Iodine Value Effect on Biodiesel Engine Performance Analysis Report
VerifiedAdded on 2023/06/15

Student id:149179676
Biodiesel from Vegetable Oil/ Animal Fat
Hamad Almuaini
Title: The effect of iodine value of Biodiesel blends from inedible vegetable on engine
performance, combustion and emission
Paraphrase This Document

Student id:149179676
1.Introduction
There are limited nonrenewable sources of energy thus they will eventually deplete. However,
they have hazardous side effects such as carbon emissions leading to global warming. As a
result, there is a need to generate energy from renewable sources which have no danger of
exhaustion. Fossil fuels are examples of energy from nonrenewable sources while biofuels,
biomass, and biodiesel are forms of renewable energy.
Fossil fuels are carbon-based sources of fuel such as oil, coal, and natural gas. They are referred
to as nonrenewable as they take more than a million years to be formed in the earth through
decayed animals and plants. Typically, they are formed through geological processes. Though
the fossil fuels are relatively cheap compared to other forms of energy they have significant
adverse effects such as toxic and global warming emissions.
Biofuels are forms of renewable energy which are produced through modern biological processes
such as agriculture and anaerobic digestion. Their production involves carbon fixation in plants
by the use of photosynthesis [25]. Biofuels were identified as the green alternative of the fossil
fuels; still, they have an impact to the environment as they involve burning of the plants.
Moreover, it requires an enormous collection of plants to only produce a little energy thus it
faces huge competition from the food requirement.
Biomass entails obtaining energy from burning wood and other organic matter. When the organic
matter is burned, it releases energy it store from the sun through the process of photosynthesis. In
fact, biomass energy is related to biofuels as they originate from living organisms [25].
Noteworthy, biomass is convertible to other usable forms of energy such as methane gas or
transportation fuels such as ethanol and biodiesel.
Biodiesel is a vegetable oil or animal fat-based diesel fuel produced through the process of
transesterification. Oil crops such as rapeseed, soybeans, and palm are the most sources of the
biodiesel energy [26]. However, these sources are expensive to obtain due to competition from
the industrial industry. As a result, biodiesel is produced from waste vegetable oil sourced from
restaurants and industrial food producers. The use of the waste vegetables makes the biodiesel
cost effective thus it can compete with the typical fossil fuels.

Student id:149179676
There are various approaches through which biodiesel can be obtained from vegetable oil and
animal fat. A base catalyzed transesterification of the oil is the most common and economical
process as it requires low pressures and temperatures and it has a high percentage yield [27].
Two different types of catalysts can be used for this transesterification – Homogeneous catalysts
and Heterogeneous catalysts. The use of homogeneous alkaline catalysts is conventional in this
transesterification reaction [31]. There are certain drawbacks of using these catalysts in the
reaction. The process requires intensive energy and excess amount of water is produced. It is
extremely difficult to separate this excess water produced. On the other hand, the consumption of
energy is much less with the use of heterogeneous catalysts and the production of water is also
less and can be separated and reused [32]. Other methods that could be utilized are direct acid
catalyzed transesterification of the oil and conversion of the oil to its fatty acids and then to
biodiesel.
Different biodiesel blends were prepared and tested on a 6-cylinder DI diesel engine. The reports
from the test results have stated that there has been a reduction in the power of the torque and the
brake. If a compression ignition engine can be supplied with biodiesel fuel, then the compression
ratio of the engine will be increased, injection timing of the machine will be advanced and
opening pressure of the injector will be higher and this might improve the performance of the
engine. With the help of the biodiesel oils, there will be significant reduction in the particulate
matters such as emissions of carbon monoxide, carbon dioxide and hydrocarbons [33].
Biodiesel use has various benefits. Foremost, the fuel is a carbon-neutral source of energy in that
the fuel produces no net output of carbon. This is achieved because the carbon dioxide released
from the combustion is offset by the carbon dioxide absorbed when the oil crops are grown.
Therefore, it has no impact on global warming or toxic emissions [26]. Moreover, the fuel is
safer than the fossil fuel. It has less damage than petroleum if spilled or released to the
environment and it is less combustible. The biodiesel flashpoint is more than 130°C compare to
petroleum’s 52°C. The biodiesel fuel also improves the engine operational efficiency. It achieves
this by the ability to improve fuel lubricity which is essential in keeping engine moving parts
from wearing prematurely.
Biodiesel is a blended fuel between biological sources vegetables oil or animal fats with
petroleum diesel. The biodiesel is made by a chemical process called Transterification where
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Student id:149179676
glycerin is separated from the animal fats or vegetable oil leaving two products one is methyl
ester and the second is glycerin.
Nonrenewable sources of energy are not finite and will run out one day. However, renewable
sources ensure that these sources remain useful to the present and future generation. Among the
many sources of emerging renewable sources of fuel is biodiesel which apart from being
renewable is also environmentally cleaner compared to nonrenewable diesel obtained from
extracted oil. Thus the sustainability of biodiesel is not limited to its continued availability
independent of geopolitical concerns but also the protection of the environment due to some of
the extraction techniques used for nonrenewable sources. Methods like fracking and offshore
drilling coupled with the degradation and pollution occasioned by oil spills make biodiesel a
better choice as a source of clean and green fuel.
Hydrogenation is the process of converting unsaturated fatty acid to saturated fatty acid.
Multiple methods are used in order to check the saturation level in fatty acid; among them, one is
a determination of iodine level of fats. In analytical chemistry, the unsaturation of fats, oil, and
wax is measured by taking the iodine value of the compound. The number of grams of iodine
consumed by 100g of fats is considered as iodine value or iodine number. Furthermore, physical
and chemical properties of fuels are important to determine the quality of fuels, combustion
characteristics it is also known as fuel characterization test (Heating value, flash point, iodine
number, etc.…). However, the aim of this report is to study and testing in engine the different
types of biodiesel by mixing chicken fat which has low iodine value with non-edible vegetable
oil cotton seed which has high iodine value. Moreover, after fuel characterization will test in
engine the biodiesel with different blend percentage (20/80%, 40/60%, 60/40%, 80/20%,
100/0%), compare biodiesel results with diesel result and to analyze the performance,
combustion and emission result to understand the effect of iodine value.
2.Aim and Objectives
It is important to think about other sources of energy that can substitute nonrenewable sources.
Besides, the environmental cost of utilizing nonrenewable sources of energy is having a negative
impact on the quality of life through air pollution and increased carbon emission. However,
biodiesel vegetable oil provides an alternative of a clean and green source of energy that is
Paraphrase This Document

Student id:149179676
renewable. Nonetheless, biodiesel from vegetable sources has also come under intense pressure
because the majority of it has been produced from food sources. Moreover, each type of oil or
animal fat has different degree of saturation level it can be saturation and unsaturation in other
word oil vegetables or animal fats with high iodine value and low iodine value. Furthermore,
Saturation fatty acid contain only single bond between carbon atom and unsaturated fatty acid
contain double bonds between the carbons in addition into the single bonds that present in the
fatty acid chain. However, In UK 14111 test standard has maximum iodine value of vegetable oil
and animal fat is 120. Consequently, the aim of this study is to understand effects of iodine value
(Degree of unsaturation) of biodiesel on engine performance, combustion characteristics, and
emissions.
The objectives:
Investigate possible biodiesel feedstocks according to their iodine values and availability
Provide biodiesel from selected feedstocks (for ex: one with high iodine value (inedible
vegetable oil) and one with low iodine value (Animal fat)
Blend the biodiesel at different percentage
Fuel Characterization
Engine for the test fuels
Analyse the performance, Combustion and emission result to understand effects of iodine
value
3. Different Biodiesel Feedstock
The earliest alternative fuel is biodiesel fuel. The molecules are combined to make the fats and
oils called as triglycerides. Multiple long chains are joined together to make up each of the
glycerides. This chain might be composed of 8 to 22 carbons which are attached to the backbone.
The chains of fatty acids make up the Biodiesel; which is chemically bonded to a single
methanol molecule [1]. The final biodiesel product does not contain glycerol molecule; this is
removed by implementing multiple methods. Fatty acid methyl ester (FAME) is usually
considered as biodiesel. The byproduct of this process possess thousands of industrial chemical

Student id:149179676
uses, and also commonly used in a household. The braked fatty acid chain is termed as “free
fatty acid (FFA)”. FFAs are commonly considered as the desirable biodiesel feedstock; still, it
requires conversion processes [2]. Biodiesel feedstock is further divided into different categories,
with respect to the source of fatty acid, as follow:
1.Oil Feedstock: is the most common raw material for biodiesel fuel is considered as Rapeseed
and soybean oil. The ninety percent of all biodiesel fuel is generated by Soybean oil as the
feedstock in the UK [3]. Furthermore, it can be taken from pennycress and jatropha; crops like
mustard, flax, sunflower, palm oil, coconut, hemp is considered as the good resource of Soybean
oil.
2.Waste vegetable oil (WVO): is the discarded oil from the restaurants and food stores are also a
popular and important biodiesel fuel feedstock. Researchers find that the waste vegetable oil is
the best resource and raw material for the biodiesel fuel feedstock [4].
3.Algae: is waste materials and sewage are utilized for the growth of algae, without making use
of land. These are also used as the feedstock for biodiesel fuel [5].
4.(Oil from halophytes): Halophytes such as Salicornia bigelovii is another important source of
feedstock for biodiesel fuel, which can be grown by utilizing saltwater in coastal areas [6]; as
coastal areas are unable to grow conventional crops. Similar to the yields of the fresh water
irrigation, they produce yields equal to them.
3.1 Inedible Vegetable Oils
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Student id:149179676
Inedible vegetable oil is an alternative source of biodiesel fuel. A great quantity of inedible
vegetable oil is used as the feedstock; like jatropha, Karanja, mahua, linseed, rubber seed,
cottonseed and neem oils etc. Fuels are being modified such as jatropha is blended with other oil
or fuels. For this reason, jatropha oil is the leading among inedible vegetable oils, commercially.
A big difference among the properties of edible oil and biodiesel fuel feedstock is found and
characterized. According to the environmental aspects, the biodiesel generally causes the
increment in NOx emission which in results decrease reg HC, CO and PM emission for the
implication of biodiesel.
3.1.1Castor Oil
Castor oil is produced from the Castor seed plant which is found in East Africa, parts of the
Mediterranean region and Indian subcontinent. The plant has a high yield of recoverable oil
which stands at 42% [7]. Also, castor oil is commercially important due to its high percentage of
unsaturated fatty acids and an iodine value of between 82-90 in its unprocessed form [8].
Coupled with its other qualities, castor oil is ideal for the production of biodiesel on a
commercial scale.
3.1.2 Jatropha
The Jatropha plant is also priced as a source of raw material for the production of biodiesel. Like
the Castor plant, Jatropha has a recoverable oil content of approximately 47% making its large-
scale commercial cultivation economically viable for the production of vegetable oil. Among its
many qualities, jatropha oil has a high iodine value of 4.78 per 100g due to the high
concentration of unsaturated fatty acids [9]. Thus, these iodine values make it ideal for the
production of biodiesel.
3.1.3 Pongamia
The Milletia piñata plant which is found in South East Asia is the source of Pongamia oil. Due to
its varied distribution, the plant has different names based on localities like Karanja, Kuningan,
and Honge [10]. However, the seeds have a yield of up to 39% recoverable oil that has made it
an important source of vegetable oil ideal for the production of biodiesel. Apart from the other
Paraphrase This Document

Student id:149179676
physical characteristics of the Pongamia oil, it has an iodine value of between 86-91 [11] thus
making it very similar to castor oil in composition.
3.1.4 Cottonseed
Cottonseed is a crop which is relatively smaller than any other crop. This is obtained from cotton
plants and commercially used for the production of oil. There are two major category of cotton,
crop for fiber and crop for seed. The seed crop takes part in the production of cotton seed oil
[12]. This produced oil is further used in many types of industries. The various uses of
cottonseed oil include the use of this oil in food. This oil can be extracted very easily and thus it
is a cheap vegetable oil. Due to the lesser price of this oil, this is of huge demand in America as
cottonseed oil is mostly produced in America. But this oil in its true form is not edible. To make
it edible, refinement has to be done. This oil is also used in cosmetics mostly as body oils or even
for the manufacture of cleansing products, eye liners, lipsticks, lip balms etc. This widely used in
the manufacture of cosmetics due to its lower price and neutral taste. Cottonseed oil is also used
as a biodiesel. There is an extremely superior property of lubricity in cottonseed oil [36]. There
are a lot of other unique components in cottonseed oil. The oil is extremely useful in reducing oil
oxidation due to the presence of a natural anti-oxidant named gossypol and carotene. The oil can
also be formulated and used to show improvements in the process of combustion due to its
properties. The properties possessed by biodiesel are mostly inhibited from the parent oil and
thus, the production of biodiesel from cottonseed oil is also expected to obtain oxidative stability
and enhance the performance of the engines [37]. Approximately, biodiesel produced by the 50%
of the cottonseed is 148.3 million gallons. This produces a more competitive oil market for the
producers, ginners and oil mills as the cotton seed oil biodiesel has been potentially hit the
market [13]. Additionally, the dependency on foreign oil has been reduced by the production of

Student id:149179676
such biodiesel. This has increased the utilization of renewable source for the production [14].
Biodiesel is produced from cottonseed oil with the use of calcined egg shells as catalyst in the
process of transesterification. In the process, palmitic oil and linoleic acid is produced in plenty.
Biodiesel that are partially purified have been found to have a flash point and a fire point and
with a calorific value which is quite high. This type of biodiesel has shown less emissions of
carbon monoxide (CO) and hydrocarbons (HC) which in turn has shown improvements in the
performances in the engines [34]. Biodiesel was produced from vegetable oils which were non-
edible with the help of the process named transesterification in the presence of a catalyst. The
molar ratio for this process is 6:1. With 1 percent concentration in the catalyst, a yield of 93
percent of biodiesel was produced. If the concentration of the catalyst was increased, then soap
was formed from it instead of biodiesel [35]. Thus, in production of biodiesel from cottonseed
oil, the concentration of the catalyst is extremely important. It was also important to understand
the suitability of the biodiesel produced from cottonseed oil as fuels in engines. Thus fueling of
CI engine was performed with different varieties of cottonseed oil with the aim to understand the
suitability of the oil as fuel. This study involved normal cottonseed oil, preheated cottonseed oil,
trans esterified cottonseed oil as well as orange oil-DEE-diesel blended cottonseed oil. From the
study, better engine performance was observed from the preheated cottonseed oil and the
blended cottonseed oil with orange oil and DEE diesel.
3.1.5 Croton
The most extensive flowering plant genus in the family of sprung euphorbiaceae is Croton [15].
This is also an important biodiesel fuel feedstock.
3.1.6 Microalgae
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Student id:149179676
Microalgae have become a major resource in the pursuit of renewable energy that is sustainable
and environmentally safe. Because of its quick growth, the algae in this class not only offer a raw
material for renewable energy but also absorb pollutants such as phosphates. Among its other
qualities, microalgae have an iodine content of 20.90 g I2/100 g following ASTM D6751 test
method [16]. Thus, with iodine values of less than 100, microalgae make a good resource for
biodiesel production.
3.1.7 Rubber seed
This is extracted with hexane at room temperature to give rubber seed oil of 24wt%. The
extracted oil was analyzed and considered as feedstock for biodiesel. The composition and the
key properties are taken with respect to the composition of fatty acid [17].
3.2 Animal Fats
An alkyl ester of fatty acid, commonly known as biodiesel is of prime interest nowadays as this
is a renewable source of energy and is also environment friendly. Mostly production of biodiesel
is made from vegetable oils which are of high quality. It has been observed that these high
quality vegetable oils are quite costly and accounts for more than 85 percent of the cost of
production of biodiesel [38]. Thus, a lot of studies has been conducted on production of biodiesel
from cheaper feedstock such as frying oils or animal fats. Approximately, one-third of the oil
produced is from animal fats. The fats which are used to produce oil can also be used as
feedstock for biodiesel fuels. This may include:
beef tallow
pork lard
chicken fat
Paraphrase This Document

Student id:149179676
The most attractive feedstock for biodiesel is animal fats because of the sustainable lower cost as
compared to the vegetable oil. Partially, this is limited as compared to the vegetable oil, as it is
limited in the market. Mostly, the fats of animals are edible by humans, that’s why it is short in
the market as compared to the vegetable oils [18]. For the food of pets and animal feed, this
animal fat is also utilized. Moreover, for the industrial use such as soap production, animal’s fats
are used as the raw material. For this purpose, most of the domestic animal fats are exported.
3.2.1 Beef Tallow, Chicken Fat & Pork Lard
About 40% of the saturated fatty acid is beef Tallow and Pork lard, which is the sum of
“myristic, palmitic and stearic acids”; while fat of chicken is minimal than pork lard and it is
about 30-33%. In comparison to the vegetable oil, soybeans oil is 14% and canola oil is 6% in
the saturated fatty acid. Hence, tallow, chicken fats, and lard are physically solid at room
temperature, while others are usually liquids. Liquid fatty oils are viscous and approximately
near to solids [19].
Extraction of chicken fat is made from feather meal which is in turn obtained from chicken
wastes such as feathers of chicken, blood, offal and trims after the process of rendition.
According to previous studies, in 2006 there was a projection of 10.5 million chicken production
in China. This gave an estimation of production of 11 percent of the total body fat. Thus, there
was 115500 tons of chicken fat. Thus, every year, there is a huge number of chicken producing
even a larger volume of fat which is considered as a waste. Moreover, due to the development of
a recent consciousness in health, humans have started to avoid consumption of chicken fat. Thus,
chicken fat can be used as a feed stock for the production of biodiesel in a cheaper cost as
compared to other high quality vegetable oils [39].
Student id:149179676
Chicken fats contain fatty acid, which comprises of 100ppm of Sulphur; this sulphur is mainly
originated from amino acids. This amino acid is associated with the protein molecules of the
fatty acid present in chicken fats. Usually, chicken fats are obtained by the process of rendering.
Among the animal’s fats, chicken fats possess high level of linoleic acid, and also contain a
greater amount of omega-6 fatty acid. The level of this acid is 17.9% and 22.8%, respectively.
Due to the presence of fatty acids, the production of biodiesel in the presence of an alkali catalyst
with the process of transesterification is not performed directly. This is mainly because the fatty
acids can react with the alkali and form soap which will be resulting in emulsification and the
process will be failure. Thus, a two-step process has been adopted involving an esterification
process with the help of acid as a catalyst and a transesterification process with alkali as catalyst
and combining the two [39].
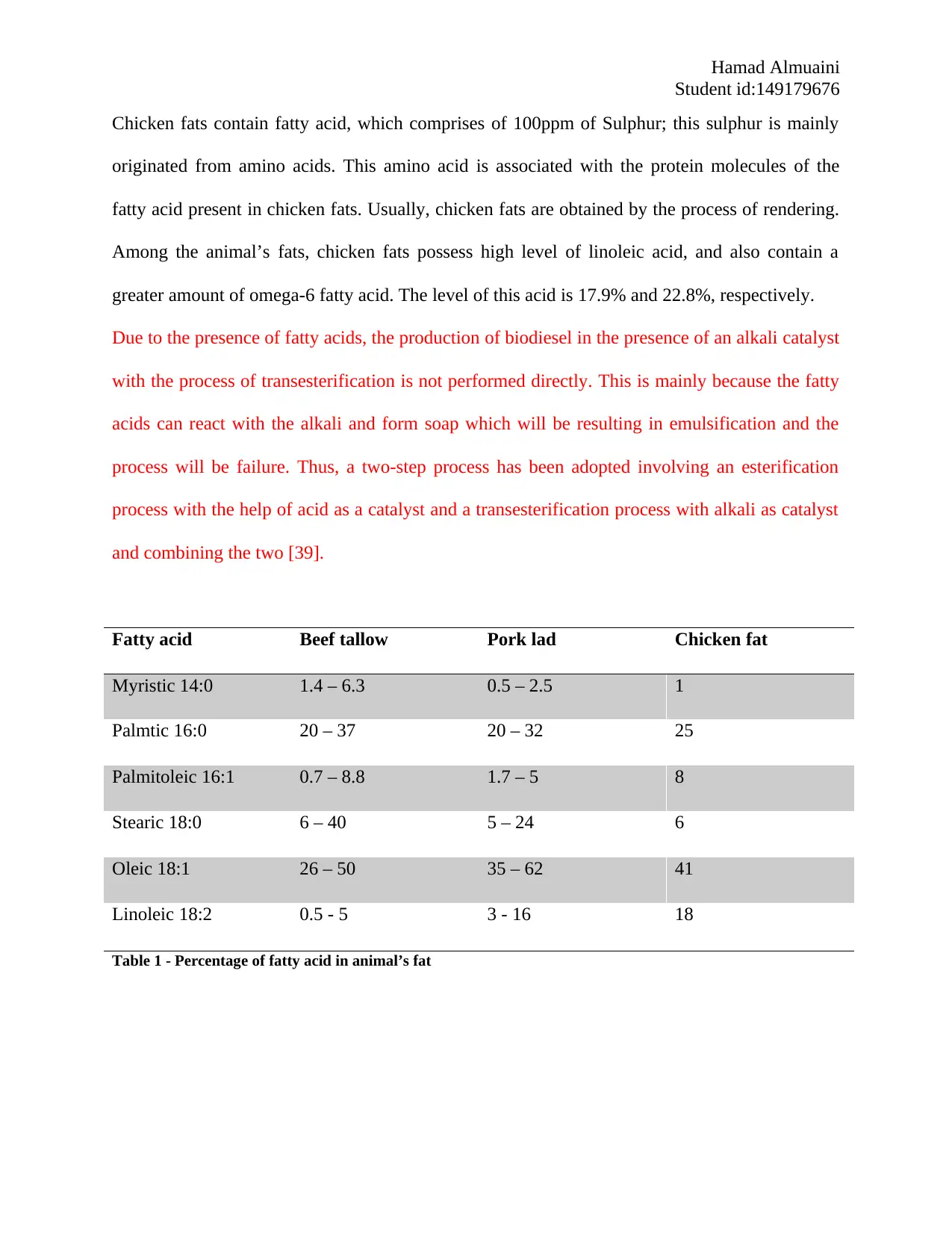
Fatty acid Beef tallow Pork lad Chicken fat
Myristic 14:0 1.4 – 6.3 0.5 – 2.5 1
Palmtic 16:0 20 – 37 20 – 32 25
Palmitoleic 16:1 0.7 – 8.8 1.7 – 5 8
Stearic 18:0 6 – 40 5 – 24 6
Oleic 18:1 26 – 50 35 – 62 41
Linoleic 18:2 0.5 - 5 3 - 16 18
Table 1 - Percentage of fatty acid in animal’s fat
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Student id:149179676
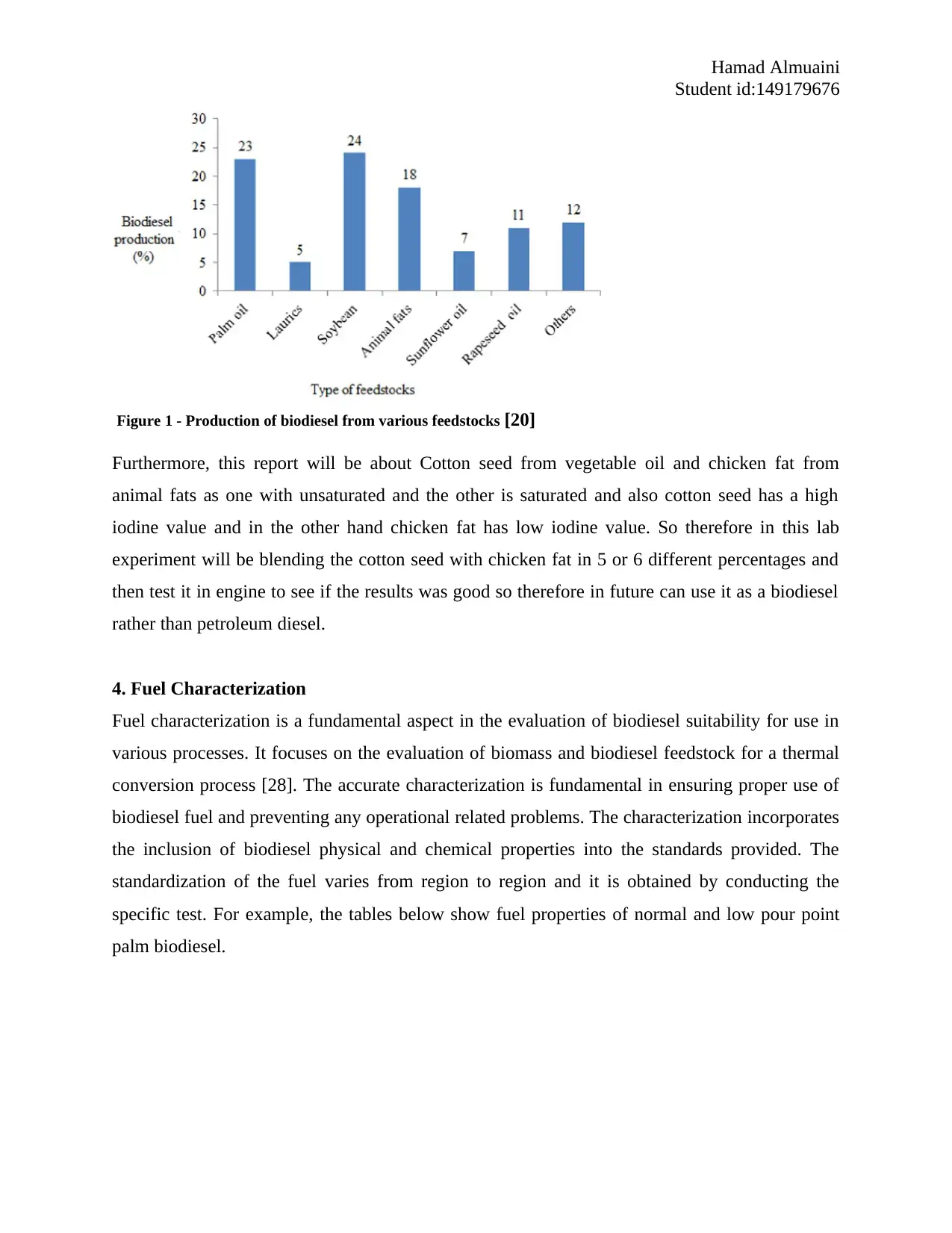
Figure 1 - Production of biodiesel from various feedstocks [20]
Furthermore, this report will be about Cotton seed from vegetable oil and chicken fat from
animal fats as one with unsaturated and the other is saturated and also cotton seed has a high
iodine value and in the other hand chicken fat has low iodine value. So therefore in this lab
experiment will be blending the cotton seed with chicken fat in 5 or 6 different percentages and
then test it in engine to see if the results was good so therefore in future can use it as a biodiesel
rather than petroleum diesel.
4. Fuel Characterization
Fuel characterization is a fundamental aspect in the evaluation of biodiesel suitability for use in
various processes. It focuses on the evaluation of biomass and biodiesel feedstock for a thermal
conversion process [28]. The accurate characterization is fundamental in ensuring proper use of
biodiesel fuel and preventing any operational related problems. The characterization incorporates
the inclusion of biodiesel physical and chemical properties into the standards provided. The
standardization of the fuel varies from region to region and it is obtained by conducting the
specific test. For example, the tables below show fuel properties of normal and low pour point
palm biodiesel.
Paraphrase This Document
Student id:149179676
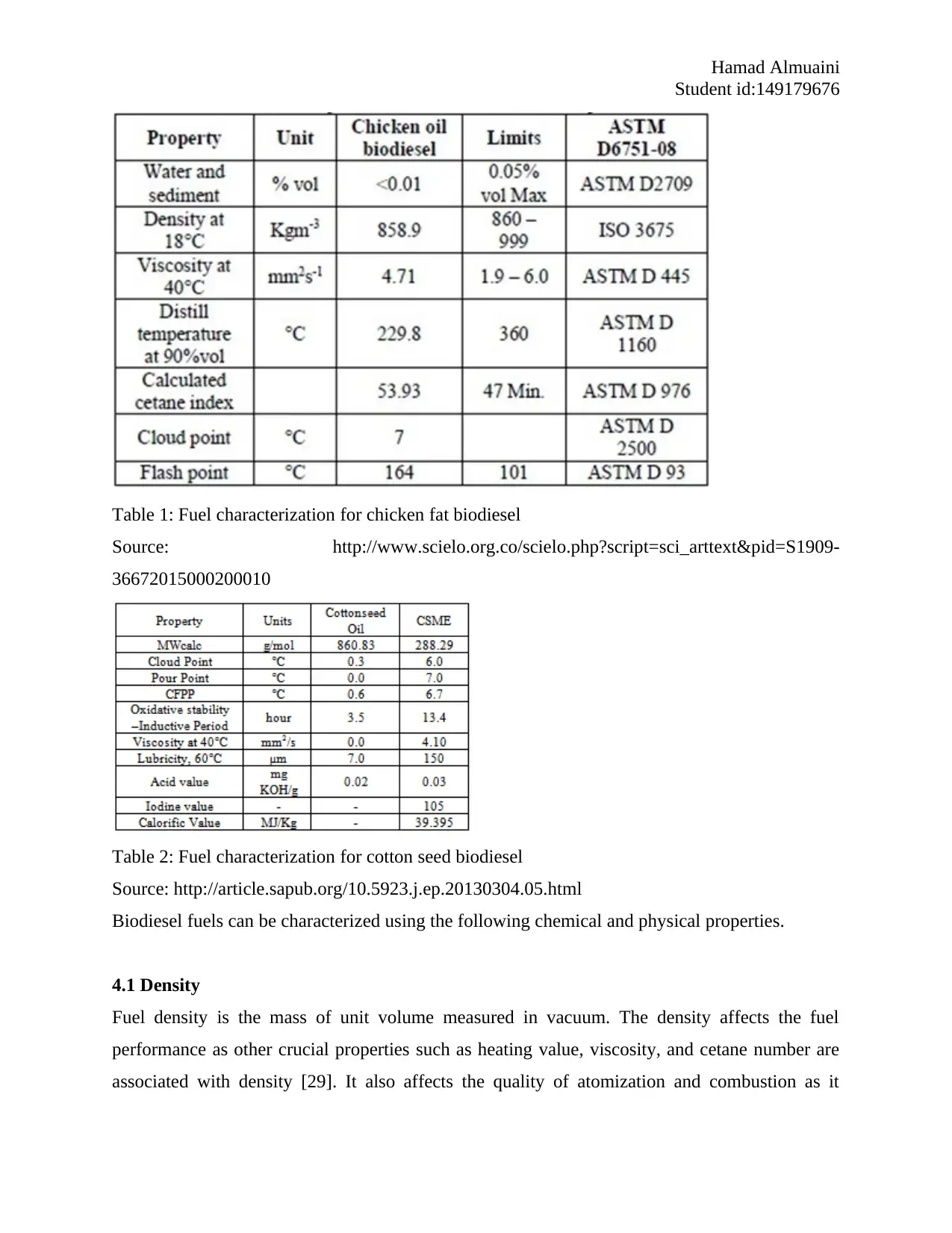
Table 1: Fuel characterization for chicken fat biodiesel
Source: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S1909-
36672015000200010
Table 2: Fuel characterization for cotton seed biodiesel
Source: http://article.sapub.org/10.5923.j.ep.20130304.05.html
Biodiesel fuels can be characterized using the following chemical and physical properties.
4.1 Density
Fuel density is the mass of unit volume measured in vacuum. The density affects the fuel
performance as other crucial properties such as heating value, viscosity, and cetane number are
associated with density [29]. It also affects the quality of atomization and combustion as it

Student id:149179676
influences the fuel mass that gets to the combustion chamber. Additionally, obtaining the density
is vital in the manufacturing, storage, and distribution of the biodiesel. In esters, it depends on
molar mass, free fatty acid content, water content, and temperature. In general, the biodiesel
density is higher than that of diesel. The density of a fuel can be measured using the standard
tests and at a temperature of 15°C [30]. A hydrometer is used for this purpose.
4.2 Viscosity of Biodiesel
It is the property to resist the relative movement tendency of fuel composing layers due to
intermolecular attraction forces. It highly impacts the ease of starting an engine, the spray
quality, and the penetration of the injected jet. Therefore, fuels with low viscosity provide a very
fine spray which may lead to insufficient penetration and the formation of black smoke. On the
other hand, a highly viscous fuel results to formation of big drops leading to a blue smoke due to
interruption of the combustion reaction [29]. Additionally, too high viscosity may cause
operational problems at low temperatures. This is because viscosity increases with reducing the
temperature. Viscosity also influences the lubricity nature of biodiesel fuels. It is measured using
a viscometer at a temperature of 40°C.
4.3 Flash Point (FP)
It is the minimum temperature in which the fuel will ignite on the application of an igniting
source. It is fundamental in classifying biodiesel fuels for the purposes of transportations and
storage conditions. For example, fuels with very low flash points may cause explosions while in
storage or transportation due to the ambient temperatures. As the flash point is indirectly
proportional to alcohol content it is used to determine the presence of methanol or ethanol in
fuels [29]. Noteworthy, the minimum flash point of the fuels differs in various regions.
4.4 Acid Value
It is the mass of potassium hydroxide in milligrams that is required to neutralize the acidic
constituents of one gram sample. The acid value, also known as the neutralization number, is
utilized to quantify the presence of acid moieties in a fuel sample. Therefore, it is a measure of
the content of fatty acids in the biodiesel thus determining the corrosiveness of the fuel, the
presence of water, and filter clogging [29]. A high acid value can cause functioning problems
and fuel filter clogging. Moreover, it is used to measure freshness as fuels stored for a long term
will have high acid value.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Student id:149179676
6. Methodologies
6.1 process of oil extraction [20]
Melting: melting chicken fat by heating between 150 – 180 °C in oven for 30 – 35
minutes was suitable to extract oil
Soxhelt extraction: the solvent used in this method is n-hexane to extract the oil from
feedstock by heating on a certain temperature constantly
Microwaves heating: the interaction between the molecular and the
electromagnetic field within the solvent and feedstock improve and
reduce the time used to extract oil that can reach to 7 minutes which is a
lot less than conventional heating that can reach up to 90 minutes.
Ultrasound assisted: which uses the oscillating sound pressure waves with
frequency higher than the human hearing range.
Supercritical carbon dioxide, were alcohol is maintained at high
temperature and pressure to extract oil. In this method 90% of the oil is
extracted within 60 minutes.
6.2 Methodology and experimental procedure for biodiesel production
Waste chicken fat and skin were bought from Kismet halal market Coventry road at cheaper cost
and the skin was manually de-feathered and thoroughly washed with tap water to remove
unwanted things like blood and bones. The waste chicken fat skin was cut into small pieces
before melting at 150 - 180 °C depend upon the medium of extraction, filtered or centrifuged to
separate the solid substances from extracted oil. If the FFA value of extracting oil is less than
2%, Esterification is not required, otherwise if FFA was more than 2% Esterification is required.
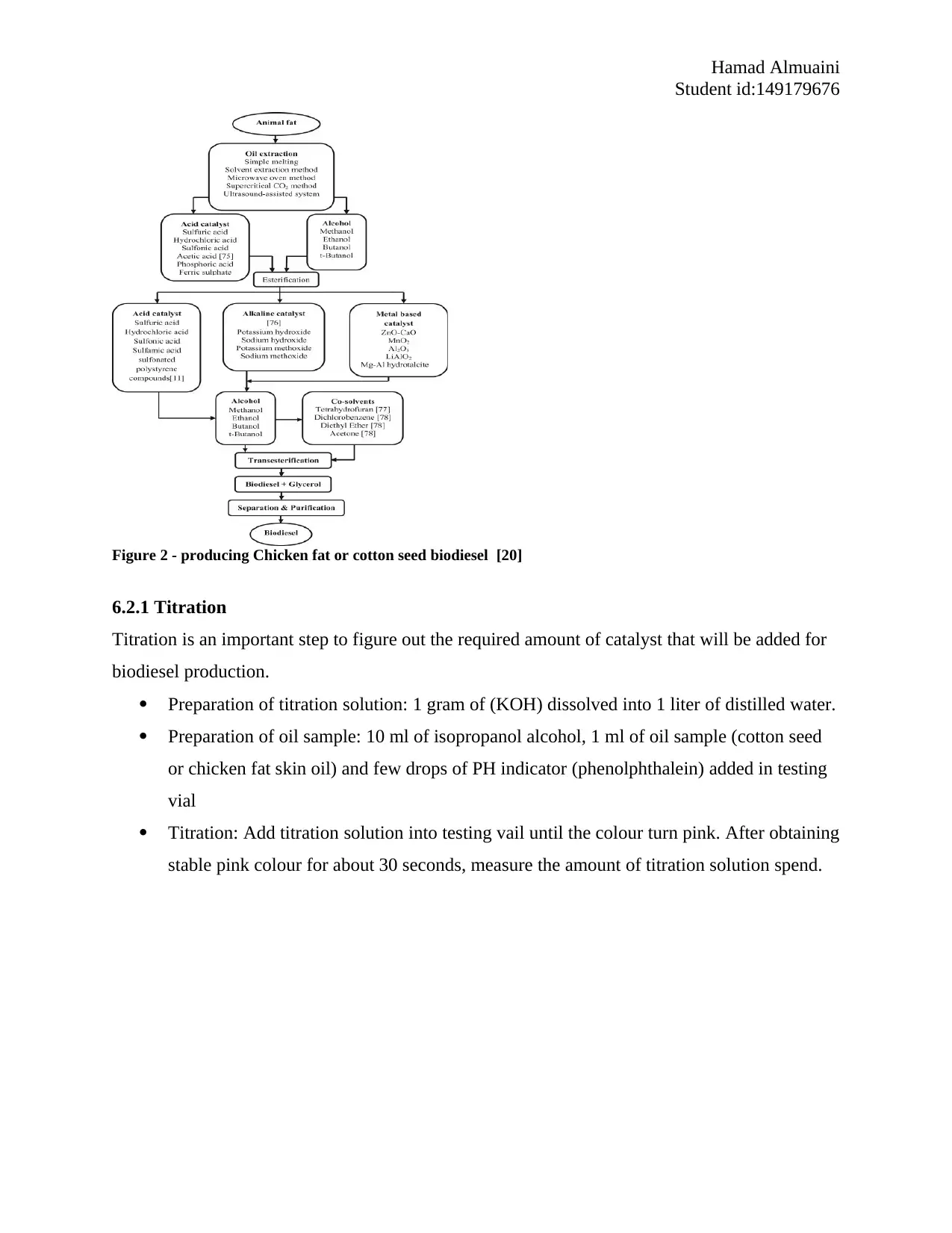
Furthermore, figure 2 illustrate the stage process of producing biodiesel of chicken fat or cotton
seed.
Paraphrase This Document
Student id:149179676
Figure 2 - producing Chicken fat or cotton seed biodiesel [20]
6.2.1 Titration
Titration is an important step to figure out the required amount of catalyst that will be added for
biodiesel production.
Preparation of titration solution: 1 gram of (KOH) dissolved into 1 liter of distilled water.
Preparation of oil sample: 10 ml of isopropanol alcohol, 1 ml of oil sample (cotton seed
or chicken fat skin oil) and few drops of PH indicator (phenolphthalein) added in testing
vial
Titration: Add titration solution into testing vail until the colour turn pink. After obtaining
stable pink colour for about 30 seconds, measure the amount of titration solution spend.

Student id:149179676
Figure 3 - Titration test [21]
Calculation of the amount of catalyst is required to produce biodiesel is depending on few
parameters. At first, batch of oil that will converted into biodiesel has to be known. Furthermore,
result obtained from titration T is important. Moreover, base value which is different for each
catalyst needs to take into account. KOH base value is 7.0, this is the amount of catalyst required
for per liter of brand new oil to produce biodiesel.
6.2.2 Transesterification
Transesterification is commonly used method to obtain methyl esters from cotton seed oil,
chicken fat oil and other feedstocks.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
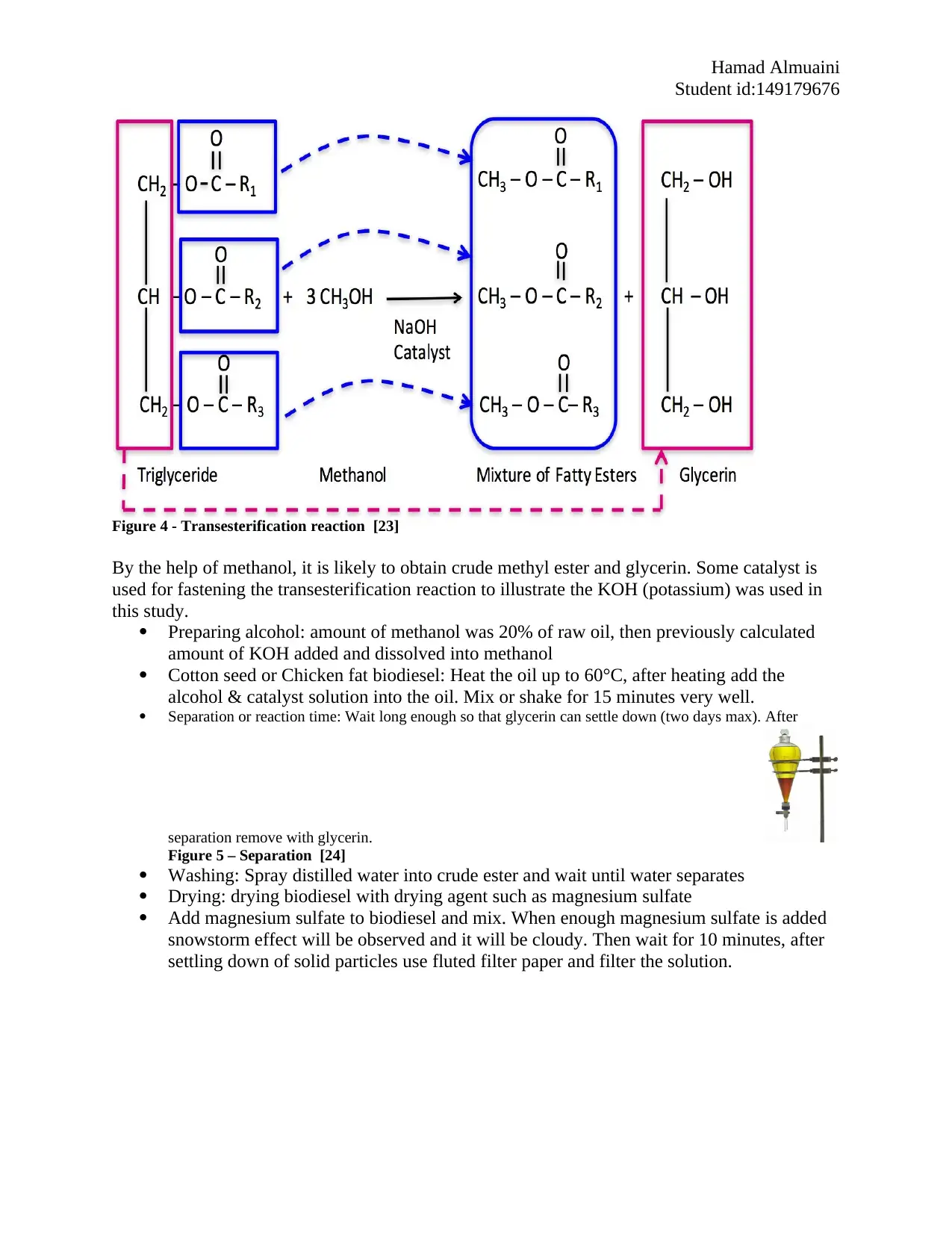
Student id:149179676
Figure 4 - Transesterification reaction [23]
By the help of methanol, it is likely to obtain crude methyl ester and glycerin. Some catalyst is
used for fastening the transesterification reaction to illustrate the KOH (potassium) was used in
this study.
Preparing alcohol: amount of methanol was 20% of raw oil, then previously calculated
amount of KOH added and dissolved into methanol
Cotton seed or Chicken fat biodiesel: Heat the oil up to 60°C, after heating add the
alcohol & catalyst solution into the oil. Mix or shake for 15 minutes very well.
Separation or reaction time: Wait long enough so that glycerin can settle down (two days max). After
separation remove with glycerin.
Figure 5 – Separation [24]
Washing: Spray distilled water into crude ester and wait until water separates
Drying: drying biodiesel with drying agent such as magnesium sulfate
Add magnesium sulfate to biodiesel and mix. When enough magnesium sulfate is added
snowstorm effect will be observed and it will be cloudy. Then wait for 10 minutes, after
settling down of solid particles use fluted filter paper and filter the solution.
Paraphrase This Document

Student id:149179676
6.3 Blending cotton seed and chicken biodiesel in different percentage
Due to the higher viscosity and poor volatility animal fat (chicken fat) cannot be directly use in
diesel engine. Furthermore, in this study there will be 7 different blending percentages of 150ml
biodiesel from cotton seed (V) and chicken fat biodiesel (A) as it shown in figure 6. For
example, 60% of cotton seed in 150ml will be (60/100) x 150ml = 90ml, for chicken fat 40% of
150ml will be (40/100) x 150 = 60ml.
Figure 6 - Blending Biodiesel Cotton seed (V) with Chicken fat (A)
Student id:149179676
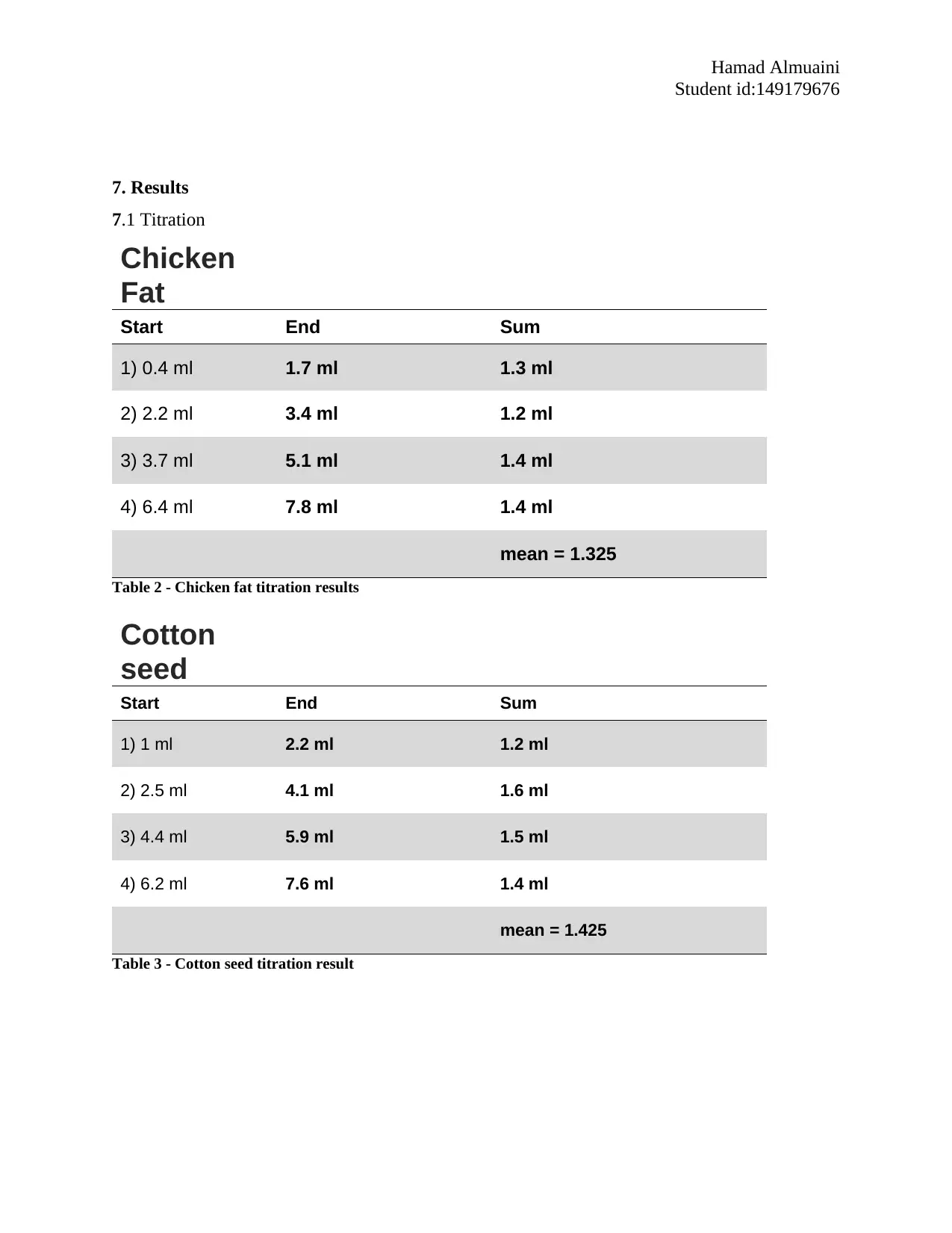
7. Results
7.1 Titration
Chicken
Fat
Start End Sum
1) 0.4 ml 1.7 ml 1.3 ml
2) 2.2 ml 3.4 ml 1.2 ml
3) 3.7 ml 5.1 ml 1.4 ml
4) 6.4 ml 7.8 ml 1.4 ml
mean = 1.325
Table 2 - Chicken fat titration results
Cotton
seed
Start End Sum
1) 1 ml 2.2 ml 1.2 ml
2) 2.5 ml 4.1 ml 1.6 ml
3) 4.4 ml 5.9 ml 1.5 ml
4) 6.2 ml 7.6 ml 1.4 ml
mean = 1.425
Table 3 - Cotton seed titration result
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Student id:149179676
7.2 Biodiesel production calculations
5.2.1 Chicken fat results
To produce 1000ml of chicken fat biodiesel
20% of oil Methanol = 200ml
5.2.2 Cotton seed results
To produce 1100 ml of cotton seed biodiesel
20% of oil Methanol = 220ml
Paraphrase This Document

Student id:149179676
6.References
[1] Gui, M.M., Lee, K.T. and Bhatia, S., 2008. Feasibility of edible oil vs. non-edible oil vs.
waste edible oil as biodiesel feedstock. Energy, 33(11), pp.1646-1653.
[2] Leung, Dennis Y.C. et al. "A Review on Biodiesel Production Using Catalyzed
Transesterification." Applied Energy, vol. 87, no. 4, 2010, pp. 1083-1095.
[3] Canakci, M. and Van Gerpen, J., 2001. Biodiesel production from oils and fats with high free
fatty acids. Transactions of the ASAE, 44(6), p.1429.
[4] Refaat, A.A., 2010. Different techniques for the production of biodiesel from waste vegetable
oil. International Journal of Environmental Science & Technology, 7(1), pp.183-213.
[5] Campbell, M.N., 2008. Biodiesel: algae as a renewable source for liquid fuel. Guelph
Engineering Journal, 1(1), pp.2-7.
[6] Ruan, C.J., Li, H., Guo, Y.Q., Qin, P., Gallagher, J.L., Seliskar, D.M., Lutts, S. and Mahy,
G., 2008. Kosteletzkya virginica, an agroecoengineering halophytic species for
alternative agricultural production in China's east coast: Ecological adaptation and
benefits, seed yield, oil content, fatty acid and biodiesel properties. Ecological
Engineering, 32(4), pp.320-328.
[7] Sattanathan, R. “Production of Biodiesel from Castor Oil with its Performance and Emission
Test.” International Journal of Science and Research, vol. 4, no. 1, 2015, pp. 273-279.
[8] Goldemberg J. Renewable energy: regional potentials and priorities. The Johannersburg
Renewable Energy Coalition1st International Conference in Brussels; Jun 4, 2003
http://www.castor-international.nl/cmdata/documents/Castor-oil-Ph--Eur-virgin-
(printbare-versie-op-briefpapier-sjabloon).pdf. Accessed Oct 19 2017
[9] Mohammed, Nurudeen et al. “Jatropha Curcas Oil Characterization and Its Significance for
Feedstock Selection in Biodiesel Production.” 2014 5th International Conference on
Food Engineering and Biotechnology IPCBEE, vol. 65, 2014, pp. 57-62.
[10] Karmee, S.K. and Chadha, A., 2005. Preparation of biodiesel from crude oil of Pongamia
pinnata. Bioresource technology, 96(13), pp.1425-1429.
[11] Bobade S.N. and Khyade V.B. “Detail study on the Properties of Pongamia Pinnata
(Karanja) for the Production of Biofuel.” Research Journal of Chemical Sciences, vol. 2,
no. 7, 2012, pp.16-20.

Student id:149179676
[12] Pant KS, Kumar D, Gairola S. Seed oil content variation in jatropha curcas L. in different
altitudinal ranges and site conditions in H.P. India Lyonia. 2006;11:31–34
[13] Nabi, M.N., Rahman, M.M. and Akhter, M.S., 2009. Biodiesel from cotton seed oil and its
effect on engine performance and exhaust emissions. Applied Thermal
Engineering, 29(11), pp.2265-2270.
[14] Tiwari AK, Kumar A, Raheman H. Biodiesel production from Jatropha oil (Jatropha
Carcus) with high free fatty acids: an optimized process. Biomass and
Bioenergy. 2007;31:569–575
[15] Kafuku, G. and Mbarawa, M., 2010. Biodiesel production from Croton megalocarpus oil
and its process optimization. Fuel, 89(9), pp.2556-2560.
[16] [19] Kwangdinata, Raymond et al. “Production of Biodiesel from Lipid of
Phytoplanktonchaetoceros Calcitransthrough Ultrasonic Method.” The Scientific World
Journal, vol. 2014, 2014, pp.1-5.
[17] Ahmad, J., Yusup, S., Bokhari, A. and Kamil, R.N.M., 2014. Study of fuel properties of
rubber seed oil based biodiesel. Energy Conversion and Management, 78, pp.266-275.
[18] Gürü, M., Artukoğlu, B.D., Keskin, A. and Koca, A., 2009. Biodiesel production from
waste animal fat and improvement of its characteristics by synthesized nickel and
magnesium additive. Energy Conversion and Management, 50(3), pp.498-502.
[20] A comprehensive review of low cost biodiesel production from waste chicken fat. M.
Kirubakaran, V. Arul Mozhi Selvan . 2018.⁎
[21] Clarkson, Dr Richard. Concentration of Solutions . Chemistry rules. [Online] 5 1, 2012.
[Cited: 2 5, 2018.] http://www.chemistryrules.me.uk/middle/concentration.htm#title.
[23] Caroline Burgess Clifford, Senior Research Associate, Energy Institute, The Pennsylvania
State University. 9.2 The Reaction of Biodiesel: Transesterification. e-education. [Online] 2017.
[Cited: 2 7, 2018.] https://www.e-education.psu.edu/egee439/node/684.
[24] Tech, Rick Da. Single Stage Base Recipes. make biodiesel . [Online] 2018. [Cited: 2 7,
2018.] http://www.make-biodiesel.org/Biodiesel-Recipes/single-stage-base-recipes.html
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Student id:149179676
[25] Naik, S. N ꎬ , et al. "Production of first and second generation biofuels: a comprehensive
review." Renewable and Sustainable Energy Reviews 14.2 (2010): 578-597.
[26] Balat, Mustafa. "Potential alternatives to edible oils for biodiesel production–A review of
current work." Energy Conversion and Management 52.2 (2011): 1479-1492.
[27] Leung, Dennis YC, Xuan Wu, and M. K. H. Leung. "A review on biodiesel production
using catalyzed transesterification." Applied Energy 87.4 (2010): 1083-1095.
[28] Sharma, Y. C., B. Singh, and S. N. Upadhyay. "Advancements in development and
characterization of biodiesel: a review." Fuel 87.12 (2008): 2355-2373.
[29] Barabás, István, and Ioan-Adrian Todoruț. "Biodiesel quality, standards and
properties." Biodiesel-quality, Emissions and By-products. InTech, 2011.
[30] Indhumathi, P., Syed Shabudeen PS, and U. S. Shoba. "A Method for production and
characterization of biodiesel from green micro algae." International Journal of Bio-
Science and Bio-Technology 6.5 (2014): 111-122.
[31] Kumar, Ajeet, S. K. Shukla, and J. V. Tierkey. "A review of research and policy on using
different biodiesel oils as fuel for CI engine." Energy Procedia 90 (2016): 292-304.
[32] Kumar, M. Vijay, A. Veeresh Babu, and P. Ravi Kumar. "The impacts on combustion,
performance and emissions of biodiesel by using additives in direct injection diesel
engine." Alexandria Engineering Journal (2017).
[33] Nabi, Md Nurun, Md Mustafizur Rahman, and Md Shamim Akhter. "Biodiesel from cotton
seed oil and its effect on engine performance and exhaust emissions." Applied Thermal
Engineering 29.11-12 (2009): 2265-2270.
Paraphrase This Document

Student id:149179676
[34] Sinha, Duple, and S. Murugavelh. "Biodiesel production from waste cotton seed oil using
low cost catalyst: Engine performance and emission characteristics." Perspectives in
science 8 (2016): 237-240.
[35] Atabani, A. E., et al. "Non-edible vegetable oils: a critical evaluation of oil extraction, fatty
acid compositions, biodiesel production, characteristics, engine performance and
emissions production." Renewable and sustainable energy reviews 18 (2013): 211-245.
[36] Chauhan, Bhupendra Singh, Naveen Kumar, and Haeng Muk Cho. "A study on the
performance and emission of a diesel engine fueled with Jatropha biodiesel oil and its
blends." Energy 37.1 (2012): 616-622.
[37] Mofijur, M., et al. "Evaluation of biodiesel blending, engine performance and emissions
characteristics of Jatropha curcas methyl ester: Malaysian perspective." Energy 55
(2013): 879-887.
[38] Fan, Xiaohu, et al. "Engine performance test of cottonseed oil biodiesel." The Open Fuels &
Energy Science Journal 1.7 (2008).
[39] Shi, Wenying, et al. "Biodiesel production from waste chicken fat with low free fatty acids
by an integrated catalytic process of composite membrane and sodium
methoxide." Bioresource technology 139 (2013): 316-322.

Student id:149179676
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
© 2024 | Zucol Services PVT LTD | All rights reserved.





