Dysregulated Pathways due to Gut Dysbiosis
VerifiedAdded on 2022/10/04
|13
|2553
|8
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: INTEGRATIVE BIOCHEMICAL ANALYSIS
INTEGRATIVE BIOCHEMICAL ANALYSIS
Name of the Student:
Name of the University:
Author note:
INTEGRATIVE BIOCHEMICAL ANALYSIS
Name of the Student:
Name of the University:
Author note:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1INTEGRATIVE BIOCHEMICAL ANALYSIS
Table of Contents
1. Introduction......................................................................................................................2
2. Discussion........................................................................................................................2
2.1. Dysregulated Pathways due to Gut Dysbiosis..........................................................2
2.1.1. Obesity and Diet................................................................................................2
2.1.2. Lifestyle.............................................................................................................4
2.1.3. Clinical History..................................................................................................5
2.1.4. Family History and Implications.......................................................................5
2.2. Dietary Nutritional Advice.......................................................................................5
2.3. Lifestyle Advice........................................................................................................6
4. Conclusion.......................................................................................................................7
5. References........................................................................................................................8
6. Appendices....................................................................................................................11
Table of Contents
1. Introduction......................................................................................................................2
2. Discussion........................................................................................................................2
2.1. Dysregulated Pathways due to Gut Dysbiosis..........................................................2
2.1.1. Obesity and Diet................................................................................................2
2.1.2. Lifestyle.............................................................................................................4
2.1.3. Clinical History..................................................................................................5
2.1.4. Family History and Implications.......................................................................5
2.2. Dietary Nutritional Advice.......................................................................................5
2.3. Lifestyle Advice........................................................................................................6
4. Conclusion.......................................................................................................................7
5. References........................................................................................................................8
6. Appendices....................................................................................................................11

2INTEGRATIVE BIOCHEMICAL ANALYSIS
1. Introduction
Susan has symptoms of anxiety, stress, urination, fatigue, abdominal pain, bloating. This
paper aims to discuss the impact of biochemical pathway dysregulation, dietary habits and family
history on Susan’s diagnosis of gut dysbiosis and the role of lifestyle and nutritional
interventions as mitigation strategies.
2. Discussion
2.1. Dysregulated Pathways due to Gut Dysbiosis
Gut dysbiosis results in hepatic inflammation, translocation of dysbiosis mediators and
hindrances to gut endothelial barrier as key contributors of Non-Alcoholic Fatty Liver Disease
(NAFLD) in individuals (Aragonès et al., 2019).
2.1.1. Obesity and Diet
Susan’s anthropometric measurements indicate obesity due to her diet of processed
meats, packaged juices and biscuits and fast foods (Zhao et al., 2017). Gut microbiota ferment
the surplus energy acquired from such a diet into short chain fatty acids (SCFAs) like butyrate,
acetate and propionate. SCFA leads to gut dysbiosis via small intestinal bacterial overgrowth
(SIBO) (Paolellaet al., 2014). Paolella et al., (2014) evidence the role of obesity in metabolic
dysregulation via adipogenesis – SCFA generated due to fermentation of surplus calories results
in activation of Gpr43 and Gpr40 and resultant uncontrolled metabolic conversion of pre-
adipocytes to adipocytes. The resultant adiposity stimulates macrophage and phagocytic
inflammation, alteration and even gut bacterial death and thus gut dysbiosis. Low grade
inflammation due to excessive adiposity further stimulates pro-inflammatory cytokine
1. Introduction
Susan has symptoms of anxiety, stress, urination, fatigue, abdominal pain, bloating. This
paper aims to discuss the impact of biochemical pathway dysregulation, dietary habits and family
history on Susan’s diagnosis of gut dysbiosis and the role of lifestyle and nutritional
interventions as mitigation strategies.
2. Discussion
2.1. Dysregulated Pathways due to Gut Dysbiosis
Gut dysbiosis results in hepatic inflammation, translocation of dysbiosis mediators and
hindrances to gut endothelial barrier as key contributors of Non-Alcoholic Fatty Liver Disease
(NAFLD) in individuals (Aragonès et al., 2019).
2.1.1. Obesity and Diet
Susan’s anthropometric measurements indicate obesity due to her diet of processed
meats, packaged juices and biscuits and fast foods (Zhao et al., 2017). Gut microbiota ferment
the surplus energy acquired from such a diet into short chain fatty acids (SCFAs) like butyrate,
acetate and propionate. SCFA leads to gut dysbiosis via small intestinal bacterial overgrowth
(SIBO) (Paolellaet al., 2014). Paolella et al., (2014) evidence the role of obesity in metabolic
dysregulation via adipogenesis – SCFA generated due to fermentation of surplus calories results
in activation of Gpr43 and Gpr40 and resultant uncontrolled metabolic conversion of pre-
adipocytes to adipocytes. The resultant adiposity stimulates macrophage and phagocytic
inflammation, alteration and even gut bacterial death and thus gut dysbiosis. Low grade
inflammation due to excessive adiposity further stimulates pro-inflammatory cytokine

3INTEGRATIVE BIOCHEMICAL ANALYSIS
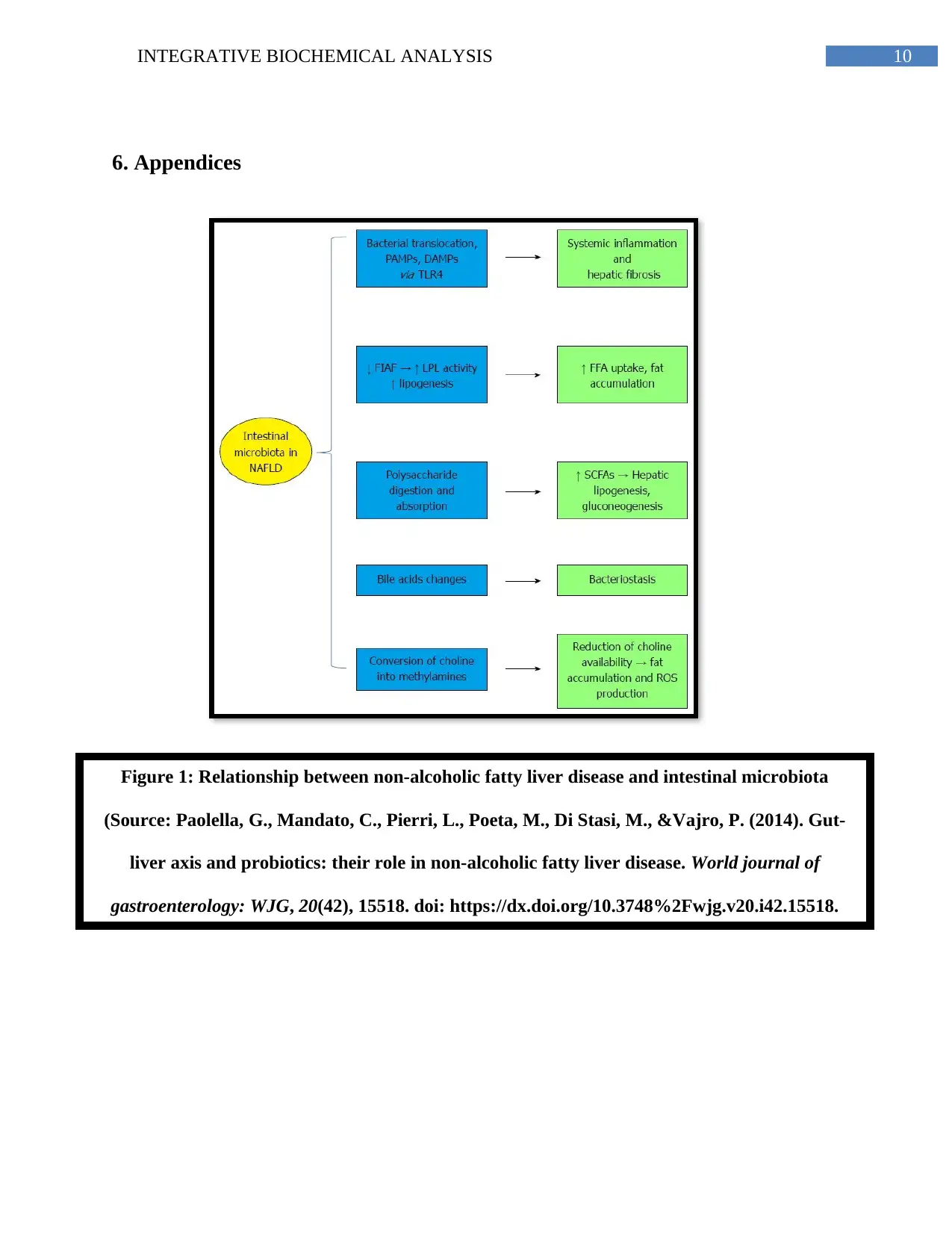
production, hepatocyte damage, disturbed fatty acid metabolism and excessive intrahepatic
circulation further leading to steasosis and NAFLD (Telle-Hansen, Holven&Ulven, 2018)
(Appendix, Figure 1).
SIBO leads to decreased motility and transit time and explains Susan’s abdominal
cramping, bloating, diarrhea and nausea (Choi& Chang, 2016). Hepatic conjugation of bile acids
via glycine or taurine initiate flow of bile which further results in metabolism and excretion of
free fatty acids, free cholesterol and detoxified xenobiotics. SIBO has been further linked to
premature deconjugation of bile acids and disturbance cholesterol and biliary detoxification
leading to enterohepatic and systemtic circulation of cholesterol resulting in steasosis, NAFLD
and hypercholesterol - indicated by Susan’s excessive blood cholesterol (Kvit et al., 2019).
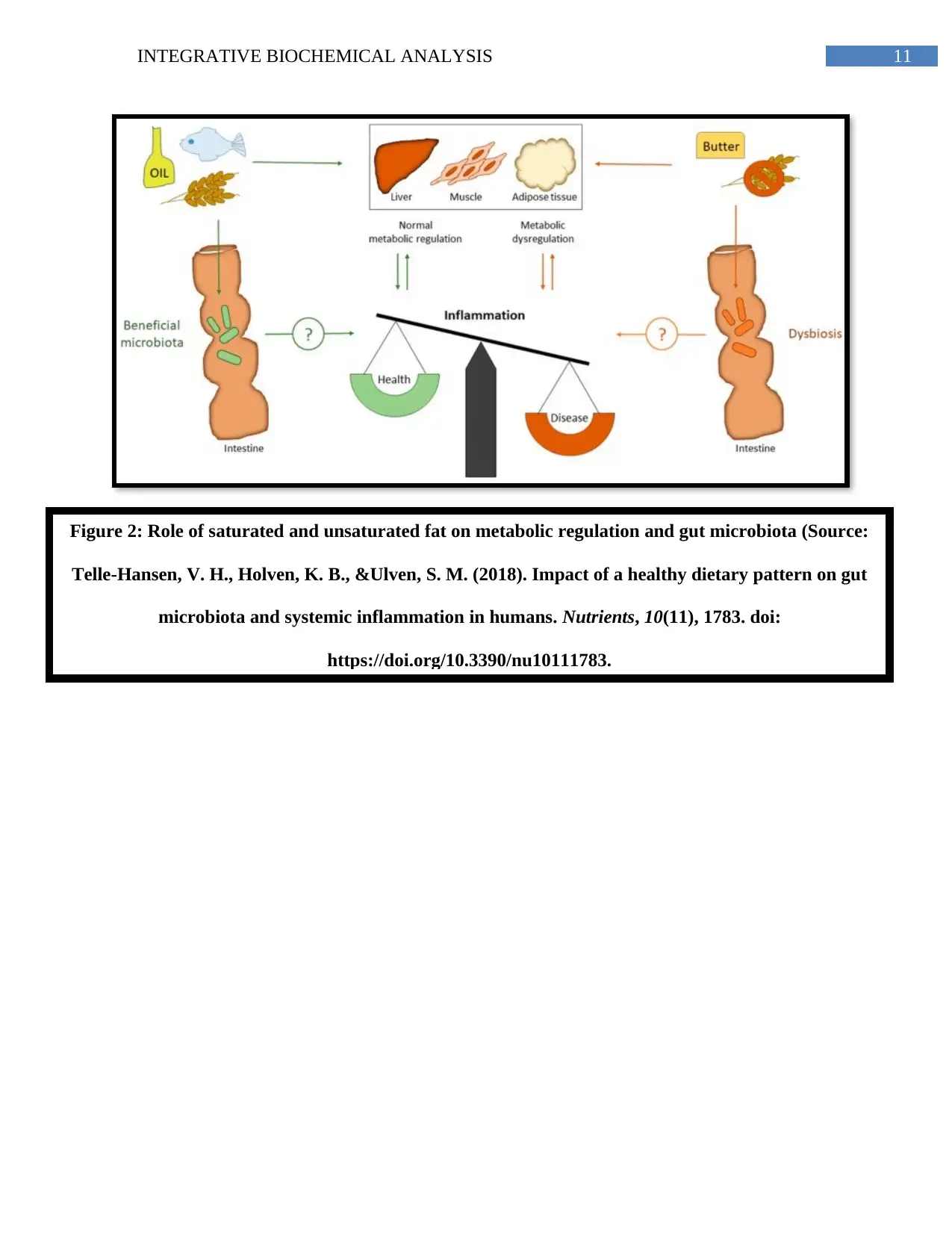
Another key contributor of gut dysbiosis and NAFLD is a high saturated fat diet from
takeaway meals and processed meats – observed in Susan’s diet. A high saturated fat diet results
in fatty streak accumulation in the tissues and endothelium which further trigger LDL oxidation,
inflammatory response by macrophages resulting in cellular damage, metabolic dysregulation,
and alteration of microbial colonies (Telle-Hansen, Holven&Ulven, 2018) (Appendix, Figure 2).
The alteration of gut microbial colonies can be due to the impact of fatty acids, obtained from
high saturated fat intake, in the disruption of enzymatic, nutrient absorption and energy
production generated by the gastrointestinal tract and gut microbiota (Coelho, Cândido&Alfenas,
2018).
2.1.2. Lifestyle
Perfumes as well as processed foods contain sulfites – an additive incorporate to restrict
spoilage and contamination by bacterial colonies. This occurs by substituting the electrophilic
positions of biomolecules within gut bacterial cells resulting in inhibition of microbial enzymes
production, hepatocyte damage, disturbed fatty acid metabolism and excessive intrahepatic
circulation further leading to steasosis and NAFLD (Telle-Hansen, Holven&Ulven, 2018)
(Appendix, Figure 1).
SIBO leads to decreased motility and transit time and explains Susan’s abdominal
cramping, bloating, diarrhea and nausea (Choi& Chang, 2016). Hepatic conjugation of bile acids
via glycine or taurine initiate flow of bile which further results in metabolism and excretion of
free fatty acids, free cholesterol and detoxified xenobiotics. SIBO has been further linked to
premature deconjugation of bile acids and disturbance cholesterol and biliary detoxification
leading to enterohepatic and systemtic circulation of cholesterol resulting in steasosis, NAFLD
and hypercholesterol - indicated by Susan’s excessive blood cholesterol (Kvit et al., 2019).
Another key contributor of gut dysbiosis and NAFLD is a high saturated fat diet from
takeaway meals and processed meats – observed in Susan’s diet. A high saturated fat diet results
in fatty streak accumulation in the tissues and endothelium which further trigger LDL oxidation,
inflammatory response by macrophages resulting in cellular damage, metabolic dysregulation,
and alteration of microbial colonies (Telle-Hansen, Holven&Ulven, 2018) (Appendix, Figure 2).
The alteration of gut microbial colonies can be due to the impact of fatty acids, obtained from
high saturated fat intake, in the disruption of enzymatic, nutrient absorption and energy
production generated by the gastrointestinal tract and gut microbiota (Coelho, Cândido&Alfenas,
2018).
2.1.2. Lifestyle
Perfumes as well as processed foods contain sulfites – an additive incorporate to restrict
spoilage and contamination by bacterial colonies. This occurs by substituting the electrophilic
positions of biomolecules within gut bacterial cells resulting in inhibition of microbial enzymes
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

4INTEGRATIVE BIOCHEMICAL ANALYSIS
responsible for NADPH and ATP secretion and thus, cellular death. Susan loves perfumes which
may increase her intake of sulfites and alter her gut microbial growth, resultant dysbiosis and
NAFLD (Irwin et al., 2017). Additionally, terpenoids and terpenes – prevalent in perfume
essential oils, exhibit antimicrobial activity by rupturing bacterial plasma membrane (Guimarães
et al., 2019). Alcohol intake by Susan, can induce dehydration of microbial cell membranes
coupled with reduced alcohol dehydrogenase functioning in females, toxic acetaldehyde
production resulting in gut dysbiosis and NAFLD. Further, excess alcohol results in increased
insulin resistance and Alanine Amino Tranferase, Alkaline Phosphatase and Amino Tranferase
levels, indicative of NALFD (Engen et al., 2015).
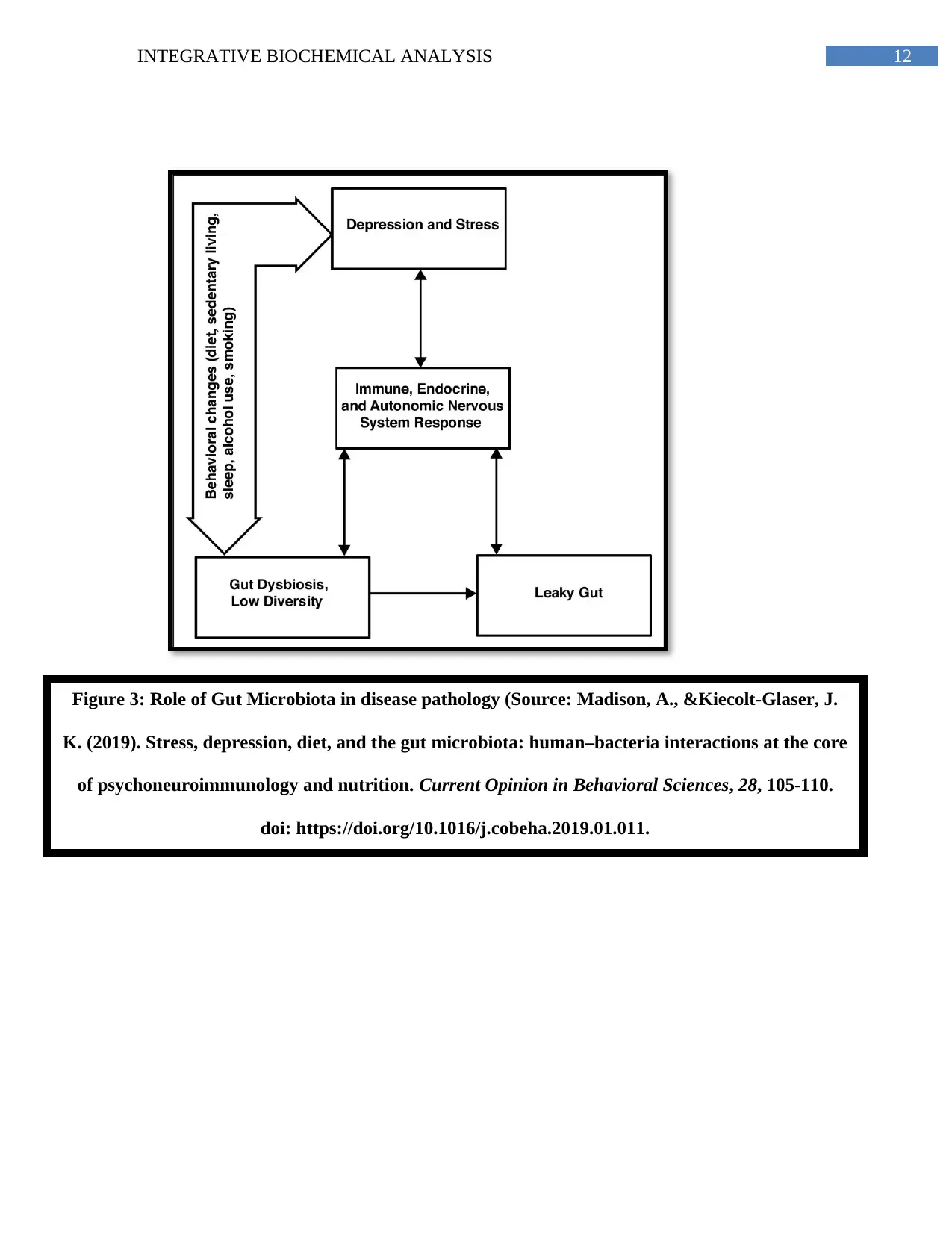
Gut microbiota works in close conjunction with one’s circadian rhythm which is why,
individuals with tight occupational schedules, stress, and disrupted sleep encounter a ‘leaky gut’
(Li et al., 2018). Anxiety has been linked to excessive cortisol –– the hormone causing
inflammation, calorie conservation, lipogenesis and gut microbial changes. Susan’s busy work
hours and sleeplessness thus contributes to gut dysbiosis and NAFLD (Madison &Kiecolt-
Glaser, 2019) (Appendix, Figure 3).
2.1.3. Clinical History
Susan’s medical history demonstrates antibiotic consumption which may cause dysbiosis.
Antibiotics alter gut microbial activity disruption of inter-species interactions and functioning of
microbiome induced lipid metabolic pathways resulting in gut dysbiosis and the resultant
adiposity and NAFLD (Yoon& Yoon, 2018).
responsible for NADPH and ATP secretion and thus, cellular death. Susan loves perfumes which
may increase her intake of sulfites and alter her gut microbial growth, resultant dysbiosis and
NAFLD (Irwin et al., 2017). Additionally, terpenoids and terpenes – prevalent in perfume
essential oils, exhibit antimicrobial activity by rupturing bacterial plasma membrane (Guimarães
et al., 2019). Alcohol intake by Susan, can induce dehydration of microbial cell membranes
coupled with reduced alcohol dehydrogenase functioning in females, toxic acetaldehyde
production resulting in gut dysbiosis and NAFLD. Further, excess alcohol results in increased
insulin resistance and Alanine Amino Tranferase, Alkaline Phosphatase and Amino Tranferase
levels, indicative of NALFD (Engen et al., 2015).
Gut microbiota works in close conjunction with one’s circadian rhythm which is why,
individuals with tight occupational schedules, stress, and disrupted sleep encounter a ‘leaky gut’
(Li et al., 2018). Anxiety has been linked to excessive cortisol –– the hormone causing
inflammation, calorie conservation, lipogenesis and gut microbial changes. Susan’s busy work
hours and sleeplessness thus contributes to gut dysbiosis and NAFLD (Madison &Kiecolt-
Glaser, 2019) (Appendix, Figure 3).
2.1.3. Clinical History
Susan’s medical history demonstrates antibiotic consumption which may cause dysbiosis.
Antibiotics alter gut microbial activity disruption of inter-species interactions and functioning of
microbiome induced lipid metabolic pathways resulting in gut dysbiosis and the resultant
adiposity and NAFLD (Yoon& Yoon, 2018).

5INTEGRATIVE BIOCHEMICAL ANALYSIS
2.1.4. Family History and Implications
Gut dysbiosis and NAFLD hinders liver functioning and detoxification. As researched by
Cline (2015), hepatic detoxification occurs via phase 1 toxicant transformation into hydrophilic
forms via cytochrome p450 enzymes and phase II conjugation of toxicants into soluble excretory
forms via intrahepatic antioxidants like glutathione, glucuronide, sulfate, glycine, proline, serine
and glutamine. Lack of antioxidant intake and NAFLD decreases glutathione and p450 enzymes
essential for oxidation and conjugation (Masarone et al., 2018). This leads to accumulation of
reactive oxygen species, inflammation and diabetes, cardiovascular conditions and cancer. These
may be further aggravated by family history and personal habits of nutritionally imbalanced diet
consumption (Paolella et al., 2014). Susan’s diagnostic results, diet, diabetes symptoms and
family history indicate risk of metabolic dysregulation and disease.
2.2. Dietary Nutritional Advice
Susan must consume probiotics like yoghurt and milk-based beverages fortified with
Actinomycetes,Bifidobacteri, Bacillus and Clostridium. Such foods restore dominance in
existing microbiome resulting in repair of intestinal barrier damages and mitigation of gut
dysbiosis symptoms of diarrhea and cramping as observed in Susan (Paolella et al.,
2014).
Susan must consume high fiber foods like green leafy vegetables, wholegrains and fruits,
since, as per Kieffer, Martin and Adams (2016), fiber rich foods improve gut microbial
functioning and proliferation via enhanced metabolism of nitrogen by gut bacteria and
increased interaction between gut microbial colonies. Soluble fiber sources like pectin,
found in beets, apples and oatmeal increase colonic transit time, lipid absorption and
fecal excretion and resultant stimulation of intrahepatic bile detoxification pathways,
2.1.4. Family History and Implications
Gut dysbiosis and NAFLD hinders liver functioning and detoxification. As researched by
Cline (2015), hepatic detoxification occurs via phase 1 toxicant transformation into hydrophilic
forms via cytochrome p450 enzymes and phase II conjugation of toxicants into soluble excretory
forms via intrahepatic antioxidants like glutathione, glucuronide, sulfate, glycine, proline, serine
and glutamine. Lack of antioxidant intake and NAFLD decreases glutathione and p450 enzymes
essential for oxidation and conjugation (Masarone et al., 2018). This leads to accumulation of
reactive oxygen species, inflammation and diabetes, cardiovascular conditions and cancer. These
may be further aggravated by family history and personal habits of nutritionally imbalanced diet
consumption (Paolella et al., 2014). Susan’s diagnostic results, diet, diabetes symptoms and
family history indicate risk of metabolic dysregulation and disease.
2.2. Dietary Nutritional Advice
Susan must consume probiotics like yoghurt and milk-based beverages fortified with
Actinomycetes,Bifidobacteri, Bacillus and Clostridium. Such foods restore dominance in
existing microbiome resulting in repair of intestinal barrier damages and mitigation of gut
dysbiosis symptoms of diarrhea and cramping as observed in Susan (Paolella et al.,
2014).
Susan must consume high fiber foods like green leafy vegetables, wholegrains and fruits,
since, as per Kieffer, Martin and Adams (2016), fiber rich foods improve gut microbial
functioning and proliferation via enhanced metabolism of nitrogen by gut bacteria and
increased interaction between gut microbial colonies. Soluble fiber sources like pectin,
found in beets, apples and oatmeal increase colonic transit time, lipid absorption and
fecal excretion and resultant stimulation of intrahepatic bile detoxification pathways,

6INTEGRATIVE BIOCHEMICAL ANALYSIS
mitigation of adipose-associated inflammation and control of gut dysbiosis and NAFLD
symptoms.
Susan must consume carotenes, vitamin B3, polyphenols, curcumin and anthocyanins
which are abundant in salmon, tuna, chicken breast, turmeric, green tea, onions, garlic
and colorful fruits and vegetables. Vitamin B3 and antioxidants combat free radical
formation and improve hepatic detoxification functioning (Cline, 2015). Ascorbic acid
and quercetin increase glutathione and CYP1A1, CYP1A2 and UGT – required for
detoxification. Susan can find these in citrus fruits, cruciferous vegetables and potaoes,
carrots, apples and onions (Paolella et al., 2014).
2.3. Lifestyle Advice
According to Smart et al., (2016), aerobic and resistance training improves metabolism
and insulin sensitivity resulting in improved liver enzyme functioning, intrahepatic circulation,
reduced intrahepatic fat and reduced circulation of free fatty acids. Weekly 150 to 300 minutes of
exercise can be beneficial for Susan (Department of Health, 2019).
4. Conclusion
Thus, nutritional intake determines the efficiency of essential biochemical pathways. One
must consume a diverse range of nutrients to ensure adequate detoxification within the body. In
conclusion, along with consumption of an antioxidant rich diet and engagement in mild to
moderate physical activity, Susan must also be advised to engage in exercise.
mitigation of adipose-associated inflammation and control of gut dysbiosis and NAFLD
symptoms.
Susan must consume carotenes, vitamin B3, polyphenols, curcumin and anthocyanins
which are abundant in salmon, tuna, chicken breast, turmeric, green tea, onions, garlic
and colorful fruits and vegetables. Vitamin B3 and antioxidants combat free radical
formation and improve hepatic detoxification functioning (Cline, 2015). Ascorbic acid
and quercetin increase glutathione and CYP1A1, CYP1A2 and UGT – required for
detoxification. Susan can find these in citrus fruits, cruciferous vegetables and potaoes,
carrots, apples and onions (Paolella et al., 2014).
2.3. Lifestyle Advice
According to Smart et al., (2016), aerobic and resistance training improves metabolism
and insulin sensitivity resulting in improved liver enzyme functioning, intrahepatic circulation,
reduced intrahepatic fat and reduced circulation of free fatty acids. Weekly 150 to 300 minutes of
exercise can be beneficial for Susan (Department of Health, 2019).
4. Conclusion
Thus, nutritional intake determines the efficiency of essential biochemical pathways. One
must consume a diverse range of nutrients to ensure adequate detoxification within the body. In
conclusion, along with consumption of an antioxidant rich diet and engagement in mild to
moderate physical activity, Susan must also be advised to engage in exercise.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7INTEGRATIVE BIOCHEMICAL ANALYSIS
5. References
Aragonès, G., González-García, S., Aguilar, C., Richart, C., &Auguet, T. (2019). Gut
Microbiota-Derived Mediators as Potential Markers in Nonalcoholic Fatty Liver
Disease. BioMed research international, 2019. doi:
https://doi.org/10.1155/2019/8507583.
Choi, C. H., & Chang, S. K. (2016). Role of small intestinal bacterial overgrowth in functional
gastrointestinal disorders. Journal of neurogastroenterology and Motility, 22(1), 3. doi:
https://dx.doi.org/10.5056%2Fjnm15196.
Coelho, O. G. L., Cândido, F. G., &Alfenas, R. D. C. G. (2018). Dietary fat and gut microbiota:
mechanisms involved in obesity control. Critical reviews in food science and nutrition, 1-
9. doi: https://doi.org/10.1080/10408398.2018.1481821.
Department of Health. (2019). Department of Health | Australia's Physical Activity and
Sedentary Behaviour Guidelines and the Australian 24-Hour Movement Guidelines.
Retrieved 16 October 2019, from
https://www1.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-
phys-act-guidelines.
Engen, P. A., Green, S. J., Voigt, R. M., Forsyth, C. B., &Keshavarzian, A. (2015). The
gastrointestinal microbiome: alcohol effects on the composition of intestinal
microbiota. Alcohol research: current reviews, 37(2), 223. Retrieved from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4590619/pdf/arcr-37-2-223.pdf.
5. References
Aragonès, G., González-García, S., Aguilar, C., Richart, C., &Auguet, T. (2019). Gut
Microbiota-Derived Mediators as Potential Markers in Nonalcoholic Fatty Liver
Disease. BioMed research international, 2019. doi:
https://doi.org/10.1155/2019/8507583.
Choi, C. H., & Chang, S. K. (2016). Role of small intestinal bacterial overgrowth in functional
gastrointestinal disorders. Journal of neurogastroenterology and Motility, 22(1), 3. doi:
https://dx.doi.org/10.5056%2Fjnm15196.
Coelho, O. G. L., Cândido, F. G., &Alfenas, R. D. C. G. (2018). Dietary fat and gut microbiota:
mechanisms involved in obesity control. Critical reviews in food science and nutrition, 1-
9. doi: https://doi.org/10.1080/10408398.2018.1481821.
Department of Health. (2019). Department of Health | Australia's Physical Activity and
Sedentary Behaviour Guidelines and the Australian 24-Hour Movement Guidelines.
Retrieved 16 October 2019, from
https://www1.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-
phys-act-guidelines.
Engen, P. A., Green, S. J., Voigt, R. M., Forsyth, C. B., &Keshavarzian, A. (2015). The
gastrointestinal microbiome: alcohol effects on the composition of intestinal
microbiota. Alcohol research: current reviews, 37(2), 223. Retrieved from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4590619/pdf/arcr-37-2-223.pdf.

8INTEGRATIVE BIOCHEMICAL ANALYSIS
Guimarães, A. C., Meireles, L. M., Lemos, M. F., Guimarães, M. C. C., Endringer, D. C.,
Fronza, M., & Scherer, R. (2019). Antibacterial Activity of Terpenes and Terpenoids
Present in Essential Oils. Molecules, 24(13), 2471. doi:
https://doi.org/10.3390/molecules24132471.
Irwin, S. V., Fisher, P., Graham, E., Malek, A., &Robidoux, A. (2017). Sulfites inhibit the
growth of four species of beneficial gut bacteria at concentrations regarded as safe for
food. PloS one, 12(10), e0186629. doi: https://doi.org/10.1371/journal.pone.0186629.
Kieffer, D. A., Martin, R. J., & Adams, S. H. (2016). Impact of dietary fibers on nutrient
management and detoxification organs: gut, liver, and kidneys. Advances in Nutrition,
7(6), 1111-1121. doi: https://doi.org/10.3945/an.116.013219.
Kvit, K. B., Kharchenko, N. V., Kharchenko, V. V., Chornenka, O. I., Chornovus, R. I.,
Dorofeeva, U. S., ... &Slaba, O. M. (2019). The role of small intestinal bacterial
overgrowth in the pathogenesis of hyperlipidemia. Wiadomoscilekarskie (Warsaw,
Poland: 1960), 72(4), 645-649. Retrieved from:
http://medlist.org/pdf/wl/2019_04_27.pdf.
Li, Y., Hao, Y., Zhang, B., & Fan, F. (2018). The role of microbiome in insomnia, circadian
disturbance and depression. Frontiers in psychiatry, 9, 669. doi:
https://doi.org/10.3389/fpsyt.2018.00669.
Madison, A., &Kiecolt-Glaser, J. K. (2019). Stress, depression, diet, and the gut microbiota:
human–bacteria interactions at the core of psychoneuroimmunology and
nutrition. Current Opinion in Behavioral Sciences, 28, 105-110. doi:
https://doi.org/10.1016/j.cobeha.2019.01.011.
Guimarães, A. C., Meireles, L. M., Lemos, M. F., Guimarães, M. C. C., Endringer, D. C.,
Fronza, M., & Scherer, R. (2019). Antibacterial Activity of Terpenes and Terpenoids
Present in Essential Oils. Molecules, 24(13), 2471. doi:
https://doi.org/10.3390/molecules24132471.
Irwin, S. V., Fisher, P., Graham, E., Malek, A., &Robidoux, A. (2017). Sulfites inhibit the
growth of four species of beneficial gut bacteria at concentrations regarded as safe for
food. PloS one, 12(10), e0186629. doi: https://doi.org/10.1371/journal.pone.0186629.
Kieffer, D. A., Martin, R. J., & Adams, S. H. (2016). Impact of dietary fibers on nutrient
management and detoxification organs: gut, liver, and kidneys. Advances in Nutrition,
7(6), 1111-1121. doi: https://doi.org/10.3945/an.116.013219.
Kvit, K. B., Kharchenko, N. V., Kharchenko, V. V., Chornenka, O. I., Chornovus, R. I.,
Dorofeeva, U. S., ... &Slaba, O. M. (2019). The role of small intestinal bacterial
overgrowth in the pathogenesis of hyperlipidemia. Wiadomoscilekarskie (Warsaw,
Poland: 1960), 72(4), 645-649. Retrieved from:
http://medlist.org/pdf/wl/2019_04_27.pdf.
Li, Y., Hao, Y., Zhang, B., & Fan, F. (2018). The role of microbiome in insomnia, circadian
disturbance and depression. Frontiers in psychiatry, 9, 669. doi:
https://doi.org/10.3389/fpsyt.2018.00669.
Madison, A., &Kiecolt-Glaser, J. K. (2019). Stress, depression, diet, and the gut microbiota:
human–bacteria interactions at the core of psychoneuroimmunology and
nutrition. Current Opinion in Behavioral Sciences, 28, 105-110. doi:
https://doi.org/10.1016/j.cobeha.2019.01.011.

9INTEGRATIVE BIOCHEMICAL ANALYSIS
Masarone, M., Rosato, V., Dallio, M., Gravina, A. G., Aglitti, A., Loguercio, C., ...&Persico, M.
(2018). Role of oxidative stress in pathophysiology of nonalcoholic fatty liver
disease. Oxidative medicine and cellular longevity, 2018. doi:
https://doi.org/10.1155/2018/9547613.
Paolella, G., Mandato, C., Pierri, L., Poeta, M., Di Stasi, M., &Vajro, P. (2014). Gut-liver axis
and probiotics: their role in non-alcoholic fatty liver disease. World journal of
gastroenterology: WJG, 20(42), 15518. doi:
https://dx.doi.org/10.3748%2Fwjg.v20.i42.15518.
Telle-Hansen, V. H., Holven, K. B., &Ulven, S. M. (2018). Impact of a healthy dietary pattern
on gut microbiota and systemic inflammation in humans. Nutrients, 10(11), 1783. doi:
https://doi.org/10.3390/nu10111783.
Yoon, M. Y., & Yoon, S. S. (2018). Disruption of the gut ecosystem by antibiotics. Yonsei
medical journal, 59(1), 4-12. doi: https://doi.org/10.3349/ymj.2018.59.1.4.
Zhao, L., Zhang, Q., Ma, W., Tian, F., Shen, H., & Zhou, M. (2017). A combination of quercetin
and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut
microbiota. Food & function, 8(12), 4644-4656. doi: 10.1039/C7FO01383C.
Masarone, M., Rosato, V., Dallio, M., Gravina, A. G., Aglitti, A., Loguercio, C., ...&Persico, M.
(2018). Role of oxidative stress in pathophysiology of nonalcoholic fatty liver
disease. Oxidative medicine and cellular longevity, 2018. doi:
https://doi.org/10.1155/2018/9547613.
Paolella, G., Mandato, C., Pierri, L., Poeta, M., Di Stasi, M., &Vajro, P. (2014). Gut-liver axis
and probiotics: their role in non-alcoholic fatty liver disease. World journal of
gastroenterology: WJG, 20(42), 15518. doi:
https://dx.doi.org/10.3748%2Fwjg.v20.i42.15518.
Telle-Hansen, V. H., Holven, K. B., &Ulven, S. M. (2018). Impact of a healthy dietary pattern
on gut microbiota and systemic inflammation in humans. Nutrients, 10(11), 1783. doi:
https://doi.org/10.3390/nu10111783.
Yoon, M. Y., & Yoon, S. S. (2018). Disruption of the gut ecosystem by antibiotics. Yonsei
medical journal, 59(1), 4-12. doi: https://doi.org/10.3349/ymj.2018.59.1.4.
Zhao, L., Zhang, Q., Ma, W., Tian, F., Shen, H., & Zhou, M. (2017). A combination of quercetin
and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut
microbiota. Food & function, 8(12), 4644-4656. doi: 10.1039/C7FO01383C.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
10INTEGRATIVE BIOCHEMICAL ANALYSIS
6. Appendices
Figure 1: Relationship between non-alcoholic fatty liver disease and intestinal microbiota
(Source: Paolella, G., Mandato, C., Pierri, L., Poeta, M., Di Stasi, M., &Vajro, P. (2014). Gut-
liver axis and probiotics: their role in non-alcoholic fatty liver disease. World journal of
gastroenterology: WJG, 20(42), 15518. doi: https://dx.doi.org/10.3748%2Fwjg.v20.i42.15518.
6. Appendices
Figure 1: Relationship between non-alcoholic fatty liver disease and intestinal microbiota
(Source: Paolella, G., Mandato, C., Pierri, L., Poeta, M., Di Stasi, M., &Vajro, P. (2014). Gut-
liver axis and probiotics: their role in non-alcoholic fatty liver disease. World journal of
gastroenterology: WJG, 20(42), 15518. doi: https://dx.doi.org/10.3748%2Fwjg.v20.i42.15518.
11INTEGRATIVE BIOCHEMICAL ANALYSIS
Figure 2: Role of saturated and unsaturated fat on metabolic regulation and gut microbiota (Source:
Telle-Hansen, V. H., Holven, K. B., &Ulven, S. M. (2018). Impact of a healthy dietary pattern on gut
microbiota and systemic inflammation in humans. Nutrients, 10(11), 1783. doi:
https://doi.org/10.3390/nu10111783.
Figure 2: Role of saturated and unsaturated fat on metabolic regulation and gut microbiota (Source:
Telle-Hansen, V. H., Holven, K. B., &Ulven, S. M. (2018). Impact of a healthy dietary pattern on gut
microbiota and systemic inflammation in humans. Nutrients, 10(11), 1783. doi:
https://doi.org/10.3390/nu10111783.
12INTEGRATIVE BIOCHEMICAL ANALYSIS
Figure 3: Role of Gut Microbiota in disease pathology (Source: Madison, A., &Kiecolt-Glaser, J.
K. (2019). Stress, depression, diet, and the gut microbiota: human–bacteria interactions at the core
of psychoneuroimmunology and nutrition. Current Opinion in Behavioral Sciences, 28, 105-110.
doi: https://doi.org/10.1016/j.cobeha.2019.01.011.
Figure 3: Role of Gut Microbiota in disease pathology (Source: Madison, A., &Kiecolt-Glaser, J.
K. (2019). Stress, depression, diet, and the gut microbiota: human–bacteria interactions at the core
of psychoneuroimmunology and nutrition. Current Opinion in Behavioral Sciences, 28, 105-110.
doi: https://doi.org/10.1016/j.cobeha.2019.01.011.
1 out of 13
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
