Engineering Research Methods Assignment 2022
VerifiedAdded on 2022/10/11
|17
|3609
|12
Assignment
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

ENGINEERING RESEARCH METHODS 1
ENGINEERING RESEARCH METHODS
By Name
Course
Instructor
Institution
Location
Date
ENGINEERING RESEARCH METHODS
By Name
Course
Instructor
Institution
Location
Date
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

ENGINEERING RESEARCH METHODS 2
ABSTRACT
Due to the urge for the reduction of environmental pollution, there is a higher need to
develop a fuel which can be used to start a vehicle. For this proposal, there will be the use of
seawater which will be employed in the generation of hydrogen gas. Basically, this technology
uses of seawater to start the vehicle engine. This concept is driven from the urge to use free fuel
in powering the Automotive. Nevertheless, this technology is still at its experimental stages to
validate if it is true that seawater can really be employed to power vehicle successfully. This
proposal is hence employed to scrutinize if really vehicles can operate on seawater.
ABSTRACT
Due to the urge for the reduction of environmental pollution, there is a higher need to
develop a fuel which can be used to start a vehicle. For this proposal, there will be the use of
seawater which will be employed in the generation of hydrogen gas. Basically, this technology
uses of seawater to start the vehicle engine. This concept is driven from the urge to use free fuel
in powering the Automotive. Nevertheless, this technology is still at its experimental stages to
validate if it is true that seawater can really be employed to power vehicle successfully. This
proposal is hence employed to scrutinize if really vehicles can operate on seawater.

ENGINEERING RESEARCH METHODS 3
TABLE OF CONTENT
ABSTRACT....................................................................................................................................................2
TABLE OF CONTENT.....................................................................................................................................3
TABLE OF FIGURES.......................................................................................................................................3
INTRODUCTION.......................................................................................................................................3
PROBLEM STATEMENT..........................................................................................................................5
PROPOSED SOLUTION............................................................................................................................5
METHODOLOGY......................................................................................................................................5
A summary of the process of seawater in starting of car.........................................................................9
PROPOSAL TIME-LINE..........................................................................................................................10
PROPOSAL ANALYSIS..........................................................................................................................11
MATHEMATICAL CONUNDRUM TEST........................................................................................................12
GANTT CHART.......................................................................................................................................13
CONCLUSION.........................................................................................................................................14
References.................................................................................................................................................15
TABLE OF FIGURES
Figure 1: Showing hydrolysis of seawater to produce hydrogen as a fuel...................................................6
Figure 2: Showing the process of using hydrogen ions to start a car...........................................................8
Figure 3: Showing hydrogen from its storage point to the propulsion point for the fuel cell technology....9
Figure 4: Showing the seawater hydrolysis to generate hydrogen which will be used to start a car..........10
Figure 5: Showing the project Gantt chart.................................................................................................13
INTRODUCTION
TABLE OF CONTENT
ABSTRACT....................................................................................................................................................2
TABLE OF CONTENT.....................................................................................................................................3
TABLE OF FIGURES.......................................................................................................................................3
INTRODUCTION.......................................................................................................................................3
PROBLEM STATEMENT..........................................................................................................................5
PROPOSED SOLUTION............................................................................................................................5
METHODOLOGY......................................................................................................................................5
A summary of the process of seawater in starting of car.........................................................................9
PROPOSAL TIME-LINE..........................................................................................................................10
PROPOSAL ANALYSIS..........................................................................................................................11
MATHEMATICAL CONUNDRUM TEST........................................................................................................12
GANTT CHART.......................................................................................................................................13
CONCLUSION.........................................................................................................................................14
References.................................................................................................................................................15
TABLE OF FIGURES
Figure 1: Showing hydrolysis of seawater to produce hydrogen as a fuel...................................................6
Figure 2: Showing the process of using hydrogen ions to start a car...........................................................8
Figure 3: Showing hydrogen from its storage point to the propulsion point for the fuel cell technology....9
Figure 4: Showing the seawater hydrolysis to generate hydrogen which will be used to start a car..........10
Figure 5: Showing the project Gantt chart.................................................................................................13
INTRODUCTION

ENGINEERING RESEARCH METHODS 4
This technology has some potential of vehicle operating on the seawater since it contains
some salts which make it possible to attain hydrogen through hydrolysis. From several
publications, it has been seen that water can be hydrolyzed to produce hydrogen gas. Even
though it is seen that it is water which is pumped into the vehicle as fuel but water cannot burn to
propel the engine hence the water will have to be hydrolyzed. The technology of the hydrogen-
fuelled cars is in its initial stages where it has been implemented in some countries in the world
like the United Kingdom where the Transport of London ( ToL) is having some buses operating
on hydrogen gas as fuel.
This technology is very significant to our environment since when hydrogen burns it only
gives out water as the product as opposed to the fossil fuel which gives out carbon ( IV ) oxide
together with other products which are very toxic to our environment and can sometimes result
to cancer (Yamada, 2010). The equation of the combustion of hydrogen is illustrated using the
following equation 1;
H2 +O2 H2O . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
This concept involves dissolving this salty water in magnesium in a fuel cell in order to generate
hydrogen ions which will hence move to the carbon cathode electrode which will then help in
generation of electrical current to help start a vehicle (Gewirth, 2010). In some cases, carbon
may be blocked as a result of hydrogen mitigation to the cathode and become polarized but this
is an exceptional case where carbon is porous. The air would diffuse down the carbon and this
would make oxygen to combine with hydrogen and form water so that the electrical effect can
continue to produce power to the engine (García, 2013). This is illustrated using the following
diagram (figure 1) below;
This technology has some potential of vehicle operating on the seawater since it contains
some salts which make it possible to attain hydrogen through hydrolysis. From several
publications, it has been seen that water can be hydrolyzed to produce hydrogen gas. Even
though it is seen that it is water which is pumped into the vehicle as fuel but water cannot burn to
propel the engine hence the water will have to be hydrolyzed. The technology of the hydrogen-
fuelled cars is in its initial stages where it has been implemented in some countries in the world
like the United Kingdom where the Transport of London ( ToL) is having some buses operating
on hydrogen gas as fuel.
This technology is very significant to our environment since when hydrogen burns it only
gives out water as the product as opposed to the fossil fuel which gives out carbon ( IV ) oxide
together with other products which are very toxic to our environment and can sometimes result
to cancer (Yamada, 2010). The equation of the combustion of hydrogen is illustrated using the
following equation 1;
H2 +O2 H2O . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
This concept involves dissolving this salty water in magnesium in a fuel cell in order to generate
hydrogen ions which will hence move to the carbon cathode electrode which will then help in
generation of electrical current to help start a vehicle (Gewirth, 2010). In some cases, carbon
may be blocked as a result of hydrogen mitigation to the cathode and become polarized but this
is an exceptional case where carbon is porous. The air would diffuse down the carbon and this
would make oxygen to combine with hydrogen and form water so that the electrical effect can
continue to produce power to the engine (García, 2013). This is illustrated using the following
diagram (figure 1) below;
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

ENGINEERING RESEARCH METHODS 5
PROBLEM STATEMENT
For many years fossil fuels have been used with the help of a battery to start a car, the use
of this old technology possess some disadvantages of pollutions and cost. From the World
Health Organization (WHO) about 4.2 million deaths are recorded due to air pollution. Some of
these air pollutions originate from the vehicles which operate on fossil fuel. Therefore there is a
higher need to help reduce the air pollution which will, in turn, reduces the deaths due to air
pollution.
PROPOSED SOLUTION
It is hence very vital to come up with a way of reducing the emission of harmful gases
into the environment. The cars operate every day and they have become parts of our life,
therefore we cannot avoid their operation but rather make them operate in a very clean way. This
proposal thus suggests the use of seawater in starting and running the vehicles through hydrogen
fuel cells. The operation of this technology proposed will hence be based on pure seawater which
will produce hydrogen gas and used in hydrogen fuel cells.
METHODOLOGY
The hydrogen gas produced is the one which will be used as a fuel in fuel cells to help in
starting the vehicle. From the diagram below it is very easy to obtain hydrogen from water;
PROBLEM STATEMENT
For many years fossil fuels have been used with the help of a battery to start a car, the use
of this old technology possess some disadvantages of pollutions and cost. From the World
Health Organization (WHO) about 4.2 million deaths are recorded due to air pollution. Some of
these air pollutions originate from the vehicles which operate on fossil fuel. Therefore there is a
higher need to help reduce the air pollution which will, in turn, reduces the deaths due to air
pollution.
PROPOSED SOLUTION
It is hence very vital to come up with a way of reducing the emission of harmful gases
into the environment. The cars operate every day and they have become parts of our life,
therefore we cannot avoid their operation but rather make them operate in a very clean way. This
proposal thus suggests the use of seawater in starting and running the vehicles through hydrogen
fuel cells. The operation of this technology proposed will hence be based on pure seawater which
will produce hydrogen gas and used in hydrogen fuel cells.
METHODOLOGY
The hydrogen gas produced is the one which will be used as a fuel in fuel cells to help in
starting the vehicle. From the diagram below it is very easy to obtain hydrogen from water;
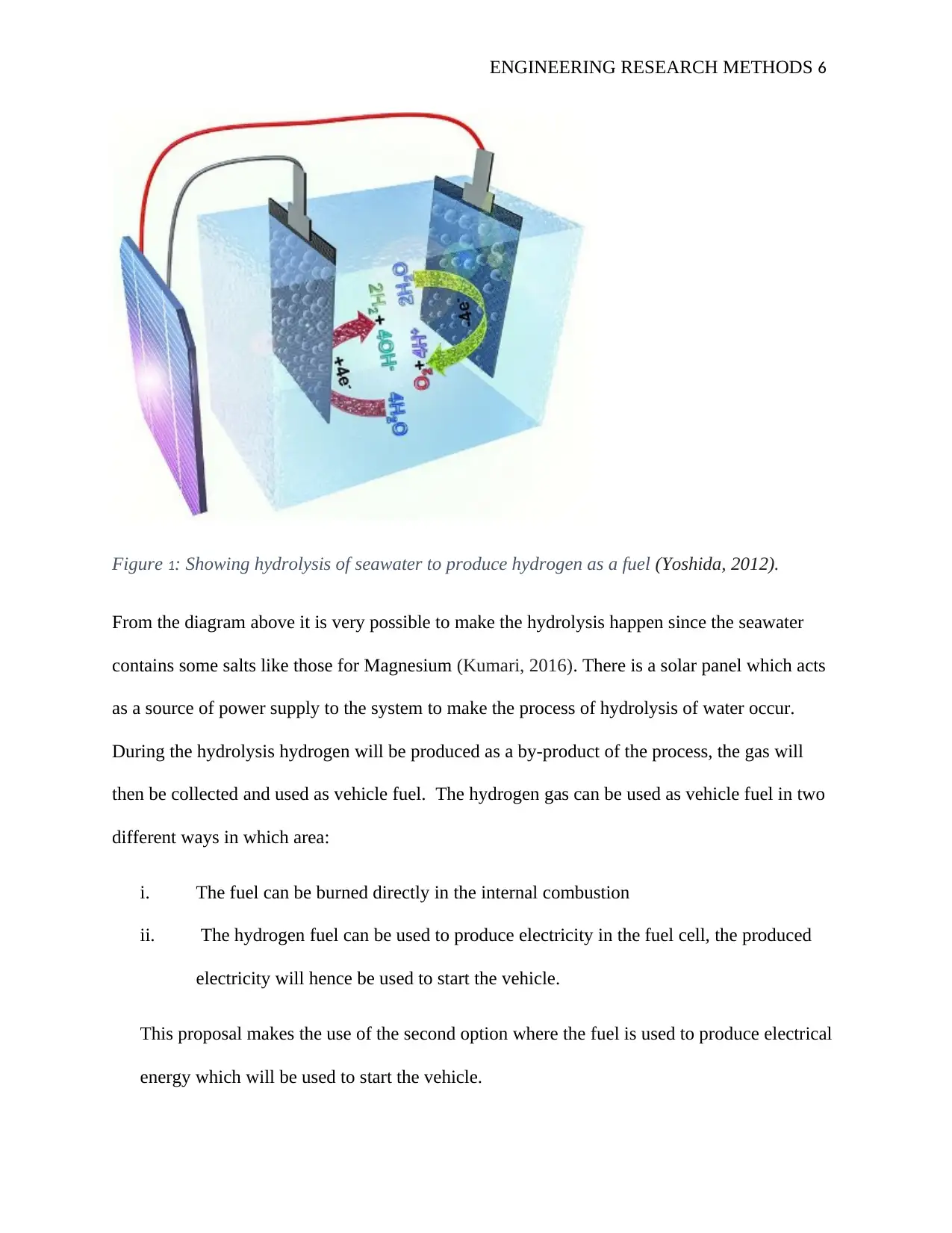
ENGINEERING RESEARCH METHODS 6
Figure 1: Showing hydrolysis of seawater to produce hydrogen as a fuel (Yoshida, 2012).
From the diagram above it is very possible to make the hydrolysis happen since the seawater
contains some salts like those for Magnesium (Kumari, 2016). There is a solar panel which acts
as a source of power supply to the system to make the process of hydrolysis of water occur.
During the hydrolysis hydrogen will be produced as a by-product of the process, the gas will
then be collected and used as vehicle fuel. The hydrogen gas can be used as vehicle fuel in two
different ways in which area:
i. The fuel can be burned directly in the internal combustion
ii. The hydrogen fuel can be used to produce electricity in the fuel cell, the produced
electricity will hence be used to start the vehicle.
This proposal makes the use of the second option where the fuel is used to produce electrical
energy which will be used to start the vehicle.
Figure 1: Showing hydrolysis of seawater to produce hydrogen as a fuel (Yoshida, 2012).
From the diagram above it is very possible to make the hydrolysis happen since the seawater
contains some salts like those for Magnesium (Kumari, 2016). There is a solar panel which acts
as a source of power supply to the system to make the process of hydrolysis of water occur.
During the hydrolysis hydrogen will be produced as a by-product of the process, the gas will
then be collected and used as vehicle fuel. The hydrogen gas can be used as vehicle fuel in two
different ways in which area:
i. The fuel can be burned directly in the internal combustion
ii. The hydrogen fuel can be used to produce electricity in the fuel cell, the produced
electricity will hence be used to start the vehicle.
This proposal makes the use of the second option where the fuel is used to produce electrical
energy which will be used to start the vehicle.

ENGINEERING RESEARCH METHODS 7
The reaction of hydrolysis of water as in figure 1 above is illustrated in equations below;
Anode reaction:
2 H2O () → O2 (g) + 4 H+(aq) + 4e− . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Cathode Reaction :
2 H2O(l) + 2e− → H2(g) + 2 OH−(aq)
These two equations will give the overall equation as below;
2H2O (l) → 2 H2(g) + O2(g) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
This technology will hence work perfectly with the availability of the hydrogen ions at the anode
electrode. Once the hydrogen ions are generated in the hydrolysis process the electrical energy
generated sometimes can be stored in a battery which will then employed in the starting of the
car (Gao, 2013). But in some cases, the electrical energy generated can be used directly to start
the car. For this proposal, the produced hydrogen is used directly to start the vehicle and the
battery here is used for the hydrolysis of water (Fukuzumi, 2017). This is illustrated using the
diagram below;
The reaction of hydrolysis of water as in figure 1 above is illustrated in equations below;
Anode reaction:
2 H2O () → O2 (g) + 4 H+(aq) + 4e− . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Cathode Reaction :
2 H2O(l) + 2e− → H2(g) + 2 OH−(aq)
These two equations will give the overall equation as below;
2H2O (l) → 2 H2(g) + O2(g) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
This technology will hence work perfectly with the availability of the hydrogen ions at the anode
electrode. Once the hydrogen ions are generated in the hydrolysis process the electrical energy
generated sometimes can be stored in a battery which will then employed in the starting of the
car (Gao, 2013). But in some cases, the electrical energy generated can be used directly to start
the car. For this proposal, the produced hydrogen is used directly to start the vehicle and the
battery here is used for the hydrolysis of water (Fukuzumi, 2017). This is illustrated using the
diagram below;
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
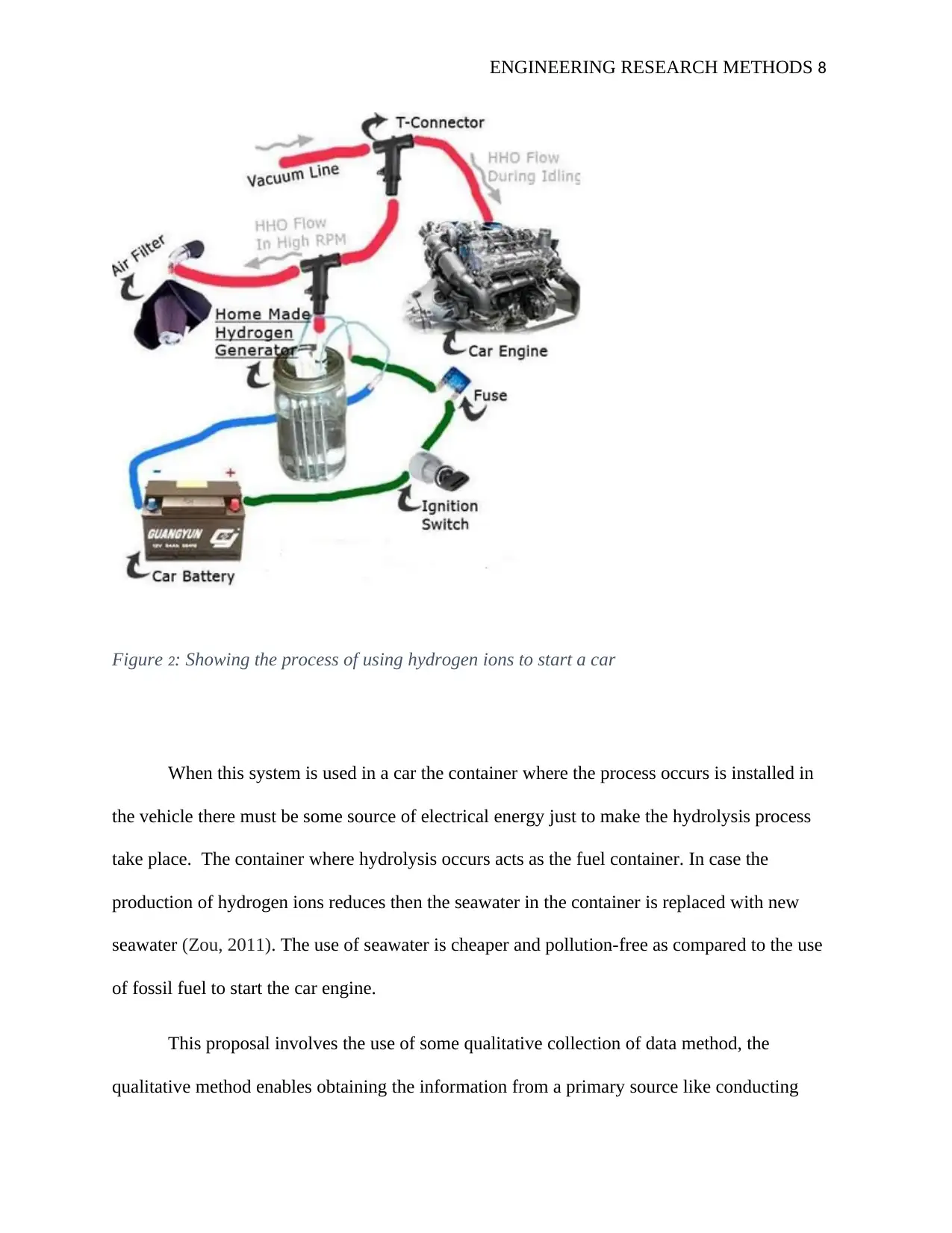
ENGINEERING RESEARCH METHODS 8
Figure 2: Showing the process of using hydrogen ions to start a car
When this system is used in a car the container where the process occurs is installed in
the vehicle there must be some source of electrical energy just to make the hydrolysis process
take place. The container where hydrolysis occurs acts as the fuel container. In case the
production of hydrogen ions reduces then the seawater in the container is replaced with new
seawater (Zou, 2011). The use of seawater is cheaper and pollution-free as compared to the use
of fossil fuel to start the car engine.
This proposal involves the use of some qualitative collection of data method, the
qualitative method enables obtaining the information from a primary source like conducting
Figure 2: Showing the process of using hydrogen ions to start a car
When this system is used in a car the container where the process occurs is installed in
the vehicle there must be some source of electrical energy just to make the hydrolysis process
take place. The container where hydrolysis occurs acts as the fuel container. In case the
production of hydrogen ions reduces then the seawater in the container is replaced with new
seawater (Zou, 2011). The use of seawater is cheaper and pollution-free as compared to the use
of fossil fuel to start the car engine.
This proposal involves the use of some qualitative collection of data method, the
qualitative method enables obtaining the information from a primary source like conducting

ENGINEERING RESEARCH METHODS 9
some interview to have information from the manufactures of the automobiles about the use of
seawater to generate hydrogen for starting of the car (Simamora, 2012). The concept behind the
development of this technology receives a good recommendation from the interviewees. And
most interviewees believe that the implementation of the seawater in starting a car will highly
help in the reduction of air pollution and also reduce the cost of operating the car.
Figure 3: Showing hydrogen from its storage point to the propulsion point for fuel cell
technology(Dodds, 2015 )
From figure 3 above the hydrogen fuel tank is filled with the seawater which must be salty and
the seawater is filled in the seawater filling port. The hydrogen produced is then employed in
starting the car (Tollefson, 2010).
A summary of the process of seawater in starting of the car
A source of power (battery) is connected at the anode to the cathode via the seawater
(electrolyte). When the power is switched on the seawater will split into hydrogen ions (positive
some interview to have information from the manufactures of the automobiles about the use of
seawater to generate hydrogen for starting of the car (Simamora, 2012). The concept behind the
development of this technology receives a good recommendation from the interviewees. And
most interviewees believe that the implementation of the seawater in starting a car will highly
help in the reduction of air pollution and also reduce the cost of operating the car.
Figure 3: Showing hydrogen from its storage point to the propulsion point for fuel cell
technology(Dodds, 2015 )
From figure 3 above the hydrogen fuel tank is filled with the seawater which must be salty and
the seawater is filled in the seawater filling port. The hydrogen produced is then employed in
starting the car (Tollefson, 2010).
A summary of the process of seawater in starting of the car
A source of power (battery) is connected at the anode to the cathode via the seawater
(electrolyte). When the power is switched on the seawater will split into hydrogen ions (positive
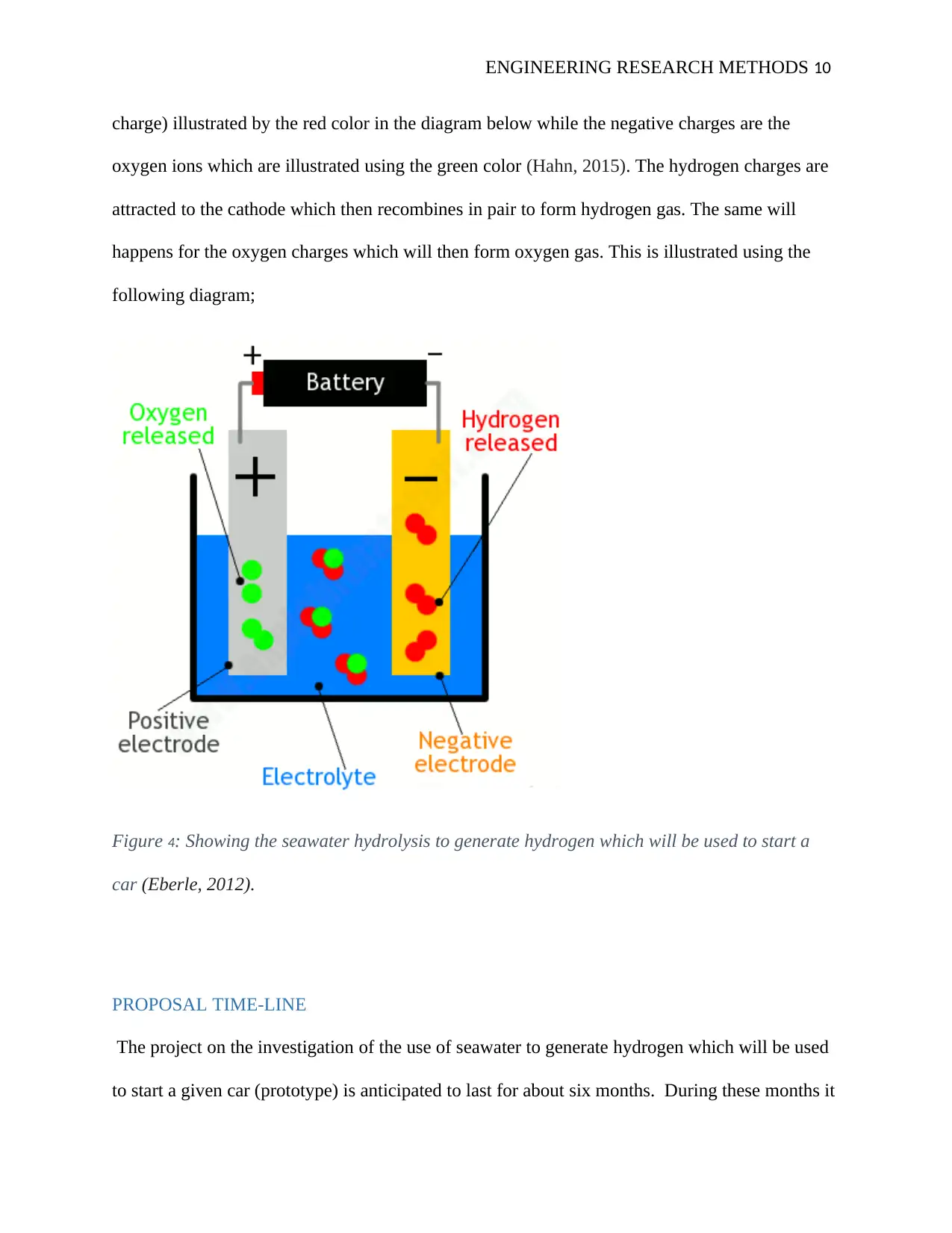
ENGINEERING RESEARCH METHODS 10
charge) illustrated by the red color in the diagram below while the negative charges are the
oxygen ions which are illustrated using the green color (Hahn, 2015). The hydrogen charges are
attracted to the cathode which then recombines in pair to form hydrogen gas. The same will
happens for the oxygen charges which will then form oxygen gas. This is illustrated using the
following diagram;
Figure 4: Showing the seawater hydrolysis to generate hydrogen which will be used to start a
car (Eberle, 2012).
PROPOSAL TIME-LINE
The project on the investigation of the use of seawater to generate hydrogen which will be used
to start a given car (prototype) is anticipated to last for about six months. During these months it
charge) illustrated by the red color in the diagram below while the negative charges are the
oxygen ions which are illustrated using the green color (Hahn, 2015). The hydrogen charges are
attracted to the cathode which then recombines in pair to form hydrogen gas. The same will
happens for the oxygen charges which will then form oxygen gas. This is illustrated using the
following diagram;
Figure 4: Showing the seawater hydrolysis to generate hydrogen which will be used to start a
car (Eberle, 2012).
PROPOSAL TIME-LINE
The project on the investigation of the use of seawater to generate hydrogen which will be used
to start a given car (prototype) is anticipated to last for about six months. During these months it
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

ENGINEERING RESEARCH METHODS 11
is only the testing of the engine and not the building of other parts of the car. Actually, once the
engine is able to work on the seawater then the other parts will just operate effectively (O'hayre,
2016) . During this time of six months, the data will be collected from interviews and some
crucial analysis of the operation of the engine on the seawater will be conducted. The data is
collected through primary (e.g. interviews) and secondary sources (e.g. publications).
PROPOSAL ANALYSIS
The analysis of the operation of this kind of technology will basically depend on the
information obtained from both secondary and primary source of data collection (Sobrino, 2010).
From the collected data it will be seen if actually, the seawater operates effectively and if
individuals like its operation in starting the cars (Blomen, 2013). In the analysis, it will be
checked how long it takes to starts a car and compares this time with how long it takes for the
fossil-fuelled cars (Huang, 2017). The efficiency of the operations of the seawater engine starters
will then be checked against the fossils typed engine and a conclusion will be made if actually,
we need to use the seawater to start the car engine or if it is not effective and we just need to use
the old technique.
During the analysis, the causes of deaths which can be prevented from the air pollution
will also be analyzed and be checked if actually, the reduced cases of deaths are significant
which will then promote the use of seawater to start the cars (Simamora, 2013). During the
analysis, the amount of salt in the water is established and then the suitable salt required to be
used in the seawater but in case the salt content is not enough in the seawater then some salts can
be literally added to make it work effectively (Offer, 2010). Basically the effectiveness of the
seawater (hydrogen fuel) during the analysis is conducted on a prototype engine to check if it
will really operate on the actual engine.
is only the testing of the engine and not the building of other parts of the car. Actually, once the
engine is able to work on the seawater then the other parts will just operate effectively (O'hayre,
2016) . During this time of six months, the data will be collected from interviews and some
crucial analysis of the operation of the engine on the seawater will be conducted. The data is
collected through primary (e.g. interviews) and secondary sources (e.g. publications).
PROPOSAL ANALYSIS
The analysis of the operation of this kind of technology will basically depend on the
information obtained from both secondary and primary source of data collection (Sobrino, 2010).
From the collected data it will be seen if actually, the seawater operates effectively and if
individuals like its operation in starting the cars (Blomen, 2013). In the analysis, it will be
checked how long it takes to starts a car and compares this time with how long it takes for the
fossil-fuelled cars (Huang, 2017). The efficiency of the operations of the seawater engine starters
will then be checked against the fossils typed engine and a conclusion will be made if actually,
we need to use the seawater to start the car engine or if it is not effective and we just need to use
the old technique.
During the analysis, the causes of deaths which can be prevented from the air pollution
will also be analyzed and be checked if actually, the reduced cases of deaths are significant
which will then promote the use of seawater to start the cars (Simamora, 2013). During the
analysis, the amount of salt in the water is established and then the suitable salt required to be
used in the seawater but in case the salt content is not enough in the seawater then some salts can
be literally added to make it work effectively (Offer, 2010). Basically the effectiveness of the
seawater (hydrogen fuel) during the analysis is conducted on a prototype engine to check if it
will really operate on the actual engine.

ENGINEERING RESEARCH METHODS 12
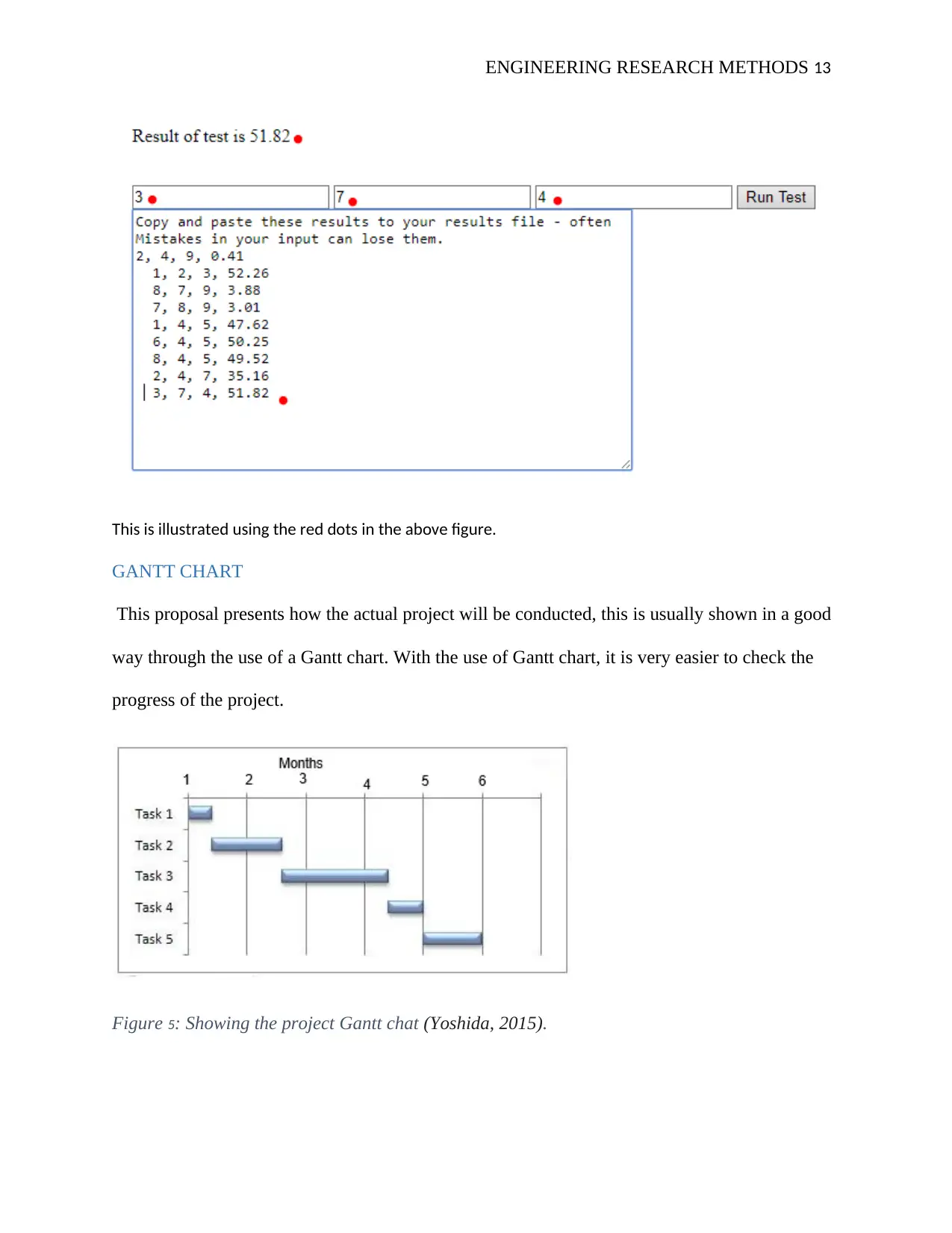
MATHEMATICAL CONUNDRUM TEST
The Mathematical conundrum test is like a mathematical puzzle, and in this project, it is conducted and
the results are given below;
From different values used in running the test of the conundrum used randomly the below are the
results given in the fourth row with the first three rows are the conundrum test input values;
2, 4, 9, 0.41
1, 2, 3, 52.26
8, 7, 9, 3.88
7, 8, 9, 3.01
1, 4, 5, 47.62
6, 4, 5, 50.25
8, 4, 5, 49.52
2, 4, 7, 35.16
7, 4, 8, 21.32
0.7, 0.4, 1, 34.52
2, 4, 1, 33.6
3, 7, 9, 3.28
8, 9, 10, 0
3, 7, 4, 51.82
Basically the test running and the output is given as below
MATHEMATICAL CONUNDRUM TEST
The Mathematical conundrum test is like a mathematical puzzle, and in this project, it is conducted and
the results are given below;
From different values used in running the test of the conundrum used randomly the below are the
results given in the fourth row with the first three rows are the conundrum test input values;
2, 4, 9, 0.41
1, 2, 3, 52.26
8, 7, 9, 3.88
7, 8, 9, 3.01
1, 4, 5, 47.62
6, 4, 5, 50.25
8, 4, 5, 49.52
2, 4, 7, 35.16
7, 4, 8, 21.32
0.7, 0.4, 1, 34.52
2, 4, 1, 33.6
3, 7, 9, 3.28
8, 9, 10, 0
3, 7, 4, 51.82
Basically the test running and the output is given as below
ENGINEERING RESEARCH METHODS 13
This is illustrated using the red dots in the above figure.
GANTT CHART
This proposal presents how the actual project will be conducted, this is usually shown in a good
way through the use of a Gantt chart. With the use of Gantt chart, it is very easier to check the
progress of the project.
Figure 5: Showing the project Gantt chat (Yoshida, 2015).
This is illustrated using the red dots in the above figure.
GANTT CHART
This proposal presents how the actual project will be conducted, this is usually shown in a good
way through the use of a Gantt chart. With the use of Gantt chart, it is very easier to check the
progress of the project.
Figure 5: Showing the project Gantt chat (Yoshida, 2015).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ENGINEERING RESEARCH METHODS 14
CONCLUSION
In conclusion, this proposal proposes the use of seawater in the starting of the car engine.
As discussed above this technology is made possible due to the fact that the seawater contains
some salts which will act as an electrolyte in the electrolysis process. In the electrolysis, process
hydrogen is produced which will then be employed in generating electricity for starting the car
engine. The use of hydrogen (from seawater) is a technology which is focused on reducing air
pollution and make the cheaper fuel for starting car engine. What is required is just the seawater
having some salt solution in it and some small source of power to make the process of
electrolysis to happen.
The data will then be collected to get the views of individuals like the users on the
effectiveness of the use of the seawater. The data collected through interview will be very
significant in deducing if we really need to use the seawater to help reduce some air pollution
and reduces the chances of contacting some air related diseases like lung cancer among other
types of cancers. The proposal stipulates a relatively long time of six months for the actual
project to be completed. This time is longer to allow testing to be conducted and some interview
to help get the views of the public if they think this fuel is good to be used. The actual project
will be conducted on a prototype to check if the seawater could actually be used in the real car to
help start the car. The prototype is believed to operate the same ways as the actual car hence if
the seawater works in the experimental prototype then it will definitely work in the actual car.
CONCLUSION
In conclusion, this proposal proposes the use of seawater in the starting of the car engine.
As discussed above this technology is made possible due to the fact that the seawater contains
some salts which will act as an electrolyte in the electrolysis process. In the electrolysis, process
hydrogen is produced which will then be employed in generating electricity for starting the car
engine. The use of hydrogen (from seawater) is a technology which is focused on reducing air
pollution and make the cheaper fuel for starting car engine. What is required is just the seawater
having some salt solution in it and some small source of power to make the process of
electrolysis to happen.
The data will then be collected to get the views of individuals like the users on the
effectiveness of the use of the seawater. The data collected through interview will be very
significant in deducing if we really need to use the seawater to help reduce some air pollution
and reduces the chances of contacting some air related diseases like lung cancer among other
types of cancers. The proposal stipulates a relatively long time of six months for the actual
project to be completed. This time is longer to allow testing to be conducted and some interview
to help get the views of the public if they think this fuel is good to be used. The actual project
will be conducted on a prototype to check if the seawater could actually be used in the real car to
help start the car. The prototype is believed to operate the same ways as the actual car hence if
the seawater works in the experimental prototype then it will definitely work in the actual car.

ENGINEERING RESEARCH METHODS 15
References
Blomen, L.J. and Mugerwa, M.N. eds., 2013. Fuel cell systems. Springer Science & Business
Media.
Dodds, P.E., Staffell, I., Hawkes, A.D., Li, F., Grünewald, P., McDowall, W. and Ekins, P.,
2015. Hydrogen and fuel cell technologies for heating: A review. International journal of
hydrogen energy, 40(5), pp.2065-2083.
Eberle, U., Müller, B. and Von Helmolt, R., 2012. Fuel cell electric vehicles and hydrogen
infrastructure: status 2012. Energy & Environmental Science, 5(10), pp.8780-8798.
Fukuzumi, S., Lee, Y.M. and Nam, W., 2017. Fuel production from seawater and fuel cells using
seawater. ChemSusChem, 10(22), pp.4264-4276.
Gao, W., Feng, X., Pei, A., Gu, Y., Li, J. and Wang, J., 2013. Seawater-driven magnesium based
Janus micromotors for environmental remediation. Nanoscale, 5(11), pp.4696-4700.
García, P., Torreglosa, J.P., Fernández, L.M. and Jurado, F., 2013. Control strategies for high-
power electric vehicles powered by hydrogen fuel cell, battery and supercapacitor. Expert
Systems with Applications, 40(12), pp.4791-4804.
Gewirth, A.A. and Thorum, M.S., 2010. Electroreduction of dioxygen for fuel-cell applications:
materials and challenges. Inorganic chemistry, 49(8), pp.3557-3566.
Hahn, R., Mainert, J., Glaw, F. and Lang, K.D., 2015. Sea water magnesium fuel cell power
supply. Journal of Power Sources, 288, pp.26-35.
References
Blomen, L.J. and Mugerwa, M.N. eds., 2013. Fuel cell systems. Springer Science & Business
Media.
Dodds, P.E., Staffell, I., Hawkes, A.D., Li, F., Grünewald, P., McDowall, W. and Ekins, P.,
2015. Hydrogen and fuel cell technologies for heating: A review. International journal of
hydrogen energy, 40(5), pp.2065-2083.
Eberle, U., Müller, B. and Von Helmolt, R., 2012. Fuel cell electric vehicles and hydrogen
infrastructure: status 2012. Energy & Environmental Science, 5(10), pp.8780-8798.
Fukuzumi, S., Lee, Y.M. and Nam, W., 2017. Fuel production from seawater and fuel cells using
seawater. ChemSusChem, 10(22), pp.4264-4276.
Gao, W., Feng, X., Pei, A., Gu, Y., Li, J. and Wang, J., 2013. Seawater-driven magnesium based
Janus micromotors for environmental remediation. Nanoscale, 5(11), pp.4696-4700.
García, P., Torreglosa, J.P., Fernández, L.M. and Jurado, F., 2013. Control strategies for high-
power electric vehicles powered by hydrogen fuel cell, battery and supercapacitor. Expert
Systems with Applications, 40(12), pp.4791-4804.
Gewirth, A.A. and Thorum, M.S., 2010. Electroreduction of dioxygen for fuel-cell applications:
materials and challenges. Inorganic chemistry, 49(8), pp.3557-3566.
Hahn, R., Mainert, J., Glaw, F. and Lang, K.D., 2015. Sea water magnesium fuel cell power
supply. Journal of Power Sources, 288, pp.26-35.

ENGINEERING RESEARCH METHODS 16
Huang, M., Ouyang, L., Ye, J., Liu, J., Yao, X., Wang, H., Shao, H. and Zhu, M., 2017.
Hydrogen generation via hydrolysis of magnesium with seawater using Mo, MoO 2, MoO 3 and
MoS 2 as catalysts. Journal of Materials Chemistry A, 5(18), pp.8566-8575.
Kumari, S., White, R.T., Kumar, B. and Spurgeon, J.M., 2016. Solar hydrogen production from
seawater vapor electrolysis. Energy & Environmental Science, 9(5), pp.1725-1733.
Offer, G.J., Howey, D., Contestabile, M., Clague, R. and Brandon, N.P., 2010. Comparative
analysis of battery electric, hydrogen fuel cell and hybrid vehicles in a future sustainable road
transport system. Energy policy, 38(1), pp.24-29.
O'hayre, R., Cha, S.W., Colella, W. and Prinz, F.B., 2016. Fuel cell fundamentals. John Wiley &
Sons.
Schlapbach, L. and Züttel, A., 2011. Hydrogen-storage materials for mobile applications.
In Materials for sustainable energy: a collection of peer-reviewed research and review articles
from nature publishing group (pp. 265-270).
Simamora, A.J., Chang, F.C., Wang, H.P., Yang, T.C., Wei, Y.L. and Lin, W.K., 2013. H2 fuels
from photocatalytic splitting of seawater affected by Nano-TiO2 promoted with CuO and
NiO. International Journal of Photoenergy, 2013.
Simamora, A.J., Hsiung, T.L., Chang, F.C., Yang, T.C., Liao, C.Y. and Wang, H.P., 2012.
Photocatalytic splitting of seawater and degradation of methylene blue on CuO/nano
TiO2. International Journal of Hydrogen Energy, 37(18), pp.13855-13858.
Huang, M., Ouyang, L., Ye, J., Liu, J., Yao, X., Wang, H., Shao, H. and Zhu, M., 2017.
Hydrogen generation via hydrolysis of magnesium with seawater using Mo, MoO 2, MoO 3 and
MoS 2 as catalysts. Journal of Materials Chemistry A, 5(18), pp.8566-8575.
Kumari, S., White, R.T., Kumar, B. and Spurgeon, J.M., 2016. Solar hydrogen production from
seawater vapor electrolysis. Energy & Environmental Science, 9(5), pp.1725-1733.
Offer, G.J., Howey, D., Contestabile, M., Clague, R. and Brandon, N.P., 2010. Comparative
analysis of battery electric, hydrogen fuel cell and hybrid vehicles in a future sustainable road
transport system. Energy policy, 38(1), pp.24-29.
O'hayre, R., Cha, S.W., Colella, W. and Prinz, F.B., 2016. Fuel cell fundamentals. John Wiley &
Sons.
Schlapbach, L. and Züttel, A., 2011. Hydrogen-storage materials for mobile applications.
In Materials for sustainable energy: a collection of peer-reviewed research and review articles
from nature publishing group (pp. 265-270).
Simamora, A.J., Chang, F.C., Wang, H.P., Yang, T.C., Wei, Y.L. and Lin, W.K., 2013. H2 fuels
from photocatalytic splitting of seawater affected by Nano-TiO2 promoted with CuO and
NiO. International Journal of Photoenergy, 2013.
Simamora, A.J., Hsiung, T.L., Chang, F.C., Yang, T.C., Liao, C.Y. and Wang, H.P., 2012.
Photocatalytic splitting of seawater and degradation of methylene blue on CuO/nano
TiO2. International Journal of Hydrogen Energy, 37(18), pp.13855-13858.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

ENGINEERING RESEARCH METHODS 17
Sobrino, F.H., Monroy, C.R. and Pérez, J.L.H., 2010. Critical analysis on hydrogen as an
alternative to fossil fuels and biofuels for vehicles in Europe. Renewable and Sustainable Energy
Reviews, 14(2), pp.772-780.
Tollefson, J., 2010. Hydrogen vehicles: fuel of the future?. Nature News, 464(7293), pp.1262-
1264.
Yamada, Y., Fukunishi, Y., Yamazaki, S.I. and Fukuzumi, S., 2010. Hydrogen peroxide as
sustainable fuel: electrocatalysts for production with a solar cell and decomposition with a fuel
cell. Chemical Communications, 46(39), pp.7334-7336.
Yoshida, T. and Kojima, K., 2015. Toyota MIRAI fuel cell vehicle and progress toward a future
hydrogen society. The Electrochemical Society Interface, 24(2), pp.45-49.
Zou, M.S., Yang, R.J., Guo, X.Y., Huang, H.T., He, J.Y. and Zhang, P., 2011. The preparation of
Mg-based hydro-reactive materials and their reactive properties in seawater. International
Journal of Hydrogen Energy, 36(11), pp.6478-6483.
Sobrino, F.H., Monroy, C.R. and Pérez, J.L.H., 2010. Critical analysis on hydrogen as an
alternative to fossil fuels and biofuels for vehicles in Europe. Renewable and Sustainable Energy
Reviews, 14(2), pp.772-780.
Tollefson, J., 2010. Hydrogen vehicles: fuel of the future?. Nature News, 464(7293), pp.1262-
1264.
Yamada, Y., Fukunishi, Y., Yamazaki, S.I. and Fukuzumi, S., 2010. Hydrogen peroxide as
sustainable fuel: electrocatalysts for production with a solar cell and decomposition with a fuel
cell. Chemical Communications, 46(39), pp.7334-7336.
Yoshida, T. and Kojima, K., 2015. Toyota MIRAI fuel cell vehicle and progress toward a future
hydrogen society. The Electrochemical Society Interface, 24(2), pp.45-49.
Zou, M.S., Yang, R.J., Guo, X.Y., Huang, H.T., He, J.Y. and Zhang, P., 2011. The preparation of
Mg-based hydro-reactive materials and their reactive properties in seawater. International
Journal of Hydrogen Energy, 36(11), pp.6478-6483.
1 out of 17
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.

