MAE 204 Thermodynamics HW #6: Piston-Cylinder, Mixing Chamber Analysis
VerifiedAdded on 2022/10/04
|6
|487
|12
Homework Assignment
AI Summary
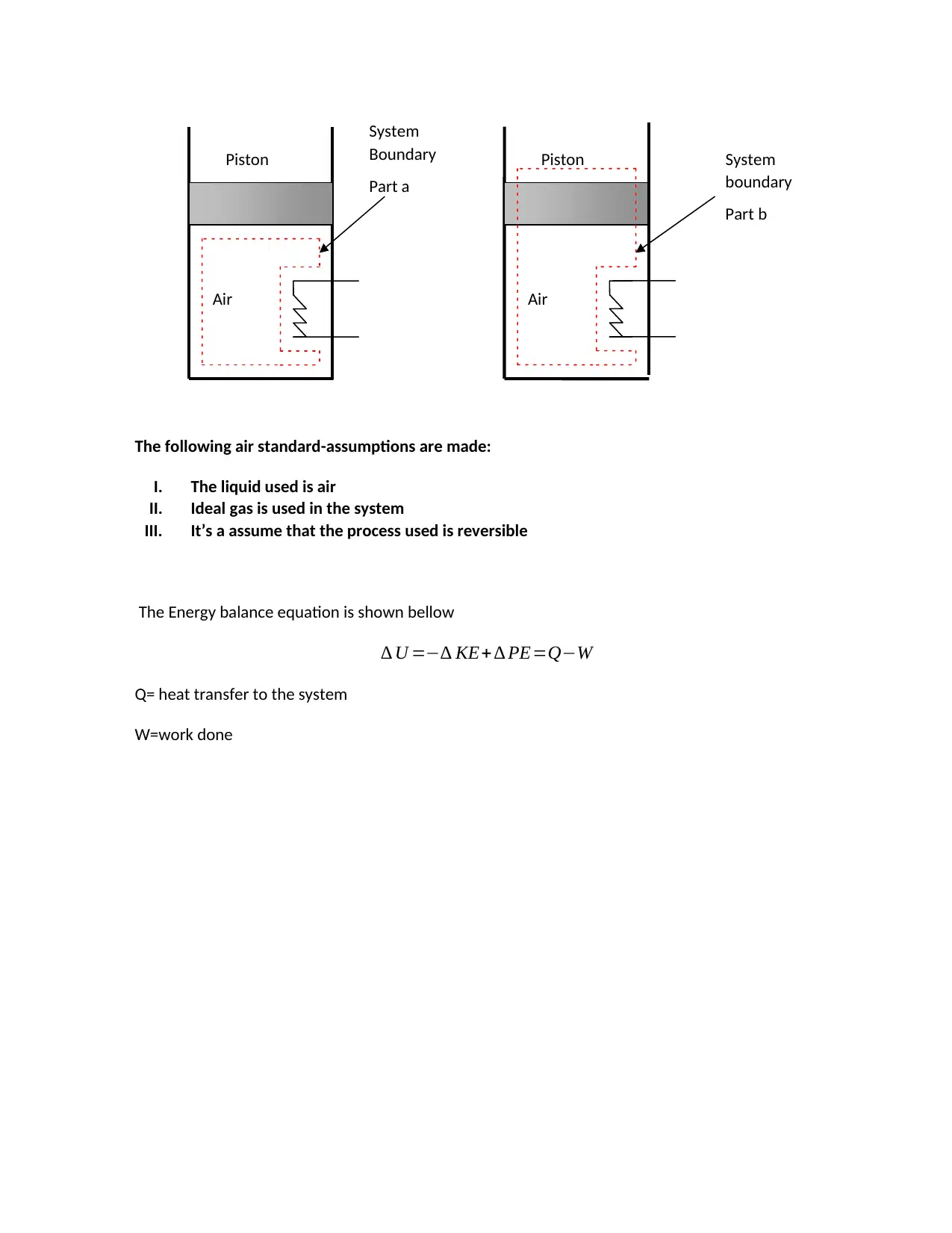
This document presents the complete solutions for MAE 204 Homework #6, a mechanical engineering assignment focusing on thermodynamics. The assignment includes two primary problems. The first problem analyzes air compression within a closed piston-cylinder system, requiring students to draw a schematic, state assumptions, and write the energy balance equation. It then asks for the determination of work and heat transfer under both isothermal and polytropic process conditions (n=1.3). The second problem involves a well-insulated water mixing chamber operating at steady state, with two inlets and one outlet. Students must determine the phase description, temperature, and specific volume of the outlet water, considering given mass flow rates, temperatures, pressures, and the use of a mixing paddle. The solutions demonstrate the application of thermodynamic principles, including energy balance, and the use of thermodynamic tables for property determination.
1 out of 6












![[object Object]](/_next/static/media/star-bottom.7253800d.svg)