectives
5. Kane,
Hoskinand
Bennett
2015 Cancer induced bone pain Not relevant to this
studies aim and
objectives
2.7 Quality appraisal
It has been common practice among the academics to critically appraise the extracted studies
before the process of examining the studies for evaluation of the information presented in the
study. There are many different tools or frame work that can be utilised for this assessment of
the studies such as GRADE (Gugiu and RisteiGugiu 2010)and John Hopkins nursing evidence-
based practice rating scale (JHLEBP) (Newhouse et al. 2007). However, for this REA, GRADE
tool had been utilised for the assessment for the purpose of critically appraises the included
studies. In addition to this, level of hierarchy and level of evidence have also been employed for
the assessment of the included research articles. Guidelines that have been used for the level of
hierarchy and level of evidence have been presented in the Appendix 2 and Appendix 1
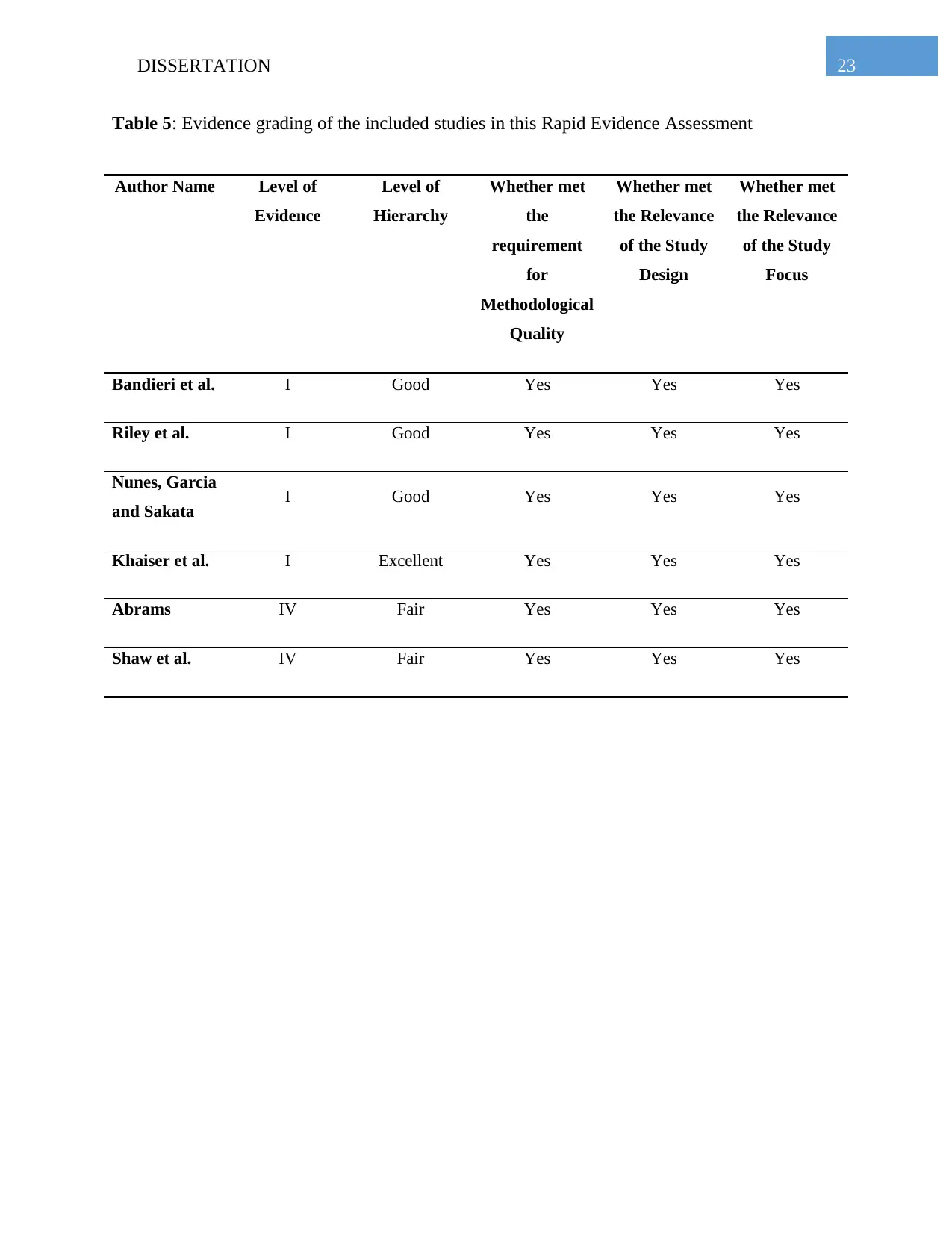
respectively. The quality appraisal of the included study has been presented in the Table 5 below.
pain control and reduce opioid requirements?
2. Bruce et al. 2018 Preferences for medical marijuana over
prescription medications among persons living
with chronic conditions: Alternative,
complementary, and tapering uses.
Not relevant to this
studies aim and
objectives
3. Paice 2018 Navigating cancer pain management in the
midst of the opioid epidemic
Not relevant to this
studies aim and
objectives
4. Singh
andChaturvedi
2015 Complementary and alternative medicine in
cancer pain management: a systematic review
Not relevant to this
studies aim and
objectives
5. Kane,
Hoskinand
Bennett
2015 Cancer induced bone pain Not relevant to this
studies aim and
objectives
2.7 Quality appraisal
It has been common practice among the academics to critically appraise the extracted studies
before the process of examining the studies for evaluation of the information presented in the
study. There are many different tools or frame work that can be utilised for this assessment of
the studies such as GRADE (Gugiu and RisteiGugiu 2010)and John Hopkins nursing evidence-
based practice rating scale (JHLEBP) (Newhouse et al. 2007). However, for this REA, GRADE
tool had been utilised for the assessment for the purpose of critically appraises the included
studies. In addition to this, level of hierarchy and level of evidence have also been employed for
the assessment of the included research articles. Guidelines that have been used for the level of
hierarchy and level of evidence have been presented in the Appendix 2 and Appendix 1
respectively. The quality appraisal of the included study has been presented in the Table 5 below.
23DISSERTATION
Table 5: Evidence grading of the included studies in this Rapid Evidence Assessment
Author Name Level of
Evidence
Level of
Hierarchy
Whether met
the
requirement
for
Methodological
Quality
Whether met
the Relevance
of the Study
Design
Whether met
the Relevance
of the Study
Focus
Bandieri et al. I Good Yes Yes Yes
Riley et al. I Good Yes Yes Yes
Nunes, Garcia
and Sakata I Good Yes Yes Yes
Khaiser et al. I Excellent Yes Yes Yes
Abrams IV Fair Yes Yes Yes
Shaw et al. IV Fair Yes Yes Yes
Table 5: Evidence grading of the included studies in this Rapid Evidence Assessment
Author Name Level of
Evidence
Level of
Hierarchy
Whether met
the
requirement
for
Methodological
Quality
Whether met
the Relevance
of the Study
Design
Whether met
the Relevance
of the Study
Focus
Bandieri et al. I Good Yes Yes Yes
Riley et al. I Good Yes Yes Yes
Nunes, Garcia
and Sakata I Good Yes Yes Yes
Khaiser et al. I Excellent Yes Yes Yes
Abrams IV Fair Yes Yes Yes
Shaw et al. IV Fair Yes Yes Yes

24DISSERTATION
Chapter 3
3. Results
3.1 Summary of the included studies
In all total 6 studies were incorporated in this Rapid Evidence Assessment. Among them, three
studies related to the cannabis used as pain management in cancer patient while other three are
based on use of morphine as a pain management in cancer patient. Out of the six studies, three of
them are RCTs, one systematic review, one cohort study and one review. The details of these
studies can be found in the Table 3.
3.2 Summary of the excluded studies and rationale behind exclusion
From the extracted and retrieved study, 5 studies were excluded. These studies were excluded
due to the irrelevance of the studies in relation to the research question of this rapid evidence
assessment. The description of these studies can be found on the Table 4 above.
3.3 Description of the study findings
3.3.1 Bandieri et al. 2016
In an open 28-day, randomized monitored multi-centre trial, and adolescents receiving either a
mild opioid or low-dose morphine was allocated mild cancer suffering. The main results were
amount of respondents, which were characterized by numerical ratings as clients with a 20%
decrease in pain intensity. In total, 240 cancer participants were included in the research (122 in
the weak opioid and 118 in the low morphine category). The main results happened in the low
Chapter 3
3. Results
3.1 Summary of the included studies
In all total 6 studies were incorporated in this Rapid Evidence Assessment. Among them, three
studies related to the cannabis used as pain management in cancer patient while other three are
based on use of morphine as a pain management in cancer patient. Out of the six studies, three of
them are RCTs, one systematic review, one cohort study and one review. The details of these
studies can be found in the Table 3.
3.2 Summary of the excluded studies and rationale behind exclusion
From the extracted and retrieved study, 5 studies were excluded. These studies were excluded
due to the irrelevance of the studies in relation to the research question of this rapid evidence
assessment. The description of these studies can be found on the Table 4 above.
3.3 Description of the study findings
3.3.1 Bandieri et al. 2016
In an open 28-day, randomized monitored multi-centre trial, and adolescents receiving either a
mild opioid or low-dose morphine was allocated mild cancer suffering. The main results were
amount of respondents, which were characterized by numerical ratings as clients with a 20%
decrease in pain intensity. In total, 240 cancer participants were included in the research (122 in
the weak opioid and 118 in the low morphine category). The main results happened in the low
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

25DISSERTATION
dose morphine unit at 88.2 and 57.7% (odd danger, 6.18; 95% CI, 3.12 to 12.24; P less than
0.001). The main result was in the low dose community. In the low-dose morphine cluster, the
proportion of respondents was greater at 1 week. In the cluster of low dose morphine it was
considerably greater (P less than 0.001) for statistical significant (greater than or equal to 30
percent) and extremely significant (greater than or equal to 50 per cent) pain decrease from
baseline. In the weak- opioid community a shift in the therapy allocated was more common due
to insufficient analgesia. Patients were better off as a particular disease in the morphine category
based on the overall symptom rating of the Edmonton Symptom Assessment System. The
negative impacts in both organizations were comparable.13 individuals (eight in a M and five in
the WO group) were not included in this assessment from the initial randomized cohort of 240
individuals, because they left the survey for different times before the first measurement of the
main endpoint. 88.2 percent (97 out of 110) in the M band and 54.7 percent (64 of 117) of WO
groups (Odds Ratio, 6.18; 95 percent CI, 3.12 to 12.24; P = .001) accomplished the main start
point with a 20 percent pain decrease or more from baseline. This outcome was not modified by
complete adjustment for baseline covariates. The overall situation of the clients was superior in
morphine (average score, 10; IQR, 6 to 15) compared to the low- opioid community (average
score, 19; IQR, 10 to 17; P, 001).). The overall ESAS rating was superior in children. Both
medicines have been well tolerated. Only five nurses in each community stopped therapy due to
negative impacts or tolerability, respectively (three and two patients per group). There were no
variations between the two organizations in the severity and frequency of opioid reactions.
3.3.2 Riley et al. 2015
Patients suffering from cancer related discomfort were alerted to either oral oxycodone or
morphine as first-line therapy in this potential, open label, randomized monitored study with a
dose morphine unit at 88.2 and 57.7% (odd danger, 6.18; 95% CI, 3.12 to 12.24; P less than
0.001). The main result was in the low dose community. In the low-dose morphine cluster, the
proportion of respondents was greater at 1 week. In the cluster of low dose morphine it was
considerably greater (P less than 0.001) for statistical significant (greater than or equal to 30
percent) and extremely significant (greater than or equal to 50 per cent) pain decrease from
baseline. In the weak- opioid community a shift in the therapy allocated was more common due
to insufficient analgesia. Patients were better off as a particular disease in the morphine category
based on the overall symptom rating of the Edmonton Symptom Assessment System. The
negative impacts in both organizations were comparable.13 individuals (eight in a M and five in
the WO group) were not included in this assessment from the initial randomized cohort of 240
individuals, because they left the survey for different times before the first measurement of the
main endpoint. 88.2 percent (97 out of 110) in the M band and 54.7 percent (64 of 117) of WO
groups (Odds Ratio, 6.18; 95 percent CI, 3.12 to 12.24; P = .001) accomplished the main start
point with a 20 percent pain decrease or more from baseline. This outcome was not modified by
complete adjustment for baseline covariates. The overall situation of the clients was superior in
morphine (average score, 10; IQR, 6 to 15) compared to the low- opioid community (average
score, 19; IQR, 10 to 17; P, 001).). The overall ESAS rating was superior in children. Both
medicines have been well tolerated. Only five nurses in each community stopped therapy due to
negative impacts or tolerability, respectively (three and two patients per group). There were no
variations between the two organizations in the severity and frequency of opioid reactions.
3.3.2 Riley et al. 2015
Patients suffering from cancer related discomfort were alerted to either oral oxycodone or
morphine as first-line therapy in this potential, open label, randomized monitored study with a

26DISSERTATION
chosen cross over stage. Until the person indicated appropriate pain control, dose was titrated
separately. The alternative opioid has been moved in patients who have failed to react to the
first-line opium (be they due to insufficient analgesia or negative impacts unacceptable). The
recruitment of two hundred patients was considered in this investigation. Intention to treat
and analyze the number of patients reacting to morphine (62 per cent) or oxycodone (67 per cent)
was used as a first-line opioid which was not significantly distinguished by intention to treat
(total number of patients = 198, morphine = 98, oxycodone =100). Simultaneous changes to
either morphine (67 per cent) or oxycodone (52 per cent) have not been observed in the ensuing
reaction. An assessment of a protocol showed that when both opioids were accessible a 95
percent reaction frequency was accessible. In first-line respondents or non- responders there was
no distinction in negative response rates among oxycodone and morphine. The most prevalent
cause for the first line opioid change was intolerable side effects with sleepiness as the most
prevalent negative response. Although dose increases were observed, some nurses (8 per
cent) turned because of unrestrained pain, and the remaining 28 per cent had an uninhibited pain
mix and intolerable side impacts. The average pre- contaminated dose of morphine was 60 mg
(range 30 to 260 mg) with a median post-contamination dose of 60 mg (range 20 to130 mg) in
respondents randomized to morphine. Five patients with morphine were assumed to have a
severe negative event (five constipations). There was a presumed severe response to eight
oxycodone patients (two constipations, three confusions, vomiting, and nausea, somnolence and
delusions, and secondary virus poisoning to the opioid). Seven people who received morphine
encountered unprecedented severe negative occurrences (arthritis, other negative responses to
medicines, falling, lower gastrointestinal bleeding, infectious aneurysm, mistake in the
prescription of medicines and severe coronary syndrome).
chosen cross over stage. Until the person indicated appropriate pain control, dose was titrated
separately. The alternative opioid has been moved in patients who have failed to react to the
first-line opium (be they due to insufficient analgesia or negative impacts unacceptable). The
recruitment of two hundred patients was considered in this investigation. Intention to treat
and analyze the number of patients reacting to morphine (62 per cent) or oxycodone (67 per cent)
was used as a first-line opioid which was not significantly distinguished by intention to treat
(total number of patients = 198, morphine = 98, oxycodone =100). Simultaneous changes to
either morphine (67 per cent) or oxycodone (52 per cent) have not been observed in the ensuing
reaction. An assessment of a protocol showed that when both opioids were accessible a 95
percent reaction frequency was accessible. In first-line respondents or non- responders there was
no distinction in negative response rates among oxycodone and morphine. The most prevalent
cause for the first line opioid change was intolerable side effects with sleepiness as the most
prevalent negative response. Although dose increases were observed, some nurses (8 per
cent) turned because of unrestrained pain, and the remaining 28 per cent had an uninhibited pain
mix and intolerable side impacts. The average pre- contaminated dose of morphine was 60 mg
(range 30 to 260 mg) with a median post-contamination dose of 60 mg (range 20 to130 mg) in
respondents randomized to morphine. Five patients with morphine were assumed to have a
severe negative event (five constipations). There was a presumed severe response to eight
oxycodone patients (two constipations, three confusions, vomiting, and nausea, somnolence and
delusions, and secondary virus poisoning to the opioid). Seven people who received morphine
encountered unprecedented severe negative occurrences (arthritis, other negative responses to
medicines, falling, lower gastrointestinal bleeding, infectious aneurysm, mistake in the
prescription of medicines and severe coronary syndrome).

27DISSERTATION
3.3.3Nunes, Garcia and Sakata 2014
The goal of this research was to assess morphine, as alternative to the guidelines of the WHO or
World Health Organization, as principal drug for the therapy of mild cancer suffering in
advanced and/ or metastatic individuals. Sixty individuals without opioid treatment, aged 18,
randomized to two groups, with no opioid treatment. Group 1 patients got analgesic ladder
medicinal products; Group 2 patients got morphine as their primary analgesic medicament, and
Group 1, the first mild opiates, and powerful opiates, the third phase. The effectiveness and
tolerability of first morphine use were assessed for three months every two weeks. The
population information organizations were comparable. The quality of life, the pain intensity, the
physical capabilities, saturation with therapy, the need to supplement, and the amount of
morphine did notice any significant difference among organizations. In Group 1, there was a
high frequency of pain (p = 0.0088), drowsiness of pain (p = 0.0005), constipation of pain of the
second visit (p = 0.0071) and drowsiness of the final visit (p = 0.05) and drowsiness. The pain
intensity in the two groups decreased in this research, which indicates that the methods work.
Another research showed better pain control and fulfilment than the standard group in those
clients who received powerful opioids but with more negative impacts. It is feasible to achieve a
stronger analgesic impact by combining paracetamol and morphine. In other studies, pain
management was improved by the mixture of powerful opiums and non- opioids. During the
course of this research, there was no deterioration in quality of living and physical capability but
the adverse effect of the illness was evident. No variation in patient satisfaction was found in this
research, which is a major evaluation. The occurrence of negative events is another important
issue. In another research, but more negative impacts were reported for clients getting powerful
optimal opioids. In individuals with standard treatment, the incidence of nausea was reduced and
3.3.3Nunes, Garcia and Sakata 2014
The goal of this research was to assess morphine, as alternative to the guidelines of the WHO or
World Health Organization, as principal drug for the therapy of mild cancer suffering in
advanced and/ or metastatic individuals. Sixty individuals without opioid treatment, aged 18,
randomized to two groups, with no opioid treatment. Group 1 patients got analgesic ladder
medicinal products; Group 2 patients got morphine as their primary analgesic medicament, and
Group 1, the first mild opiates, and powerful opiates, the third phase. The effectiveness and
tolerability of first morphine use were assessed for three months every two weeks. The
population information organizations were comparable. The quality of life, the pain intensity, the
physical capabilities, saturation with therapy, the need to supplement, and the amount of
morphine did notice any significant difference among organizations. In Group 1, there was a
high frequency of pain (p = 0.0088), drowsiness of pain (p = 0.0005), constipation of pain of the
second visit (p = 0.0071) and drowsiness of the final visit (p = 0.05) and drowsiness. The pain
intensity in the two groups decreased in this research, which indicates that the methods work.
Another research showed better pain control and fulfilment than the standard group in those
clients who received powerful opioids but with more negative impacts. It is feasible to achieve a
stronger analgesic impact by combining paracetamol and morphine. In other studies, pain
management was improved by the mixture of powerful opiums and non- opioids. During the
course of this research, there was no deterioration in quality of living and physical capability but
the adverse effect of the illness was evident. No variation in patient satisfaction was found in this
research, which is a major evaluation. The occurrence of negative events is another important
issue. In another research, but more negative impacts were reported for clients getting powerful
optimal opioids. In individuals with standard treatment, the incidence of nausea was reduced and
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

28DISSERTATION
the times of opioid were adapted to measure pain seriousness. The prevalence of adverse impacts
was greater in this research when morphine was the first drug to be administered that is
compatible with the WHO ladder validation. In the event of lower doses of dipyrone or
paracetamol and morphine for Group 2 patients, it is possible for the incidence of dizziness and
drowsiness to be compared to that with the WHO ladder. The first injection of morphine in
Group 2 for all women was resolved and the frequency of negative reactions may have increased.
The interdependence of initial dose on the basis of the strength of the discomfort can decrease
negative impacts with a gradual increase.
3.3.4Khaiser et al. 2016
This scope assessment directed at identifying therapeutic dosing policies in acute or chronic
circumstances for distinct types of marijuana. Four databases– Embase, MEDLINE, PsycInfo
and Cochrane Central – conducted a literature search. Other papers, assessments, and Health
Canada records were recognized through the hand-search. A total of 11 items have been chosen
to be included. In separate pain circumstances, seven controlled, randomized trials evaluated the
use of smoked marijuana: three vaporized cannabis studies and one retrospective observational
survey examined various cannabis formulations. Small sample sizes, brief engagement times,
and high dosage variation are limiting the current literature that supports smoked- vaporized
cannabis. There is still restricted evidence for oral marijuana types (oils, brownies). Smoking
marijuana should be vaporized, with a progressively starting tolerance of 3-4 inhalations daily
with around 45 seconds amid inhalation, using a low delta9-tetrahydrocannabinol (THC)
potential (e.g. 1 per cent THC). The same approach can be used to evaluate pain relief versus a
side impact profile while keeping at least 2 hours of intervals amid individual doses. Two studies
have examined the relief of severe discomfort with smoked marijuana. In the first study, 15
the times of opioid were adapted to measure pain seriousness. The prevalence of adverse impacts
was greater in this research when morphine was the first drug to be administered that is
compatible with the WHO ladder validation. In the event of lower doses of dipyrone or
paracetamol and morphine for Group 2 patients, it is possible for the incidence of dizziness and
drowsiness to be compared to that with the WHO ladder. The first injection of morphine in
Group 2 for all women was resolved and the frequency of negative reactions may have increased.
The interdependence of initial dose on the basis of the strength of the discomfort can decrease
negative impacts with a gradual increase.
3.3.4Khaiser et al. 2016
This scope assessment directed at identifying therapeutic dosing policies in acute or chronic
circumstances for distinct types of marijuana. Four databases– Embase, MEDLINE, PsycInfo
and Cochrane Central – conducted a literature search. Other papers, assessments, and Health
Canada records were recognized through the hand-search. A total of 11 items have been chosen
to be included. In separate pain circumstances, seven controlled, randomized trials evaluated the
use of smoked marijuana: three vaporized cannabis studies and one retrospective observational
survey examined various cannabis formulations. Small sample sizes, brief engagement times,
and high dosage variation are limiting the current literature that supports smoked- vaporized
cannabis. There is still restricted evidence for oral marijuana types (oils, brownies). Smoking
marijuana should be vaporized, with a progressively starting tolerance of 3-4 inhalations daily
with around 45 seconds amid inhalation, using a low delta9-tetrahydrocannabinol (THC)
potential (e.g. 1 per cent THC). The same approach can be used to evaluate pain relief versus a
side impact profile while keeping at least 2 hours of intervals amid individual doses. Two studies
have examined the relief of severe discomfort with smoked marijuana. In the first study, 15

29DISSERTATION
healthy participants experienced intradermal, induced capsaicin skin pain from four uniform
marijuana inhalations were used by Wallace et al. (2007). When capsaicin was taken in
premature marijuana (20 minutes), there had not been any distinction in pain perceptions
between the marijuana potentials (small [2 per cent THC], intermediate [4 per cent THC],
elevated [8 per cent THC] or placebo). High amounts of marijuana were also correlated with
increasing mild to moderate side impacts as opposed to other doses, such as dizziness and
drowsiness. The elevated dose (9.4 per cent THC) marijuana in comparison to placebo (p =
0.023) accomplished approximately an 11 per cent reduction in daily pain intensity. The
remaining strengths (2.5 per cent, 6 per cent) yielded in significant and mild declines. More
sleepiness, stronger sleep and less wakefulness (p < 0.05) were combined with greater dose, but
no accounts of confusion or disorientation that were seriously cognitive. In the evaluation
research of HIV neuropathy, the number ranking scales (clinically important reduction) relative
to placebo of smoked marijuana use were revealed to be > 30 per cent decreased in pain
intensity. There is some proof of marijuana in discomfort and spasticity associated with MS.
Patient declines of 4 per cent with single inhalation of THC marijuana have been recorded by
Corey-Bloom et al. (2012) for three days of therapy. The modified Ashworth scale was
decreased by 2.74 marks, which was a significance of stronger spasticity than placebo (p
<0.0001). The pain assessed by VAS was reduced in comparison with placebo by an average of
5.28 marks (p = 0.008). Five members retired owing to treatment negative impacts (euphoria,
dizziness, and exhaustion) in the intervention arm. The use of morphine as first therapy drug did
not support a stronger analgesic impact, which was higher incidence of negative impacts than the
ladder suggested by the World Health Organization.
healthy participants experienced intradermal, induced capsaicin skin pain from four uniform
marijuana inhalations were used by Wallace et al. (2007). When capsaicin was taken in
premature marijuana (20 minutes), there had not been any distinction in pain perceptions
between the marijuana potentials (small [2 per cent THC], intermediate [4 per cent THC],
elevated [8 per cent THC] or placebo). High amounts of marijuana were also correlated with
increasing mild to moderate side impacts as opposed to other doses, such as dizziness and
drowsiness. The elevated dose (9.4 per cent THC) marijuana in comparison to placebo (p =
0.023) accomplished approximately an 11 per cent reduction in daily pain intensity. The
remaining strengths (2.5 per cent, 6 per cent) yielded in significant and mild declines. More
sleepiness, stronger sleep and less wakefulness (p < 0.05) were combined with greater dose, but
no accounts of confusion or disorientation that were seriously cognitive. In the evaluation
research of HIV neuropathy, the number ranking scales (clinically important reduction) relative
to placebo of smoked marijuana use were revealed to be > 30 per cent decreased in pain
intensity. There is some proof of marijuana in discomfort and spasticity associated with MS.
Patient declines of 4 per cent with single inhalation of THC marijuana have been recorded by
Corey-Bloom et al. (2012) for three days of therapy. The modified Ashworth scale was
decreased by 2.74 marks, which was a significance of stronger spasticity than placebo (p
<0.0001). The pain assessed by VAS was reduced in comparison with placebo by an average of
5.28 marks (p = 0.008). Five members retired owing to treatment negative impacts (euphoria,
dizziness, and exhaustion) in the intervention arm. The use of morphine as first therapy drug did
not support a stronger analgesic impact, which was higher incidence of negative impacts than the
ladder suggested by the World Health Organization.

30DISSERTATION
3.3.5 Abrams 2016
In cancer patients, neuropathic pain is definitely a problem. In a systematic assessment of 6
randomised, placebo-controlled, double blind cannabinoid studies (5 focusing on neuropathic
pain), proof for the use, in connection with traditional analgesics, of low- dose medical
marijuana in refractive neuropathic pain was discovered. Five studies of inhaled marijuana have
also been examined in patients with peripheral HIV- related neuropathy and have again shown a
beneficial impact on marijuana relative to placebo. In a latest small study, a dose-reaction impact
for vaporized marijuana was shown in the relief of diabetes-peripheral neuropathic suffering.
Pre- clinical trials in rodent models showed that cannabinoids could stop peripheral neuropathy.
The activation of cb1 and cb2 receptor cuts out the growth of peripheral neuropathy with
vincristine induced in mice. Anandamide (endocannabinoid) and a fatty- acid hydrolase inhibitor
that metabolizes anandamide attenuated chemotherapy -caused peripheral neuropathy in mouse
getting regular cisplatin. Pre-treatment of Cannabidiol prevents neuropathy caused by
paclitaxelin. To date, a hybrid placebo controlled test of nabiximols is the only human research
of a cannabis based medication in peripheral neuropathy. In general, the pain ratings recorded
with nabiximols and placebo were not distinct. On a scale of 0–10, 5 respondents revealed a
decrease of neuropathic pain of more than 2 points. This finding indicates that 5 patients should
receive sublingual training for 1 (an appropriate number-needed treatment for a neuropathic
disorder) in order to experience the clinical advantage, indicating that further research into
marijuana in chemotherapy-induced peripheral neuropathy is warranted. Many cancer patients
clinically profit from marijuana being added to their pain regimes. While the impact on
peripheral neuropathy induced by chemotherapy was not evident, other types of cancer-related
suffering tend to be reacting. Patients whose well-meant oncologists or palliative care group have
3.3.5 Abrams 2016
In cancer patients, neuropathic pain is definitely a problem. In a systematic assessment of 6
randomised, placebo-controlled, double blind cannabinoid studies (5 focusing on neuropathic
pain), proof for the use, in connection with traditional analgesics, of low- dose medical
marijuana in refractive neuropathic pain was discovered. Five studies of inhaled marijuana have
also been examined in patients with peripheral HIV- related neuropathy and have again shown a
beneficial impact on marijuana relative to placebo. In a latest small study, a dose-reaction impact
for vaporized marijuana was shown in the relief of diabetes-peripheral neuropathic suffering.
Pre- clinical trials in rodent models showed that cannabinoids could stop peripheral neuropathy.
The activation of cb1 and cb2 receptor cuts out the growth of peripheral neuropathy with
vincristine induced in mice. Anandamide (endocannabinoid) and a fatty- acid hydrolase inhibitor
that metabolizes anandamide attenuated chemotherapy -caused peripheral neuropathy in mouse
getting regular cisplatin. Pre-treatment of Cannabidiol prevents neuropathy caused by
paclitaxelin. To date, a hybrid placebo controlled test of nabiximols is the only human research
of a cannabis based medication in peripheral neuropathy. In general, the pain ratings recorded
with nabiximols and placebo were not distinct. On a scale of 0–10, 5 respondents revealed a
decrease of neuropathic pain of more than 2 points. This finding indicates that 5 patients should
receive sublingual training for 1 (an appropriate number-needed treatment for a neuropathic
disorder) in order to experience the clinical advantage, indicating that further research into
marijuana in chemotherapy-induced peripheral neuropathy is warranted. Many cancer patients
clinically profit from marijuana being added to their pain regimes. While the impact on
peripheral neuropathy induced by chemotherapy was not evident, other types of cancer-related
suffering tend to be reacting. Patients whose well-meant oncologists or palliative care group have
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

31DISSERTATION
taken elevated amounts of opioids at the end of their lives often think that they are completely
unable to contact their loved ones in their valuable rest due to modified awareness. Many have
effectively withdrawn or withdrawn their opiate by incorporating marijuana to their lifestyle.
Although the most probable analgesic impacts would appear in THC-dominant marijuana strains,
patients often report important reductions in discomfort caused by mainly CBD - rich strains.
Although CBD simply does not attach to the receptor cb1, it blocks the acid - fibre binding factor
that carries an intracellular fatty amide hydrolysing of the endocannabinoid, thus permitting the
continuance of the endogenously complex cannabinoid.
3.3.6 Shaw et al. 2017
A single Canadian health care supplier has investigated the effectiveness of marijuana therapy
for cancer patients undergoing marijuana therapy. Data from a voluntary internet study
composed of population data, present medical circumstances, diseases appearance and
seriousness and quality of life (QOL). Four and 10 months following original use follow-up (FU)
studies were finished. Among the cancer patients, the most common types were primary
gastrointestinal tumours (17.7 percent, n = 29), the breast (13.4 per cent, n = 22), lymphomas and
leukemia (13.4 percent, n = 22), and the prostate (7.3 per cent, n = 12), have been reported by
164 patients with current or previous diagnoses of cancer (7.3 per cent, n = 22), gynecology and
lungs (7.3 per cent, n = 12). The findings were not statistically important while increases in
frequently reported diseases such as pain, depression, anxiety, fatigue, and sleep issues were
seen. Patients ' capacity to deal with suffering in 4-month FU (p < 0.0001) has been shown to be
statistically important. The most reported excellent QOL (66.7 per cent), was stable from
baseline to FU for 4 months. Only sleep experience was enhanced with statistical importance of
related QOL variables (p = 0.02). The dry mouth, psychosis, reduced concentration and memory,
taken elevated amounts of opioids at the end of their lives often think that they are completely
unable to contact their loved ones in their valuable rest due to modified awareness. Many have
effectively withdrawn or withdrawn their opiate by incorporating marijuana to their lifestyle.
Although the most probable analgesic impacts would appear in THC-dominant marijuana strains,
patients often report important reductions in discomfort caused by mainly CBD - rich strains.
Although CBD simply does not attach to the receptor cb1, it blocks the acid - fibre binding factor
that carries an intracellular fatty amide hydrolysing of the endocannabinoid, thus permitting the
continuance of the endogenously complex cannabinoid.
3.3.6 Shaw et al. 2017
A single Canadian health care supplier has investigated the effectiveness of marijuana therapy
for cancer patients undergoing marijuana therapy. Data from a voluntary internet study
composed of population data, present medical circumstances, diseases appearance and
seriousness and quality of life (QOL). Four and 10 months following original use follow-up (FU)
studies were finished. Among the cancer patients, the most common types were primary
gastrointestinal tumours (17.7 percent, n = 29), the breast (13.4 per cent, n = 22), lymphomas and
leukemia (13.4 percent, n = 22), and the prostate (7.3 per cent, n = 12), have been reported by
164 patients with current or previous diagnoses of cancer (7.3 per cent, n = 22), gynecology and
lungs (7.3 per cent, n = 12). The findings were not statistically important while increases in
frequently reported diseases such as pain, depression, anxiety, fatigue, and sleep issues were
seen. Patients ' capacity to deal with suffering in 4-month FU (p < 0.0001) has been shown to be
statistically important. The most reported excellent QOL (66.7 per cent), was stable from
baseline to FU for 4 months. Only sleep experience was enhanced with statistical importance of
related QOL variables (p = 0.02). The dry mouth, psychosis, reduced concentration and memory,

32DISSERTATION
and sleepiness were side impacts of marijuana usage. The pain was rated in average by 1 to 10
patients and FU for 4 months, with a rating from 1 to 4, a mild amount of pain, and pain from 5
to 7, a moderation of 8 to 10, and serious pain. In 75 per cent (n = 140) of 160 cancer- related
patients, recurring suffering was present at baseline. The percentage of clients reporting suffering
both in the baseline and in the four-month FEU (n = 24) decreased from 45.8 per cent (n = 11) to
16.7 per cent (n = 4), but statistically important variations were not observed (p = 0.06). Because
few nurses replied at peak and at 10-month FU, the outcomes were not included with the 10-
month FU result. The capacity to handle suffering in both baseline and 4-month FU was recorded
by 12 nurses. Seven nurses recorded very hard baseline compared with 0 four-month FU
patients. The results showed that the capacity to deal with Baseline Pain was statistically
significantly improved to 4 months of FU (n = 12, p <0.0001). Table 2 shows improvements in
the stress ratings and the capacity to cope with pain in baseline and FU patients. Anxiety,
depression, fatigue, sleep issues, and weakness were the most prevalent symptoms reported by
nurses. Changes to 4- month FU signs with reaction divided into enhancement (measuring, mild
or mild), no alter, and decline (measurement, mild, or mild). Patients recorded symptom
modifications at FU for 4 months and also assessed the scope of the diseases. Among
participants who were on both a basic and a four-month Fu, 83.3% (n = 24) improvement of
anxiety, 58.3% improvement in depression (n = 16), 25.0 per cent improvement in fatigue
exhaustion (n = 15), 66.7 per cent improvement in sleep (n = 16) and 41.7 per cent improvement
in vulnerability (n = 15) improvement of anxiety.
and sleepiness were side impacts of marijuana usage. The pain was rated in average by 1 to 10
patients and FU for 4 months, with a rating from 1 to 4, a mild amount of pain, and pain from 5
to 7, a moderation of 8 to 10, and serious pain. In 75 per cent (n = 140) of 160 cancer- related
patients, recurring suffering was present at baseline. The percentage of clients reporting suffering
both in the baseline and in the four-month FEU (n = 24) decreased from 45.8 per cent (n = 11) to
16.7 per cent (n = 4), but statistically important variations were not observed (p = 0.06). Because
few nurses replied at peak and at 10-month FU, the outcomes were not included with the 10-
month FU result. The capacity to handle suffering in both baseline and 4-month FU was recorded
by 12 nurses. Seven nurses recorded very hard baseline compared with 0 four-month FU
patients. The results showed that the capacity to deal with Baseline Pain was statistically
significantly improved to 4 months of FU (n = 12, p <0.0001). Table 2 shows improvements in
the stress ratings and the capacity to cope with pain in baseline and FU patients. Anxiety,
depression, fatigue, sleep issues, and weakness were the most prevalent symptoms reported by
nurses. Changes to 4- month FU signs with reaction divided into enhancement (measuring, mild
or mild), no alter, and decline (measurement, mild, or mild). Patients recorded symptom
modifications at FU for 4 months and also assessed the scope of the diseases. Among
participants who were on both a basic and a four-month Fu, 83.3% (n = 24) improvement of
anxiety, 58.3% improvement in depression (n = 16), 25.0 per cent improvement in fatigue
exhaustion (n = 15), 66.7 per cent improvement in sleep (n = 16) and 41.7 per cent improvement
in vulnerability (n = 15) improvement of anxiety.

33DISSERTATION
Chapter 4
4. Discussion
4.1 Discussion on morphine as a pain management among cancer patients
In their research, Nunes, Garcia and Sakata (2014) found that pain intensity in both teams
decreased, indicating efficacy of the methods. In a second research, the individuals getting strong
opioids were better controlled by discomfort and satisfied with higher negative impacts than the
standard community. During the course of this research, the quality of life and physical ability
were not affected but the adverse effect of the illness was not reflected. In a second research the
quality of life and physical function were gradually worsened although the nurses received
powerful opioids had a stronger command of the suffering. The patient satisfaction, which is a
significant type of evaluation, did not differ in this research. The occurrence of negative impacts
is another significant point. Another research showed more satisfaction but had more negative
impacts in clients getting powerful Opioids. In individuals with standard therapy, the frequency
of nausea was decreased and the doses of opioids adapted according to pain intensity. This
research shows a greater prevalence of negative impacts when morphine was the first medicine
to be administered in accordance with the WHO ladder approval.
Riley et al. (2015) have noted that all pain indexes in the opioid responder have increased
considerably with oxycodone and morphine when used on the first and second lines. In primary
opioid responder and non-responder pain results (P value below 0.05 for average pain, worse
pain, and percentage relief) there were a statistically important distinction. The most prevalent
reason for first-line opioid change was intolerable side impacts, with dizziness as the most
Chapter 4
4. Discussion
4.1 Discussion on morphine as a pain management among cancer patients
In their research, Nunes, Garcia and Sakata (2014) found that pain intensity in both teams
decreased, indicating efficacy of the methods. In a second research, the individuals getting strong
opioids were better controlled by discomfort and satisfied with higher negative impacts than the
standard community. During the course of this research, the quality of life and physical ability
were not affected but the adverse effect of the illness was not reflected. In a second research the
quality of life and physical function were gradually worsened although the nurses received
powerful opioids had a stronger command of the suffering. The patient satisfaction, which is a
significant type of evaluation, did not differ in this research. The occurrence of negative impacts
is another significant point. Another research showed more satisfaction but had more negative
impacts in clients getting powerful Opioids. In individuals with standard therapy, the frequency
of nausea was decreased and the doses of opioids adapted according to pain intensity. This
research shows a greater prevalence of negative impacts when morphine was the first medicine
to be administered in accordance with the WHO ladder approval.
Riley et al. (2015) have noted that all pain indexes in the opioid responder have increased
considerably with oxycodone and morphine when used on the first and second lines. In primary
opioid responder and non-responder pain results (P value below 0.05 for average pain, worse
pain, and percentage relief) there were a statistically important distinction. The most prevalent
reason for first-line opioid change was intolerable side impacts, with dizziness as the most
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

34DISSERTATION
frequent side effect. Although some nurses (8 per cent) changed due to uncontrolled pain, the
remaining 28 per cent were accompanied by a mix of intolerable and uncontrolled pain. In first-
line responders and non-responders (P value greater than 0.05; Mann-Whitney U test), there was
no distinction in negative response rates amid oxycodone and morphine. The most prevalent
negative response in those who changed from first line opioid was drowning accompanied by
pain or hallucinations or confusion or bafflement. In clients who obtained a healthy clinical
reaction whether morphine or oxycodone has been used in the first line of opioid, there was no
important distinction. When used as first line switches from oxycodone and morphine, there was
no significant distinction in reaction between morphine and oxycodone. Time change between
the opioids was no different as well. This information indicates that morphine and oxycodone are
equally effective in establishing superiority. The applicability of the outcomes in day -to -day
clinical exercise within a clinical practical context of a non- elected group of cancer patients is
strong. Given the heterogeneous community of patients, the large variety of doses needed is not
surprising. Dose conversion difficulties have been well recorded particularly when changing
between opioids.
Study exhibited that low dose morphine was a preliminary and sufficient degree of analgesics in
comparison with Step II Opioids for moderate cancer suffering with fair tolerance and a
beneficial effect on general well-being. Strong opioids are extremely controlled in most nations,
Andoncologists, family doctors, and internists may prefer to prescribe reduced regulatory
opioids. The data indicate that this intermediate stage could be more expensive and less effective.
A three-step ladder is available as the grounds for the therapy of cancer suffering in the present
WHO recommendation. A 2-step method is described by new guidelines, including that of the
EAPC. The conclusion of this research, which is in favour of beginning straight with one stage
frequent side effect. Although some nurses (8 per cent) changed due to uncontrolled pain, the
remaining 28 per cent were accompanied by a mix of intolerable and uncontrolled pain. In first-
line responders and non-responders (P value greater than 0.05; Mann-Whitney U test), there was
no distinction in negative response rates amid oxycodone and morphine. The most prevalent
negative response in those who changed from first line opioid was drowning accompanied by
pain or hallucinations or confusion or bafflement. In clients who obtained a healthy clinical
reaction whether morphine or oxycodone has been used in the first line of opioid, there was no
important distinction. When used as first line switches from oxycodone and morphine, there was
no significant distinction in reaction between morphine and oxycodone. Time change between
the opioids was no different as well. This information indicates that morphine and oxycodone are
equally effective in establishing superiority. The applicability of the outcomes in day -to -day
clinical exercise within a clinical practical context of a non- elected group of cancer patients is
strong. Given the heterogeneous community of patients, the large variety of doses needed is not
surprising. Dose conversion difficulties have been well recorded particularly when changing
between opioids.
Study exhibited that low dose morphine was a preliminary and sufficient degree of analgesics in
comparison with Step II Opioids for moderate cancer suffering with fair tolerance and a
beneficial effect on general well-being. Strong opioids are extremely controlled in most nations,
Andoncologists, family doctors, and internists may prefer to prescribe reduced regulatory
opioids. The data indicate that this intermediate stage could be more expensive and less effective.
A three-step ladder is available as the grounds for the therapy of cancer suffering in the present
WHO recommendation. A 2-step method is described by new guidelines, including that of the
EAPC. The conclusion of this research, which is in favour of beginning straight with one stage

35DISSERTATION
three opioid, can lead to the WHO recommendations change, needs to be further verified by
stage IIIb / phase IV research. The second step will simplify the treatment and perhaps improve
cancer paint control for nurses.
4.2 Discussion on Cannabis as a pain management among cancer patients
Dosing recommendation: Controlled studies showed the effectiveness of THC doses from 1% to
9% in neuropathic pain environments. The finest dosing approach in this band is that up to 3-4
vaporized doses per day (divided in 45 seconds by the Foltin operation), begin with reduced
THC levels depending on patient background (for example 1-2 percent THC) (Khaiser et al.
2016). Patients with native cannabis should begin the lower THC dose (i.e., 1% THC), to avoid
undesirable side effects and titrate to pain relief (Health Canada 2016). Cannabis-naive patients
should be recommended for at least 30 minutes to distinguish inhalations to evaluate adverse
effects and avoid overdose. The same suggestions can be pursued for non-neuropathic
discomfort as above. Dronabinol or Marinol ® (oral synthesized THC capsules in sesame oil) as
a referenced reference can be dosed with Cannabis Oil because there is no clinical testing
(Health Canada 2016). Marinol ® average is 20 mg of THC daily, and the quantities vary from
2.5 mg to a peak of 210 mg of THC daily. As for marijuana consumed, at the smallest level (i.e.
2.5 mg THC) acids should be applied and then titrated (Health Canada, 2016). Although medical
marijuana is not approved in most nations as a medicinal agent, it is accessible on a medical
prescription for nurses. Current research investigation on Cannabis mainly concerns smoked and
vaporized methods of shipment and very little proof of oral types such as brownies and oils is
available (Khaiser et al. 2016). Tobacco smoking is not an ideal arrangement because of the use
of poisonous by-products. The trials recognized in the evaluation are restricted to big variation in
dose approaches, tiny sample size, and brief cycles of action. Standardized
three opioid, can lead to the WHO recommendations change, needs to be further verified by
stage IIIb / phase IV research. The second step will simplify the treatment and perhaps improve
cancer paint control for nurses.
4.2 Discussion on Cannabis as a pain management among cancer patients
Dosing recommendation: Controlled studies showed the effectiveness of THC doses from 1% to
9% in neuropathic pain environments. The finest dosing approach in this band is that up to 3-4
vaporized doses per day (divided in 45 seconds by the Foltin operation), begin with reduced
THC levels depending on patient background (for example 1-2 percent THC) (Khaiser et al.
2016). Patients with native cannabis should begin the lower THC dose (i.e., 1% THC), to avoid
undesirable side effects and titrate to pain relief (Health Canada 2016). Cannabis-naive patients
should be recommended for at least 30 minutes to distinguish inhalations to evaluate adverse
effects and avoid overdose. The same suggestions can be pursued for non-neuropathic
discomfort as above. Dronabinol or Marinol ® (oral synthesized THC capsules in sesame oil) as
a referenced reference can be dosed with Cannabis Oil because there is no clinical testing
(Health Canada 2016). Marinol ® average is 20 mg of THC daily, and the quantities vary from
2.5 mg to a peak of 210 mg of THC daily. As for marijuana consumed, at the smallest level (i.e.
2.5 mg THC) acids should be applied and then titrated (Health Canada, 2016). Although medical
marijuana is not approved in most nations as a medicinal agent, it is accessible on a medical
prescription for nurses. Current research investigation on Cannabis mainly concerns smoked and
vaporized methods of shipment and very little proof of oral types such as brownies and oils is
available (Khaiser et al. 2016). Tobacco smoking is not an ideal arrangement because of the use
of poisonous by-products. The trials recognized in the evaluation are restricted to big variation in
dose approaches, tiny sample size, and brief cycles of action. Standardized

36DISSERTATION
Cannabis surveys regarding orally administer route (oils, brownies etc.) are not fundamentally
existing, and the capacity to draw significant findings is limited. A small therapy can be chosen
and titrated to an appropriate dose to alleviate the pain and enhance function with minimal
negative impacts (Khaiser et al. 2016).
Administration route: Evidence suggests oral administration is faster, more long- term and
smaller in the blood than inhaled medicine (Khaiser et al. 2016). THC peak plasma
concentrations may be 5 times as low as oral paths by tobacco (Khaiser et al. 2016). Although
some cognitive change in cannabis-laced Brownies was found to be 2.8 per cent THC, this
occurred after intake between 2.5 - 3.5 hours (Khaiser et al. 2016). Therefore, inhaled forms
(approximately 15 minutes) compared to verbal administration (Khaiser et al. 2016) quickly
occur the typical side impact of THC, euphoria and other beneficial reinforcing events such as
relaxation and improved sensory feelings. The inter-subjective variation of Oral Cannabis can
also lead to individual physiological variations, such as the frequency of gastrointestinal intake,
degrees of first pass metabolism and the genetic making of the participants. The most favoured
way of administration is since the inhalation leads to a straightforward and quick start of pain
relief. THC-related negative neurological or psychoactive impacts (e.g., sedation, dysphoria, and
low concentration) with inhaled marijuana are nevertheless established to have a demonstrable
connection (Deshpande et al. 2015).
Side effects: In their research, Shaw et al., (2017) noted that in nine clients, dry mouths,
psychoactive impacts, reduced memory, reduced concentre, and sleepiness, were the most
prevalent side impacts encountered owing to the use of marijuana, as well. Specifically: 4 out of
9 dry throat (44.4 per cent), psychoactive impacts (44.4 per cent), and reduced memory (44.4 per
Cannabis surveys regarding orally administer route (oils, brownies etc.) are not fundamentally
existing, and the capacity to draw significant findings is limited. A small therapy can be chosen
and titrated to an appropriate dose to alleviate the pain and enhance function with minimal
negative impacts (Khaiser et al. 2016).
Administration route: Evidence suggests oral administration is faster, more long- term and
smaller in the blood than inhaled medicine (Khaiser et al. 2016). THC peak plasma
concentrations may be 5 times as low as oral paths by tobacco (Khaiser et al. 2016). Although
some cognitive change in cannabis-laced Brownies was found to be 2.8 per cent THC, this
occurred after intake between 2.5 - 3.5 hours (Khaiser et al. 2016). Therefore, inhaled forms
(approximately 15 minutes) compared to verbal administration (Khaiser et al. 2016) quickly
occur the typical side impact of THC, euphoria and other beneficial reinforcing events such as
relaxation and improved sensory feelings. The inter-subjective variation of Oral Cannabis can
also lead to individual physiological variations, such as the frequency of gastrointestinal intake,
degrees of first pass metabolism and the genetic making of the participants. The most favoured
way of administration is since the inhalation leads to a straightforward and quick start of pain
relief. THC-related negative neurological or psychoactive impacts (e.g., sedation, dysphoria, and
low concentration) with inhaled marijuana are nevertheless established to have a demonstrable
connection (Deshpande et al. 2015).
Side effects: In their research, Shaw et al., (2017) noted that in nine clients, dry mouths,
psychoactive impacts, reduced memory, reduced concentre, and sleepiness, were the most
prevalent side impacts encountered owing to the use of marijuana, as well. Specifically: 4 out of
9 dry throat (44.4 per cent), psychoactive impacts (44.4 per cent), and reduced memory (44.4 per
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

37DISSERTATION
cent), 3 out of 3 sleepiness (33.3 per cent), and 2 patients reported decrease in concentration
(22.2 per cent).
cent), 3 out of 3 sleepiness (33.3 per cent), and 2 patients reported decrease in concentration
(22.2 per cent).

38DISSERTATION
Chapter 5
5. Conclusion
5.1 Summary
In short, cannabis and cannabinoids are helpful in the therapy of cancer diseases and diseases.
Exciting pre-clinical evidence implies that cannabinoids are efficient not only in treating
chemotherapy but in preventing peripheral neuropathy induced by chemotherapy. In pain relief,
cannabinoids may be synergistic with opioids. The cannabis security profile with general
tolerable and short-lived side impacts is appropriate. Cannabinoids, which are probably most
remarkable in key nervous systems, could have immediate tumour activation. There is still no
clinical information available on the cancer impact of cannabis concentrate. Clinical information
on cannabis impacts is not available at this time. Researchers have found, although scarce, proof
to promote use of cannabis pharmacotherapy in some of the clinical situations to be useful in
people residing with cancer, and be out of their lives. If a person with severe distress and his or
her health care provider, for instance, operate together without achievement in first-line and
second-line therapies, the testing of cannabis or cannabinoids may be a sensible next step. The
associated rise in cannabis studies is needed in the light of growing discussions on the merits of
medical and recreational marijuana policy.
There is a difference that people getting the morphine as first treatment have more negative
impacts at start of treatment, and that both are similar techniques of pain handling developed
cancer patients. The use of a powerful opioid can be a more suitable indicator in chosen clients
Chapter 5
5. Conclusion
5.1 Summary
In short, cannabis and cannabinoids are helpful in the therapy of cancer diseases and diseases.
Exciting pre-clinical evidence implies that cannabinoids are efficient not only in treating
chemotherapy but in preventing peripheral neuropathy induced by chemotherapy. In pain relief,
cannabinoids may be synergistic with opioids. The cannabis security profile with general
tolerable and short-lived side impacts is appropriate. Cannabinoids, which are probably most
remarkable in key nervous systems, could have immediate tumour activation. There is still no
clinical information available on the cancer impact of cannabis concentrate. Clinical information
on cannabis impacts is not available at this time. Researchers have found, although scarce, proof
to promote use of cannabis pharmacotherapy in some of the clinical situations to be useful in
people residing with cancer, and be out of their lives. If a person with severe distress and his or
her health care provider, for instance, operate together without achievement in first-line and
second-line therapies, the testing of cannabis or cannabinoids may be a sensible next step. The
associated rise in cannabis studies is needed in the light of growing discussions on the merits of
medical and recreational marijuana policy.
There is a difference that people getting the morphine as first treatment have more negative
impacts at start of treatment, and that both are similar techniques of pain handling developed
cancer patients. The use of a powerful opioid can be a more suitable indicator in chosen clients

39DISSERTATION
with serious suffering. Morphine and oxycodone can therefore be included in health centres as
vital opioids for improving results.
5.2 Limitations
This research has its limits and a certain biasness cannot be ignored, although the standardized
Rapid Evidence Assessment method was adopted for improving accuracy. The rigorous
integration requirements of studies published in English and carried out by nations in Western
part of the globe prevented major study in other regions such as sub Saharan African or non –
English - speaking groups. The brief time frame for Rapid Evidence Assessment s can also
contribute to restricted performance evaluation and evaluation. The Rapid Evidence
Assessment strategy is not a complete systematic evaluation, but provides an outline of the
quality of the evidence of the pain management of morphine and cannabis among cancer
patients.
5.3 Recommendations
One of the recommendations would be to would be investigating in study which exhibiting the
ability of cannabis actually lowering the risk for neuropathy or to avert it from evolving in first
place using animal model.
The main limitation of few studies used in this investigation is its unfeasibility of the usage of a
double blind design. Further investigations are recommended to assess possibilities for the
analgesic hierarchy.
with serious suffering. Morphine and oxycodone can therefore be included in health centres as
vital opioids for improving results.
5.2 Limitations
This research has its limits and a certain biasness cannot be ignored, although the standardized
Rapid Evidence Assessment method was adopted for improving accuracy. The rigorous
integration requirements of studies published in English and carried out by nations in Western
part of the globe prevented major study in other regions such as sub Saharan African or non –
English - speaking groups. The brief time frame for Rapid Evidence Assessment s can also
contribute to restricted performance evaluation and evaluation. The Rapid Evidence
Assessment strategy is not a complete systematic evaluation, but provides an outline of the
quality of the evidence of the pain management of morphine and cannabis among cancer
patients.
5.3 Recommendations
One of the recommendations would be to would be investigating in study which exhibiting the
ability of cannabis actually lowering the risk for neuropathy or to avert it from evolving in first
place using animal model.
The main limitation of few studies used in this investigation is its unfeasibility of the usage of a
double blind design. Further investigations are recommended to assess possibilities for the
analgesic hierarchy.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

40DISSERTATION
Declaration of conflicting interest
With regard to the studies, authoring or publishing of this rapid evidence assessment, there are
no potential conflicts of interest. The authors of this review got no economic aid for this
investigation.
Declaration of conflicting interest
With regard to the studies, authoring or publishing of this rapid evidence assessment, there are
no potential conflicts of interest. The authors of this review got no economic aid for this
investigation.

41DISSERTATION
6. References
Abrams, D.I. and Guzman, M., 2015. Cannabis in cancer care. Clinical Pharmacology &
Therapeutics, 97(6), pp.575-586.
Abrams, D.I., 2016. Integrating cannabis into clinical cancer care. Current Oncology, 23(Suppl
2), p.S8.
Bandieri, E., Romero, M., Ripamonti, C.I., Artioli, F., Sichetti, D., Fanizza, C., Santini, D.,
Cavanna, L., Melotti, B., Conte, P.F. and Roila, F., 2016. Randomized trial of low-dose
morphine versus weak opioids in moderate cancer pain. J ClinOncol, 34(5), pp.436-42.
Bettany-Saltikov, J., 2012. How to do a systematic literature review in nursing: a step-by-step
guide. McGraw-Hill Education (UK).
Blake, A., Wan, B.A., Malek, L., DeAngelis, C., Diaz, P., Lao, N., Chow, E. and O’Hearn, S.,
2017. A selective review of medical cannabis in cancer pain management. Annals of palliative
medicine, 6(2), pp.S215-S222.
Booth, A., Sutton, A. and Papaioannou, D., 2016. Systematic approaches to a successful
literature review. Sage.
Borgelt, L.M., Franson, K.L., Nussbaum, A.M. and Wang, G.S., 2013. The P harmacologic and
C linical E ffects of M edical C annabis. Pharmacotherapy: The Journal of Human
Pharmacology and Drug Therapy, 33(2), pp.195-209.
6. References
Abrams, D.I. and Guzman, M., 2015. Cannabis in cancer care. Clinical Pharmacology &
Therapeutics, 97(6), pp.575-586.
Abrams, D.I., 2016. Integrating cannabis into clinical cancer care. Current Oncology, 23(Suppl
2), p.S8.
Bandieri, E., Romero, M., Ripamonti, C.I., Artioli, F., Sichetti, D., Fanizza, C., Santini, D.,
Cavanna, L., Melotti, B., Conte, P.F. and Roila, F., 2016. Randomized trial of low-dose
morphine versus weak opioids in moderate cancer pain. J ClinOncol, 34(5), pp.436-42.
Bettany-Saltikov, J., 2012. How to do a systematic literature review in nursing: a step-by-step
guide. McGraw-Hill Education (UK).
Blake, A., Wan, B.A., Malek, L., DeAngelis, C., Diaz, P., Lao, N., Chow, E. and O’Hearn, S.,
2017. A selective review of medical cannabis in cancer pain management. Annals of palliative
medicine, 6(2), pp.S215-S222.
Booth, A., Sutton, A. and Papaioannou, D., 2016. Systematic approaches to a successful
literature review. Sage.
Borgelt, L.M., Franson, K.L., Nussbaum, A.M. and Wang, G.S., 2013. The P harmacologic and
C linical E ffects of M edical C annabis. Pharmacotherapy: The Journal of Human
Pharmacology and Drug Therapy, 33(2), pp.195-209.

42DISSERTATION
Bruce, D., Brady, J.P., Foster, E. and Shattell, M., 2018. Preferences for medical marijuana over
prescription medications among persons living with chronic conditions: Alternative,
complementary, and tapering uses. The Journal of Alternative and Complementary
Medicine, 24(2), pp.146-153.
Corey-Bloom, J., Wolfson, T., Gamst, A., Jin, S., Marcotte, T.D., Bentley, H. and Gouaux, B.,
2012. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled
trial. Cmaj, 184(10), pp.1143-1150.
Daly, J., Willis, K., Small, R., Green, J., Welch, N., Kealy, M. and Hughes, E., 2007. A
hierarchy of evidence for assessing qualitative health research. Journal of clinical
epidemiology, 60(1), pp.43-49.
Davies, K.S., 2011. Formulating the evidence based practice question: a review of the
frameworks. Evidence Based Library and Information Practice, 6(2), pp.75-80.
Deshpande, A., Mailis-Gagnon, A., Zoheiry, N. and Lakha, S.F., 2015. Efficacy and adverse
effects of medical marijuana for chronic noncancer pain: Systematic review of randomized
controlled trials. Canadian Family Physician, 61(8), pp.e372-e381.
Bruce, D., Brady, J.P., Foster, E. and Shattell, M., 2018. Preferences for medical marijuana over
prescription medications among persons living with chronic conditions: Alternative,
complementary, and tapering uses. The Journal of Alternative and Complementary
Medicine, 24(2), pp.146-153.
Corey-Bloom, J., Wolfson, T., Gamst, A., Jin, S., Marcotte, T.D., Bentley, H. and Gouaux, B.,
2012. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled
trial. Cmaj, 184(10), pp.1143-1150.
Daly, J., Willis, K., Small, R., Green, J., Welch, N., Kealy, M. and Hughes, E., 2007. A
hierarchy of evidence for assessing qualitative health research. Journal of clinical
epidemiology, 60(1), pp.43-49.
Davies, K.S., 2011. Formulating the evidence based practice question: a review of the
frameworks. Evidence Based Library and Information Practice, 6(2), pp.75-80.
Deshpande, A., Mailis-Gagnon, A., Zoheiry, N. and Lakha, S.F., 2015. Efficacy and adverse
effects of medical marijuana for chronic noncancer pain: Systematic review of randomized
controlled trials. Canadian Family Physician, 61(8), pp.e372-e381.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

43DISSERTATION
Evans, D., 2003. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare
interventions. Journal of clinical nursing, 12(1), pp.77-84.
Flick, U., 2018. An introduction to qualitative research. Sage Publications Limited.
Ganann, R., Ciliska, D. and Thomas, H., 2010. Expediting systematic reviews: methods and
implications of rapid reviews. Implementation Science, 5(1), p.56.
Garud, M.S., Oza, M.J., Gaikwad, A.B. and Kulkarni, Y.A., 2017. Natural Remedies for
Treatment of Cancer Pain. In Nutritional Modulators of Pain in the Aging Population (pp. 101-
106). Academic Press.
Gugiu, P.C. and RisteiGugiu, M., 2010. A critical appraisal of standard guidelines for grading
levels of evidence. Evaluation & the Health Professions, 33(3), pp.233-255.
Haby, M.M., Chapman, E., Clark, R., Barreto, J., Reveiz, L. and Lavis, J.N., 2016. What are the
best methodologies for rapid reviews of the research evidence for evidence-informed decision
making in health policy and practice: a rapid review. Health research policy and systems, 14(1),
p.83.
Health Canada 2016. Access to Cannabis for Medical Purposes Regulations - Daily Amount Fact
Sheet (Dosage) - Canada.ca. Canada.ca. Available at:
https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/information-
medical-practitioners/cannabis-medical-purposes-regulations-daily-amount-fact-sheet-
dosage.html [Accessed 31 May 2019].
Kane, C.M., Hoskin, P. and Bennett, M.I., 2015. Cancer induced bone pain. bmj, 350, p.h315.
Evans, D., 2003. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare
interventions. Journal of clinical nursing, 12(1), pp.77-84.
Flick, U., 2018. An introduction to qualitative research. Sage Publications Limited.
Ganann, R., Ciliska, D. and Thomas, H., 2010. Expediting systematic reviews: methods and
implications of rapid reviews. Implementation Science, 5(1), p.56.
Garud, M.S., Oza, M.J., Gaikwad, A.B. and Kulkarni, Y.A., 2017. Natural Remedies for
Treatment of Cancer Pain. In Nutritional Modulators of Pain in the Aging Population (pp. 101-
106). Academic Press.
Gugiu, P.C. and RisteiGugiu, M., 2010. A critical appraisal of standard guidelines for grading
levels of evidence. Evaluation & the Health Professions, 33(3), pp.233-255.
Haby, M.M., Chapman, E., Clark, R., Barreto, J., Reveiz, L. and Lavis, J.N., 2016. What are the
best methodologies for rapid reviews of the research evidence for evidence-informed decision
making in health policy and practice: a rapid review. Health research policy and systems, 14(1),
p.83.
Health Canada 2016. Access to Cannabis for Medical Purposes Regulations - Daily Amount Fact
Sheet (Dosage) - Canada.ca. Canada.ca. Available at:
https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/information-
medical-practitioners/cannabis-medical-purposes-regulations-daily-amount-fact-sheet-
dosage.html [Accessed 31 May 2019].
Kane, C.M., Hoskin, P. and Bennett, M.I., 2015. Cancer induced bone pain. bmj, 350, p.h315.

44DISSERTATION
Khaiser, M., Peng, M., Ahrari, S., Pasetka, M. and DeAngelis, C. (2016). Medical cannabis
dosing strategies in pain-related conditions: A scoping review of current literature. 9:449–463.
Lynch, M.E., Cesar-Rittenberg, P. and Hohmann, A.G., 2014. A double-blind, placebo-
controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for
treatment of chemotherapy-induced neuropathic pain. Journal of pain and symptom
management, 47(1), pp.166-173.
Melnyk, B.M. and Fineout-Overholt, E. eds., 2011. Evidence-based practice in nursing &
healthcare: A guide to best practice. Lippincott Williams & Wilkins.
Mercadante, S., Aielli, F., Adile, C., Costanzi, A. and Casuccio, A., 2016. Fentanyl pectin nasal
spray versus oral morphine in doses proportional to the basal opioid regimen for the management
of breakthrough cancer pain: a comparative study. Journal of pain and symptom
management, 52(1), pp.27-34.
Newhouse, R.P., Dearholt, S.L., Poe, S.S., Pugh, L.C. and White, K.M., 2007. Johns Hopkins
nursing evidence-based practice model and guidelines. Indianapolis, IN: Sigma Theta Tau
International Honor Society of Nursing.
Nunes, B.C., Garcia, J.B.D.S. and Sakata, R.K., 2014. Morphine as first medication for treatment
of cancer pain. Revistabrasileira de anestesiologia, 64(4), pp.236-240.
Paice, J.A., 2018. Navigating cancer pain management in the midst of the opioid
epidemic. Oncology, 32(8).
Khaiser, M., Peng, M., Ahrari, S., Pasetka, M. and DeAngelis, C. (2016). Medical cannabis
dosing strategies in pain-related conditions: A scoping review of current literature. 9:449–463.
Lynch, M.E., Cesar-Rittenberg, P. and Hohmann, A.G., 2014. A double-blind, placebo-
controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for
treatment of chemotherapy-induced neuropathic pain. Journal of pain and symptom
management, 47(1), pp.166-173.
Melnyk, B.M. and Fineout-Overholt, E. eds., 2011. Evidence-based practice in nursing &
healthcare: A guide to best practice. Lippincott Williams & Wilkins.
Mercadante, S., Aielli, F., Adile, C., Costanzi, A. and Casuccio, A., 2016. Fentanyl pectin nasal
spray versus oral morphine in doses proportional to the basal opioid regimen for the management
of breakthrough cancer pain: a comparative study. Journal of pain and symptom
management, 52(1), pp.27-34.
Newhouse, R.P., Dearholt, S.L., Poe, S.S., Pugh, L.C. and White, K.M., 2007. Johns Hopkins
nursing evidence-based practice model and guidelines. Indianapolis, IN: Sigma Theta Tau
International Honor Society of Nursing.
Nunes, B.C., Garcia, J.B.D.S. and Sakata, R.K., 2014. Morphine as first medication for treatment
of cancer pain. Revistabrasileira de anestesiologia, 64(4), pp.236-240.
Paice, J.A., 2018. Navigating cancer pain management in the midst of the opioid
epidemic. Oncology, 32(8).

45DISSERTATION
Peters, M.D., Godfrey, C.M., Khalil, H., McInerney, P., Parker, D. and Soares, C.B., 2015.
Guidance for conducting systematic scoping reviews. International journal of evidence-based
healthcare, 13(3), pp.141-146.
Riley, J., Branford, R., Droney, J., Gretton, S., Sato, H., Kennett, A., Oyebode, C., Thick, M.,
Wells, A., Williams, J. and Welsh, K., 2015. Morphine or oxycodone for cancer-related pain? A
randomized, open-label, controlled trial. Journal of pain and symptom management, 49(2),
pp.161-172.
Shaw, E., Chow, E., Wolt, A. and Slaven, M., 2017. The use of medical cannabis in cancer
patients. Journal of Pain Management, 10(4), pp.353-362.
Shinde, S., Gordon, P., Sharma, P., Gross, J. and Davis, M.P., 2015. Use of non-opioid
analgesics as adjuvants to opioid analgesia for cancer pain management in an inpatient palliative
unit: does this improve pain control and reduce opioid requirements?. Supportive Care in
Cancer, 23(3), pp.695-703.
Silverman, D. ed., 2016. Qualitative research. Sage.
Singh, P. and Chaturvedi, A., 2015. Complementary and alternative medicine in cancer pain
management: a systematic review. Indian journal of palliative care, 21(1), p.105.
Smith, V., Devane, D., Begley, C.M. and Clarke, M., 2011. Methodology in conducting a
systematic review of systematic reviews of healthcare interventions. BMC medical research
methodology, 11(1), p.15.
Peters, M.D., Godfrey, C.M., Khalil, H., McInerney, P., Parker, D. and Soares, C.B., 2015.
Guidance for conducting systematic scoping reviews. International journal of evidence-based
healthcare, 13(3), pp.141-146.
Riley, J., Branford, R., Droney, J., Gretton, S., Sato, H., Kennett, A., Oyebode, C., Thick, M.,
Wells, A., Williams, J. and Welsh, K., 2015. Morphine or oxycodone for cancer-related pain? A
randomized, open-label, controlled trial. Journal of pain and symptom management, 49(2),
pp.161-172.
Shaw, E., Chow, E., Wolt, A. and Slaven, M., 2017. The use of medical cannabis in cancer
patients. Journal of Pain Management, 10(4), pp.353-362.
Shinde, S., Gordon, P., Sharma, P., Gross, J. and Davis, M.P., 2015. Use of non-opioid
analgesics as adjuvants to opioid analgesia for cancer pain management in an inpatient palliative
unit: does this improve pain control and reduce opioid requirements?. Supportive Care in
Cancer, 23(3), pp.695-703.
Silverman, D. ed., 2016. Qualitative research. Sage.
Singh, P. and Chaturvedi, A., 2015. Complementary and alternative medicine in cancer pain
management: a systematic review. Indian journal of palliative care, 21(1), p.105.
Smith, V., Devane, D., Begley, C.M. and Clarke, M., 2011. Methodology in conducting a
systematic review of systematic reviews of healthcare interventions. BMC medical research
methodology, 11(1), p.15.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

46DISSERTATION
Wallace, M., Schulteis, G., Atkinson, J.H., Wolfson, T., Lazzaretto, D., Bentley, H., Gouaux, B.
and Abramson, I., 2007. Dose-dependent effects of smoked cannabis on capsaicin-induced pain
and hyperalgesia in healthy volunteers. Anesthesiology: The Journal of the American Society of
Anesthesiologists, 107(5), pp.785-796.
Wiffen, P.J., Wee, B. and Moore, R.A., 2016. Oral morphine for cancer pain. Cochrane
Database of Systematic Reviews, (4).
Wiffen, P.J., Wee, B., Derry, S., Bell, R.F. and Moore, R.A., 2017. Opioids for cancer pain‐an
overview of Cochrane reviews. Cochrane Database of Systematic Reviews, (7).
Wildridge, V. and Bell, L., 2002. How CLIP became ECLIPSE: a mnemonic to assist in
searching for health policy/management information. Health Information & Libraries
Journal, 19(2), pp.113-115.
Wong, G., Greenhalgh, T., Westhorp, G., Buckingham, J. and Pawson, R., 2013. RAMESES
publication standards: realist syntheses. BMC medicine, 11(1), p.21.
Wallace, M., Schulteis, G., Atkinson, J.H., Wolfson, T., Lazzaretto, D., Bentley, H., Gouaux, B.
and Abramson, I., 2007. Dose-dependent effects of smoked cannabis on capsaicin-induced pain
and hyperalgesia in healthy volunteers. Anesthesiology: The Journal of the American Society of
Anesthesiologists, 107(5), pp.785-796.
Wiffen, P.J., Wee, B. and Moore, R.A., 2016. Oral morphine for cancer pain. Cochrane
Database of Systematic Reviews, (4).
Wiffen, P.J., Wee, B., Derry, S., Bell, R.F. and Moore, R.A., 2017. Opioids for cancer pain‐an
overview of Cochrane reviews. Cochrane Database of Systematic Reviews, (7).
Wildridge, V. and Bell, L., 2002. How CLIP became ECLIPSE: a mnemonic to assist in
searching for health policy/management information. Health Information & Libraries
Journal, 19(2), pp.113-115.
Wong, G., Greenhalgh, T., Westhorp, G., Buckingham, J. and Pawson, R., 2013. RAMESES
publication standards: realist syntheses. BMC medicine, 11(1), p.21.
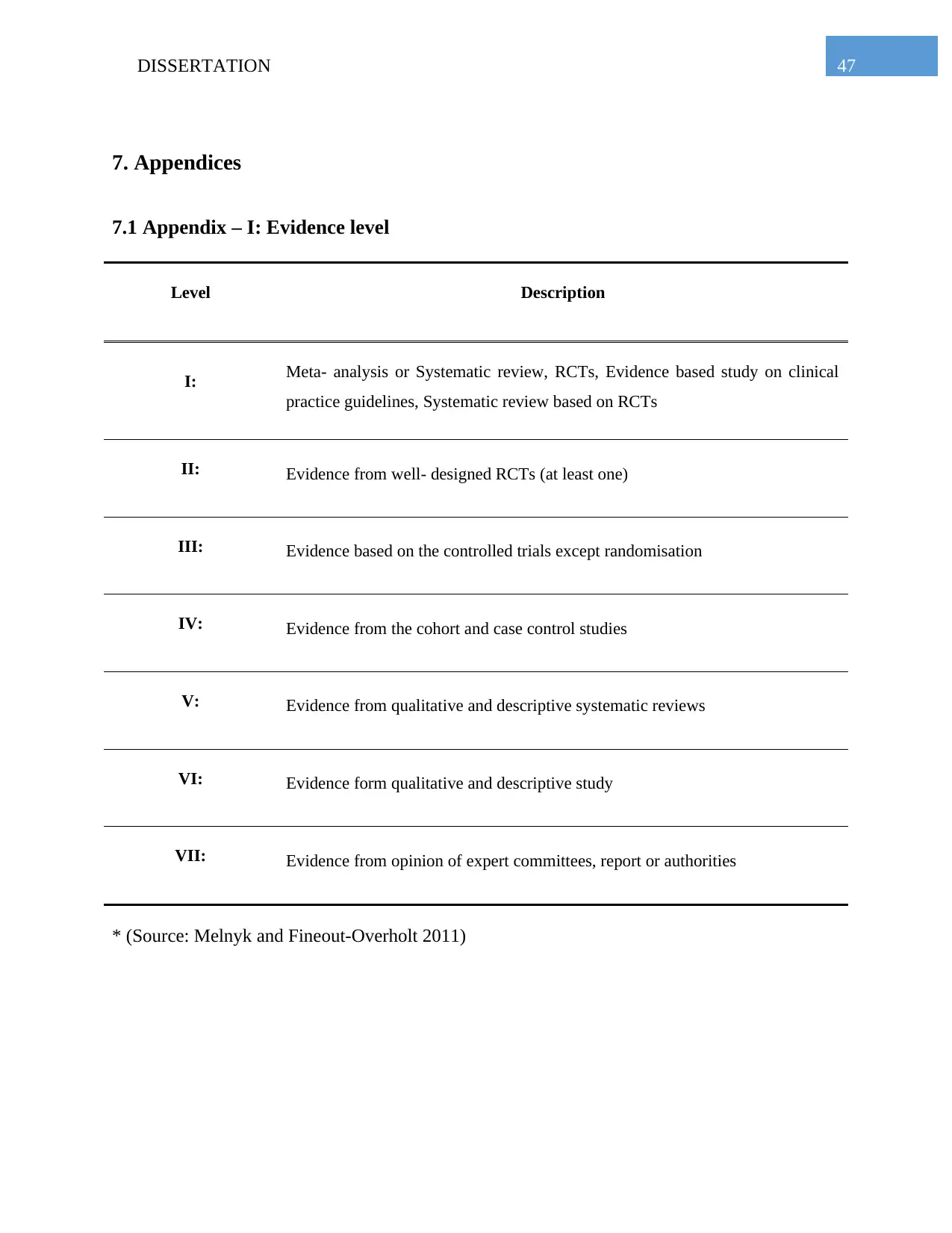
47DISSERTATION
7. Appendices
7.1 Appendix – I: Evidence level
Level Description
I: Meta- analysis or Systematic review, RCTs, Evidence based study on clinical
practice guidelines, Systematic review based on RCTs
II: Evidence from well- designed RCTs (at least one)
III: Evidence based on the controlled trials except randomisation
IV: Evidence from the cohort and case control studies
V: Evidence from qualitative and descriptive systematic reviews
VI: Evidence form qualitative and descriptive study
VII: Evidence from opinion of expert committees, report or authorities
* (Source: Melnyk and Fineout-Overholt 2011)
7. Appendices
7.1 Appendix – I: Evidence level
Level Description
I: Meta- analysis or Systematic review, RCTs, Evidence based study on clinical
practice guidelines, Systematic review based on RCTs
II: Evidence from well- designed RCTs (at least one)
III: Evidence based on the controlled trials except randomisation
IV: Evidence from the cohort and case control studies
V: Evidence from qualitative and descriptive systematic reviews
VI: Evidence form qualitative and descriptive study
VII: Evidence from opinion of expert committees, report or authorities
* (Source: Melnyk and Fineout-Overholt 2011)
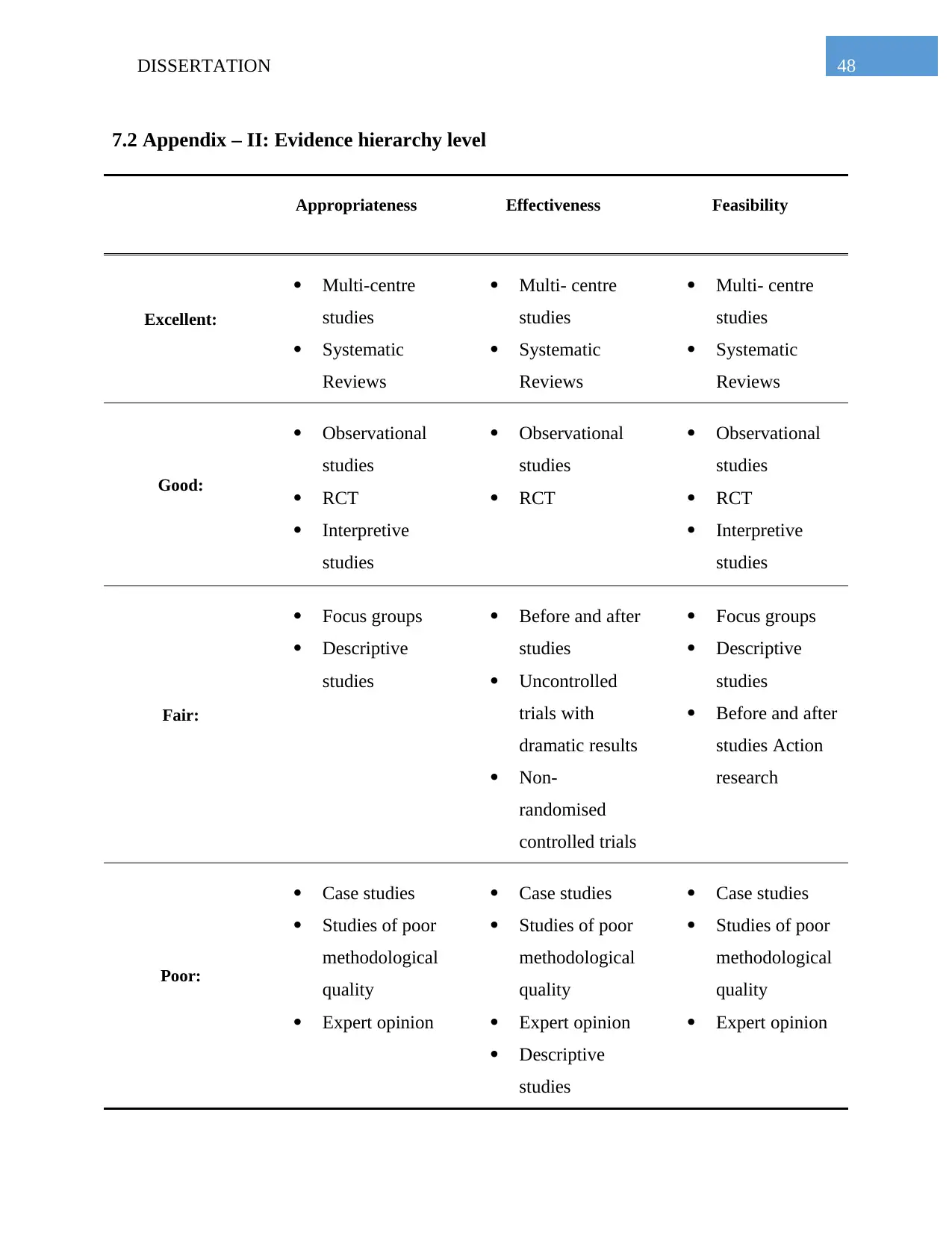
48DISSERTATION
7.2 Appendix – II: Evidence hierarchy level
Appropriateness Effectiveness Feasibility
Excellent:
Multi-centre
studies
Systematic
Reviews
Multi- centre
studies
Systematic
Reviews
Multi- centre
studies
Systematic
Reviews
Good:
Observational
studies
RCT
Interpretive
studies
Observational
studies
RCT
Observational
studies
RCT
Interpretive
studies
Fair:
Focus groups
Descriptive
studies
Before and after
studies
Uncontrolled
trials with
dramatic results
Non-
randomised
controlled trials
Focus groups
Descriptive
studies
Before and after
studies Action
research
Poor:
Case studies
Studies of poor
methodological
quality
Expert opinion
Case studies
Studies of poor
methodological
quality
Expert opinion
Descriptive
studies
Case studies
Studies of poor
methodological
quality
Expert opinion
7.2 Appendix – II: Evidence hierarchy level
Appropriateness Effectiveness Feasibility
Excellent:
Multi-centre
studies
Systematic
Reviews
Multi- centre
studies
Systematic
Reviews
Multi- centre
studies
Systematic
Reviews
Good:
Observational
studies
RCT
Interpretive
studies
Observational
studies
RCT
Observational
studies
RCT
Interpretive
studies
Fair:
Focus groups
Descriptive
studies
Before and after
studies
Uncontrolled
trials with
dramatic results
Non-
randomised
controlled trials
Focus groups
Descriptive
studies
Before and after
studies Action
research
Poor:
Case studies
Studies of poor
methodological
quality
Expert opinion
Case studies
Studies of poor
methodological
quality
Expert opinion
Descriptive
studies
Case studies
Studies of poor
methodological
quality
Expert opinion
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

49DISSERTATION
* (Source: Evans 2003)
* (Source: Evans 2003)

50DISSERTATION
7.3 Appendix – III: Evidence hierarchy level for qualitative studies
Level Description
I: Studies whose outcome can be generalised; Sample collected from diverse
population
II: Single theoretical framework based concept studies
III: Descriptive studies (sample collected from narrowly diverse population)
IV: Solitary case study
* (Source: Daly et al. 2007)
7.3 Appendix – III: Evidence hierarchy level for qualitative studies
Level Description
I: Studies whose outcome can be generalised; Sample collected from diverse
population
II: Single theoretical framework based concept studies
III: Descriptive studies (sample collected from narrowly diverse population)
IV: Solitary case study
* (Source: Daly et al. 2007)
1 out of 51
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.





