The Effects of Vitamin D and Retinoic Acid on HLA-DR Expression Level
VerifiedAdded on 2023/02/01
|43
|14738
|66
Thesis and Dissertation
AI Summary
This dissertation investigates the effects of Vitamin D and Retinoic Acid on the expression level of HLA-DR, a key component of the Major Histocompatibility Complex (MHC). The study provides an overview of MHC molecules, their role in adaptive and innate immunity, and the differences between MHC class I and class II. It explores the association of MHC molecules with inflammatory and autoimmune diseases. The research methodology includes qualitative and quantitative approaches, secondary data analysis, and examination of Vitamin D and KG-1 cells along with ATRA. The findings suggest that Retinoic acid plays a crucial role in modulating the switch between regulatory and inflammatory T-cells, indicating mutual communication between pathways. Future research will focus on the types of communication existing between HLA-DR and vitamin D.

Dissertation
THE EFFECTS OF VITAMIN D AND RETINOIC ACID ON THE
EXPRESSION LEVEL OF HLA-DR
THE EFFECTS OF VITAMIN D AND RETINOIC ACID ON THE
EXPRESSION LEVEL OF HLA-DR
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Abstract
The term MHC refers to a major histocompatibility complex molecule which is used to
control and monitor the cell-mediated adaptive process. There are major two kinds of the
immune system used to exist in the human body, for example, adaptive immunity and innate
immunity. It is identified that MHC molecules support to examine T-cell antigen receptors
and it also provides a platform for indicating peptide antigens. Moreover, human leukocyte
antigen also helps for controlling the amount of MHC presented antigens which destroy
cancerous cells. This research paper identified the key factors linked with MHC molecules
and provided in-depth analysis of the research topic. There are two types of MHC classes
used such as class I and class II and this study also compared both MHC class I and class II.
The molecules of MHC class I consists of one membrane spanning alpha chain which is
encoded by the MHC gene. MHC class II molecules, it consists of two membrane-spanning
chains, alpha and beta, but their sizes are similar. Several molecules of MHC are directly
linked with inflammatory diseases and also increased risk of autoimmune. The study
involved vitamin D and KG-1 cells along with ATRA. Vitamin D is defined as “sunshine
vitamin” which is produced in the skin, in response to the direct sunlight. It is analysed that
that RA (Retinoic Acid) plays a vital role in the homeostatic control of the human immune
system In this research study, there are various kinds of research methodologies will be used
for example qualitative and quantitative research design, sampling technique, data analysis
approach, data collection method. Moreover, a secondary process will be used for obtaining
related data and information about the research topic. This paper shows the impact of vitamin
D and critically reviewed MHC molecules. It is concluded that Retinoic acid plays a critical
role in modulating the process of switch between the regulatory and inflammatory T-cells. It
is suggested that mutual communication exists between the two pathways where activation of
one pathway stops the other pathway from improving the level of expression. In future
The term MHC refers to a major histocompatibility complex molecule which is used to
control and monitor the cell-mediated adaptive process. There are major two kinds of the
immune system used to exist in the human body, for example, adaptive immunity and innate
immunity. It is identified that MHC molecules support to examine T-cell antigen receptors
and it also provides a platform for indicating peptide antigens. Moreover, human leukocyte
antigen also helps for controlling the amount of MHC presented antigens which destroy
cancerous cells. This research paper identified the key factors linked with MHC molecules
and provided in-depth analysis of the research topic. There are two types of MHC classes
used such as class I and class II and this study also compared both MHC class I and class II.
The molecules of MHC class I consists of one membrane spanning alpha chain which is
encoded by the MHC gene. MHC class II molecules, it consists of two membrane-spanning
chains, alpha and beta, but their sizes are similar. Several molecules of MHC are directly
linked with inflammatory diseases and also increased risk of autoimmune. The study
involved vitamin D and KG-1 cells along with ATRA. Vitamin D is defined as “sunshine
vitamin” which is produced in the skin, in response to the direct sunlight. It is analysed that
that RA (Retinoic Acid) plays a vital role in the homeostatic control of the human immune
system In this research study, there are various kinds of research methodologies will be used
for example qualitative and quantitative research design, sampling technique, data analysis
approach, data collection method. Moreover, a secondary process will be used for obtaining
related data and information about the research topic. This paper shows the impact of vitamin
D and critically reviewed MHC molecules. It is concluded that Retinoic acid plays a critical
role in modulating the process of switch between the regulatory and inflammatory T-cells. It
is suggested that mutual communication exists between the two pathways where activation of
one pathway stops the other pathway from improving the level of expression. In future

research, the authors will describe the kinds of communication exists between HLA-DR and
vitamin D.
Keywords: vitamin D, MHC, HLA-DR, T-cells, and immune system
vitamin D.
Keywords: vitamin D, MHC, HLA-DR, T-cells, and immune system
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

1. Introduction Chapter
1.1 MHC molecules and immunity
The cell-mediated adaptive immunity system is basically regulated by MHC, which is a
Major Histo-compatibility Complex molecule (Giles et al. 2015). The immune system of the
body fights the pathogens that succeed in invading the normal tissues. Immune system
protects the human body in various ways against bacteria, viruses, parasites, cancerous cell or
any other. Pathogens enter into the body in an invasive way.Two types of immune system
exist, innate immunity and adaptive immunity. Innate immune defences are mediated by
white blood cells or WBC, such as granulocytes, monocytes/macrophages and natural killer
cells, and by antibacterial proteins, such as acute phase and complement proteins, circulating
in blood.But, in case of adaptive immunity, some specific defences against an invader are
developed (Rock, Reits and Neefjes 2016). However, several forms of adaptive immunity are
there and they are humoral and cell mediated. In order to destroy the antigens, antibodies
appear in the body fluids in case of humoral adaptive immunity system but, in cell mediated
immunity system cells can destroy other cells become active. They destroy all the disease
infected cells (Rock, Reits and Neefjes 2016).
1.1.1 Function of MHC
Some specific functions of MHC molecules have been found by the researchers. MHC
moleculeshelps to introduce T-cell antigen receptors (and, in parallel, B-cell antigen receptors
for B cells) at an early stage. Inside the human body cells, proteins are broken down into
short fragments (Giles et al. 2015). Those short fragmented proteins can be displayed as
peptide antigens by MHC molecules. The self peptide, derived from the own proteins, as well
as the foreign peptides derived from the invading pathogens is displayed by MHC molecules
1.1 MHC molecules and immunity
The cell-mediated adaptive immunity system is basically regulated by MHC, which is a
Major Histo-compatibility Complex molecule (Giles et al. 2015). The immune system of the
body fights the pathogens that succeed in invading the normal tissues. Immune system
protects the human body in various ways against bacteria, viruses, parasites, cancerous cell or
any other. Pathogens enter into the body in an invasive way.Two types of immune system
exist, innate immunity and adaptive immunity. Innate immune defences are mediated by
white blood cells or WBC, such as granulocytes, monocytes/macrophages and natural killer
cells, and by antibacterial proteins, such as acute phase and complement proteins, circulating
in blood.But, in case of adaptive immunity, some specific defences against an invader are
developed (Rock, Reits and Neefjes 2016). However, several forms of adaptive immunity are
there and they are humoral and cell mediated. In order to destroy the antigens, antibodies
appear in the body fluids in case of humoral adaptive immunity system but, in cell mediated
immunity system cells can destroy other cells become active. They destroy all the disease
infected cells (Rock, Reits and Neefjes 2016).
1.1.1 Function of MHC
Some specific functions of MHC molecules have been found by the researchers. MHC
moleculeshelps to introduce T-cell antigen receptors (and, in parallel, B-cell antigen receptors
for B cells) at an early stage. Inside the human body cells, proteins are broken down into
short fragments (Giles et al. 2015). Those short fragmented proteins can be displayed as
peptide antigens by MHC molecules. The self peptide, derived from the own proteins, as well
as the foreign peptides derived from the invading pathogens is displayed by MHC molecules
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

(Rock, Reits and Neefjes 2016). HLA (human leukocyte antigen) present in the MHC of
human body also helps in monitoring the amount of MHC-presented antigens that destroys
cancerous cells which displays increased amount of self-antigens (Moutsianas et al. 2015).
There are a vast number of potential peptide targets but the number of MHC proteins is
limited and due to this purpose MHC proteins are highly effective in binding several types of
peptides. Moreover, MHC proteins have the capability of binding peptides of different kinds
and even of different structures. Due to this unique property, MHC proteins are different from
other proteins or molecules.Tissue allorecognition is another function of MHC and it plays a
major role in preventing successful transplantation of organ (Moutsianas et al. 2015).
1.1.2MHC and antigen presentation
MHC is useful in controlling the process in which the immune system of human body detects
as well as responds to some specific antigens. The MHC molecules also control the antigen
specificity of T-cell recognition (Giles et al. 2015). There are two different classes of MHC
molecules, class I as well as class II. Both the classes have similarity in function of involving
the delivery of very short peptides into the surface of cell recognition and it basically takes
place by CD8+ and CD4+ T cells respectively. It is possible to stimulate some specific T-
cells by MHC class I which is basically located on all cells which are nucleated(Cho et al.
2015).
1.1.3 Difference between MHC class I and MHC class II
MHC is known to be highly polymorphic and in the immune function, the role of it
significant. In E. jankowskii, low level of MHC polymorphism was revealed and it was
similar to that in E.Cioides. There is a difference between the two classes of MHC. The
properties are not similar. The class I is the glycoproteins, which are expressed upon the
surface of all the nucleated cells. The main role of the class I MHC is the presentations of
human body also helps in monitoring the amount of MHC-presented antigens that destroys
cancerous cells which displays increased amount of self-antigens (Moutsianas et al. 2015).
There are a vast number of potential peptide targets but the number of MHC proteins is
limited and due to this purpose MHC proteins are highly effective in binding several types of
peptides. Moreover, MHC proteins have the capability of binding peptides of different kinds
and even of different structures. Due to this unique property, MHC proteins are different from
other proteins or molecules.Tissue allorecognition is another function of MHC and it plays a
major role in preventing successful transplantation of organ (Moutsianas et al. 2015).
1.1.2MHC and antigen presentation
MHC is useful in controlling the process in which the immune system of human body detects
as well as responds to some specific antigens. The MHC molecules also control the antigen
specificity of T-cell recognition (Giles et al. 2015). There are two different classes of MHC
molecules, class I as well as class II. Both the classes have similarity in function of involving
the delivery of very short peptides into the surface of cell recognition and it basically takes
place by CD8+ and CD4+ T cells respectively. It is possible to stimulate some specific T-
cells by MHC class I which is basically located on all cells which are nucleated(Cho et al.
2015).
1.1.3 Difference between MHC class I and MHC class II
MHC is known to be highly polymorphic and in the immune function, the role of it
significant. In E. jankowskii, low level of MHC polymorphism was revealed and it was
similar to that in E.Cioides. There is a difference between the two classes of MHC. The
properties are not similar. The class I is the glycoproteins, which are expressed upon the
surface of all the nucleated cells. The main role of the class I MHC is the presentations of

peptide antigens into the TC (cytotoxic T) cells. The molecules of MHC class I consists of
one membrane spanning alpha chain which is encoded by MHC gene and one beta chain,
which is encoded by the beta2 microglobulin gene (Cho et al. 2015). It also presents foreign
intracellular antigens. But, in case of MHC class II molecules, it consists of two membrane
spanning chains, alpha and beta, but their sizes are similar and both are produced by the
MHC genes. The glycoproteins of MHC class II is present only on some specialized antigen
presenting cells. It also presents 14-18 amino acid peptides which is greater than MHC I. The
class II of MHC also presents foreign extracellular antigen that induces antibody production
as well. The inflammatory response increases the blood flow to the inflammatory area and it
brings immune cells to the site(Van der Meijden et al. 2016). All these properties distinguish
MHC class II from the properties of the MHC class I. Again, MHC class II is basically is a
class of major histo-compatibility complex molecules. These are generally found only on
antigen-presenting cells that includes mononeuclear phagocytes, dendritic cells, etc. All the
cells are extremely important in initiating the immune responses. MHC, the group of genes is
also useful in encoding the proteins found on the surface of cells, and it helps in the
recognition of antigens. At the same time it also determines the histo-compatibility (Giles et
al. 2015). MHC molecule is generally found in human chromosome and can be termed as
human leukocyte antigen (HLA).
one membrane spanning alpha chain which is encoded by MHC gene and one beta chain,
which is encoded by the beta2 microglobulin gene (Cho et al. 2015). It also presents foreign
intracellular antigens. But, in case of MHC class II molecules, it consists of two membrane
spanning chains, alpha and beta, but their sizes are similar and both are produced by the
MHC genes. The glycoproteins of MHC class II is present only on some specialized antigen
presenting cells. It also presents 14-18 amino acid peptides which is greater than MHC I. The
class II of MHC also presents foreign extracellular antigen that induces antibody production
as well. The inflammatory response increases the blood flow to the inflammatory area and it
brings immune cells to the site(Van der Meijden et al. 2016). All these properties distinguish
MHC class II from the properties of the MHC class I. Again, MHC class II is basically is a
class of major histo-compatibility complex molecules. These are generally found only on
antigen-presenting cells that includes mononeuclear phagocytes, dendritic cells, etc. All the
cells are extremely important in initiating the immune responses. MHC, the group of genes is
also useful in encoding the proteins found on the surface of cells, and it helps in the
recognition of antigens. At the same time it also determines the histo-compatibility (Giles et
al. 2015). MHC molecule is generally found in human chromosome and can be termed as
human leukocyte antigen (HLA).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
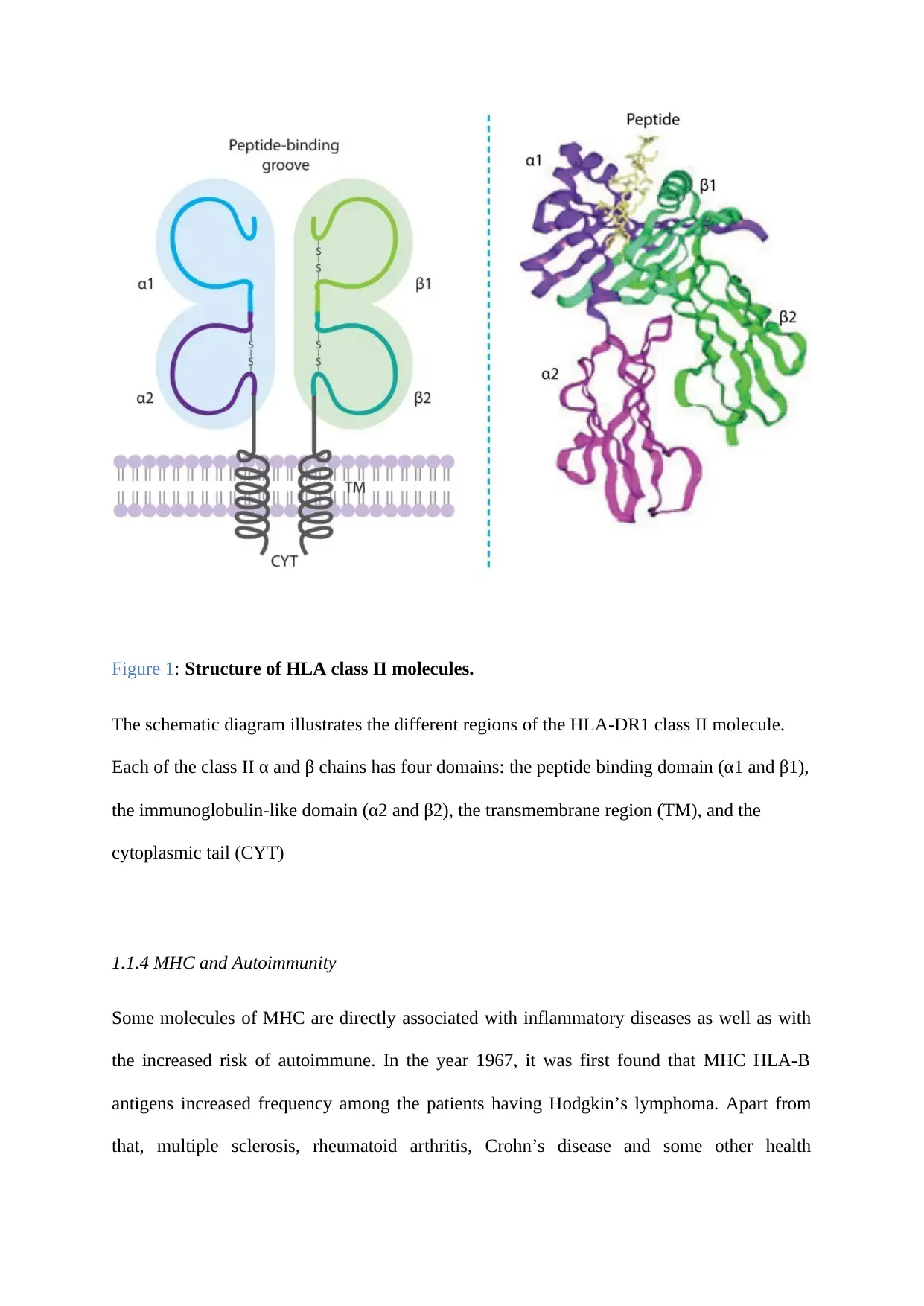
Figure 1: Structure of HLA class II molecules.
The schematic diagram illustrates the different regions of the HLA-DR1 class II molecule.
Each of the class II α and β chains has four domains: the peptide binding domain (α1 and β1),
the immunoglobulin-like domain (α2 and β2), the transmembrane region (TM), and the
cytoplasmic tail (CYT)
1.1.4 MHC and Autoimmunity
Some molecules of MHC are directly associated with inflammatory diseases as well as with
the increased risk of autoimmune. In the year 1967, it was first found that MHC HLA-B
antigens increased frequency among the patients having Hodgkin’s lymphoma. Apart from
that, multiple sclerosis, rheumatoid arthritis, Crohn’s disease and some other health
The schematic diagram illustrates the different regions of the HLA-DR1 class II molecule.
Each of the class II α and β chains has four domains: the peptide binding domain (α1 and β1),
the immunoglobulin-like domain (α2 and β2), the transmembrane region (TM), and the
cytoplasmic tail (CYT)
1.1.4 MHC and Autoimmunity
Some molecules of MHC are directly associated with inflammatory diseases as well as with
the increased risk of autoimmune. In the year 1967, it was first found that MHC HLA-B
antigens increased frequency among the patients having Hodgkin’s lymphoma. Apart from
that, multiple sclerosis, rheumatoid arthritis, Crohn’s disease and some other health
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

conditions of human being are also associated with some specific MHC molecules(Hauser et
al. 2017).In an analysis, conducted by the association of MHC disease revealed that a
susceptibility of shared disease is there to the alleles that arise from HLA-DR4 haplotypes.
Simultaneously, the analysis indicates that there is a common as well as disease specific
association between autoimmunity and the MHC. The exact and specific mechanism behind
the autoimmunity and MHC molecules has not properly been found in the researches but it
potentially reflects a breakdown in tolerance to self-antigens in the antigen presentation of
normal MHC class II. Therefore, some specific class II alleles work as the determinants of
auto-antigen targeting (Cho et al. 2015).
1.1.5 MHC and tissue allorecognition
1.1.5.1Transplant rejection
Allorecognition is basically the capability of an organism that helps in distinguishing its
tissues from those of another organism. This distinguishing is possible within the same
species also plays the important role in the implication of transplantation. Various risks are
there in organ transplantation and one of them is alloresponse, and in this condition,
histoincompatible antigen is identified as well as recognized and it also produces an adaptive
immune response by employing allospecific T-cells (Hauser et al. 2017). All these things can
lead to the direct rejection of all the tissues that are transplanted. But, the direct involvement
of MHC into the mechanism of allorecognition helps in this regard. Here, the T-cell identifies
the determinants on the donor. MHC molecules always display a type of antigenic
determinant that is termed as epitope. T cells have the ability to identify the epitopes
presented by particular allelic variant of MHC molecules. But, if the epitopes are presented
by allelic variants of another MHC molecule, then it is not possible for the T-cells to
recognize those (Van der Meijden et al. 2016).
al. 2017).In an analysis, conducted by the association of MHC disease revealed that a
susceptibility of shared disease is there to the alleles that arise from HLA-DR4 haplotypes.
Simultaneously, the analysis indicates that there is a common as well as disease specific
association between autoimmunity and the MHC. The exact and specific mechanism behind
the autoimmunity and MHC molecules has not properly been found in the researches but it
potentially reflects a breakdown in tolerance to self-antigens in the antigen presentation of
normal MHC class II. Therefore, some specific class II alleles work as the determinants of
auto-antigen targeting (Cho et al. 2015).
1.1.5 MHC and tissue allorecognition
1.1.5.1Transplant rejection
Allorecognition is basically the capability of an organism that helps in distinguishing its
tissues from those of another organism. This distinguishing is possible within the same
species also plays the important role in the implication of transplantation. Various risks are
there in organ transplantation and one of them is alloresponse, and in this condition,
histoincompatible antigen is identified as well as recognized and it also produces an adaptive
immune response by employing allospecific T-cells (Hauser et al. 2017). All these things can
lead to the direct rejection of all the tissues that are transplanted. But, the direct involvement
of MHC into the mechanism of allorecognition helps in this regard. Here, the T-cell identifies
the determinants on the donor. MHC molecules always display a type of antigenic
determinant that is termed as epitope. T cells have the ability to identify the epitopes
presented by particular allelic variant of MHC molecules. But, if the epitopes are presented
by allelic variants of another MHC molecule, then it is not possible for the T-cells to
recognize those (Van der Meijden et al. 2016).

1.1.5.2 Tolerance
The development of the T-cells always depends on the interaction with the MHC molecules.
Studies revealed that during the development in the thymus, 98% T cells die due to the
process of falling selection. Two stages are there in which the first or initial stage is the
positive selection, and in this stage, T cells are found to be interacting with the self MHC in
the thymus but the second stage is the negative selection in which T cells, interacting with
MHC are removed strongly (Giles et al. 2015). Different MHC variants will present different
peptides. Someone else’s (allogeneic) MHC variants and the peptides they present will differ
from those to which the transplant recipient’s T cells are tolerant. The T cells recognise both
types of difference. Autoimmune disease is a phenomenon in the natural world, albeit that it
is a disease state rather than part of normal physiology(Moutsianas et al. 2015).
1.2 Vitamin D
There are various kinds of vitamins. Human body requires many vitamins and one of them is
Vitamin d, which is commonly referred as “sunshine vitamin” because it is generally
produced in the skin, in response to the direct sunlight. During the exposure to the sunlight,
the ultraviolet ray present in it is mainly responsible for photolyzing 7-dehydrocholesterol
(Mokry et al. 2015). There are many functions of vitamin D in improving the health of human
being. This vitamin is soluble in fat and it consists of the compound vitamins of Vitamin D1,
D2, D3. When body is exposed to sunlight it naturally produces vitamin D and also some
food supplements are there in the market to ensure the adequate amount of vitamin D in the
blood. Vitamin D, which is mainly obtained from the direct sun exposure, supplement and
food, is found to be biologically inert and in order to get activated it has to undergo two
different steps of hydroxylation inside the human body.The first stage of the hydroxylation
occurs in theliver, and in this stage, the vitamin D is converted into 25-hydroxyvitamin D,
The development of the T-cells always depends on the interaction with the MHC molecules.
Studies revealed that during the development in the thymus, 98% T cells die due to the
process of falling selection. Two stages are there in which the first or initial stage is the
positive selection, and in this stage, T cells are found to be interacting with the self MHC in
the thymus but the second stage is the negative selection in which T cells, interacting with
MHC are removed strongly (Giles et al. 2015). Different MHC variants will present different
peptides. Someone else’s (allogeneic) MHC variants and the peptides they present will differ
from those to which the transplant recipient’s T cells are tolerant. The T cells recognise both
types of difference. Autoimmune disease is a phenomenon in the natural world, albeit that it
is a disease state rather than part of normal physiology(Moutsianas et al. 2015).
1.2 Vitamin D
There are various kinds of vitamins. Human body requires many vitamins and one of them is
Vitamin d, which is commonly referred as “sunshine vitamin” because it is generally
produced in the skin, in response to the direct sunlight. During the exposure to the sunlight,
the ultraviolet ray present in it is mainly responsible for photolyzing 7-dehydrocholesterol
(Mokry et al. 2015). There are many functions of vitamin D in improving the health of human
being. This vitamin is soluble in fat and it consists of the compound vitamins of Vitamin D1,
D2, D3. When body is exposed to sunlight it naturally produces vitamin D and also some
food supplements are there in the market to ensure the adequate amount of vitamin D in the
blood. Vitamin D, which is mainly obtained from the direct sun exposure, supplement and
food, is found to be biologically inert and in order to get activated it has to undergo two
different steps of hydroxylation inside the human body.The first stage of the hydroxylation
occurs in theliver, and in this stage, the vitamin D is converted into 25-hydroxyvitamin D,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

and again, the second stage of the hydroxylation occurs primarily inside the kidney and forms
the physiologically active 1, known as calcitriol (Mokry et al. 2015). The major function of
vitamin D is to maintain the calcium and phosphate level of blood. Even, recent research
suggests that proper intake of vitamin D provides protection from osteoporosis, hypertension
and several other autoimmune diseases.
1.2.1 Role of Vitamin D
Vitamin is essential in promoting calcium absorption in the gut and also maintains the
adequate concentration of serum calcium and phosphate which helps in enabling the normal
mineralization of bones and again it prevents hypocalcemic tetany as well. Sufficiency of
Vitamin D inside the human body prevents rickets in children and osteomalacia among
adults. Vitamin D has various other roles in the human body, as it is associated with the
immune system. It has the ability to modulate the innate as well as the adaptive immune
responses. Deficiency of vitamin D is responsible for increased susceptibility to infection.It
also contributes to the cell growth and reduction of inflammation (Mokry et al. 2015). There
is an association between the vitamin D deficiency and multiple sclerosis. It has been found
in many studies that the women who take vitamin D as dietary supplements are less likely to
be suffered from multiple sclerosis and even, the incidence is less than 33% when compared
to the women who are not taking vitamin D as dietary supplement. Many researchers are in
the progress of investigating the therapeutic effects of the vitamin D in preventing multiple
sclerosis (Kreiter et al. 2015).
1.2.2 Properties of Vitamin D
Vitamin D has the properties of vitamin as well as hormone. It is highly necessary for the
mineral homeostasis and the proper and well formation of the bones. Two different forms
vitamin D are there which includes ergocalciferol and cholecalciferol. The ergocalciferol is
the physiologically active 1, known as calcitriol (Mokry et al. 2015). The major function of
vitamin D is to maintain the calcium and phosphate level of blood. Even, recent research
suggests that proper intake of vitamin D provides protection from osteoporosis, hypertension
and several other autoimmune diseases.
1.2.1 Role of Vitamin D
Vitamin is essential in promoting calcium absorption in the gut and also maintains the
adequate concentration of serum calcium and phosphate which helps in enabling the normal
mineralization of bones and again it prevents hypocalcemic tetany as well. Sufficiency of
Vitamin D inside the human body prevents rickets in children and osteomalacia among
adults. Vitamin D has various other roles in the human body, as it is associated with the
immune system. It has the ability to modulate the innate as well as the adaptive immune
responses. Deficiency of vitamin D is responsible for increased susceptibility to infection.It
also contributes to the cell growth and reduction of inflammation (Mokry et al. 2015). There
is an association between the vitamin D deficiency and multiple sclerosis. It has been found
in many studies that the women who take vitamin D as dietary supplements are less likely to
be suffered from multiple sclerosis and even, the incidence is less than 33% when compared
to the women who are not taking vitamin D as dietary supplement. Many researchers are in
the progress of investigating the therapeutic effects of the vitamin D in preventing multiple
sclerosis (Kreiter et al. 2015).
1.2.2 Properties of Vitamin D
Vitamin D has the properties of vitamin as well as hormone. It is highly necessary for the
mineral homeostasis and the proper and well formation of the bones. Two different forms
vitamin D are there which includes ergocalciferol and cholecalciferol. The ergocalciferol is
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

used as food additive and the cholecalciferol is the naturally occuraing Vitamin D, which is
directly synthesized in the skin with exposure to sunlight and some specific food
supplements. According to studies, it triggers the immune cells of body and produces
antibodies(Palacios and Gonzalez 2014). Therefore, vitamin D has a great contribution to the
overall immunity system of human body. Evidences from more than 500 studies support the
fact that vitamin D plays huge role in the immune system of human being. It assists in the
maintenance of muscle comfort and joints. There is adequate amount of vitamin D in the
sunlight and exposure to sunlight and some specific dietary food supplements can help in
supplying sufficient vitamin D to the human being but studies in the United States National
Centres and a report regarding the Health Statistics states that approximately 70% of
individuals suffer from vitamin D deficiency (Palacios and Gonzalez 2014). The people of
vitamin D deficient include elderly as well as the breastfed infants as they cannot get the
sufficient amount of sun exposure. In addition, people who have fat mal-absorption syndrome
such as inflammatory bowel disease, cystic fibrosis and others also are at risk of suffering
from the vitamin D deficiency. Usage of some specific medications such as carbamazepine
and phenytoin can increase the metabolism rate of vitamin D inside the human body. All
these agents also help in increasing the hepatic metabolism of vitamin D to the compounds
that are inactive and at the same time it reduces calcium absorption. People, especially
women mainly suffer from this syndrome due to not having sufficient amount of calcium
absorption inside body (Matzaraki et al. 2017).
In the year 2008, the American Academy of Dermatology provided a new version of position
statement on the vitamin D and regarding the role of it in the maintenance of optimal health
after reviewing evidences. American Academy of Paediatrics recommends 400 IU per day of
vitamin D3 taken with food for children. In some cases, higher doses of vitamin D is
necessary for human being and such patients should always be referred for further assistance
directly synthesized in the skin with exposure to sunlight and some specific food
supplements. According to studies, it triggers the immune cells of body and produces
antibodies(Palacios and Gonzalez 2014). Therefore, vitamin D has a great contribution to the
overall immunity system of human body. Evidences from more than 500 studies support the
fact that vitamin D plays huge role in the immune system of human being. It assists in the
maintenance of muscle comfort and joints. There is adequate amount of vitamin D in the
sunlight and exposure to sunlight and some specific dietary food supplements can help in
supplying sufficient vitamin D to the human being but studies in the United States National
Centres and a report regarding the Health Statistics states that approximately 70% of
individuals suffer from vitamin D deficiency (Palacios and Gonzalez 2014). The people of
vitamin D deficient include elderly as well as the breastfed infants as they cannot get the
sufficient amount of sun exposure. In addition, people who have fat mal-absorption syndrome
such as inflammatory bowel disease, cystic fibrosis and others also are at risk of suffering
from the vitamin D deficiency. Usage of some specific medications such as carbamazepine
and phenytoin can increase the metabolism rate of vitamin D inside the human body. All
these agents also help in increasing the hepatic metabolism of vitamin D to the compounds
that are inactive and at the same time it reduces calcium absorption. People, especially
women mainly suffer from this syndrome due to not having sufficient amount of calcium
absorption inside body (Matzaraki et al. 2017).
In the year 2008, the American Academy of Dermatology provided a new version of position
statement on the vitamin D and regarding the role of it in the maintenance of optimal health
after reviewing evidences. American Academy of Paediatrics recommends 400 IU per day of
vitamin D3 taken with food for children. In some cases, higher doses of vitamin D is
necessary for human being and such patients should always be referred for further assistance

of physicians and they should never follow the process of self-treatment as the dosage of
vitamin D must be determined by an expert physician only (Thompson et al. 2017). US food
and Nutrition Board has also set an upper limit for the intake of vitamin D, in order to avoid
the toxicity caused by the over intake of it. According to them the upper intake of vitamin D
is 2000 IU per day for the individuals older than one year and for the infants the upper limit is
1000 IU. Again, some studies reported that an estimated number of more than 6.3 million
children of United States are lacking the adequate amount of vitamin D (Lefevre 2015).
According to evidences, 7 out of 10 children in the United States have low level of vitamin D
which puts them in the risk of rickets, cardiovascular disease and weak bones (Campos et al.
2015).
1.2.3 Multiple Sclerosis and Vitamin D
Multiple sclerosis is basically a complex trait in the medical term and in this disease, the
allelic variation in the MHC class II is found to be exerting the strongest effect on the genetic
risk.The risk of developing an autoimmune disease, such as MS, is determined by a number
of genetic and environmental factors (Van der Meijden et al. 2016).Some strong
epidemiological information about multiple sclerosis provides evidence that environmental
factor has major contribution to the incidence as well as prevalence of the disease and
sunlight or vitamin D is the main environmental factor in this regard (Dendrou, Fugger and
Friese 2015). Growing evidences indicate that insufficient sun exposure or lack of vitamin D
is one of the greatest reasons of Multiple Sclerosis (MS). This is a very complex neurological
disease and it is related to the strong genetic component. Studies show that there is a massive
involvement of vitamin D in the nervous as well as in the immune system of human being.
Some circumstantial evidence show that MS patients are vitamin D deficient and dietary
vitamin intake is helpful in reducing all the risks regarding MS (Gorman et al. 2016).
vitamin D must be determined by an expert physician only (Thompson et al. 2017). US food
and Nutrition Board has also set an upper limit for the intake of vitamin D, in order to avoid
the toxicity caused by the over intake of it. According to them the upper intake of vitamin D
is 2000 IU per day for the individuals older than one year and for the infants the upper limit is
1000 IU. Again, some studies reported that an estimated number of more than 6.3 million
children of United States are lacking the adequate amount of vitamin D (Lefevre 2015).
According to evidences, 7 out of 10 children in the United States have low level of vitamin D
which puts them in the risk of rickets, cardiovascular disease and weak bones (Campos et al.
2015).
1.2.3 Multiple Sclerosis and Vitamin D
Multiple sclerosis is basically a complex trait in the medical term and in this disease, the
allelic variation in the MHC class II is found to be exerting the strongest effect on the genetic
risk.The risk of developing an autoimmune disease, such as MS, is determined by a number
of genetic and environmental factors (Van der Meijden et al. 2016).Some strong
epidemiological information about multiple sclerosis provides evidence that environmental
factor has major contribution to the incidence as well as prevalence of the disease and
sunlight or vitamin D is the main environmental factor in this regard (Dendrou, Fugger and
Friese 2015). Growing evidences indicate that insufficient sun exposure or lack of vitamin D
is one of the greatest reasons of Multiple Sclerosis (MS). This is a very complex neurological
disease and it is related to the strong genetic component. Studies show that there is a massive
involvement of vitamin D in the nervous as well as in the immune system of human being.
Some circumstantial evidence show that MS patients are vitamin D deficient and dietary
vitamin intake is helpful in reducing all the risks regarding MS (Gorman et al. 2016).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 43
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





